Abstract
STUDY QUESTION
Is increasing the intensity of high-intensity focused ultrasound (HIFU) by 30% in the treatment of rectal endometriosis a safe procedure?
SUMMARY ANSWER
This study demonstrates the safety of a 30% increase in the intensity of HIFU in the treatment of rectal endometriosis, with no Clavien–Dindo Grade III complications overall, and namely no rectovaginal fistulae.
WHAT IS KNOWN ALREADY
A feasibility study including 20 patients with rectal endometriosis demonstrated, with no severe complications, a significant improvement in digestive disorders, dysmenorrhoea, dyspareunia, and health status, although the volume of the endometriosis nodule did not appear to be reduced.
STUDY DESIGN, SIZE, DURATION
A prospective multicentre cohort study was conducted between 2020 and 2022 with 60 patients with symptomatic rectal endometriosis. Following the failure of medical treatment, HIFU treatment was offered as an alternative to surgery.
PARTICIPANTS/MATERIALS, SETTING, METHODS
As the main objective of this study was to examine safety, all adverse events observed during the 6 months of follow-up were analysed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) and Clavien–Dindo classifications. Secondary objectives included evaluating the evolution of symptoms using validated questionnaires: gynaecological and digestive pain symptoms with a visual analogue scale, health status with the Medical Outcomes Study 36-item Short Form (SF-36) questionnaire, average post-operative daily pain level, and analgesic medication required in the 10 days following treatment. MRI was also performed at Day 1 to detect early complications. Finally, we performed a blinded MRI review of the evolution of the nodule at 6 months post-treatment.
MAIN RESULTS AND THE ROLE OF CHANCE
The procedure was performed under spinal anaesthesia for 30% of the patients. The median duration of treatment was 32 min. Fifty-five patients left the hospital on Day 1. MRI scans performed on Day 1 did not highlight any early-onset post-operative complication. Using the Clavien–Dindo classification, we listed 56.7% Grade I events, 3.4% Grade II events, and no events Grade III or higher. At 1, 3, and 6 months, all gynaecologic, digestive and general symptoms, as well as health status, had significantly improved. The evolution of the nodule was also significant (P < 0.001) with a 28% decrease in volume.
LIMITATIONS, REASONS FOR CAUTION
The main objective was safety and not effectiveness. The study was not randomized and there was no control group.
WIDER IMPLICATIONS OF THE FINDINGS
HIFU treatment for rectal endometriosis results in an improvement of symptoms with low morbidity; as such, for selected patients, it could be a valuable alternative to surgical approaches following the failure of medical treatment.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by the company EDAP TMS. Professors Dubernard and Rousset are consultants for EDAP TMS. Dubernard received travel support from EDAP-TMS. Dr F. Chavrier received industrial grants from EDAP-TMS. He has developed a device for generating focused ultrasonic waves with reduced treatment time. This device has been patented by EDAP-TMS. Dr Lafon received industrial grants from EDAP-TMS; he declares that EDAP-TMS provided funding directly to INSERM to support a young researcher chair in therapeutic ultrasound, which is unrelated to the current study.
TRIAL REGISTRATION NUMBER
ClinicalTrials.gov identifier NCT04494568.
Keywords: rectal endometriosis, deep infiltrating endometriosis, high-intensity focused ultrasound, safety, gynaecological symptoms, gastrointestinal symptoms, health status, Medical Outcomes Study 36-item Short Form
Introduction
Deep infiltrating rectal endometriosis (rectal DIE) affects 8–12% of women with intestinal endometriosis (Seracchioli et al., 2007; Wills et al., 2008). Over 80% of these digestive cases have a rectal or lower sigmoid involvement (Chapron et al., 2006). Rectal endometriosis lesions often extend from the torus uterinum and/or utero-sacral ligaments (USL) and may also spread to the posterior vaginal cuff (Abrão et al., 2015). Together, this results in a large fibrotic endometriosis lesion between the rectal and uterine area, causing debilitating painful symptoms (Chapron et al., 2004). Patients with rectal DIE experience more symptoms than other forms of endometriosis. They report dysmenorrhoea, deep dyspareunia, and gastrointestinal disorders including bloating, alternating diarrhoea and constipation, rectal cramping, and pain on defecation. Medical treatment can be used, but it is contraceptive and often only effective in the short term, with poor tolerability. Consequently, surgical treatment is often preferred (Nezhat et al., 2018) and usually involves the resection of all deep infiltrating lesions, rather than being limited to the digestive locations, as in most cases all lesions merge into one (Chapron et al., 2004). While surgical solutions improve pain symptoms (Byrne et al., 2018; Vercellini et al., 2021), subsequent morbidity is significant, with acute adverse events in 10% of cases and abnormal bladder voiding in up to 29% of patients (Minelli et al., 2009; Ballester et al., 2011). Accordingly, the surgery tends to be performed in specialized reference centres (Roman et al., 2013; Dunselman et al., 2014). Given the morbidity and the functional consequences of the disease, experts are striving to offer less radical surgical solutions, such as shaving or discoid resection, to avoid segmental resection where possible (Darwish and Roman, 2016).
High-intensity focused ultrasound (HIFU) is a widely used gynaecological treatment for uterine fibroids (Tsai et al., 2021). HIFU is used to target the lesion with extreme precision, leaving nearby healthy tissue largely untouched. We demonstrated the feasibility of HIFU treatment for rectal endometriosis in a sample of 20 patients (Philip et al., 2020b). In that study, we observed a significant improvement in painful symptoms, as well as the patients’ health status, while morbidity remained low. The volume of the endometriosis nodule, however, did not appear to be reduced. Therefore, and to attempt to demonstrate the physical effect of the HIFU on the endometriotic nodule, we decided to increase the intensity of the treatment by 30%. The main objective of this prospective multicentre study was to verify the safety of a 30% increase in treatment intensity and the secondary objectives were the efficacy on gynaecologic and digestive symptoms and on the patients’ health status.
Materials and methods
This prospective multicentre safety study, ENDO-HIFU-R1 (ClinicalTrials.gov identifier NCT04494568) was conducted between September 2020 and August 2022 across four endometriosis referral centres in France: Croix-Rousse University Hospital (Lyon), Angers University Hospital (Angers), Kremlin-Bicêtre University Hospital (Paris), and a private hospital, the Tivoli-Ducos clinic (Bordeaux). Participating centres were selected based on their recognized expertise in the diagnosis and management of endometriosis, as well as their involvement in a programme dedicated to developing HIFU treatment in France for benign gynaecological pathologies (myomas and adenomyosis). The geographic spread of the centres also allowed recruitment of patients more widely.
Ethics approval
The study was approved by the ethics committee (CPP SUD-EST I, 2020-61) and the French National Medical Safety Agency (ANSM; IDRCB 2020-A00467-32). All the participants involved in the study gave their written informed consent.
Study population and participants
In each centre, consecutive patients matching the profile described below were recruited if they met the inclusion criteria. The study included patients aged ≥25 years with gastrointestinal endometriosis with rectal involvement and experiencing symptoms; treatment was offered as an alternative to surgical management after the failure or the poor tolerance of medical treatment in patients with no history of rectal surgery. Patients were required to make no changes to their medical and hormonal treatment throughout the follow-up (6 months) so that the HIFU treatment would remain the only factor influencing symptoms. Patients with no desire for a pregnancy within 6 months of treatment were selected, so that MRI could be performed at the end of the study to assess the evolution of the rectal lesion. As Focal One® (FO) treatment is ultrasound-guided (Philip et al., 2020a), the rectal lesion needed to be visible by ultrasound at the diagnosis. Final confirmation of the patients’ inclusion was based on MRI criteria according to the ENZIAN classification (Burla et al., 2019). A centralized reading of the MRI scans by an expert radiologist (E.M.) following a specific protocol was used to verify the inclusion criteria were met and thus validate each patient’s recruitment to the study. A dedicated MRI protocol was performed following the sequences recommended by the European Society of Urogenital Radiology guidelines (Bazot et al., 2017). Additional T2-weighted MRI sequences with thin axial and perpendicular slices to the rectal nodule were performed with the use of semi-automatic software (Olea®; Olea Medical, La Ciotat, France) to assess their volume. Moreover, sagittal diffusion, sagittal dynamic contrast-enhanced fat-saturated T1-weighted, and axial oblique contrast-enhanced T1 Water Fat Shift focused on the rectal nodule were performed to assess post-HIFU complications. The same process was followed for each of the three MRI scans planned in the study protocol (pre-operatively, on Day 1, and 6-months post-treatment). A definitive diagnosis of intestinal endometriosis was confirmed by a thickening of the bowel wall (mainly >3 mm) on T2-weighted studies, by an iso- or hypointense (relative to the myometrium) nodular, mass or plaque-like bowel wall thickening, and by an associated obliteration of the normal hypointense signal of the wall interrupting the normal aspect (Bazot et al., 2004). The distance between the superior extremity of the lesion and the anal margin (AM) was also measured by MRI to determine the location of the lesions according to the following scale: high rectum (within 10–15 cm of the AM), middle rectum (5–10 cm of the AM), and low rectum (0–5 cm of the AM) (Lee et al., 2019). Moreover, due to the volume of the rectal probe, the percentage of stenosis of the digestive lumen needed to be <50%. With the MRI, we were also able to identify other areas of deep endometriosis involvement, whether posterior (uterosacral ligaments, torus uterinum, vagina, parametrium) or surrounding the bladder, using MRI ENZIAN (Maciel et al., 2023).
Treatments and assessments
The FO device (EDAP TMS, Vaulx-en-Velin, France) is a real-time ultrasound-guided robotic device approved for transrectal HIFU treatment of prostate cancer (Crouzet et al., 2010; Rischmann et al., 2015; Bakavicius et al., 2022). The treatment is performed through the rectal wall. FO treatment was performed in an operating room, under either spinal or general anaesthesia. We first localized and assessed the volume of the endometriosis lesion, both the rectal nodule and other locations of DIE (USL, torus uterinum, posterior vaginal cuff) when they were visible with the imaging transducer. The lesion then was divided in a series of transverse slices at 1.7-mm intervals by the therapeutic transducer. For each slice, the operator manually defined the contour of the area to be treated, which matched the limits of the endometriosis lesion. Then the FO software automatically dispensed the burst of the HIFU beam to cover the target volume entirely (Fig. 1). To prevent the risk of rectovaginal fistulae (RVF), a safety margin of 3 mm from the rectal mucosae was automatically applied. When the volume treated was below 90% of the pretreatment scanning volume, it was considered a partial treatment. The HIFU procedures were performed by all the surgeons involved in the study. The teams in Paris, Angers, and Bordeaux were trained by the Lyon surgeons (G.D., S.W. and C.-A.P.) and supervised by them when administering the treatments.
Figure 1.
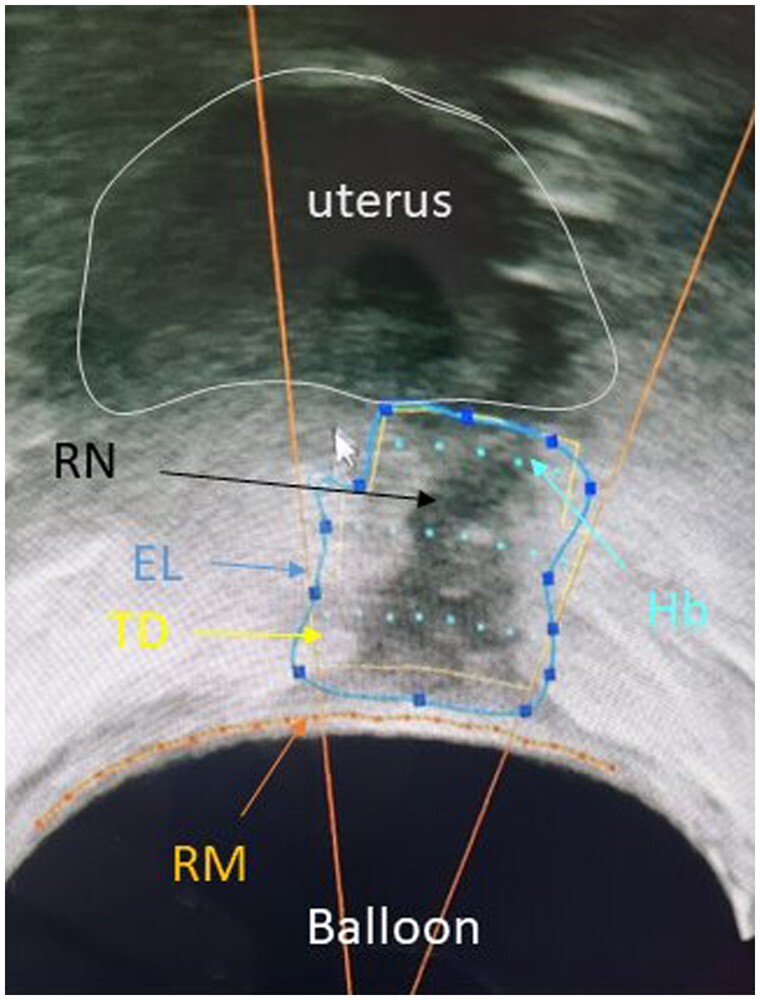
High-intensity focused ultrasound energy plan on a transversal slice; image obtained during treatment for rectal endometriosis. Balloon around the probe. RM, rectal mucosae; RN, rectal nodule developed from the right uterosacral ligament; EL, limits of endometrial nodule, defined manually by the clinician. The number of HIFU beams required to treat the area is based directly on this contouring. TD, limits of the thermal diffusion depending on number of HIFU beams (yellow line); Hb, HIFU beams; HIFU, high-intensity focused ultrasound.
Compared with the feasibility study, HIFU intensity was increased by 30%. As the main objective was safety, all adverse events reported during the 6-month follow-up period were analysed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v5.0), as well as post-operative complications according to the Clavien–Dindo classification (Clavien et al., 2009). We also planned an MRI on Day 1 to identify the treatment area and look for potential early complications. The secondary objective was the evolution of symptoms, assessed by comparing the answers to the validated questionnaires before treatment and 1-, 3-, and 6-months post-treatment in association with consultations with the doctor who performed the treatment in each centre. We used a validated visual analogue scale to assess gynaecological and gastrointestinal symptoms (Dubernard et al., 2006; Daraï et al., 2010; Ballester et al., 2011). Health status was assessed using the Medical Outcomes Study 36-item Short Form (MOS-SF-36) questionnaire (Dubernard et al., 2008a; Bourdel et al., 2019), while average daily post-operative pain levels and analgesic medication requirements were assessed during the first 10 days post-treatment by analysing the data collected in patient diaries. Specific symptoms were evaluated at each follow-up time point: anal continence with the Wexner questionnaire, constipation with the Knowles–Eccersley–Scott Symptom (KESS) questionnaire, urinary function and continence with the Urinary Symptom Profile (USP) questionnaire, and sexual function with the Female Sexual Function Index (FSFI) questionnaire.
An assessment of the evolution of the nodule was also performed. Pretreatment and 6-month post-treatment MRI scans were centralized for a blinded review (in terms of patient identification and MRI timing). An independent radiologist (P.R.) specialized in endometriosis, who was not involved in the selection of the patients, measured the volume of the nodules. Surveys were sent electronically to the patients using Castor ePRO (Castor, Amsterdam).
Statistical analysis
A statistical analysis was conducted using IBM SPSS Statistics, Release 28 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp). Binary and categorical data were presented with counts and percentages, continuous data with summary statistics (N, mean, median, SD, min, max, and 95% CI for specific outcomes). Normal distribution was assessed using the Kolmogorov–Smirnov test. When appropriate, pre- and post-paired comparisons were assessed with either a paired sample Student’s t-test or a Wilcoxon test. A Type I error rate (α level) of 5% (P < 0.05) was used for statistical significance. Safety analyses were presented as the number of adverse events reported, with the corresponding number of patients experiencing events. Serious adverse events were detailed individually.
Results
Sixty-five patients were initially included in the study. We reported 5 (8%) cases of inability to proceed owing to severe uterosacral ligaments retraction and/or a stenosis of the rectal lumen by the rectal nodule, which limited the progression of the probe. The study therefore included 60 treated patients. The clinical history of the patients and the characterization of the endometriotic lesions are reported in Table 1. The mean age of the patients was 35.7 years. Overall, 41 patients (68.3%) were using contraception at baseline. Severe posterior deep infiltrating lesions were included in the study with multiple locations. The rectal nodules (based on the midpoint) were located in the upper rectum for 38 patients (63.3%) and in the mid-rectum for the remaining patients. Other DIE localizations most commonly observed on the MRI were the USL (90.0%), vagina (73.3%), and torus uterinum (70.0%). The description of endometriotic lesions according to the ENZIAN classification is reported in Supplementary Table S1.
Table 1.
Baseline patient and endometriosis characteristics.
| Patient characteristics (N = 60) | Mean ± SD | (Median) | [Range] |
|---|---|---|---|
| First symptoms | |||
| Age at onset (years) | 22.9 ± 9.6 | (21.2) | [11; 50] |
| Time from onset (years) | 12.4 ± 8.2 | (11.4) | [0.8; 32] |
| Diagnosis | |||
| Age at diagnosis (years) | 31.0 ± 6.1 | (30.5) | [19; 51] |
| Time to diagnosis (years) | 4.9 ± 4.9 | (3.5) | [0.1; 23] |
| Before intervention | |||
| Age at intervention (years) | 35.9 ± 6.2 | (35.4) | [26; 53] |
| BMI | 24.2 ± 4.1 | (23.8) | [17; 36] |
| Gravidity | 0.95 ± 1.16 | (1.0) | [0; 4] |
| Parity | 0.73 ± 0.90 | (0.0) | [0; 3] |
| Pregnancy plan | 12 | (20.0) | |
| Infertility | 15 | (25.0) | |
| Hormonal treatment | |||
| No treatment | 17 | (28.4) | |
| Non-hormonal treatment | 5 | (8.3) | |
| Hormonal treatment | 38 | (63.3) | |
| Other endometriosis locations | |||
| Vagina | 44 | (73.3) | |
| Torus uterinum | 42 | (70.0) | |
| Uterosacral ligaments (L or R) | 54 | (90.0) | |
| Endometrioma (L or R) | 15 | (25.0) | |
| Bladder | 1 | (1.7) | |
| Other | 13 | (21.7) | |
| External adenomyosis | 11 | (18.6) | |
| Prior endometriosis surgery | 15 | (25.0) | |
| Prior abdominal surgery | 25 | (41.7) | |
| Nodule characteristics | |||
| Height (mm) | 2.6 ± 0.9 | (2.4) | [1; 4.8] |
| Width (mm) | 2.0 ± 0.6 | (1.9) | [1.1; 3.6] |
| Thickness (mm) | 1.0 ± 0.5 | (0.9) | [0.5; 3.4] |
| Volume (mm3) | 2.7 ± 2.4 | (2.2) | [0.3; 14.0] |
| Nodule position from rectum (based pre-therapeutic MRI) | |||
| Lower limit (mm) | 9.4 ± 1.6 | (9.0) | [5.0; 13] |
| Mid-point (mm) | 10.7 ± 1.5 | (10.9) | [6.9; 14] |
| Upper limit (mm) | 12.1 ± 1.6 | (12.0) | [8.7; 15] |
| Nodule in mid rectum | 22 | (36.7) | |
| Nodule in upper rectum | 38 | (63.3) |
Treatment procedures are reported in Table 2. Median procedure duration, from probe insertion to probe removal, including planification and treatment, was 32 min (range, 17–89). In addition to the rectal lesion, HIFU targeted the torus uterinum in 70.0% of cases, USL in 41.7%, and the vagina in 11.7%. Fifty-five (91.7%) patients left the hospital on Day 1 after the MRI had been performed.
Table 2.
Treatment procedures for the patients with rectal endometriosis.
| Treatment procedure (N = 60) | Mean ± SD | (Median) | [Range] |
|---|---|---|---|
| Anaesthesia | |||
| General | 42 | (70.0) | |
| Spinal | 18 | (30.0) | |
| HIFU session | |||
| Treatment completion | |||
| Complete | 51 | (85.0) | |
| Partial | 9 | (15.0) | |
| Treatment duration (hh:mm) | 0:35 ± 0:13 | (0:32) | [17; 89] |
| Operating time (hh:mm) | 1:26 ± 0:21 | (1:24) | [40; 168] |
| Nights in hospital | 0.95 ± 0.29 | (1) | [0; 2] |
| Ambulatory | 4 | (6.7) | |
| One night | 55 | (91.7) | |
| Two nights | 1 | (1.7) | |
| Sick leave duration (days), N = 43 | 9.6 ± 5.1 | (11) | [2; 21] |
| Treated lesions | |||
| Firing duration (mm:ss) | 2:30 ± 1:37 | (0:35; 9:34) | [0:17; 1:29] |
| Number of elementary lesions | 150 ± 97 | (131) | [35; 584] |
| Location | |||
| Rectum | 60 | (100.0) | |
| Torus uterinum | 42 | (70.0) | |
| Vagina | 7 | (11.7) | |
| Uterosacral ligaments | 25 | (41.7) |
HIFU, high-intensity focused ultrasound.
Adverse events according to the safety evaluation are reported in Table 3. Of the 36 (60.0%) patients with related adverse events, 34 (56.7%) were Grade I events and 2 (3.3%) were Grade II. No event Grade III or higher was observed. The most commonly reported gastrointestinal disorders (43.3% of patients) included rectal pain (15.0%), constipation (13.3%), diarrhoea (10.0%), and rectal haemorrhage (8.3%). Among the reproductive system disorders, vaginal haemorrhage was reported in 11.7% of patients, and pelvic pain and vaginal discharge in 6.7% each.
Table 3.
Related adverse events according to the System Organ Class and Clavien–Dindo classifications.
| SOC and Clavien–Dindo (N = 60) | Clavien–Dindo Grade I |
Clavien–Dindo Grade II |
Clavien–Dindo Grade III (%) |
|||
|---|---|---|---|---|---|---|
| Event (%) | Subject (%) | Event (%) | Subject (%) | Event (%) | Subject (%) | |
| Any | 76 (92.7) | 34 (56.7) | 4 (4.9) | 2 (3.3) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 43 (52.4) | 26 (43.3) | ||||
| Reproductive system and breast disorders | 15 (18.3) | 11(18.3) | ||||
| General disorders and admin, site conditions | 11 (13.4) | 9 (15.0) | ||||
| Renal and urinary disorders | 3 (3.7) | 3 (5.0) | 2 (2.4) | 1 (1.7) | ||
| Infections and infestations | 2 (2.4) | 1 (1.7) | ||||
| Skin and subcutaneous disorders | 1 (1.2) | 1 (1.7) | ||||
| Musculoskeletal and connective tissue disorders | 1 (1.2) | 1 (1.7) | ||||
| Nervous system disorders | 1 (1.2) | 1 (1.7) | ||||
| Psychiatric disorders | 1 (1.2) | 1 (1.7) | ||||
Clavien–Dindo was not applicable for two complications: one case of diarrhoea the day before treatment (probably due to the implementation of the study procedures) and one case of cervical stiches (due to a Museux clamp required during the HIFU procedure).
HIFU, high-intensity focused ultrasound.
Of the two patients with Clavien–Dindo Grade II complications, one was a combined vaginal and bladder infection and one was a bladder atony with severe voiding dysfunction; for the latter, the procedure was performed under spinal anaesthesia and the patient presented a bladder globe of 700 cc after removal of the bladder catheter, worsening a pre-operative urinary voiding dysfunction.
According to patient diaries, a moderate level of post-operative pain was reported, with a median level of 3 on Day 1 (Supplementary Fig. S1). A progressive reduction of post-operative pain within the first 10 days after treatment reached a median of 0 from Day 8. Type ≥2 analgesics were used by 25% of patients immediately after the procedure, and by fewer than 20% after Day 5. For the 48 patients in employment, the mean sick leave duration was 9.6 days (range, 2–21).
No early complication was reported on the MRI performed on Day 1. Immediate efficacy was not available for one patient because of artefacts. For the others, the devascularization area and modification of the nodule were observed in 51 (86.4%) and 49 (83.0%) patients, respectively. As expected, most of the MRI modifications involved the pelvic fat (63.3%), rectal wall (65%), and vagina (13.3%).
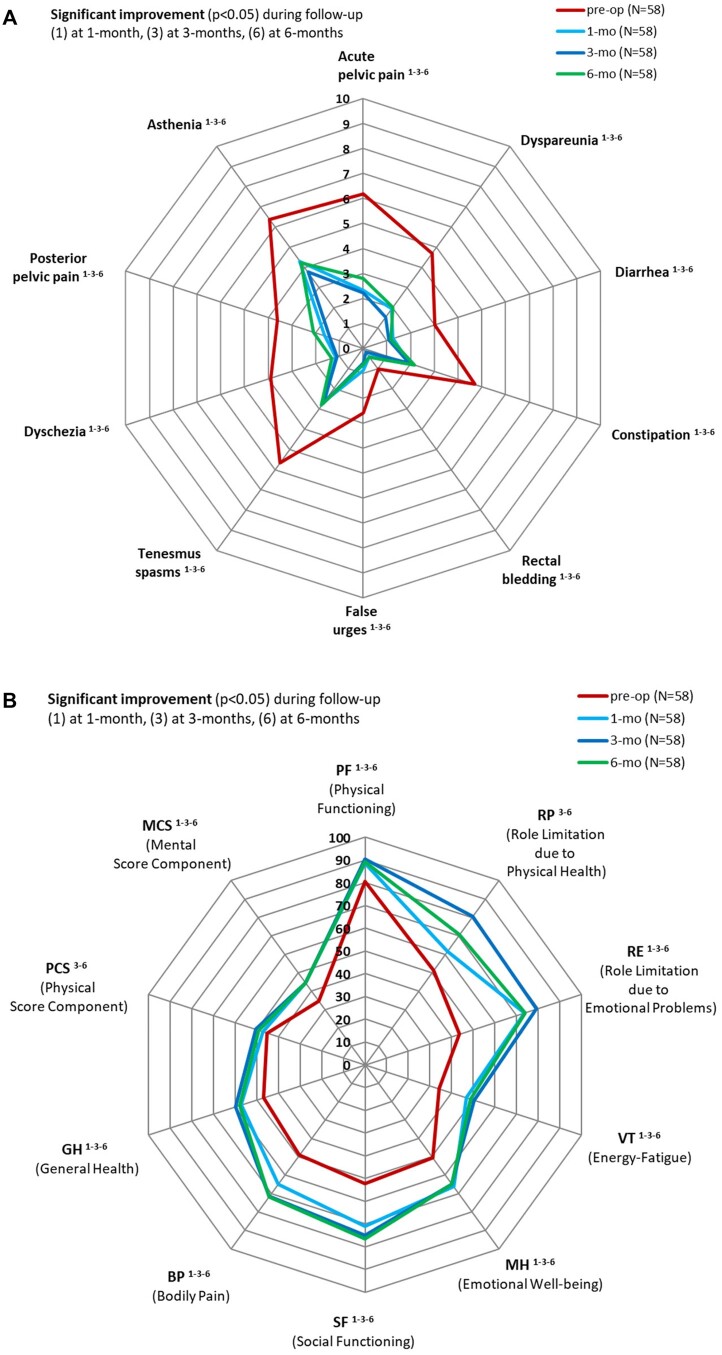
Symptom evolution is reported in Table 4 and Fig. 2A. Among the 60 patients treated, two had an unplanned pregnancy during the follow-up period; both were removed from the paired evaluations. All the gynaecologic and gastrointestinal symptoms showed significant improvement at all follow-up evaluation points. This improvement was maintained for all symptoms, with no significant variation between 1 and 3 months, 1 and 6 months, or 3 and 6 months (except for dyspareunia, P = 0.035). More specifically, for the gynaecologic symptoms, the proportion of patients having reported a symptom diminution during follow-up was 91.8%, 89.8%, and 81.6% for acute pelvic pain and 88.4%, 90.7%, and 83.7% for dyspareunia at 1, 3, and 6 months, respectively. In the same way, for digestive symptoms, a high proportion of patients (from 85.7% to 97.1%) reported a symptom diminution for any of the six digestive symptoms components at all follow-up evaluations.
Table 4.
Evolution of symptoms using a visual analogue scale—paired results.
| HIFU (N = 58) |
|||
|---|---|---|---|
| Mean ± SD (median) | [IQR] | (95% CI) | |
| Gynaecological symptoms | |||
| Acute pelvic pain/dysmenorrhoea | |||
| Pre-op | 6.2 ± 3.1 (7) | [5; 8.3] | (5.4; 7.0) |
| Six months | 2.8 ± 3.0 (2) | [0; 5] | (2.0; 3.6) |
| Difference | −3.4 ± 3.8 (−3) | [−7; 0] | (−4.4; −2.4) |
| P-value | <0.001 | ||
| Dyspareunia | |||
| Pre-op | 4.7 ± 3.3 (6) | [0; 7.3] | (3.8; 5.6) |
| Six months | 2.0 ± 2.9 (0) | [0; 3.3] | (1.3; 2.8) |
| Difference | −2.7 ± 3.3 (−3) | [−5.3; 0] | (−3.5; −1.8) |
| P-value | <0.001 | ||
| Gastrointestinal symptoms | |||
| Diarrhoea | |||
| Pre-op | 3.0 ± 3.3 (2.5) | [0; 6] | (2.2; 3.9) |
| Six months | 1.2 ± 2.3 (0) | [0; 1.3] | (0.6; 1.8) |
| Difference | −1.9 ± 3.1 (0) | [−4; 0] | (−2.7; −1.1) |
| P-value | <0.001 | ||
| Constipation | |||
| Pre-op | 4.7 ± 3.7 (5.5) | [0; 8] | (3.7; 5.7) |
| Six months | 2.2 ± 2.7 (0) | [0; 4] | (1.5; 2.9) |
| Difference | −2.5 ± 3.3 (−2) | [−4; 0] | (−3.4; −1.7) |
| P-value | <0.001 | ||
| Rectal bleeding | |||
| Pre-op | 1.1 ± 2.5 (0) | [0; 0] | (0.4; 1.7) |
| Six months | 0.4 ± 1.6 (0) | [0; 0] | (0.0; 0.9) |
| Difference | −0.6 ± 2.6 (0) | [0; 0] | (−1.3; 0.0) |
| P-value | 0.035 | ||
| False urges | |||
| Pre-op | 2.6 ± 3.0 (0) | [0; 5] | (1.8; 3.4) |
| Six months | 0.6 ± 1.9 (0) | [0; 0] | (0.2; 1.1) |
| Difference | −1.9 ± 2.8 (0) | [−4; 0] | (−2.7; −1.2) |
| P-value | <0.001 | ||
| Tenesmus/spasms | |||
| Pre-op | 5.7 ± 3.0 (6) | [4.8; 8] | (4.9; 6.5) |
| Six months | 2.8 ± 2.9 (3) | [0; 5] | (2.1; 3.6) |
| Difference | −2.8 ± 3.3 (−2.5) | [−6; 0] | (−3.7; −2.0) |
| P-value | <0.001 | ||
| Pain on defecation | |||
| Pre-op | 3.9 ± 3.7 (4) | [0; 7] | (2.9; 4.9) |
| Six months | 1.3 ± 2.1 (0) | [0; 2.3] | (0.8; 1.9) |
| Difference | −2.6 ± 3.1 (−2) | [−5; 0] | (−3.4; −1.7) |
| P-value | <0.001 | ||
| General symptoms | |||
| Post-pelvic pain | |||
| Pre-op | 3.6 ± 3.8 (3) | [0; 7] | (2.6; 4.6) |
| Six months | 2.1 ± 2.8 (0) | [0; 3.3] | (1.4; 2.8) |
| Difference | −1.5 ± 3.7 (0) | [−5; 0] | (−2.5; −0.5) |
| P-value | 0.004 | ||
| Asthenia | |||
| Pre-op | 6.4 ± 2.6 (7) | [5; 8] | (5.7; 7.1) |
| Six months | 4.2 ± 3.0 (4) | [1.8; 7] | (3.5; 5.0) |
| Difference | −2.1 ± 3.1 (−2) | [−4.3; 0] | (−2.9; −1.3) |
| P-value | <0.001 | ||
HIFU, high-intensity focused ultrasound; IQR, interquartile range.
Figure 2.
Evolution of symptoms and health status at 1, 3, and 6 months after treatment by high-intensity focused ultrasound for rectal endometriosis. (A) Symptoms, measured by visual analogue scale (1–10); (B) health status, measured by Medical Outcomes Study 36-item Short Form questionnaire. Pre-op, pre-operative; 1-mo, 1 month; 3-mo, 3 months; 6-mo, 6 months.
According to the health status analysis based on the SF-36 questionnaire (Table 5 and Fig. 2B), the proportion of patients having reported an improvement at 6 months was 65.5% and 84.5% for Physical score and Mental score components, respectively. A significant improvement was observed since the 1-month visit, except for Role limitation due to Physical Health (RP) and Physical Component Scale, which were significantly improved from the 3-months evaluation. This quality-of-life improvement was maintained for all the items during follow-up with no significant variation between 1 and 3 months, 1 and 6 months, and 3 and 6 months, except for RP, Social Functioning, Bodily Pain and Physical Component Scale, where the improvement still increased after the 1-month visit. Urinary symptoms (USP questionnaire) were also improved for urinary urgency at 6 months and incomplete bladder voiding at 1 and 6 months (Supplementary Table S2).
Table 5.
Evolution of health status according to the MOS-SF-36 questionnaire—paired results.
| MOS-SF-36 health status | HIFU (N = 58) |
||
|---|---|---|---|
| Mean ± SD (median) | [IQR] | (95% CI) | |
| PF: physical functioning | |||
| Pre-op | 80.6 ± 21.4 (90) | [70; 95] | (75; 86) |
| Six months | 89.1 ± 14.1 (93) | [85; 100] | (85; 93) |
| Difference | 8.5 ± 16.2 (5) | [−5; 15] | (4.3; 12.8) |
| P-value | <0.001 | ||
| RP: role limitation due to physical health | |||
| Pre-op | 51.3 ± 40.9 (50) | [0; 100] | (41; 62) |
| Six months | 70.7 ± 40.3 (100) | [25; 100] | (60; 81) |
| Difference | 19.4 ± 48.0 (0) | [0; 50] | (6.8; 32) |
| P-value | <0.001 | ||
| RE: role limitation due to emotional problems | |||
| Pre-op | 43.7 ± 37.0 (33) | [0; 67] | (34; 53) |
| Six months | 74.1 ± 38.5 (100) | [33; 00] | (64; 84) |
| Difference | 30.5 ± 44.3 (33) | [0; 67] | (18.8; 42.1) |
| P-value | <0.001 | ||
| VT: energy/fatigue | |||
| Pre-op | 34.1 ± 17.8 (30) | [20; 45] | (29; 38) |
| Six months | 48.8 ± 19.7 (50) | [50; 65] | (44; 54) |
| Difference | 14.7 ± 16.8 (15) | [0; 25] | (10.3; 19.2) |
| P-value | <0.001 | ||
| MH: emotional well-being | |||
| Pre-op | 50.6 ± 19.5 (52) | [36; 64] | (46; 56) |
| Six months | 65.0 ± 18.2 (68) | [52; 80] | (60; 70) |
| Difference | 14.3 ± 18.7 (10) | [0; 25] | (9.4; 19.3) |
| P-value | <0.001 | ||
| SF: social functioning | |||
| Pre-op | 52.4 ± 24.2 (50) | [38; 63] | (46; 59) |
| Six months | 76.5 ± 21.3 (75) | [63; 100] | (71; 82) |
| Difference | 24.1 ± 21.3 (25) | [13; 38] | (18.5; 29.7) |
| P-value | <0.001 | ||
| BP: bodily pain | |||
| Pre-op | 49.2 ± 23.7 (45) | [34; 68] | (43; 55) |
| Six months | 71.9 ± 20.0 (74) | [58; 90] | (67; 77) |
| Difference | 22.7 ± 25.2 (20) | [7; 43] | (16.1; 29.3) |
| P-value | <0.001 | ||
| GH: general health | |||
| Pre-op | 46.8 ± 19.4 (45) | [35; 65] | (42; 52) |
| Six months | 57.8 ± 18.4 (60) | [44; 70] | (53; 63) |
| Difference | 10.9 ± 14.3 (10) | [0; 25] | (7.2; 14.7) |
| P-value | <0.001 | ||
| PCS: physical component summary | |||
| Pre-op | 45.3 ± 8.9 (47) | [24; 61] | (43; 48) |
| Six months | 49.2 ± 7.0 (51) | [33; 63] | (47; 51) |
| Difference | 3.9 ± 7.5 (4) | [−9; +21] | (2; 6) |
| P-value | <0.001 | ||
| MCS: mental component summary | |||
| Pre-op | 34.5 ± 10.4 (35) | [27; 40] | (31; 37) |
| Six months | 44.3 ± 11.2 (48) | [38; 52] | (41; 47) |
| Difference | 9.8 ± 10.2 (10) | [2; 16] | (1.4; 6.6) |
| P-value | <0.001 | ||
MOS-SF-36, Medical Outcomes Study 36-item Short Form.
The FSFI, Wexner, and KESS questionnaire results are provided in Supplementary Tables S3, S4, and S5, respectively. Regarding continence (Wexner score), an improvement was observed but it was non-significant. Conversely, we observed a significant improvement in constipation at 6 months (KESS score). The FSFI score, which was below 26 in half of our patients, was shown to have significantly improved by 2.2 points (P = 0.012).
Patient perceptions were positive, with 81.0%, 89.7%, and 84.5% of patients feeling that their condition had improved at 1, 3, and 6 months, respectively (Supplementary Table S6). This satisfaction was also reflected, at 6 months, in patient acceptance of retreatment if necessary (94.8%) and in their recommendation of treatment to a friend (96.6%).
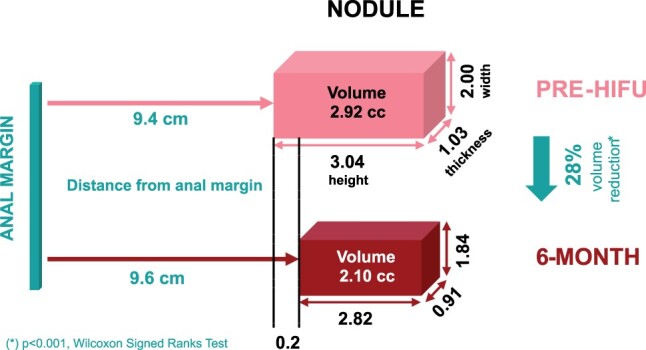
Data on assessment of the nodule at 6 months are reported in Figs 3 and 4. Among the MRI scans performed for our 60 patients, three were censored from nodule evolution analysis: two because of pregnancy and one because of the suspicion of a multiple nodule that would have skewed the analysis. Volume evolution, which was the main criteria for this blinded review, significantly decreased from 2.92 to 2.10 cc (P < 0.001).
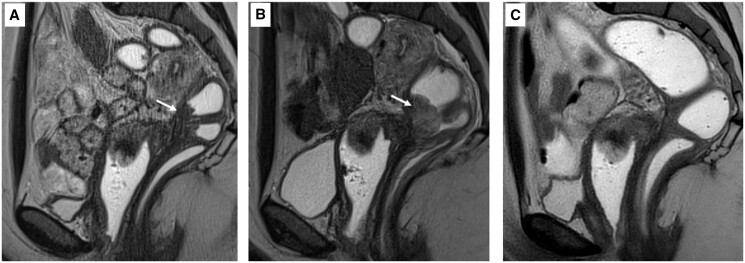
Figure 3.
Blinded revue of the nodule evolution at 6 months post-treatment (N = 57). *P<0.05. HIFU: high-intensity focused ultrasound.
Figure 4.
MRI T2 sagittal sequences performed on the same level. (A) Before treatment, (B) Day 1 after treatment, and (C) 6 months post-treatment. The rectal endometriosis nodule is indicated by the white arrow. In panel (B), the hyperintensity shows a thicker nodule and oedema corresponding to the effect of the high-intensity focused ultrasound treatment. Panel (C) shows that the nodule is no longer visible.
Discussion
This is the first prospective multicentre study to evaluate the safety of HIFU treatment for rectal endometriosis. Among its limitations, the lack of randomization and the absence of a control group, especially regarding surgery and the length of post-HIFU follow-up of the patients (6 months), can restrict the interpretation of the results within the wider context of the treatment of rectal endometriosis. In addition, we cannot exclude the possibility of a placebo effect, although a randomized study by Bergqvist et al. (1998) comparing the effects of triptorelin versus placebo on the symptoms of endometriosis demonstrated that this effect tended to dissipate from 2 months after the start of treatment. Finally, the HIFU treatment of gastrointestinal endometriosis only targets rectal lesions, sometimes associated with a torus uterinum and/or USL involvement. Though the disease is very often multifocal, this treatment does not address other endometriosis locations. However, in selected patients with rectal DIE, this multicentre prospective study confirms the very low morbidity of HIFU treatment. In this study, we corroborated results from our prior feasibility study (Philip et al., 2020b) while observing no Grade III complications even as we increased treatment intensity by 30%. This was also regardless of experience, as some physicians were administering the treatment for the first time, under guidance.
HIFU thus appears to be a safe, minimally invasive transrectal approach. Comparatively, in a longitudinal study involving 364 gastrointestinal surgical procedures, Abo et al. (2019) encountered a 14.8% rate of Grade III complications, including 3.8% cases of RVF (Abo et al., 2019). More recently, a meta-analysis by Bendifallah et al. (2021) found a 1.5% rate of RVF, of which 0.3% occurred following rectal shaving, pointing to the safety of that approach. In contrast, the rate of Grade I complications in our study was higher than generally found in relevant literature. This could be caused by the study design and specified data collection, as the study’s primary endpoint was safety, while Grade I events are often omitted from other study results because they tend to improve or resolve spontaneously with no or limited medical intervention. Moreover, we observed a significantly lower rate of Grade II complications then reported in the literature, especially for patients treated with a conservative approach. In the long run, however, the most problematic complication is voiding dysfunction (Dubernard et al., 2008b; Ballester et al., 2011, 2014), which can affect up to 19% of patients (Jayot et al., 2018) immediately after conservative surgeries. Although one patient in our study presented with severe bladder dysfunction, we otherwise observed no worsening of urinary function. Results even showed that urinary urgency and incomplete bladder voiding had significantly improved at 6 months. For the one patient who experienced a serious adverse event, the independent data and safety monitoring board concluded that this event was related to several procedures performed within the research period involving spinal anaesthesia, as well as concurrent disease (endometriosis with bilateral hypogastric plexus nerve involvement). Moreover, a review of the MRI performed on Day 1 for this patient showed that HIFU lesions were only visualized in the rectal nodule and not in the hypogastric plexus nerve areas.
In addition to low morbidity, it is worth mentioning that the duration of HIFU treatment and hospital stay are much shorter than for surgery. Comparatively, the average treatment time for the surgical management of patients was 208 min, which included removal of all endometriotic lesions (Abo et al., 2018); the average length of hospital stay was 7.5 days (Jayot et al., 2018). Moreover, the median post-operative pain score remained very low, allowing outpatient management. The low duration of the procedure and the hospital stay can also explain the significant reduction of post-operative morbidity. Therefore, HIFU treatment also appears to be a cost-effective procedure that could have health economics benefits in the future.
The study confirmed a significant improvement in the patients’ gynaecological symptoms, digestive symptoms, and health status. Currently, surgery is the standard of care for digestive endometriosis as it helps to significantly improve symptoms. In a previous prospective study involving 58 colorectal resections, we demonstrated a significant improvement of digestive symptoms (Dubernard et al., 2006). Such improvements have since been corroborated in several studies (Mabrouk et al., 2011; Ribeiro et al., 2014; Touboul et al., 2015; Comptour et al., 2019). Comparably to surgery, improvements are observed after HIFU treatment across all symptoms, dyspareunia in particular, as early as the first month post-treatment. The significant reduction of the volume of the nodule observed after a 30% increase in the intensity of the treatment can also explain the improvement in digestive symptoms. As demonstrated, the gastrointestinal disorders linked to endometriosis are not limited to infiltration of the endometriosis lesions into the wall of the digestive tract, but seem to be more complex (Roman et al., 2012). In fact, functional disorders observed in cases of digestive endometriosis are frequently compared to irritable bowel syndrome (IBS) (Chiaffarino et al., 2021). The reduction of the volume of the rectal lesions observed 6 months post-treatment, evidencing the physical effects of the HIFU, is likely to explain the decrease in constipation, better bowel function, and improvement in IBS symptoms.
Finally, a visible trend in recent years has been to limit the use of radical surgical treatments, especially in digestive diseases. Whereas digestive segmental resection used to account for nearly half of surgical approaches (Roman and FRIENDS group (French coloRectal Infiltrating ENDometriosis Study Group), 2017), it is on the decrease, and surgical management is evolving towards mostly conservative treatments. Bendifallah et al. (2021) confirmed this in their meta-analysis, finding a 30% increase in conservative treatments compared to a study by Donnez and Roman (2017). The functional nature of endometriosis explains this change in practices and the similarities in symptom improvement resulting from these varying surgical approaches (Daraï et al., 2010; Fanfani et al., 2010; De Cicco et al., 2011; Meuleman et al., 2011). HIFU treatment is in line with this evolution, as the practice can be considered as equivalent to rectal shaving in terms of the safety margin. To prevent a digestive fistula, the safety margin for the first HIFU sonications is 3 mm (Fig. 1) from the rectal mucosa. Such a margin is difficult to manage in surgery but is probably very close to what we apply in rectal shaving procedures. Consequently, considering the low morbidity associated with this treatment approach, HIFU could be offered as first line for a specifically selected population of patients. Surgery would then be used after HIFU failure or for the treatment of other endometriosis locations that are not accessible with an HIFU treatment. During the long-term follow-up period for our feasibility study, only two patients (10%) received surgery (one shaving and one segmental resection) and the procedure was not made more difficult by the fact that the patient had previously been treated with HIFU (Philip et al., 2020b).
To conclude, our study confirmed that a transrectal robotic HIFU treatment of rectal endometriosis with a 30% intensity increase remained safe while significantly improving gynaecological and digestive symptoms, as well as patients’ health status. The significant reduction in the volume of the nodule at 6 months also validates the therapeutic effect of HIFU on endometriosis lesions. To further evaluate the efficacy of the technique, it should now be investigated in a study with a higher standard of proof. A randomized double-blind study comparing HIFU treatment and a simulated treatment (sham group) is currently underway in France across nine referral centres specializing in endometriosis.
Supplementary Material
Acknowledgements
We thank the clinical research department (C. Jossan, C. Faure, J.-P. Giraud, N. Gaudin, P. Martins, M. Sané) and the R&D Department (L. Brunel, I. Scala, J. Raimbault, M. Azzarello, N. Guillen) at the EDAP TMS. We are also grateful to the medical teams of the Croix-Rousse University Hospital, Angers University Hospital, Kremlin-Bicêtre University Hospital, and Tivoli-Ducot Clinic. Volumetric analysis software was provided free of charge by Olea Medical (La Ciotat, France). Editorial assistance was provided by Agnella Izzo Matic, PhD, CMPP (AIM Biomedical LLC) and was funded by EDAP TMS.
Contributor Information
G Dubernard, Department of Gynaecology and Obstetrics, Croix-Rousse University Hospital, Hospices Civils de Lyon, Claude Bernard University, Lyon, France; Laboratory of Therapeutic Applications of Ultrasound, Claude Bernard University, Lyon, France.
E Maissiat, Department of Radiology, Croix-Rousse University Hospital, Lyon, France.
G Legendre, Department of Gynaecology and Obstetrics, Angers University Hospital, Angers, France.
T Dennis, Department of Gynaecology, Tivoli-Ducos Clinic, Bordeaux, France.
P Capmas, Department of Gynaecology and Obstetrics, Kremlin-Bicêtre University Hospital, Paris, France.
S Warembourg, Department of Gynaecology and Obstetrics, Croix-Rousse University Hospital, Hospices Civils de Lyon, Claude Bernard University, Lyon, France.
P Descamps, Department of Gynaecology and Obstetrics, Angers University Hospital, Angers, France.
F Chavrier, Laboratory of Therapeutic Applications of Ultrasound, Claude Bernard University, Lyon, France.
H Roman, Department of Gynaecology, Tivoli-Ducos Clinic, Bordeaux, France.
H Fernandez, Department of Gynaecology and Obstetrics, Kremlin-Bicêtre University Hospital, Paris, France.
E Nguyen-Ba, Department of Gynaecology and Obstetrics, Croix-Rousse University Hospital, Hospices Civils de Lyon, Claude Bernard University, Lyon, France.
B Merlot, Department of Gynaecology, Tivoli-Ducos Clinic, Bordeaux, France.
P Rousset, Department of Radiology, South Lyon University Hospital, Lyon, France.
C Lafon, Laboratory of Therapeutic Applications of Ultrasound, Claude Bernard University, Lyon, France.
Charles-André Philip, Department of Gynaecology and Obstetrics, Croix-Rousse University Hospital, Hospices Civils de Lyon, Claude Bernard University, Lyon, France; Laboratory of Therapeutic Applications of Ultrasound, Claude Bernard University, Lyon, France.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
G.D. designed the trial, selected and validated inclusion criteria for all patients, performed or supervised treatments across all participating centres, and wrote the first draft of the article. E.M. reviewed all patient MRIs across all centres and validated the inclusion of the patients. P.R. performed pretreatment and 6-month post-treatment blinded review of MRI findings. G.L., T.D., P.C., S.W., P.D., H.R., H.F., E.N.-B., B.M., and C.-A.P. selected the patients and performed the treatments in their departments. F.C. and C.L. performed the simulated treatment.
Funding
EDAP TMS. Volumetric analysis software was provided free of charge by the OLEA company.
Conflict of interest
Professors G.D. and P.R. are consultants for EDAP TMS. G.D. received travel support from EDAP-TMS. Dr F.C received industrial grants from EDAP-TMS. He has developed a device for generating focused ultrasonic waves with reduced treatment time. This device has been patented by EDAP-TMS. Dr C.L. received industrial grants from EDAP-TMS; he declares that EDAP-TMS provided funding directly to INSERM to support a young researcher chair in therapeutic ultrasound, which is unrelated to the current study.
References
- Abo C, Bendifallah S, Jayot A, Nyangoh Timoh K, Tuech J-J, Roman H, Daraï E. Discoid resection for colorectal endometriosis: results from a prospective cohort from two French tertiary referral centres. Colorectal Dis 2019;21:1312–1320. [DOI] [PubMed] [Google Scholar]
- Abo C, Moatassim S, Marty N, Saint Ghislain M, Huet E, Bridoux V, Tuech JJ, Roman H. Postoperative complications after bowel endometriosis surgery by shaving, disc excision, or segmental resection: a three-arm comparative analysis of 364 consecutive cases. Fertil Steril 2018;109:172–178.e1. [DOI] [PubMed] [Google Scholar]
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update 2015;21:329–339. [DOI] [PubMed] [Google Scholar]
- Bakavicius A, Marra G, Macek P, Robertson C, Abreu AL, George AK, Malavaud B, Coloby P, Rischmann P, Moschini M et al. Available evidence on HIFU for focal treatment of prostate cancer: a systematic review. Int Braz J Urol 2022;48:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester M, Chereau E, Dubernard G, Coutant C, Bazot M, Daraï E. Urinary dysfunction after colorectal resection for endometriosis: results of a prospective randomized trial comparing laparoscopy to open surgery. Am J Obstet Gynecol 2011;204:303.e1–303.e6. [DOI] [PubMed] [Google Scholar]
- Ballester M, Dubernard G, Wafo E, Bellon L, Amarenco G, Belghiti J, Daraï E. Evaluation of urinary dysfunction by urodynamic tests, electromyography and quality of life questionnaire before and after surgery for deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol 2014;179:135–140. [DOI] [PubMed] [Google Scholar]
- Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, Manganaro L, Buñesch L, Kido A, Togashi K et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol 2017;27:2765–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazot M, Darai E, Hourani R, Thomassin I, Cortez A, Uzan S, Buy J-N. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 2004;232:379–389. [DOI] [PubMed] [Google Scholar]
- Bendifallah S, Puchar A, Vesale E, Moawad G, Daraï E, Roman H. Surgical outcomes after colorectal surgery for endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol 2021;28:453–466. [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Bergh T, Hogström L, Mattsson S, Nordenskjöld F, Rasmussen C. Effects of triptorelin versus placebo on the symptoms of endometriosis. Fertil Steril 1998;69:702–708. [DOI] [PubMed] [Google Scholar]
- Bourdel N, Chauvet P, Billone V, Douridas G, Fauconnier A, Gerbaud L, Canis M. Systematic review of quality of life measures in patients with endometriosis. PLoS One 2019;14:e0208464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burla L, Scheiner D, Samartzis EP, Seidel S, Eberhard M, Fink D, Boss A, Imesch P. The ENZIAN score as a preoperative MRI-based classification instrument for deep infiltrating endometriosis. Arch Gynecol Obstet 2019;300:109–116. [DOI] [PubMed] [Google Scholar]
- Byrne D, Curnow T, Smith P, Cutner A, Saridogan E, Clark TJ; BSGE Endometriosis Centres. Laparoscopic excision of deep rectovaginal endometriosis in BSGE Endometriosis Centres: a multicentre prospective cohort study. BMJ Open 2018;8:e018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, Vieira M, Hasan W, Bricou A. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod 2006;21:1839–1845. [DOI] [PubMed] [Google Scholar]
- Chapron C, Chopin N, Borghese B, Malartic C, Decuypere F, Foulot H. Surgical management of deeply infiltrating endometriosis: an update. Ann N Y Acad Sci 2004;1034:326–337. [DOI] [PubMed] [Google Scholar]
- Chiaffarino F, Cipriani S, Ricci E, Mauri PA, Esposito G, Barretta M, Vercellini P, Parazzini F. Endometriosis and irritable bowel syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet 2021;303:17–25. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- Comptour A, Chauvet P, Canis M, Grémeau A-S, Pouly J-L, Rabischong B, Pereira B, Bourdel N. Patient quality of life and symptoms after surgical treatment for endometriosis. J Minim Invasive Gynecol 2019;26:717–726. [DOI] [PubMed] [Google Scholar]
- Crouzet S, Rebillard X, Chevallier D, Rischmann P, Pasticier G, Garcia G, Rouviere O, Chapelon J-Y, Gelet A. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur Urol 2010;58:559–566. [DOI] [PubMed] [Google Scholar]
- Daraï E, Dubernard G, Coutant C, Frey C, Rouzier R, Ballester M. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg 2010;251:1018–1023. [DOI] [PubMed] [Google Scholar]
- Darwish B, Roman H. Surgical treatment of deep infiltrating rectal endometriosis: in favor of less aggressive surgery. Am J Obstet Gynecol 2016;215:195–200. [DOI] [PubMed] [Google Scholar]
- De Cicco C, Corona R, Schonman R, Mailova K, Ussia A, Koninckx P. Bowel resection for deep endometriosis: a systematic review. BJOG 2011;118:285–291. [DOI] [PubMed] [Google Scholar]
- Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril 2017;108:931–942. [DOI] [PubMed] [Google Scholar]
- Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod 2006;21:1243–1247. [DOI] [PubMed] [Google Scholar]
- Dubernard G, Rouzier R, David-Montefiore E, Bazot M, Darai E. Use of the SF-36 questionnaire to predict quality-of-life improvement after laparoscopic colorectal resection for endometriosis. Hum Reprod 2008a;23:846–851. [DOI] [PubMed] [Google Scholar]
- Dubernard G, Rouzier R, David-Montefiore E, Bazot M, Daraï E. Urinary complications after surgery for posterior deep infiltrating endometriosis are related to the extent of dissection and to uterosacral ligaments resection. J Minim Invasive Gynecol 2008b;15:235–240. [DOI] [PubMed] [Google Scholar]
- Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–412. [DOI] [PubMed] [Google Scholar]
- Fanfani F, Fagotti A, Gagliardi ML, Ruffo G, Ceccaroni M, Scambia G, Minelli L. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril 2010;94:444–449. [DOI] [PubMed] [Google Scholar]
- Jayot A, Nyangoh Timoh K, Bendifallah S, Ballester M, Darai E. Comparison of laparoscopic discoid resection and segmental resection for colorectal endometriosis using a propensity score matching analysis. J Minim Invasive Gynecol 2018;25:440–446. [DOI] [PubMed] [Google Scholar]
- Lee L, de Lacy B, Gomez Ruiz M, Liberman AS, Albert MR, Monson JRT, Lacy A, Kim SH, Atallah SB. A multicenter matched comparison of transanal and robotic total mesorectal excision for mid and low-rectal adenocarcinoma. Ann Surg 2019;270:1110–1116. [DOI] [PubMed] [Google Scholar]
- Mabrouk M, Montanari G, Guerrini M, Villa G, Solfrini S, Vicenzi C, Mignemi G, Zannoni L, Frasca C, Di Donato N et al. Does laparoscopic management of deep infiltrating endometriosis improve quality of life? A prospective study. Health Qual Life Outcomes 2011;9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel C, Ferreira H, Djokovic D, Kyaw Tun J, Keckstein J, Rizzo S, Manganaro L. MRI of endometriosis in correlation with the #Enzian classification: applicability and structured report. Insights Imaging 2023;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman C, Tomassetti C, D’Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I, D’Hooghe T. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update 2011;17:311–326. [DOI] [PubMed] [Google Scholar]
- Minelli L, Fanfani F, Fagotti A, Ruffo G, Ceccaroni M, Mereu L, Landi S, Pomini P, Scambia G. Laparoscopic colorectal resection for bowel endometriosis: feasibility, complications, and clinical outcome. Arch Surg 2009;144:234–239; discussion 239. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Li A, Falik R, Copeland D, Razavi G, Shakib A, Mihailide C, Bamford H, DiFrancesco L, Tazuke S et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol 2018;218:549–562. [DOI] [PubMed] [Google Scholar]
- Philip C-A, Prouvot C, Cortet M, Bisch C, de Saint-Hilaire P, Maissiat E, Huissoud C, Dubernard G. Diagnostic performances of tridimensional rectosonography and magnetic resonance imaging in rectosigmoid endometriosis: a prospective cohort study on 101 patients. Ultrasound Med Biol 2020a;46:225–232. [DOI] [PubMed] [Google Scholar]
- Philip C-A, Warembourg S, Dairien M, Lefevre C, Gelet A, Chavrier F, Guillen N, Tonoli H, Maissiat E, Lafon C et al. Transrectal high-intensity focused ultrasound (HIFU) for management of rectosigmoid deep infiltrating endometriosis: results of Phase-I clinical trial. Ultrasound Obstet Gynecol 2020b;56:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PAA, Sekula VG, Abdalla-Ribeiro HS, Rodrigues FC, Aoki T, Aldrighi JM. Impact of laparoscopic colorectal segment resection on quality of life in women with deep endometriosis: one year follow-up. Qual Life Res 2014;23:639–643. [DOI] [PubMed] [Google Scholar]
- Rischmann P, Crouzet S, Villers A, Pasticier G, Petit J, Surga N, Bugel H, Bondil P, Mallick S, Toledano H et al. Traitement par hémi-ablation HIFU des cancers de la prostate localisés à un seul lobe (étude AFU) : résultats finaux. Prog Urol 2015;25:834. [DOI] [PubMed] [Google Scholar]
- Roman H, Bridoux V, Tuech JJ, Marpeau L, da Costa C, Savoye G, Puscasiu L. Bowel dysfunction before and after surgery for endometriosis. Am J Obstet Gynecol 2013;209:524–530. [DOI] [PubMed] [Google Scholar]
- Roman H; Friends Group (French coloRectal Infiltrating ENDometriosis Study Group). A national snapshot of the surgical management of deep infiltrating endometriosis of the rectum and colon in France in 2015: a multicenter series of 1135 cases. J Gynecol Obstet Hum Reprod 2017;46:159–165. [DOI] [PubMed] [Google Scholar]
- Roman H, Ness J, Suciu N, Bridoux V, Gourcerol G, Leroi AM, Tuech JJ, Ducrotté P, Savoye-Collet C, Savoye G. Are digestive symptoms in women presenting with pelvic endometriosis specific to lesion localizations? A preliminary prospective study. Hum Reprod 2012;27:3440–3449. [DOI] [PubMed] [Google Scholar]
- Seracchioli R, Poggioli G, Pierangeli F, Manuzzi L, Gualerzi B, Savelli L, Remorgida V, Mabrouk M, Venturoli S. Surgical outcome and long-term follow up after laparoscopic rectosigmoid resection in women with deep infiltrating endometriosis. BJOG 2007;114:889–895. [DOI] [PubMed] [Google Scholar]
- Touboul C, Ballester M, Dubernard G, Zilberman S, Thomin A, Daraï E. Long-term symptoms, quality of life, and fertility after colorectal resection for endometriosis: extended analysis of a randomized controlled trial comparing laparoscopically assisted to open surgery. Surg Endosc 2015;29:1879–1887. [DOI] [PubMed] [Google Scholar]
- Tsai M-C, Chang L-T, Tam K-W. Comparison of high-intensity focused ultrasound and conventional surgery for patients with uterine myomas: a systematic review and meta-analysis. J Minim Invasive Gynecol 2021;28:1712–1724. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Sergenti G, Buggio L, Frattaruolo MP, Dridi D, Berlanda N. Advances in the medical management of bowel endometriosis. Best Pract Res Clin Obstet Gynaecol 2021;71:78–99. [DOI] [PubMed] [Google Scholar]
- Wills HJ, Reid GD, Cooper MJW, Morgan M. Fertility and pain outcomes following laparoscopic segmental bowel resection for colorectal endometriosis: a review. Aust N Z J Obstet Gynaecol 2008;48:292–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.