Abstract
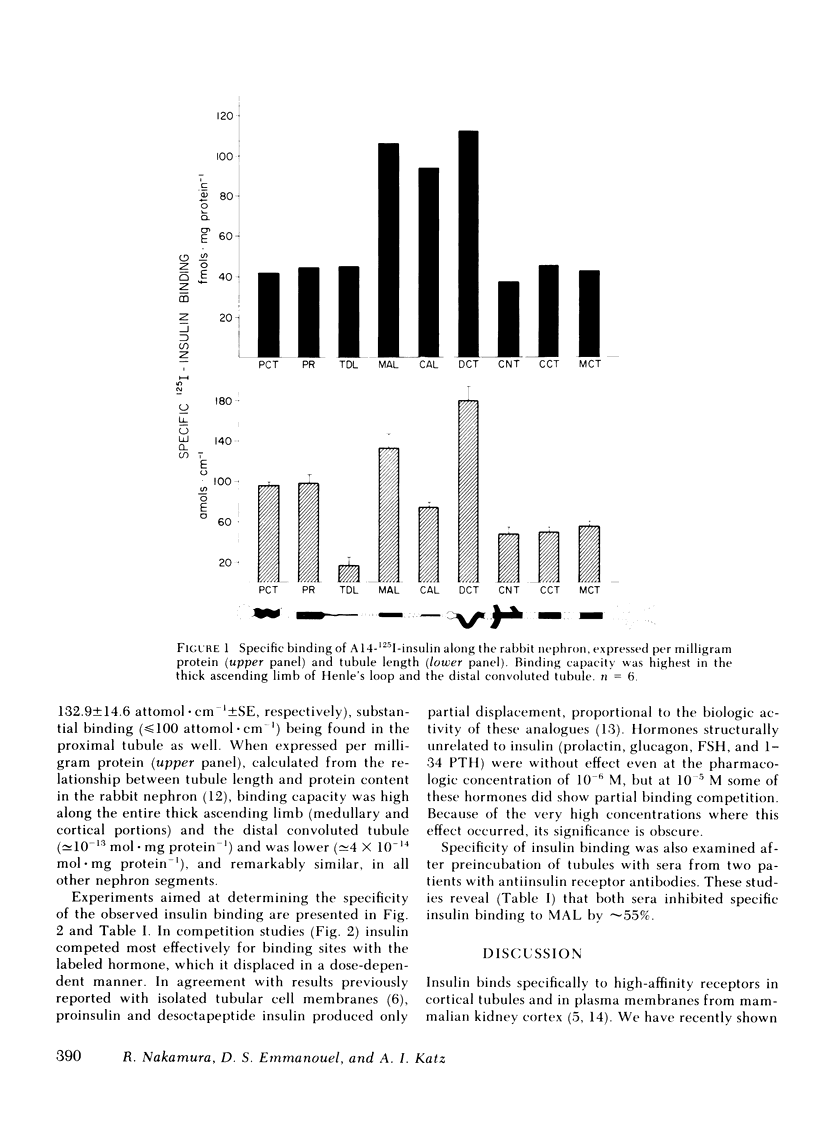
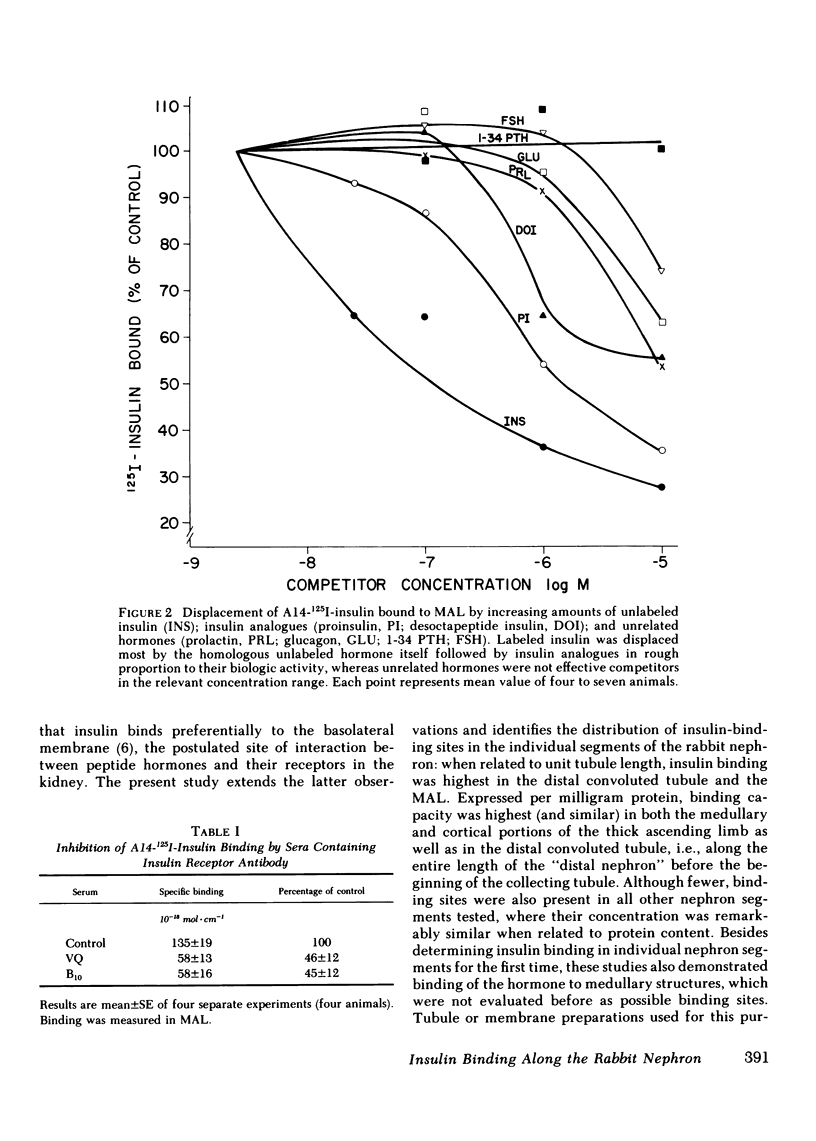
Insulin binds specifically to basolateral renal cortical membranes and modifies tubular electrolyte transport, but the target sites of this hormone in the nephron have not been identified. Using a microassay that permits measurement of hormone binding in discrete tubule segments we have determined the binding sites of 125I-insulin along the rabbit nephron. Assays were performed under conditions that minimize insulin degradation, and specific binding was measured as the difference between 125I-insulin bound in the presence or absence of excess (10(-5) M) unlabeled hormone. Insulin monoiodinated in position A14 was used in all assays. Specific insulin binding (attomol . cm-1 +/- SE) was highest in the distal convoluted tubule (180.5 +/- 15.0) and medullary thick ascending limb of Henle's loop (132.9 +/- 14.6), followed by the proximal convoluted and straight tubule. When expressed per milligram protein, insulin binding capacity was highest along the entire thick ascending limb (medullary and cortical portions) and the distal convoluted tubule, i.e., the "diluting segment" (congruent to 10(-13) mol . mg protein-1), and was lower (congruent to 4 X 10(-14) mol . mg protein-1), and remarkably similar, in all other nephron segments. Binding specificity was verified in competition studies with unlabeled insulin, insulin analogues (proinsulin and desoctapeptide insulin), and unrelated hormones (glucagon, 1-34 parathyroid hormone, prolactin, follicle-stimulating hormone). In addition, serum containing antiinsulin receptor antibody from two patients with type B insulin resistance syndrome markedly inhibited insulin binding to isolated tubules. Whether calculated per unit tubule length or protein content, insulin binding is highest in the thick ascending limb and the distal convoluted tubule, the same nephron sites where a regulatory role in sodium transport has been postulated for this hormone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D., Jr, Winston E., Hoshizaki R. J., Garland J. T., Baldwin D., Sr, Holcomb H. H., Flier J. S., Rubenstein A. Insulin-resistant diabetes with insulin receptor autoantibodies in a male patient without acanghosis nigricans. Diabetes Care. 1979 May-Jun;2(3):275–277. doi: 10.2337/diacare.2.3.275. [DOI] [PubMed] [Google Scholar]
- Blanchard R. F., Davis P. J., Blas S. D. Physical characteristics of insulin receptors on renal cell membranes. Diabetes. 1978 Feb;27(2):88–95. doi: 10.2337/diab.27.2.88. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981 Sep;21(3):165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol. 1981 Dec;241(6):F605–F611. doi: 10.1152/ajprenal.1981.241.6.F605. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I., Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Physiol. 1979 Aug;237(2):F105–F113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Lerner R. L. Binding and degradation of insulin by isolated renal cortical tubules. Endocrinology. 1980 Mar;106(3):655–662. doi: 10.1210/endo-106-3-655. [DOI] [PubMed] [Google Scholar]
- Linde S., Hansen B., Sonne O., Holst J. J., Gliemann J. Tyrosine A14[125I]monoiodoinsulin: Preparation, Biologic Properties, and long-term stability. Diabetes. 1981 Jan;30(1):1–8. doi: 10.2337/diab.30.1.1. [DOI] [PubMed] [Google Scholar]
- Mahler R. J., Szabo O. Metabolic effects of insulin on rat kidney after inhibiting degradation of the hormone. Endocrinology. 1968 Dec;83(6):1166–1172. doi: 10.1210/endo-83-6-1166. [DOI] [PubMed] [Google Scholar]
- Nizet A., Lefebvre P., Crabbé J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 1971;323(1):11–20. doi: 10.1007/BF00586561. [DOI] [PubMed] [Google Scholar]
- Talor Z., Emmanouel D. S., Katz A. I. Insulin binding and degradation by luminal and basolateral tubular membranes from rabbit kidney. J Clin Invest. 1982 May;69(5):1136–1146. doi: 10.1172/JCI110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Dons R. F., Hernandez E., Roth J., Gorden P. Insulin resistance associated with androgen excess in women with autoantibodies to the insulin receptor. Ann Intern Med. 1982 Dec;97(6):851–855. doi: 10.7326/0003-4819-97-6-851. [DOI] [PubMed] [Google Scholar]
- Vandewalle A., Wirthensohn G., Heidrich H. G., Guder W. G. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol. 1981 Jun;240(6):F492–F500. doi: 10.1152/ajprenal.1981.240.6.F492. [DOI] [PubMed] [Google Scholar]


