Abstract
Objectives
To compare clinical characteristics, including the frequency of cutaneous, extramuscular manifestations and malignancy, between adults with anti-synthetase syndrome (ASyS) and DM.
Methods
Using data regarding adults from the MYONET registry, a cohort of DM patients with anti-Mi2/-TIF1γ/-NXP2/-SAE/-MDA5 autoantibodies, and a cohort of ASyS patients with anti-tRNA synthetase autoantibodies (anti-Jo1/-PL7/-PL12/-OJ/-EJ/-Zo/-KS) were identified. Patients with DM sine dermatitis or with discordant dual autoantibody specificities were excluded. Sub-cohorts of patients with ASyS with or without skin involvement were defined based on presence of DM-type rashes (heliotrope rash, Gottron’s papules/sign, violaceous rash, shawl sign, V-sign, erythroderma, and/or periorbital rash).
Results
In total 1054 patients were included (DM, n = 405; ASyS, n = 649). In the ASyS cohort, 31% (n = 203) had DM-type skin involvement (ASyS-DMskin). A higher frequency of extramuscular manifestations, including Mechanic’s hands, Raynaud’s phenomenon, arthritis, interstitial lung disease and cardiac involvement differentiated ASyS-DMskin from DM (all P < 0.001), whereas higher frequency of any of four DM-type rashes—heliotrope rash (n = 248, 61% vs n = 90, 44%), violaceous rash (n = 166, 41% vs n = 57, 9%), V-sign (n = 124, 31% vs n = 28, 4%), and shawl sign (n = 133, 33% vs n = 18, 3%)—differentiated DM from ASyS-DMskin (all P < 0.005). Cancer-associated myositis (CAM) was more frequent in DM (n = 67, 17%) compared with ASyS (n = 21, 3%) and ASyS-DMskin (n = 7, 3%) cohorts (both P < 0.001).
Conclusion
DM-type rashes are frequent in patients with ASyS; however, distinct clinical manifestations differentiate these patients from classical DM. Skin involvement in ASyS does not necessitate increased malignancy surveillance. These findings will inform future ASyS classification criteria and patient management.
Keywords: Anti-synthetase syndrome, Dermatomyositis, Cutaneous, Rashes, Skin, Malignancy, Epidemiology, MYONET, Extramuscular
Rheumatology key messages.
Approximately one-third of patients with anti-synthetase syndrome have dermatomyositis-type cutaneous involvement.
Certain clinical manifestations differentiate patients with anti-synthetase syndrome and dermatomyositis-type cutaneous involvement from dermatomyositis.
Anti-synthetase syndrome with dermatomyositis-type cutaneous involvement is not associated with increased risk of malignancy.
Introduction
Antisynthetase syndrome (ASyS) is a clinical subtype of idiopathic inflammatory myopathy (IIM) characterized by the presence of disease-specific autoantibodies against aminoacyl-transfer RNA synthetase (ARS) including anti-Jo1, -PL12, -PL7, -EJ, -OJ, -KS, -Zo and -Ha. Clinical features of ASyS include mechanic’s hands, Raynaud’s phenomenon, interstitial lung disease (ILD), myositis, arthritis and/or fever [1–3]. Dermatomyositis (DM) is another IIM subtype distinguished by characteristic cutaneous manifestations (including Gottron’s papules/sign, erythroderma, heliotrope, violaceous, periorbital, V-sign and shawl sign rashes) with or without myositis (amyopathic) and/or ILD [1]. DM-specific autoantibodies include anti-Mi2, -TIF1γ, -SAE, -MDA5 and -NXP2 [3]. Cutaneous DM-type manifestations can also be observed in ASyS patients, and therefore the current classification criteria for DM and ASyS overlap significantly, making classification of patients with anti-ARS and associated cutaneous manifestations especially challenging [4]. An international workshop from The European Neuromuscular Centre (ENMC) further highlighted this challenge, noting that ASyS is a unique and separate subgroup from DM even in the presence of DM-type cutaneous manifestations, and recommending that such patients be classified as having ‘ASyS with DM-like rash’ and not DM [5].
Up to 28% of patients with ASyS (defined with anti-ARS) have DM-type cutaneous manifestations [6]. However, it is not clear whether ASyS patients with DM-type cutaneous manifestations resemble patients with DM, and whether they should be regarded similarly in a clinical trial setting. Furthermore, it is not known if the presence of DM-type cutaneous manifestations confers an increased risk of DM-specific extramuscular manifestations, such as malignancy. Therefore, detailed phenotyping of a cohort of patients with ASyS with DM-type cutaneous manifestations might facilitate prediction of individual patient clinical course, clarify the need for malignancy screening, and inform future ASyS classification criteria.
We aimed to investigate the clinical manifestations in patients with ASyS and cutaneous manifestations using data from an international multicentre registry (MYONET registry, previously the EuroMyositis registry) [7].
Methods
The MYONET registry
The MYONET registry was created in 2003 [7]. The questions related to the registry were formulated following a Delphi process, and consensus discussion among Rheumatology and Neurology experts led to the creation of a uniform data collection proforma for use by all participating centres. Anonymized data from the registry were downloaded on 29 November 2021, which included 4806 cases from 112 centres, in 37 countries (Supplementary Table S1, available at Rheumatology online).
ASyS and DM cohort definitions
As per registry inclusion criteria, all patients with DM met Bohan and Peter ‘definite’ or ‘probable’ diagnostic criteria [8], and all patients with ASyS met diagnostic criteria proposed by Connors et al. [9]. For this study, cohorts of patients with ASyS or DM were defined based on the presence of ARS or DM-specific autoantibodies [3]. Patients with any of the seven ARS autoantibodies (anti-Jo1, -PL12, -PL7, -EJ, -OJ, -Zo or -KS) detectable were defined as having ASyS, and patients with any of the five DM-specific autoantibodies (anti-Mi2, -TIF1γ, -SAE, -MDA5 or -NXP2) were defined as having DM. As Bohan and Peter diagnostic criteria for DM require cutaneous involvement, patients with DM sine dermatitis are not defined as DM in the registry. Five patients with both ARS and DM-specific autoantibodies were excluded. The presence of myositis-specific autoantibodies was reported by clinicians and results recorded within the registry. Methods for antibody testing varied depending on regional laboratory practices and were tabulated (Supplementary Table S2, available at Rheumatology online).
Case characteristics
Patient demographics including sex, age at diagnosis, smoking status, autoantibodies, and clinical characteristics were collated. Clinical characteristics including the presence of myopathic muscle weakness, seven DM-type cutaneous manifestations (heliotrope rash, Gottron’s papules/sign, violaceous rash, erythroderma, periorbital rash, V-sign rash and shawl sign), 11 extramuscular manifestations (periungual erythema, calcinosis, ulceration, vasculitis, mechanic’s hands, Raynaud’s phenomenon, arthritis, dysphagia, alopecia, ILD and cardiac involvement), location and number of malignancies were recorded.
Definition of ASyS with and without DM-type skin involvement sub-cohorts
Sub-cohorts of patients with ASyS with DM-type skin involvement (ASyS-DMskin) and those without DM-type skin involvement (ASyS-without-DMskin) were identified based on reported case characteristics. Patients with one or more of the DM-type cutaneous manifestation were considered to have DM-type skin involvement, and those with none considered without DM-type skin involvement. The sum of reported DM-type cutaneous manifestations out of a possible seven was calculated.
Malignancy
Within the registry, malignancy is recorded including the date of diagnosis. In this analysis we considered malignancies diagnosed within 3 years of IIM onset to be ‘cancer-associated myositis’ (CAM). The location of CAM was compared between cohorts. Skin malignancies (including benign skin lesions such as basal cell carcinomas) were excluded except for melanoma. Malignancy was recorded variably by each centre, where in the UK the registry is linked to the National Health Service (NHS) Digital service that records malignancy, whereas other centres relied on entering malignancy data manually.
Missing data
Comparing prevalence of the clinical manifestations in our cohort with previously reported data suggested that the data were missing not at random (MNAR), and that it was more likely that data were missing when the clinical characteristic was not present. Therefore, for statistical analysis imputation of missing values was considered inappropriate, and entries of clinical characteristics that were missing were considered not present. The number of missing entries for each clinical characteristic was tabulated (Supplementary Table S3, available at Rheumatology online).
Statistical analysis
Between group comparisons were assessed using descriptive statistics as appropriate, with a threshold for significance set at P < 0.05. The Benjamini–Hochberg procedure was used to adjust for multiple comparisons to create adjusted P-values [10]. Statistical analyses were performed in R version 4.1.0 and RStudio version 1.4.1106 [11].
Ethics
All patients gave informed written consent for their data to be analysed as part of this study. The MYONET (previously EuroMyositis) registry includes multiple recruiting centres in multiple countries, where ethical approvals are required and have been sought at each centre and informed consent is obtained from all included patients. All centres obtained specific ethical approval from their local ethics committees for this study.
Results
Case characteristics
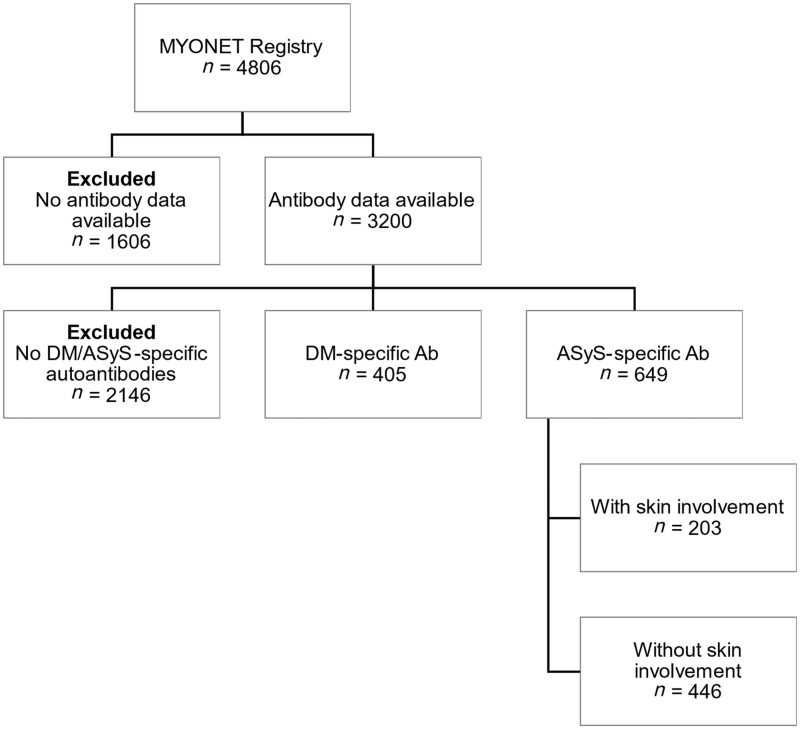
Data regarding 4806 cases were initially analysed. Patients without results of autoantibody tests available were excluded (n = 1606) leaving 3200 cases (Supplementary Table S4, available at Rheumatology online). Of these, patients without ASyS or DM-specific autoantibodies (n = 2146) were excluded. A cohort of 405 patients with DM-specific autoantibodies was identified, while 649 patients with ARS autoantibodies were identified (Fig. 1).
Figure 1.
Flowchart illustrating the patients from the MYONET registry that were included and excluded from the study. DM-specific Ab refers to Mi2, TIF1γ, SAE, MDA5 and NXP2. ASyS-specific Ab refers to Jo1, PL12, PL7, EJ, OJ, Zo and KS. ASyS: anti-synthetase syndrome
Demographics
Demographics including female sex, age at diagnosis and smoking status were compared between DM and ASyS groups. There was a significantly higher proportion of female sex in the ASyS-DMskin compared with the ASyS-without-DMskin cohorts (n = 147/203, 72% vs n = 278/446, 62%, P = 0.045). Age at diagnosis was significantly higher in the ASyS-without-DMskin cohort compared with the ASyS-DMskin cohort: 51 (interquartile range [IQR] 40–62) vs 47 (IQR 38–53) years, P = 0.005). Finally, there was a higher proportion of smokers in the ASyS cohort compared with the DM cohort (n = 197/649, 30% vs n = 96/405, 24%, P = 0.023) (Supplementary Table S5, available at Rheumatology online).
Prevalence of disease-specific autoantibodies
The most common autoantibody in the DM cohort was anti-Mi2 (n = 162/405, 40%) followed by -TIF1γ (n = 143/405, 35%), -MDA5 (n = 66/405, 16%), -SAE (n = 39/405, 10%), and -NXP2 (n = 9/405, 2%) (Supplementary Table S6, available at Rheumatology online). In the ASyS cohort the majority possessed anti-Jo1 (n = 542/649, 84%) with a lower proportion possessing other ARS: anti-PL12 (n = 41/649, 6%), -PL7 (n = 35/649, 5%), -EJ (n = 16/649, 3%), -OJ (n = 10/649, 2%) and -Zo (n = 6/649, 1%) (Supplementary Table S6, available at Rheumatology online). There were no patients with anti-Ha antibodies recorded in the registry.
Comparison of clinical characteristics between DM and ASyS cohorts
There were no significant differences in the presence of myopathic muscle weakness between DM and ASyS cohorts (Table 1). Patients in the DM cohort had a significantly higher frequency of each of the seven specified DM-type rashes compared with the ASyS cohort (Table 1). The extramuscular manifestations traditionally associated with ASyS (ILD, arthritis, Raynaud’s, mechanic’s hands) and cardiac involvement were predictably more common in this group compared with DM. Periungual erythema, ulceration, calcinosis, alopecia, vasculitis and dysphagia were more frequent in DM compared with ASyS, although there was overlap of these features across the two conditions (Table 1).
Table 1.
Clinical manifestations of disease
|
Adjusted P-value
a
|
|||||||
|---|---|---|---|---|---|---|---|
| DM (n=405) | ASyS (n=649) | ASyS-DMskin (n=203) | ASyS-without-DMskin (n=446) | DM vs ASyS | DM vs ASyS-DMskin | ASyS-DMskin vs ASyS-without-DMskin | |
| Myopathic muscle weakness, n (%) | 350 (86) | 549 (85) | 178 (88) | 371 (83) | 0.468 | 0.758 | 0.175 |
| DM-type cutaneous manifestations, n (%) | |||||||
| Heliotrope rash | 248 (61) | 90 (14) | 90 (44) | 0 (0) | <0.001 | <0.001 | |
| Gottron’s papules or sign | 254 (63) | 141 (22) | 141 (70) | 0 (0) | <0.001 | 0.152 | |
| Violaceous rash | 166 (41) | 57 (9) | 57 (28) | 0 (0) | <0.001 | 0.004 | |
| Erythroderma | 37 (9) | 15 (2) | 15 (7) | 0 (0) | <0.001 | 0.599 | |
| Periorbital rash | 97 (24) | 38 (6) | 38 (19) | 0 (0) | <0.001 | 0.207 | |
| V sign rash | 124 (31) | 28 (4) | 28 (14) | 0 (0) | <0.001 | <0.001 | |
| Shawl sign | 133 (33) | 18 (3) | 18 (9) | 0 (0) | <0.001 | <0.001 | |
| Extramuscular manifestations, n (%) | |||||||
| Periungual erythema | 148 (37) | 110 (17) | 56 (28) | 54 (12) | <0.001 | 0.0503 | <0.001 |
| Calcinosis | 22 (5) | 13 (2) | 9 (4) | 4 (1) | 0.0044 | 0.74 | <0.001 |
| Ulceration | 28 (7) | 8 (1) | 4 (2) | 4 (1) | <0.001 | 0.0272 | 0.0221 |
| Vasculitis | 11 (3) | 2 (0.3) | 0 (0) | 2 (0.4) | 0.0018 | 0.0552 | 0.533 |
| Mechanic’s hands | 45 (11) | 200 (31) | 84 (41) | 116 (26) | <0.001 | <0.001 | <0.001 |
| Raynaud’s phenomenon | 55 (14) | 252 (39) | 90 (44) | 162 (36) | <0.001 | <0.001 | 0.109 |
| Arthritis | 64 (16) | 312 (48) | 101 (50) | 211 (47) | <0.001 | <0.001 | 0.679 |
| Dysphagia | 134 (33) | 128 (20) | 47 (23) | 81 (18) | <0.001 | <0.001 | 0.254 |
| Alopecia | 47 (12) | 39 (6) | 18 (9) | 21 (5) | 0.002 | 0.417 | 0.118 |
| Interstitial lung disease | 74 (18) | 441 (68) | 126 (62) | 315 (71) | <0.001 | <0.001 | 0.091 |
| Cardiac involvement | 9 (2) | 46 (7) | 19 (9) | 27 (6) | <0.001 | <0.001 | 0.233 |
| CAM, n (%) | 67 (17) | 21 (3) | 7 (3) | 14 (3) | <0.001 | <0.001 | 1 |
Chi-square test. ASyS: antisynthetase syndrome; ASyS-DMskin: antisynthetase syndrome with skin involvement; ASyS-without-DMskin: antisynthetase syndrome without skin involvement; CAM: cancer-associated myositis.
ASyS with DM-type skin involvement sub-cohort and comparison of clinical characteristics with DM cohort
The DM cohort was compared with ASyS patients possessing DM-type rashes. Of the 649 patients in the ASyS cohort, 31% (n = 203/649) had at least one of the seven DM-type rashes indicating skin involvement. Heliotrope rash, violaceous rash, V-sign and shawl sign were significantly more frequent in the DM cohort compared with the ASyS-DMskin sub-cohort, whereas there was no difference in frequency between DM and ASyS-DMskin for the remaining three DM-type rashes (Gottron’s papules/sign, periorbital rash, erythroderma). As was observed in the overall ASyS cohort, ILD, arthritis, Raynaud’s, mechanic’s hands and cardiac involvement were significantly more frequent in the ASyS-DMskin sub-cohort, compared with the DM cohort. However, there were no significant differences in the frequency of myopathic muscle weakness, periungual erythema, calcinosis, vasculitis and alopecia in the ASyS-DMskin and DM cohorts. (Table 1).
For the DM cohort, the median number of DM-type rashes reported was 2 out of 7 (IQR 1–4), which was significantly higher than the overall ASyS cohort (median 0, IQR 0–1, P < 0.001) and comparable to the ASyS-DMskin sub-cohort (median 2, IQR 1–2, P < 0.001) (Supplementary Table S7, available at Rheumatology online).
A comparison of extramuscular manifestations between the ASyS-DMskin and ASyS-without-DMskin sub-cohorts showed that the frequency of periungual erythema, calcinosis, mechanic’s hands and ulceration was significantly higher in the ASyS-DMskin sub-cohort (Table 1).
Comparison of clinical characteristics in ASyS and in DM by antibody
In patients with ASyS, DM-type cutaneous manifestations were seen in 25% (n = 136/542) of those with anti-Jo1, 27% (n = 11/41) with -PL12, 23% (n = 8/35) with -PL7, 19% (n = 3/16) with -EJ, 40% (n = 4/10) with -OJ and 0% (n = 0/6) with -Zo antibodies (Supplementary Table S8, available at Rheumatology online). The frequency of myopathic muscle weakness, arthritis and dysphagia within the ASyS cohort was not equally distributed across the different anti-ARS antibody subtypes where the lowest frequency of myopathic muscle weakness was seen in those with anti-PL12 antibodies (46%, n = 19/41), and the highest frequency of arthritis and dysphagia was seen in those anti-Zo antibodies (67%, n = 4/6 and 50%, n = 3/6, respectively) (Supplementary Table S8, available at Rheumatology online). The frequency of periungual erythema, ulceration, mechanic’s hands, arthritis, dysphagia, alopecia and ILD, as well as the frequency of certain DM-type cutaneous manifestations (heliotrope rash, Gottron’s papules/sign, violaceous rash, periorbital rash and V-sign rash) within the DM cohort were not equally distributed across DM antibody subtypes (Supplementary Table S9, available at Rheumatology online). In those with anti-MDA5 antibodies, there was high frequency of extramuscular manifestations including calcinosis (13%, n = 8/63), mechanic’s hands (27%, n = 17/63), arthritis (38%, n = 24/63) and ILD (57%, n = 36/63). Cutaneous manifestations were generally more frequent in those with anti-TIF1γ antibodies and in those with anti-SAE antibodies and less frequent in those with anti-MDA5 and anti-Mi2 antibodies.
Comparison of CAM in disease cohorts and by antibody
The number of patients with at least one CAM was significantly higher in the DM cohort compared with the ASyS cohort (n = 67/405, 17% vs n = 21/649, 3%, Padjusted < 0.001), and in the DM cohort compared with the ASyS-DMskin cohort (n = 67/405, 17% vs n = 7/203, 3%, Padjusted < 0.001) (Table 1). There was no significant difference between the frequency of CAM in ASyS-DMskin compared with ASyS-without-DMskin cohorts (n = 7/203, 3% vs n = 14/446, 3%, Padjusted = 1) (Table 1).
Bowel (12/405, 3% vs 2/649, 0.3%, Padjusted = 0.013), breast (16/405, 4% vs 7/649, 1%, Padjusted = 0.02), lung (10/405, 3% vs 3/649, 0.5%, Padjusted = 0.03) and ovarian cancers (15/405, 4% vs 0/649, 0%, Padjusted = 0.007) were more frequently reported in DM compared with ASyS (Supplementary Table S10, available at Rheumatology online). There were no significant differences in location of CAM between DM and ASyS-DMskin, or between ASyS-DMskin and ASyS-without-DMskin cohorts (Supplementary Table S10, available at Rheumatology online). The frequency of CAM was not equally distributed between antibody subtypes, χ2 (degrees of freedom = 9, n = 737, Padjusted < 0.001), and notably the highest frequency of CAM was observed in anti-TIF1γ patients (33%, n = 46/138) (Supplementary Table S11, available at Rheumatology online).
Discussion
We identified several important findings including: (i) one-third of ASyS patients have DM-type cutaneous manifestations; (ii) DM-specific skin rashes in ASyS patients were associated with a distinct phenotype including higher frequency of mechanic’s hands, Raynaud’s phenomenon, arthritis, ILD and cardiac involvement and lower frequency of ulceration and dysphagia; and (iii) DM-specific skin rash in ASyS patients was not associated with increased risk of cancer.
First, our study demonstrates that a third of patients with ASyS have DM-type cutaneous manifestations. Our results are consistent with the previous largest published study (n = 233), which found DM-type cutaneous manifestations with a prevalence of 28% in patients with ASyS [6]. This confirms that DM-type cutaneous manifestations are observed in a substantial proportion of patients with ASyS. Interestingly, our cohort also includes patients with EJ, OJ and Zo antibodies, whereas the previous study included patients with Jo1, PL12 and PL7 [6]. Our study therefore supports previous notions that a large proportion of ASyS patients have DM-specific skin manifestations, regardless of autoantibody status. Clinicians should therefore be vigilant for DM-specific manifestations in ASyS patients and actively treat them due to their detrimental impact on quality of life [12].
Second, our study demonstrates that DM-specific rashes in ASyS patients are associated with a distinct phenotype that differentiates them from DM and from ASyS patients without DM-specific rashes. However, we also noted that increased frequency of cardiac involvement differentiated ASyS from DM, and that increased frequency of mechanic’s hands, calcinosis, ulceration and periungual erythema differentiates ASyS-DMskin from ASyS-without-DMskin, suggesting that the pathogenesis underlying ASyS-specific cutaneous manifestations may have additional vascular and endothelial aetiologies over and above that which is seen in DM-specific cutaneous manifestations. We identified clinical features including increased frequency of mechanic’s hands, Raynaud’s phenomenon, arthritis, cardiac involvement and ILD that differentiate ASyS-DMskin from DM. Therefore, clinicians should consider a diagnosis of ASyS if these clinical signs are noted in the presence of DM-type rashes. Conversely, certain DM-type rashes (heliotrope rash, V-sign, violaceous rash and shawl sign) differentiate DM from ASyS-DMskin, and were infrequently observed in ASyS. Therefore, clinicians may not need to prioritize ASyS highly in the presence of these DM-type rashes and should instead prioritize a diagnosis of DM, and ensure malignancy screening and that other disease-specific management considerations are appropriately targeted.
Third, our study assesses whether ASyS-DMskin is associated with an increased risk of CAM and found that CAM was more frequent in DM compared with ASyS, as previously reported, but that CAM was not more frequent in ASyS-DMskin compared with ASyS-without-DMskin. The surveillance of malignancy is vital in the clinical management of DM given that it is the main cause of death in patients with IIM [14]. Interestingly, ILD and presence of anti-ARS have been associated with a lower risk of CAM, suggesting that patients with ASyS may have reduced risk of CAM compared with other IIM subtypes such as DM [1, 15]. Our findings suggest that although the cutaneous manifestations in ASyS-DMskin may be driven by similar biological processes as in DM, in ASyS-DMskin this may not confer increased risk of CAM. Therefore, in clinical practice, skin involvement in ASyS need not prompt increased surveillance or investigation for CAM.
The main strength of our study is the use of international registry data which includes the largest reported cohort of patients with DM and ASyS representing patients from centres around the world with different ethnicities. This is important given that DM and ASyS are rare diseases and would be otherwise difficult to study. However, use of registry data has limitations. First, missing data is an issue which may affect the accuracy of our findings. Second, although international collaboration is a strength when studying rare diseases, variations in clinical practice may lead to variability in reporting across centres. Third, although all patients in the MYONET registry have met current IIM classification criteria, we have further defined our DM and ASyS cohorts based on the presence of autoantibodies. However, not all patients with IIM have identifiable autoantibodies, for example, one study found 28% of DM cases were seronegative, and certain rare ASyS antibodies cannot be tested for in routine clinical practice and are therefore not represented in our study [16]. Fourth, the registry relies on clinicians with an expertise in IIM to apply IIM classification criteria prior to inclusion, and case notes were not reviewed or verified, potentially introducing a degree of misclassification. Fifth, the data analysed in this study are cross-sectional meaning clinical features that develop after entry to the registry are not captured. Finally, our analysis makes no comparison with healthy or connective tissue disease populations. Therefore, we cannot draw conclusions about whether frequency of malignancy in ASyS is higher than in the general population.
In conclusion, this is the largest study to date comparing clinical manifestations in ASyS to DM, and the first study to specifically investigate a cohort with ASyS and skin manifestations akin to DM. A third of patients with ASyS have DM-type cutaneous involvement compatible with a diagnosis of DM, but although this cohort resembles DM in terms of skin rashes, there are specific clinical manifestations which differentiate the two, and risk of CAM is lower than DM and similar to ASyS patients without DM-type skin involvement. Work to elucidate the biological processes underlying clinical manifestations in these cohorts would improve our ability to classify patients and develop targeted treatments for specific disease manifestations. These findings can inform future ASyS classification criteria and improve our ability to classify patients and develop targeted treatments for specific disease manifestations.
Supplementary Material
Acknowledgements
MYONET registry collaborators
Belgium: Sophie D’Hose (University Hospital, Ghent). China: Xin Lu and Xiaolan Tian (China-Japan Friendship Hospital, Beijing). Czech Republic: Heřman Mann, Olga Kryštůfková, Lenka Pleštilová, Martin Klein, Tereza Barochová, Kateřina Kubínová (Institute of Rheumatology, Prague). Italy: Chiara Gelardi, Alberto Paladini, Mario Andrea Piga. Mexico: Mexico: Luis J. Jara (Instituto Nacional de Rehabilitación Luis Guillermo Ibarra Ibarra), Miguel A. Saavedra (Servicio de Reumatología, Hospital de Especialidades, Centro Médico La Raza, IMSS), Claudia V. Cruz-Reyes (Servicio de Reumatología, Hospital de Especialidades, Centro Médico La Raza, IMSS), Olga Vera-Lastra (Servicio de Medicina Interna, Hospital de Especialidades, Centro Médico La Raza, IMSS), Lilia Andrade-Ortega (Servicio de Reumatologia, Centro Medico Nacional 20 de Noviembre ISSSTE), Gabriel Medrano-Ramírez (Servicio de Reumatología, Hospital General de Mexico, Secretaria de Salud), Minoru Satoh (University of Occupational and Environmental Health, Kitakyushu, Japan), Mario Salazar-Páramo (Departamento de Fisiología, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara), Efrain Chavarría-Ávila (Servicio de Reumatologia 004086 del SNP CONACyT, Hospital Civil Dr Juan I Menchaca, Universidad de Guadalajara, Guadalajara, Jalisco), Andrea Aguilar-Vazquez (Instituto de Investigación en Reumatología y del Sistema Músculo Esquelético, CUCS, Universidad de Guadalajara, Guadalajara, Jalisco), Jesus-Aureliano Robles-de Anda (Instituto de Investigación en Reumatología y del Sistema Músculo Esquelético, CUCS, Universidad de Guadalajara, Guadalajara, Jalisco), Marcelo H. Petri, (Instituto de Investigación en Reumatología y del Sistema Músculo Esquelético, CUCS, Universidad de Guadalajara, Guadalajara, Jalisco). Norway: Øyvind Molberg (Oslo University Hospital, Oslo). Sweden: Maryam Dastmalchi, Antonella Notarnicola, Karina Gheorghe (all Rheumatology Unit, Karolinska University Hospital, Karolinska Institutet), Johan Rönnelid, Maria Liden (both Uppsala University, Uppsala), Balsam Hanna, (Sahlgrenska Academy, Göteborg), Awat Jalal (Örebro Hospital), Helena Hellström (Falun), Jehns Christian Martineus (Lund University). Vietnam: Nguyen Thi Ngoc Lan (Hanoi Medical University, Vietnam), Leonid Padyukov (Karolinska Institutet, Sweden). United Kingdom: Paul New (Salford Royal NHS Foundation Trust), Hazel Platt (Centre for Integrated Genomic Medical Research, University of Manchester), Simon Rothwell (Centre for Integrated Genomic Medical Research, University of Manchester). UKMYONET: Yasmeen Ahmed (Llandudno General Hospital), Raymond Armstrong (Southampton General Hospital), Robert Bernstein (Manchester Royal Infirmary), Carol Black (Royal Free Hospital, London), Simon Bowman (University Hospital, Birmingham), Ian Bruce (Manchester Royal Infirmary), Robin Butler (Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry), John Carty (Lincoln County Hospital), Chandra Chattopadhyay (Wrightington Hospital), Easwaradhas Chelliah (Wrightington Hospital), Fiona Clarke (James Cook University Hospital, Middlesborough), Peter Dawes (Staffordshire Rheumatology Centre, Stoke on Trent), Christopher Denton (Royal Free London), Joseph Devlin (Pinderfields General Hospital, Wakefield), Christopher Edwards (Southampton General Hospital), Paul Emery (Academic Unit of Musculoskeletal Disease, Leeds), John Fordham (South Cleveland Hospital, Middlesborough), Alexander Fraser (Academic Unit of Musculoskeletal Disease, Leeds), Hill Gaston (Addenbrooke’s Hospital, Cambridge), Patrick Gordon (King’s College Hospital, London), Bridget Griffiths (Freeman Hospital, Newcastle), Harsha Gunawardena (Frenchay Hospital, Bristol), Frances Hall (Addenbrooke’s Hospital, Cambridge), Michael Hanna (University College London Hospitals), Beverley Harrison (North Manchester General Hospital), Elaine Hay (Staffordshire Rheumatology Centre, Stoke on Trent), David Hilton-Jones (Oxford University Hospitals), Lesley Horden (Dewsbury District General Hospital), John Isaacs (Freeman Hospital, Newcastle), David Isenberg (University College London Hospitals), Adrian Jones (Nottingham University Hospital), Sanjeet Kamath (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Kennedy (Royal Liverpool Hospital), George Kitas (Dudley Group Hospitals Trust, Birmingham), Peter Klimiuk (Royal Oldham Hospital), Sally Knights (Yeovil District Hospital, Somerset), John Lambert (Doncaster Royal Infirmary), Peter Lanyon (Queen’s Medical Centre, Nottingham), Ramasharan Laxminarayan (Queen’s Hospital, Burton Upon Trent), Bryan Lecky (Walton Neuroscience Centre, Liverpool), Raashid Luqmani (Nuffield Orthopaedic Centre, Oxford), Pedro Machado (University College London Hospitals), Jeffrey Marks (Steeping Hill Hospital, Stockport), Michael Martin (St. James University Hospital, Leeds), Dennis McGonagle (Academic Unit of Musculoskeletal Disease, Leeds), Neil McHugh (Royal National Hospital for Rheumatic Diseases, Bath), Francis McKenna (Trafford General Hospital, Manchester), John McLaren (Cameron Hospital, Fife), Michael McMahon (Dumfries & Galloway Royal Infirmary, Dumfries), Euan McRorie (Western General Hospital, Edinburgh), Peter Merry (Norfolk & Norwich University Hospital, Norwich), Sarah Miles (Dewsbury & District General Hospital, Dewsbury), James Miller (Royal Victoria Hospital, Newcastle), Anne Nicholls (West Suffolk Hospital, Bury St. Edmunds), Jennifer Nixon (Countess of Chester Hospital, Chester), Voon Ong (Royal Free Hospital, London), Katherine Over (Countess of Chester Hospital, Chester), John Packham (Staffordshire Rheumatology Centre, Stoke on Trent), Nicolo Pipitone (King’s College Hospital, London), Michael Plant (South Cleveland Hospital, Middlesborough), Gillian Pountain (Hinchingbrooke Hospital, Huntington), Thomas Pullar (Ninewells Hospital, Dundee), Mark Roberts (Salford Royal Foundation Trust), Paul Sanders (Wythenshawe Hospital, Manchester), David Scott (King’s College Hospital, London), David Scott (Norfolk & Norwich University Hospital, Norwich), Michael Shadforth (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Sheeran (Cannock Chase Hospital, Cannock, Staffordshire), Arul Srinivasan (Broomfield Hospital, Chelmsford), David Swinson (Wrightington Hospital), Lee-Suan Teh (Royal Blackburn Hospital, Blackburn), Michael Webley (Stoke Manderville Hospital, Aylesbury), Brian Williams (University Hospital of Wales, Cardiff) and Jonathan Winer (Queen Elizabeth Hospital, Birmingham).
Contributor Information
Ryan Malcolm Hum, Centre for Musculoskeletal Research, Division of Musculoskeletal & Dermatological Sciences, The University of Manchester Faculty of Biology Medicine and Health, Manchester, UK; The University of Manchester, National Institute for Health Research Manchester Biomedical Research Centre, Manchester, UK.
James B Lilleker, Centre for Musculoskeletal Research, Division of Musculoskeletal & Dermatological Sciences, The University of Manchester Faculty of Biology Medicine and Health, Manchester, UK; Northern Care Alliance NHS Foundation Trust, Manchester Centre for Clinical Neuroscience, Salford Royal Hospital, Salford, UK.
Janine A Lamb, Division of Population Health, Health Services Research and Primary Care, The University of Manchester Faculty of Biology Medicine and Health, Epidemiology and Public Health Group, Manchester, UK.
Alexander G S Oldroyd, Centre for Musculoskeletal Research, Division of Musculoskeletal & Dermatological Sciences, The University of Manchester Faculty of Biology Medicine and Health, Manchester, UK; The University of Manchester, National Institute for Health Research Manchester Biomedical Research Centre, Manchester, UK.
Guochun Wang, Department of Rheumatology, China-Japan Friendship Hospital, Beijing, China.
Lucy R Wedderburn, Great Ormond Street Hospital for Children NHS Foundation Trust, Infection, Immunity and Inflammation, London, UK.
Louise P Diederichsen, Center for Rheumatology and Spine Diseases, Copenhagen University Hospital, Copenhagen, Denmark.
Jens Schmidt, Department of Neurology, University Medical Center Göttingen, Göttingen, Germany; Department of Neurology and Pain Treatment, Neuromuscular Center, Center for Translational Medicine, Immanuel Klinik Rüdersdorf, University Hospital of the Brandenburg Medical School Theodor Fontane, Rüdersdorf bei Berlin, Germany; Faculty of Health Sciences Brandenburg, Brandenburg Medical School Theodor Fontane, Rüdersdorf bei Berlin, Germany.
Maria Giovanna Danieli, Clinica Medica, Dipartimento di Scienze Cliniche e Molecolari, Universita Politecnica delle Marche, Ancona, Italy.
Paula Oakley, Myositis UK, Southampton, UK.
Zoltan Griger, Department of Immunology, University of Debrecen, Debrecen, Hajdú-Bihar, Hungary.
Thuy Nguyen Thi Phuong, Internal Medicine Department, Hanoi Medical University, Hanoi, Vietnam.
Chanakya Kodishala, Clinical Immunology and Rheumatology, St John's National Academy of Health Sciences, Bangalore, Karnataka, India; Department of Rheumatology, Mayo Clinic, Rochester, MN, USA.
Monica Vazquez-Del Mercado, Division de Medicina Interna, Servicio de Reumatologia, Hospital Civil Dr. Juan I. Menchaca, Universidad de Guadalajara, Guadalajara, Jalisco, Mexico.
Helena Andersson, Department of Rheumatology, Oslo University Hospital, Oslo, Norway.
Boel De Paepe, Department of Neurology, Universitair Ziekenhuis Gent, Ghent, Belgium.
Jan L De Bleecker, Department of Neurology, Universitair Ziekenhuis Gent, Ghent, Belgium.
Britta Maurer, Department of Rheumatology and Immunology, Inselspital University Hospital Bern, Bern, Switzerland.
Liza McCann, Department of Rheumatology, Alder Hey Children's NHS Foundation Trust, Liverpool, UK.
Nicolo Pipitone, Department of Rheumatology, Arcispedale Santa Maria Nuova di Reggio Emilia, Reggio Emilia, Emilia-Romagna, Italy.
Neil McHugh, Department of Rheumatology, Royal National Hospital for Rheumatic Diseases, Bath, UK; Department of Pharmacy and Pharmacology, University of Bath, Bath, UK.
Robert Paul New, MRC/ARUK Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, UK.
William E Ollier, Faculty of Science and Engineering, Manchester Metropolitan University, Manchester, UK.
Niels Steen Krogh, Zitelab Aps, Copenhagen, Denmark.
Jiri Vencovsky, Institute of Rheumatology and Department of Rheumatology, Charles University, Praha, Czech Republic.
Ingrid E Lundberg, Division of Rheumatology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Department of Gastroenterology, Dermatology, and Rheumatology, Karolinska University Hospital, Stockholm, Sweden.
Hector Chinoy, Centre for Musculoskeletal Research, Division of Musculoskeletal & Dermatological Sciences, The University of Manchester Faculty of Biology Medicine and Health, Manchester, UK; Northern Care Alliance NHS Foundation Trust, Department of Rheumatology, Salford Royal Hospital, Salford, UK.
MYONET Registry:
Sophie D’Hose, Xin Lu, Xiaolan Tian, Heřman Mann, Olga Kryštůfková, Lenka Pleštilová, Martin Klein, Tereza Barochová, Kateřina Kubínová, Chiara Gelardi, Alberto Paladini, Mario Andrea Piga, Luis J Jara, Miguel A Saavedra, Claudia V Cruz-Reyes, Olga Vera-Lastra, Lilia Andrade-Ortega, Gabriel Medrano-Ramírez, Minoru Satoh, Mario Salazar-Páramo, Efrain Chavarría-Ávila, Andrea Aguilar-Vazquez, Jesus-Aureliano Robles-de Anda, Marcelo H Petri, Øyvind Molberg, Maryam Dastmalchi, Antonella Notarnicola, Karina Gheorghe, Johan Rönnelid, Maria Liden, Balsam Hanna, Awat Jalal, Helena Hellström, Jehns Christian Martineus, Nguyen Thi Ngoc Lan, Leonid Padyukov, Paul New, Hazel Platt, Simon Rothwell, Yasmeen Ahmed, Raymond Armstrong, Robert Bernstein, Carol Black, Simon Bowman, Ian Bruce, Robin Butler, John Carty, Chandra Chattopadhyay, Easwaradhas Chelliah, Fiona Clarke, Peter Dawes, Christopher Denton, Joseph Devlin, Christopher Edwards, Paul Emery, John Fordham, Alexander Fraser, Hill Gaston, Patrick Gordon, Bridget Griffiths, Harsha Gunawardena, Frances Hall, Michael Hanna, Beverley Harrison, Elaine Hay, David Hilton-Jones, Lesley Horden, John Isaacs, David Isenberg, Adrian Jones, Sanjeet Kamath, Thomas Kennedy, George Kitas, Peter Klimiuk, Sally Knights, John Lambert, Peter Lanyon, Ramasharan Laxminarayan, Bryan Lecky, Raashid Luqmani, Pedro Machado, Jeffrey Marks, Michael Martin, Dennis McGonagle, Neil McHugh, Francis McKenna, John McLaren, Michael McMahon, Euan McRorie, Peter Merry, Sarah Miles, James Miller, Anne Nicholls, Jennifer Nixon, Voon Ong, Katherine Over, John Packham, Nicolo Pipitone, Michael Plant, Gillian Pountain, Thomas Pullar, Mark Roberts, Paul Sanders, David Scott, David Scott, Michael Shadforth, Thomas Sheeran, Arul Srinivasan, David Swinson, Lee-Suan Teh, Michael Webley, Brian Williams, and Jonathan Winer
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data will be shared upon reasonable requests to the corresponding author.
Funding
This study was supported in part by: Association Francaise Contre Les Myopathies (AFM), The European Union Sixth Framework Programme (project AutoCure; LSH-018661), European Science Foundation (ESF) in the framework of the Research Networking Programme European Myositis Network (EUMYONET), The Swedish Research Council (K2014-52X-14045-14-3) and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet; Myositis UK; Medical Research Council (MRC) UK grant MR/N003322/1; Arthritis Research UK (18474). The Czech cohort was supported by Project for Conceptual Development of Research Organization 00023728 from Ministry of Health in the Czech Republic.
This research was supported by researchers at the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC) (NIHR203308) and the National Institute for Health Research (NIHR) GOSH Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Disclosure statement: B.M. declares the following conflicts of interest: consultancies with Novartis, Boehringer Ingelheim, Janssen-Cilag and GSK, grant/research support from AbbVie, Protagen and Novartis Biomedical; speaker fees from Boehringer-Ingelheim, GSK and Novartis; as well as congress support from Medtalk, Pfizer, Roche, Actelion, Mepha and MSD, and a patent involving the use of mir-29 for the treatment of systemic sclerosis (US9247389, EP2331143). L.P.D. has received funding from Boehringer Ingelheim and has served on a data safety monitoring board for Corbus Pharmaceuticals. JLDB participates in Euro-NMD.
Ethics: All patients gave informed written consent for their data to be analysed as part of this study. The MYONET (previously EuroMyositis) registry includes multiple recruiting centres in multiple countries, where ethical approvals are required and have been sought at each centre and informed consent is obtained from all included patients. All centres obtained specific ethical approval from their local ethics committees for this study.
References
- 1. Oldroyd A, Lilleker J, Chinoy H. Idiopathic inflammatory myopathies – a guide to subtypes, diagnostic approach and treatment. Clin Med (Lond) 2017;17:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witt LJ, Curran JJ, Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med 2016;23:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol 2018;14:290–302. [DOI] [PubMed] [Google Scholar]
- 4. Lundberg IE, Tjärnlund A, Bottai M et al. ; International Myositis Classification Criteria Project consortium, The Euromyositis register and The Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland). 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mammen AL, Allenbach Y, Stenzel W, Benveniste O; ENMC 239th Workshop Study Group. 239th ENMC International Workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14-16 December 2018. Neuromuscul Disord 2020;30:70–92. [DOI] [PubMed] [Google Scholar]
- 6. Hervier B, Devilliers H, Stanciu R et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 2012;12:210–7. [DOI] [PubMed] [Google Scholar]
- 7. Lilleker JB, Vencovsky J, Wang G et al. ; All EuroMyositis Contributors. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis 2018;77:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohan A, Peter JB. Polymyositis and Dermatomyositis: (First of Two Parts). New England Journal of Medicine 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- 9. Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 2010;138:1464–74. [DOI] [PubMed] [Google Scholar]
- 10. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 11. RC Team. R: a language and environment for statistical computing. 2015. https://www.r-project.org/ (30 June 2021, date last accessed).
- 12. Hundley JL, Carroll CL, Lang W et al. Cutaneous symptoms of dermatomyositis significantly impact patients' quality of life. J Am Acad Dermatol 2006;54:217–20. [DOI] [PubMed] [Google Scholar]
- 13. Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 2011;37:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobloug GC, Svensson J, Lundberg IE, Holmqvist M. Mortality in idiopathic inflammatory myopathy: results from a Swedish nationwide population-based cohort study. Ann Rheum Dis 2018;77:40–7. [DOI] [PubMed] [Google Scholar]
- 15. Hamaguchi Y, Fujimoto M, Matsushita T et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 2013;8:e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker MJS, Oldroyd A, Roberts ME et al. The performance of the European League Against Rheumatism/American College of Rheumatology idiopathic inflammatory myopathies classification criteria in an expert-defined 10 year incident cohort. Rheumatology (Oxford) 2019;58:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared upon reasonable requests to the corresponding author.