Abstract
Quiescent primary B lymphocytes and Epstein-Barr virus (EBV)-immortalized lymphoblastoid cell lines express components of the extracellular response kinase arm of the mitogen-activated protein kinase (MAPKERK) signal transduction pathway and transmit signals through the pathway when exposed to appropriate stimuli. Although the MAPKERK pathway is activated following infection with EBV, MAPK/ERK kinase (MEK1) activity is not required to drive the proliferation of infected cells. However, MEK1 contributes to EBV latency control.
The network of mitogen-activated protein kinase (MAPK) signal transduction pathways plays an important role in regulating the response of cells to a multitude of extracellular stimuli (20, 26). The three MAPK pathways identified in humans contain similar core modules consisting of a series of sequentially acting protein kinases. The first, a MAPK kinase kinase, is activated in response to extracellular or intracellular signalling and directly phosphorylates and activates the second kinase, a MAPK kinase, which phosphorylates and activates the effector kinase, MAPK (26). Despite similarities in the overall scheme of signal transduction through these pathways, each one is activated in response to different stimuli, and their MAPK components have distinct targets. The importance of these pathways for the normal control of cell proliferation and survival is highlighted by the numerous examples of genetic changes that disrupt or alter the function of the components of these signal transduction pathways in cancer (13).
The human herpesvirus Epstein-Barr virus (EBV) overrides the normal controls of cell proliferation following the infection of quiescent B lymphocytes, driving antigen-independent proliferation of infected cells and the outgrowth of immortal lymphoblastoid cell lines (LCLs) (18, 23). EBV is also implicated in the development of several types of lymphoma, lymphoproliferative disease, and carcinoma (18, 23). It is therefore relevant to question whether EBV exploits any of the MAPK pathways during the growth transformation of infected cells. It has recently been demonstrated that two MAPK signal transduction pathways are activated by EBV: one involving the c-Jun N-terminal kinase MAPKJNK via one of the essential transforming proteins of EBV, latent membrane protein 1 (LMP1) (6–8, 11, 14), and the MAPKERK pathway (19), which is the predominant MAPK pathway used to transduce mitogenic signals. In response to mitogens, a signal is normally passed through this pathway from the MAPK kinase kinase c-raf, directing the phosphorylation of one or both of isoforms of the MAPK kinase, MEK1 and MEK2 (MEK1,2), which then phosphorylate one or both of the MAPK components, p44 ERK1 and p42 ERK2 (ERK1,2). Many potential targets for the MAPKERK pathway have been proposed; although their relevance is still under investigation, it is clear that these targets include transcription factors and protein kinases (3). A recently published study showed that the activity of ERK1,2 increases following infection of primary B lymphocytes with EBV (19). The identity of the EBV gene(s) responsible for the activation of the MAPKERK pathway has not been firmly established as yet, but studies in rodent fibroblasts implicate LMP1 (19), whereas similar experiments undertaken in a transformed human epithelial cell line found no correlation between LMP1 expression and ERK1,2 activation (14).
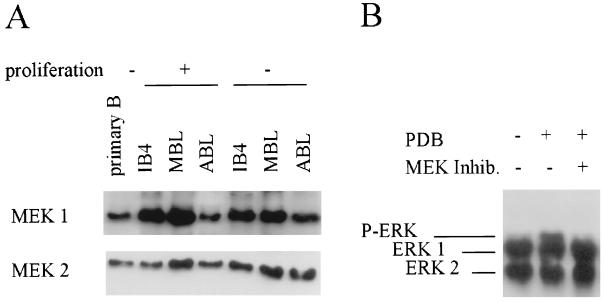
Here, we sought to explore the potential roles of signal transduction through the MAPKERK pathway in EBV-immortalized human LCLs. First we examined whether components of the MAPKERK pathway are expressed and functional in LCLs. As shown in Fig. 1, both MEK1 and MEK2 proteins are readily detected in the three representative cell lines analyzed: IB4, an established LCL originally isolated from fetal cord B lymphocytes (15), and ABL and MBL, two recently immortalized LCLs derived from adult B lymphocytes (Fig. 1A). Furthermore, both MEK1 and MEK2 proteins are also present in a population of freshly isolated human primary B lymphocytes (22, 24), which are the precursors of LCLs.
FIG. 1.
Components of the MAPKERK pathway are expressed and functional in LCLs. (A) Total protein lysates from a series of three LCLs (IB4, MBL, and ABL) and a population of freshly isolated primary B lymphocytes were fractionated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis. MEK1 and MEK2 proteins were detected with specific primary antibodies (H8 and N20, respectively; Santa Cruz) followed by horseradish peroxidase-conjugated species-specific secondary antibody and enhanced chemiluminescence (Amersham). (B) IB4 cells were exposed to PDB (1 nM) (Sigma) and the MEK kinase inhibitor PD98059 (50 μM) (New England Biolabs) for 15 min as described in the text. Total protein lysates were then fractionated as described above, and ERK1 and ERK2 proteins were detected with a specific antibody (p44/42-MAPK; New England Biolabs). The migration of ERK1 and ERK2 and the retarded migration of one of the phosphorylated ERK bands are indicated on the gel.
ERK1 and ERK2 expression was also investigated in LCLs. As seen in Fig. 1B, both ERK1 and ERK2 proteins are readily detected in the IB4 cell line. In order to establish whether signal transduction through MEK1,2 is possible in these cells, they were exposed to a known chemical activator of the MAPKERK pathway, the phorbol ester phorbol-12,13-dibutyrate (PDB). The activation of MEK1,2 was then scored by assessing the phosphorylation of ERK1,2; phosphorylated forms of ERK1,2 can be distinguished by their distinct migratory patterns in sodium dodecyl sulfate-polyacrylamide gels (Fig. 1B). The two bands observed in proliferating cells correspond to the nonphosphorylated forms of ERK1 and ERK2 (Fig. 1B, lane 1). The slower migrating band, observed within 15 min of exposure to the phorbol ester, corresponds to a phosphorylated form of ERK1,2 (Fig. 1B, lane 2). The appearance of this band shows that signals can pass successfully from PDB to ERK1,2 in these cells. The involvement of MEK1 and/or MEK2 in the process was addressed by using a highly specific chemical inhibitor of the MAPKERK pathway, PD98059 (1, 5). PD98059 binds to the inactive forms of MEK1 and MEK2 and prevents their phosphorylation and subsequent activation. PD98059 preferentially inhibits MEK1; a 10-fold higher concentration is required to inhibit MEK2. In this study, the concentration used was equal to the 50% inhibitory concentration of MEK2, so it is likely that only MEK1 is fully inhibited in vivo. However, since it is not possible to formally distinguish whether any observed inhibition by PD98059 is due to the action of MEK1 or MEK2 in this study, they are referred to as MEK1,2. In LCLs, PD98059 inhibited the phorbol ester-induced activation of ERK1,2 (Fig. 1B, lane 3, and data not shown). Taken together, these data suggest that signal transduction to ERK1,2 via MEK1,2 is functional in LCLs.
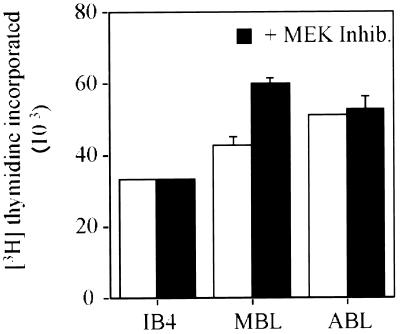
Since components of the MAPKERK signal transduction pathway are implicated in the control of cell proliferation, we sought to establish whether the pathway is required for the proliferation of LCLs in normal culture conditions. The rate of DNA synthesis was compared in LCLs cultured in the presence or absence of PD98059 for 24 h. From the data shown in Fig. 2, it is clear that PD98059 does not significantly inhibit the proliferation of LCLs. However, in other experiments when longer-term effects were investigated, a modest (30%) reduction in the rate of DNA synthesis was observed in cells treated with PD98059 for 3 days.
FIG. 2.
The proliferation of LCLs does not depend on MEK1,2 activity. IB4, MBL, and ABL cells were cultured in the presence or the absence of the MEK inhibitor PD98059 (50 μM) for 24 h. The rate of DNA synthesis was then determined using a thymidine incorporation assay and is expressed as the amount of [3H]thymidine incorporated into DNA per 6 × 104 cells within a 4-h period (9). The assays were undertaken in triplicate, and the standard deviation is shown by error bars. The data from cells cultured in the absence of the MEK inhibitor are indicated by open bars, and the corresponding data from cells cultured in the presence of the inhibitor are indicated by the solid bars.
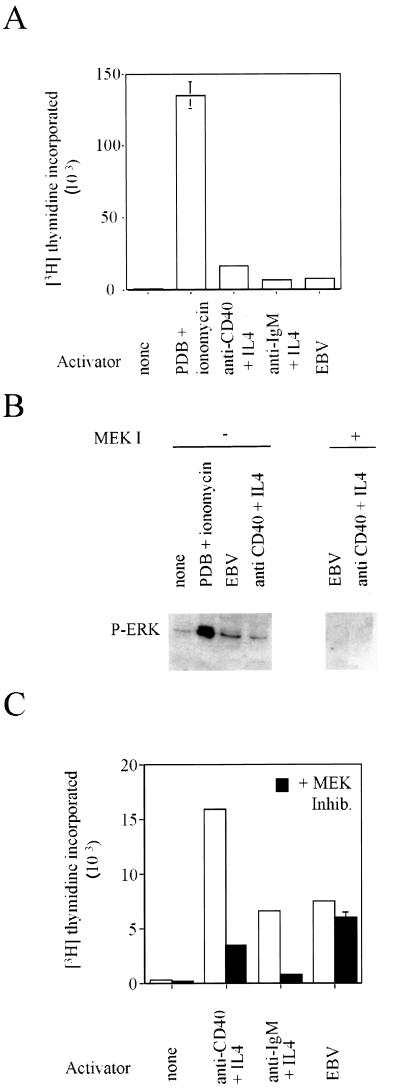
The failure of the MEK inhibitor to significantly reduce the proliferation of LCLs led us to question whether the activation of ERK1,2 observed following infection of primary B lymphocytes with EBV (19) is required for infection and cell cycle activation. We isolated human primary B lymphocytes from the peripheral circulation and subjected them to various stimuli: infection with EBV, addition of a calcium ionophore in combination with a phorbol ester, or addition of physiologically relevant activators such as anti-CD40 plus interleukin-4 (IL-4) and anti-immunoglobulin M plus IL-4 (12). In each case, the cells were stimulated to leave quiescence, to enter into the cell division cycle, and to initiate DNA synthesis within 72 h (Fig. 3A). In order to investigate the phosphorylation of ERK1,2 in primary cells, it was necessary to employ a sensitive assay using an antibody that is specific for the phosphorylated forms of ERK1,2 (Fig. 3B). The cells treated with the calcium ionophore in combination with PDB showed a dramatic increase in the level of phosphorylated ERK1,2. These cells are routinely activated one order of magnitude more effectively than those infected with EBV or activated by physiological agents (Fig. 3A). An increase in the level of phosphorylated ERK1,2 was also observed in the primary cells that had been infected with EBV or stimulated with the physiologically relevant agents anti-CD40 and IL-4 (Fig. 3B). A comparison of the kinetics of this phosphorylation showed no gross differences between the stimuli (data not shown). This independent evidence supports the published data from Roberts and Cooper showing that ERK1,2 kinase activity increases following EBV infection (19). Furthermore, incubation of the primary cells with PD98059 inhibits EBV-stimulated phosphorylation of ERK1,2 (data not shown), allowing us to expand the model to include a requirement for MEK1,2 activity.
FIG. 3.
Divergent requirements for the phosphorylation of ERK1,2 following primary B-lymphocyte stimulation and infection with EBV. Primary B lymphocytes, isolated from the peripheral circulation, were cultured in the presence or absence of the indicated activators and inhibitors. PDB (1 ng/ml), ionomycin (0.8 μg/ml), anti-CD40 (G25-8) (2), anti-immunoglobulin M (BU.1) (10), IL-4 (Sigma), and the B95-8 strain of EBV (22). (A) The rate of DNA synthesis was determined after 72 h as described in the legend to Fig. 2. (B) Total protein lysates were prepared from cells 30 min after the addition of the indicated activators and inhibitors. Phosphorylated ERK1 and ERK2 proteins were detected with a specific antibody (p44/p42 mapk-tyr204 [New England Biolabs]). The migration of phosphorylated ERK1,2 is shown on the gel. (C) The rate of DNA synthesis 72 h after the addition of the indicated stimuli was determined as described in the legend to Fig. 2. The data from cells cultured in the absence of the MEK inhibitor are indicated by open bars, and the corresponding data from cells cultured in the presence of the inhibitor are indicated by the solid bars.
The relevance of the observed activation of ERK1,2 during initial infection was then addressed by investigating whether the MEK inhibitor is able to interfere with EBV infection. The stimulation of cellular DNA synthesis occurring 3 days postinfection, which requires the expression of viral genes, was used as a measure of initial infection. Although PD98059 was not able to prevent EBV-driven DNA synthesis (Fig. 3C), in the same conditions it readily inhibited DNA synthesis stimulated by physiological agents. In these experiments, PD98059 inhibited the phosphorylation of ERK1,2 (Fig. 3B). So unless some residual and undetectable process of signal transduction to ERK1,2 remains in the PD98059-treated primary cells, this result suggests that EBV-driven and anti-CD40 plus IL-4-driven cell proliferations have different requirements for signal transduction through MEK1,2. Therefore, although we strongly support the conclusion of Roberts and Cooper (19) that the MAPKERK pathway is activated following the infection of human primary B lymphocytes with EBV, we conclude that this activation is not required for the proliferation of the infected cells.
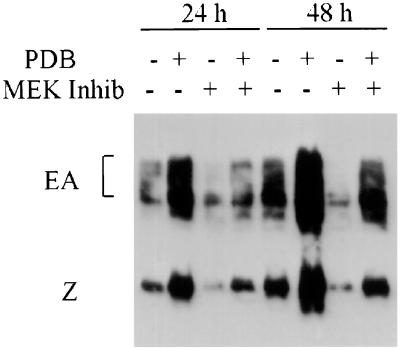
Phorbol esters are potent agents that mediate the disruption of EBV latency in LCLs (27), suggesting that the MAPKERK pathway may play a role in latency control. Phorbol esters act by direct interaction with protein kinase C (PKC) (16, 17), the activity of which is required for phorbol ester-mediated disruption of EBV latency (4). We therefore addressed whether the MAPKERK pathway is involved in the control of EBV latency. The disruption of EBV latency by phorbol esters is achieved through increased activity of a viral promoter driving the expression of the EBV gene encoding the transcription factor BZLF1, which directly activates the expression of a group of EBV lytic genes including the early antigens (EA) (18, 21, 25). A basal level of BZLF1 and EA expression can be detected in most LCLs, as illustrated for MBL cells in lanes 1 and 5 of Fig. 4. Treatment with phorbol esters causes a dramatic increase in the expression of the viral lytic genes, with the production of infectious virions within 3 to 4 days (27). Following stimulation of the cells with PDB, the expression of both BZLF1 and EA increases dramatically within 24 h, and this is sustained for at least 48 h (Fig. 4). However, the phorbol ester-mediated increase is entirely inhibited by the addition of PD98059. These data clearly demonstrate that MEK1,2 activity is required for mediating the disruption of viral latency by phorbol esters. Since PKC activity is also required for the disruption of viral latency by phorbol esters (4), these data support a model whereby phorbol ester activation of PKC signals through MEK1,2 to activate BZLF1 expression.
FIG. 4.
EBV latency disruption by phorbol ester requires MEK1,2 activity. MBL cells were cultured in the presence of PDB (1 nM) and the MEK kinase inhibitor PD98059 (50 μM) for 24 or 48 h, as indicated. Total protein lysates were then fractionated, and BZLF1 (Z) and the EBV EA were detected with a human serum, EE (4). The migrations of BZLF1 and EA are indicated on the gel.
Current models suggest that EBV exploits cellular signal transduction pathways to drive cell proliferation (23). Indeed, most comparisons between primary B lymphocytes stimulated with physiological agents or infected with EBV identified similarities in the patterns of activated genes (reviewed in reference 23). Only the life span of the cells and differences in their responses to DNA-damaging agents differentiates them. Thus, the distinct requirements for signal transduction through MEK1,2 in primary B lymphocytes stimulated with physiological agents versus those infected with EBV identifies an important distinction in the routes used by EBV and physiological agents to drive the proliferation of B lymphocytes. In addition, although the activation of the MAPKERK pathway during the initial stages of infection appears to be redundant, we have identified a role for the MAPKERK pathway in the regulation of viral latency.
Acknowledgments
This work was funded in part by grants from the Leukaemia Research Fund and the Wellcome Trust.
We thank J. Gordon and M. Rowe for reagents and S. Morley, M. Clemens, and V. Frost for helpful discussions.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;46:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Clarke E A, Ledbetter J A. Activation of human B cells mediated through two distinct cell surface differentiation antigens Bp35 and Bp50. Proc Natl Acad Sci USA. 1986;83:4494–4497. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P. The search for physiological substrates for MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 4.Davies A H, Grand R, Evans F J, Rickinson A B. Induction of Epstein-Barr virus lytic cycle by tumor-promoting and non-tumor-promoting phorbol esters requires active protein-kinase-C. J Virol. 1991;65:6838–6844. doi: 10.1128/jvi.65.12.6838-6844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliopoulos A G, Blake S, Floettmann J E, Rowe M, Young L S. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos A G, Rickinson A B. Epstein-Barr virus: LMP1 masquerades as an active receptor. Curr Biol. 1998;8:R196–R198. doi: 10.1016/s0960-9822(98)70123-x. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos A G, Young L S. Activation of the c-jun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 9.Fenton M, Bone N, Sinclair A J. The efficient and rapid import of a peptide into primary B and T lymphocytes and a lymphoblastoid cell line. J Immunol Methods. 1998;212:41–48. doi: 10.1016/s0022-1759(97)00208-1. [DOI] [PubMed] [Google Scholar]
- 10.Gordon J, Millsum M J, Flores-Rymo L, Gillis S. Regulation of resting and cycling human B lymphocytes via surface IgM and the accessory molecules interleukin-4, CD23 and CD40. Immunology. 1989;68:526–531. [PMC free article] [PubMed] [Google Scholar]
- 11.Hatzivassiliou E, Miller W E, Raab Traub N, Kieff E, Mosialos G. Fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 12.Hollyoake M, Stuhler A, Farrell P, Gordon J, Sinclair A J. The normal cell cycle activation program is exploited during the infection of quiescent primary B-lymphocytes by Epstein-Barr virus. Cancer Res. 1995;55:4784–4787. [PubMed] [Google Scholar]
- 13.Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 14.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King W, Thomas-Powell A L, Raab-Traub N, Hawke M, Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980;36:506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 17.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 18.Rickinson A, Keiff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Field’s virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 19.Roberts M L, Cooper N R. Activation of a Ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology. 1998;240:93–99. doi: 10.1006/viro.1997.8901. [DOI] [PubMed] [Google Scholar]
- 20.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair A J, Farrell P J. Epstein-Barr virus transcription factors. Cell Growth Differ. 1992;3:557–563. [PubMed] [Google Scholar]
- 22.Sinclair A J, Farrell P J. Methods to study Epstein-Barr virus and p53 status in human cells. In: Aldolph K W, editor. Methods in molecular genetics. Vol. 7. Orlando, Fla: Academic Press; 1995. pp. 89–100. [Google Scholar]
- 23.Sinclair A J, Fenton M, Delikat D. Interactions between EBV and the cell cycle control machinery. Histol Histopathol. 1998;13:461–467. doi: 10.14670/HH-13.461. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speck S H, Chatila T, Flemmington E. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 1997;5:399–405. doi: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- 26.Widmann C, Gibson S, Jarpe M B, Johnson G L. Mitogen activated protein kinase: conservation of a three kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 27.zur Hausen H, O’Neill F J, Freese U K, Hecker E. Persisting oncogenic herpesvirus induced by tumour promoter TPA. Nature. 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]