Abstract
Objectives
To evaluate a strategy designed to optimize care and increase uptake of urate-lowering therapy (ULT) during hospitalizations for gout flares.
Methods
We conducted a prospective cohort study to evaluate a strategy that combined optimal in-hospital gout management with a nurse-led, follow-up appointment, followed by handover to primary care. Outcomes, including ULT initiation, urate target attainment and re-hospitalization rates, were compared between patients hospitalized for flares in the 12 months post-implementation and a retrospective cohort of hospitalized patients from 12 months pre-implementation.
Results
One hundred and nineteen and 108 patients, respectively, were hospitalized for gout flares in the 12 months pre- and post-implementation. For patients with 6-month follow-up data available (n = 94 and n = 97, respectively), the proportion newly initiated on ULT increased from 49.2% pre-implementation to 92.3% post-implementation (age/sex-adjusted odds ratio [aOR] 11.5; 95% CI 4.36, 30.5; P < 0.001). After implementation, more patients achieved a serum urate ≤360 μmol/l within 6 months of discharge (10.6% pre-implementation vs 26.8% post-implementation; aOR 3.04; 95% CI 1.36, 6.78; P = 0.007). The proportion of patients re-hospitalized for flares was 14.9% pre-implementation vs 9.3% post-implementation (aOR 0.53; 95% CI 0.22, 1.32; P = 0.18).
Conclusion
Over 90% of patients were initiated on ULT after implementing a strategy to optimize hospital gout care. Despite increased initiation of ULT during flares, recurrent hospitalizations were not more frequent following implementation. Significant relative improvements in urate target attainment were observed post-implementation; however, for the majority of hospitalized gout patients to achieve urate targets, closer primary–secondary care integration is still needed.
Keywords: gout, crystal arthritis, hospital, admissions, urate-lowering therapy, allopurinol
Rheumatology key messages.
After optimizing hospital gout care, more than 90% of patients were initiated on urate-lowering therapy.
Significant relative improvements in urate target attainment were seen following a single, nurse-led, post-discharge appointment.
However, for the majority of hospitalized gout patients to achieve target, closer primary–secondary care integration is still needed.
Introduction
Hospitalizations for gout flares have increased markedly over the last 20 years, doubling in the USA, England and Canada [1–4]. These increases have occurred despite widespread availability of urate-lowering therapies (ULT), such as allopurinol and febuxostat. When titrated to achieve serum urate targets ≤360 μmol/l, ULT prevents flares, improves quality of life and leads to long-term reductions in hospitalizations [5–7]. International guidelines have been updated to encourage the uptake of treat-to-target ULT [8–11]. However, population-level data continue to show that ULT is initiated in only a minority of patients, while few patients achieve the urate targets necessary to prevent flares and hospitalizations [6, 12–14].
For avoidable gout admissions to be prevented, strategies are needed to optimize care and increase uptake of treat-to-target ULT in hospitalized patients. A recent systematic review found a paucity of high-quality studies in people hospitalized for gout [15]. Specifically, no prospective studies to date had evaluated strategies designed to encourage ULT uptake and prevent re-admissions in hospitalized patients [15]. We sought to address this knowledge gap.
In this study, we evaluated a strategy designed to optimize hospital gout care and increase uptake of ULT. Our strategy was modelled on a nurse-led intervention shown to be highly effective at optimizing gout management in primary care [5]. We adapted this strategy for implementation during hospitalizations for flares, and assessed outcomes including ULT initiation, serum urate target attainment and rates of re-hospitalization.
Methods
Study design and intervention
We performed a prospective cohort study at a large teaching hospital in South London, UK, which serves a population of over 1 million people. We evaluated outcomes after implementation of a strategy designed to optimize care for people hospitalized for gout flares, and compared these outcomes with a retrospective cohort of hospitalized patients from before implementation.
The intervention package consisted of two key components: (i) an in-hospital gout management pathway (Supplementary Data S1, available at Rheumatology online), based on British Society for Rheumatology (BSR), European Alliance of Associations for Rheumatology (EULAR), and American College of Rheumatology (ACR) gout management guidelines [8–10]; and (ii) a nurse-led telephone appointment performed 2 weeks after discharge.
The intervention was developed with extensive stakeholder input, following a systematic literature review [15], audit and process mapping of gout care at our hospital [16]. The management pathway was designed as a quick-reference guide on optimal gout care for use by frontline clinicians and rheumatologists. This included recommendations on: diagnostic tests (including serum urate levels and joint aspiration); rheumatologist input; flare treatments (NSAIDs, colchicine and/or corticosteroids, where appropriate); offering ULT (allopurinol first-line) to all patients unless contraindicated; initiating ULT during the acute flare; considering prophylaxis against flares during ULT initiation and titration; admission-avoidance strategies (e.g. ambulatory care units); disease education; and post-discharge advice (including treat-to-target ULT optimization, as recommended in the BSR gout management guideline [8]).
A nurse-led telephone clinic was established to provide patients with a single follow-up appointment within 2 weeks of discharge. This clinic was delivered on a weekly basis by a specialist rheumatology nurse, trained in gout management, with appointments lasting ∼30 min per patient. Objectives were to review symptoms, provide disease education, discuss flare management strategies, and provide advice to patients and their primary care team on ULT dose optimization using a treat-to-target strategy [8]. After this appointment, care was handed over to the patient’s primary care team via a clinical letter. For patients with severe gout and/or recurrent admissions, additional rheumatology outpatient follow-up could be considered.
To maximize uptake of the intervention, a multi-pronged implementation strategy was developed with implementation experts. This incorporated strategies from the Expert Recommendations for Implementing Change (ERIC) guidance [17], including digital order sets, study champions, education sessions, advertising, executive approval, quality monitoring and clinician feedback (Supplementary Data S2, available at Rheumatology online).
Study period
The study period was from 30 October 2020 to 29 April 2023. The intervention was launched on 30 October 2021. Data were collected prospectively on all patients hospitalized with gout flares in the 12-month period after intervention launch (30 October 2021–29 October 2022). Post-implementation outcomes were compared with outcomes for patients hospitalized with gout in the 12-month period prior to launch (30 October 2020–29 October 2021). All patients with linked primary care data were followed up for 6 months after discharge to review post-discharge outcomes (detailed below).
Case definitions
All emergency department (ED) attendances and admission episodes for gout flares (collectively referred to as hospitalizations) in the pre- and post-implementation periods were included. Both primary and secondary admission diagnoses of gout (e.g. flares occurring during hospitalizations for other reasons) were eligible, assuming gout was deemed the likely cause of the acute joint symptoms by the primary clinical team. Although recommended in our pathway, confirmatory joint aspiration and/or rheumatology input were not mandated, as reflects local clinical practice. Patients managed solely in an urgent care centre (primarily staffed by general practitioners rather than ED clinicians) were excluded, as the urgent care centre facility at our hospital was transferred to another institution prior to intervention launch.
Data sources
All data used in these analyses were routinely captured during clinical care. In-hospital data were extracted from electronic patient records. Post-discharge data were extracted from local care records, containing primary and secondary care data for patients with linked NHS identifiers who had not opted out of this service [18]. All data were manually validated by a rheumatologist, and pseudonymized for the purposes of analysis. Outcomes were selected a priori with stakeholder input.
Baseline characteristics
Baseline data were collected as follows: age; sex; admission type (ED attendance-only vs hospital admission); day/time of presentation (defined as out-of-hours if occurring between 9 p.m. and 9 a.m. or on a Saturday/Sunday); pre-existing gout diagnosis; pre-existing prescription for ULT (allopurinol, febuxostat, benzbromarone, sulfinpyrazone or probenecid); and baseline blood tests, if performed during the presentation (serum urate, CRP, white cell count, neutrophil count, lymphocyte count and serum creatinine).
Outcomes during hospitalization
Data were captured to ascertain whether the following outcomes occurred during the hospitalization episode: rheumatology input sought; serum urate level performed; joint aspiration performed; flare treatment(s) prescribed (NSAID, colchicine and/or oral, intramuscular, intravenous or intra-articular corticosteroids); disease education provided to patients; ULT initiated (if patient not already receiving ULT) or up-titrated (if patient already receiving ULT at a sub-optimal dose); prophylaxis prescribed (low-dose colchicine, NSAIDs or corticosteroids); gout-specific recommendations and/or follow-up on discharge.
Outcomes after hospitalization
For patients with available follow-up data, we ascertained whether the following outcomes occurred within 6 months of discharge: ULT initiation and/or up-titration; prescription of prophylaxis against flares during ULT initiation/titration; number of serum urate levels performed; attainment of serum urate targets ≤360 μmol/l and/or ≤300 μmol/l; follow-up in the gout telephone clinic and/or rheumatology outpatient clinic; re-attendance at ED and/or re-admission with a subsequent gout flare (occurring >7 days after discharge from the initial presentation).
Statistical methods
Baseline characteristics were tabulated, and between-cohort differences estimated using the chi-square test for categorical variables and an independent Student’s t-test for continuous variables. Logistic regression, with adjustment for age and sex, was used to estimate differences in categorical outcomes between pre- and post-implementation cohorts, expressed as adjusted odds ratios (aOR) with 95% CI. Linear regression, with adjustment for age and sex, was used to estimate differences in continuous outcomes, expressed as adjusted β-coefficients (aβ) with 95% CI. Kaplan–Meier survival curves were presented for repeat hospitalizations. Univariable logistic regression was performed to explore a differential impact of the intervention on patient subgroups, categorized by age, sex, admission type, time of presentation, whether the gout diagnosis was pre-existing, or whether rheumatology input was sought during hospitalization. Stata v17 (StataCorp, College Station, TX, USA) was used for all analyses. No adjustment was performed for multiple hypothesis testing, as this was an exploratory study.
Study approval and ethics
Approval to undertake this study under the remit of service evaluation was obtained from King’s College Hospital NHS Foundation Trust. No further ethical approval or written informed patient consent was required, as per UK Health Research Authority guidance.
Patient and public involvement
Patients have been closely involved in all stages of this project. Patient feedback was instrumental in conceptualizing this project, and in designing the intervention. In particular, patients emphasized the importance of a holistic, multi-faceted intervention and implementation strategy, recognizing that a single intervention was unlikely to address the multiple barriers to optimal hospital gout care. Patients will be closely involved in disseminating the findings of this study, and in developing follow-on projects.
Results
Baseline characteristics
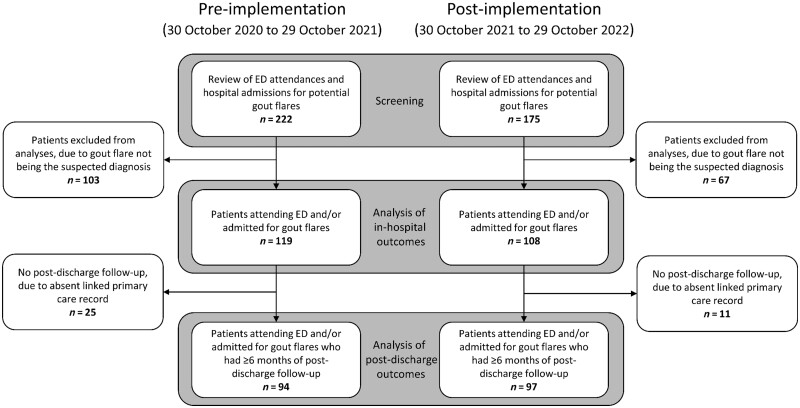
In the 12 months prior to implementation of the intervention, 119 people attended ED with gout flares, of whom 63 (52.9%) required admission to hospital. In the 12 months after implementation, 108 attended ED with gout flares, of whom 53 (49.1%) required admission. A study flowchart is shown in Fig. 1.
Figure 1.
Study flowchart depicting the pre- and post-implementation study cohorts. ED: emergency department
Baseline characteristics are shown in Table 1. Pre- and post-implementation cohorts had similar mean ages (62 vs 64 years, respectively). There were proportionally more female patients in the pre- than post-implementation cohort (26.9% vs 15.7%). The proportion of patients who had pre-existing gout diagnoses was similar in the pre- and post-implementation cohorts (66.4% vs 67.6%); 41.8% and 27.4% of known gout patients in the pre- and post-implementation cohorts, respectively, were receiving ULT prior to hospitalization. Mean serum urate levels at baseline were comparable (485 vs 487 μmol/l).
Table 1.
Baseline characteristics of the pre- and post-implementation cohorts
| Characteristic | Pre-implementation (n = 119) | Post-implementation (n = 108) | P-value |
|---|---|---|---|
| Age, mean (s.d.), years | 62 (16) | 64 (16) | 0.31 |
| Sex, n (%) | |||
| Female | 32 (26.9) | 17 (15.7) | 0.041 |
| Male | 87 (73.1) | 91 (84.3) | |
| Admission type, n (%) | |||
| Discharged from ED | 56 (47.1) | 55 (50.9) | 0.56 |
| Admitted to hospital | 63 (52.9) | 53 (49.1) | |
| Presented out-of-hours, n (%) | 59 (49.6) | 43 (39.8) | 0.14 |
| Pre-existing gout diagnosis, n (%) | 79 (66.4) | 73 (67.6) | 0.85 |
| Receiving ULT prior to hospitalization, n (%) | 33 (41.8) | 20 (27.4) | 0.063 |
| Not on ULT prior to hospitalization, n (%) | 46 (58.2) | 53 (72.6) | |
| Serum urate at baseline, mean (s.d.), μmol/l | 485 (185) | 487 (125) | 0.94 |
| CRP at baseline, mean (s.d.), mg/l | 89 (84) | 76 (79) | 0.28 |
| White cell count at baseline, mean (s.d.), ×109/l | 9.3 (3.5) | 9.5 (4.6) | 0.76 |
| Neutrophil count at baseline, mean (s.d.), ×109/l | 6.6 (3.1) | 6.5 (3.9) | 0.79 |
| Lymphocyte count at baseline, mean (s.d.), ×109/l | 1.6 (0.8) | 1.8 (0.9) | 0.14 |
| Creatinine at baseline, mean (s.d.), μmol/l | 165 (161) | 142 (116) | 0.24 |
Blood test results represent the first tests performed during the hospitalization. Inferential statistics for between-cohort differences were obtained from independent t-tests for continuous variables and chi-square tests for categorical variables. ULT: urate-lowering therapy.
Outcomes during hospitalizations
In-hospital outcomes were compared before and after implementation (Table 2). Following implementation, specialist rheumatology input was obtained more frequently in hospital (54.6% pre-implementation vs 75.9% post-implementation; aOR 2.48; 95% CI 1.37, 4.52; P = 0.003); serum urate levels were performed in more patients (66.4% vs 92.6%; aOR 6.32; 95% CI 2.75, 14.5; P < 0.001); and joint aspiration was performed more frequently (19.3% vs 47.2%; aOR 3.44; 95% CI 1.88, 6.27; P < 0.001).
Table 2.
Outcomes during hospitalizations for gout flares, comparing the pre- and post-implementation cohorts
| Outcome | Pre-implementation, n (%) (n = 119) | Post-implementation, n (%) (n = 108) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Rheumatology input during hospitalization | 65 (54.6) | 82 (75.9) | 2.48 (1.37, 4.52) | 0.003 |
| Serum urate level performed | 79 (66.4) | 100 (92.6) | 6.32 (2.75, 14.5) | <0.001 |
| Joint aspiration performed | 23 (19.3) | 51 (47.2) | 3.44 (1.88, 6.27) | <0.001 |
| Flare treatment prescribed | 111 (93.3) | 106 (98.1) | 4.46 (0.91, 21.8) | 0.065 |
| NSAIDs | 38 (31.9) | 34 (31.5) | 1.18 (0.61, 2.28) | 0.63 |
| Colchicine | 74 (62.2) | 86 (79.6) | 2.30 (1.23, 4.31) | 0.009 |
| Corticosteroids | 25 (21.0) | 40 (37.0) | 2.20 (1.21, 4.02) | 0.010 |
| Multiple flare treatments prescribed | 21 (17.6) | 49 (45.4) | 4.10 (2.20, 7.67) | <0.001 |
| Intra-articular steroid injection | 2 (1.7) | 9 (8.3) | 5.53 (1.15, 26.7) | 0.033 |
| Disease education documented prior to discharge | 27 (22.7) | 24 (22.2) | 1.00 (0.53, 1.90) | 0.99 |
| ULT initiated and/or titrated during hospitalization | 21 (17.6) | 67 (62.0) | 7.69 (4.12, 14.4) | <0.001 |
| Gout recommendations documented on discharge | 70 (58.8) | 93 (86.1) | 4.33 (2.21, 8.48) | <0.001 |
| Recommendation to initiate and/or titrate ULT after discharge | 18 (15.1) | 42 (38.9) | 3.26 (1.71, 6.19) | <0.001 |
| Recommendation for prophylaxis while titrating ULT | 23 (19.3) | 13 (12.0) | 0.54 (0.26, 1.15) | 0.11 |
| Recommendation for target serum urate level | 13 (10.9) | 28 (25.9) | 2.56 (1.23, 5.36) | 0.012 |
| Recommendation for primary care follow-up | 53 (44.5) | 43 (39.8) | 0.82 (0.48, 1.41) | 0.47 |
| Recommendation for rheumatology/gout clinic follow-up | 11 (9.2) | 73 (67.6) | 19.8 (9.34, 42.0) | <0.001 |
Odds ratios from logistic regression models are shown, with adjustment for age and sex. ULT: urate-lowering therapy.
Of pre- and post-implementation cohorts, 93.3% and 98.1%, respectively, received a guideline-recommended flare treatment. After implementation, more patients were prescribed colchicine (62.2% vs 79.6%; aOR 2.30; 95% CI 1.23, 4.31; P = 0.009), corticosteroids (21.0% vs 37.0%; aOR 2.20; 95% CI 1.21, 4.02; P = 0.010) or multiple flare treatments (17.6% vs 45.4%; aOR 4.10; 95% CI 2.20, 7.67; P < 0.001). Use of intra-articular corticosteroids increased modestly from a low baseline (1.7% vs 8.3%; aOR 5.53; 95% CI 1.15, 26.7; P = 0.033). There was no significant difference in the use of NSAIDs (31.9% vs 31.5%; aOR 1.18; 95% CI 0.61, 2.28; P = 0.63).
The proportion of patients initiated and/or up-titrated on ULT prior to discharge increased markedly following implementation, from 17.6% to 62.0% (aOR 7.69; 95% CI 4.12, 14.4; P < 0.001). After implementation, more patients were provided with gout-specific management recommendations on discharge (58.8% vs 86.1%; aOR 4.33; 95% CI 2.21, 8.48; P < 0.001). Documented evidence of disease education provision prior to discharge was low in both cohorts (22.7% vs 22.2%; aOR 1.00; 95% CI 0.53, 1.90; P = 0.99).
Outcomes after hospitalizations
Of the patients in the pre- and post-implementation cohorts, 94/119 (79.0%) and 97/108 (89.8%), respectively, had primary care follow-up data available to facilitate analyses of post-discharge outcomes (Table 3). By 6 months post-discharge, 91/97 (93.8%) of the post-implementation cohort were prescribed ULT, compared with 61/94 (64.9%) pre-implementation (aOR 7.68; 95% CI 3.02, 19.6; P < 0.001). When restricted to patients not receiving ULT prior to hospitalization, the proportion of patients who newly initiated ULT in hospital or within 6 months of discharge increased markedly after implementation, from 49.2% to 92.3% (aOR 11.5; 95% CI 4.36, 30.5; P < 0.001). Of all patients receiving ULT by 6 months, 57/61 (93.4%) and 90/91 (98.9%) of the pre- and post-implementation cohorts, respectively, were prescribed allopurinol. There was no significant difference in prophylaxis use between patients newly initiating ULT in the pre- vs post-implementation periods (25.0% vs 29.2%; aOR 1.12; 95% CI 0.42, 2.98; P = 0.81).
Table 3.
Outcomes in the 6-month period after hospitalizations for gout flares, comparing the pre- and post-implementation cohorts
| Outcome | Pre-implementation, n (%) (n = 94) | Post-implementation, n (%) (n = 97) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Receiving ULT by 6 months | 61 (64.9) | 91 (93.8) | 7.68 (3.02, 19.6) | <0.001 |
| ULT initiated in hospital or within 6 months of discharge | ||||
| Yes | 32 (49.2) | 72 (92.3) | 11.5 (4.36, 30.5) | <0.001 |
| No | 33 (50.8) | 6 (7.7) | ||
| Receiving ULT pre-admission | 29 | 19 | ||
| Prophylaxis prescribed while initiating ULT | ||||
| Yes | 8 (25.0) | 21 (29.2) | 1.12 (0.42, 2.98) | 0.81 |
| No | 24 (75.0) | 51 (70.8) | ||
| Not newly initiated on ULT | 62 | 25 | ||
| Serum urate performed at least once within 6 months | 30 (31.9) | 56 (57.7) | 2.88 (1.58, 5.25) | 0.001 |
| Serum urate ≤360 μmol/l within 6 months | 10 (10.6) | 26 (26.8) | 3.04 (1.36, 6.78) | 0.007 |
| Serum urate ≤300 μmol/l within 6 months | 5 (5.3) | 13 (13.4) | 2.65 (0.89, 7.84) | 0.079 |
| Rheumatology outpatient clinic within 6 months | 8 (8.5) | 16 (16.5) | 2.08 (0.82, 5.28) | 0.12 |
| Gout telephone clinic within 6 months | N/A | 79 (81.4) | — | — |
| Re-presented to hospital within 6 months | 14 (14.9) | 9 (9.3) | 0.53 (0.22, 1.32) | 0.18 |
Odds ratios from logistic regression models are shown, with adjustment for age and sex. A gout telephone clinic was established as part of the intervention package, and therefore was not available to patients in the pre-implementation cohort. N/A: not available; ULT: urate-lowering therapy.
Following implementation, more patients achieved a serum urate ≤360 μmol/l within 6 months of discharge: 10/94 (10.6%) pre-implementation vs 26/97 (26.8%) post-implementation (aOR 3.04; 95% CI 1.36, 6.78; P = 0.007). There was no significant difference in the proportion of patients achieving a serum urate ≤300 μmol/l within 6 months (5.3% pre-implementation vs 13.4% post-implementation; aOR 2.65; 95% CI 0.89, 7.84; P = 0.079). Mean reductions in serum urate at 6 months, relative to baseline, were 29.7 μmol/l pre-implementation vs 96.8 μmol/l post-implementation (aβ −64.3; 95% CI −128.0, −0.64; P = 0.048). The mean number of serum urate levels performed within 6 months increased from 0.5 tests pre-implementation to 1.1 tests post-implementation (aβ 0.55; 95% CI 0.14, 0.96; P = 0.009).
Of the post-implementation cohort, 79/97 (81.4%) were reviewed in the nurse-led gout telephone clinic (median time to review: 12 days), while 16/97 (16.5%) patients received rheumatology outpatient follow-up within 6 months. Prior to implementation, 8/94 (8.5%) patients received rheumatology outpatient follow-up within 6 months of discharge.
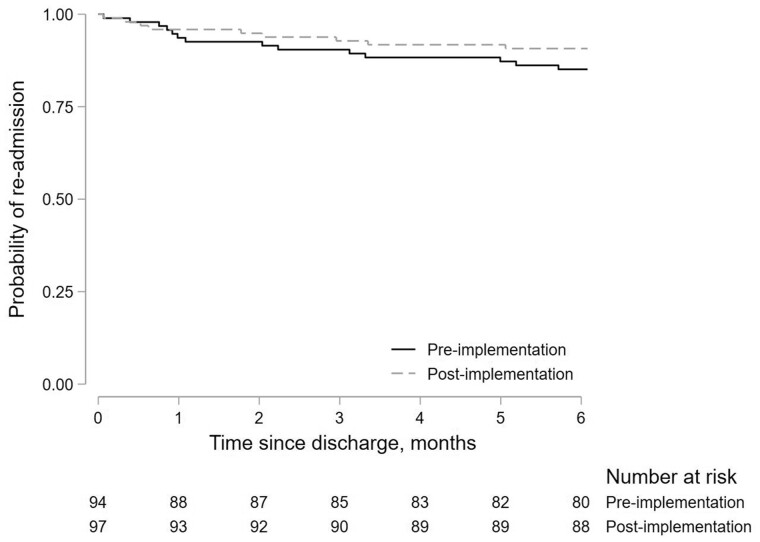
The number of patients who re-attended ED and/or were re-admitted for gout flares within 6 months of discharge was 14/94 (14.9%) pre-implementation vs 9/97 (9.3%) post-implementation (aOR 0.53; 95% CI 0.22, 1.32; P = 0.18). Survival curves for repeat hospitalizations are shown in Fig. 2.
Figure 2.
Survival curve showing the probability of re-attendance at ED and/or re-admission to hospital for gout flares following discharge. Pre-implementation (solid line) and post-implementation (dashed line) cohorts are shown. The number of patients at risk at each time point is shown in a risk table
Further analyses were performed to explore the impact of the intervention on different subgroups of patients (Supplementary Figs S1–S4, available at Rheumatology online). Odds of ULT initiation were significantly higher in the post-implementation than pre-implementation cohort, irrespective of age, sex, admission type, time of presentation, whether the gout diagnosis was pre-existing or whether rheumatology input was sought during hospitalization (Supplementary Fig. S1, available at Rheumatology online).
Discussion
Following implementation of a strategy designed to optimize care during gout hospitalizations, >90% of ULT-naïve patients were initiated on ULT—nearly double the pre-implementation baseline. Many other aspects of care improved, including urate target attainment and post-discharge follow-up. The initiation of ULT during flares did not increase recurrent hospitalizations, supporting the use of ULT in this setting.
Our intervention was modelled on one shown to be highly effective in a primary care setting. In a large randomized controlled trial in the UK, nurse-delivered patient education and treat-to-target ULT resulted in 95% of patients achieving serum urate targets within 1 year, compared with 26% with usual care [5]. Flare frequency, tophi and quality of life all improved, and the intervention was shown to be cost-effective. We adapted this intervention for implementation in a hospital setting. As well as optimizing care during patients’ hospital stays, we established a nurse-led, post-discharge clinic to facilitate disease education and provide advice on ULT optimization. This appointment was delivered as a single telephone appointment, recognizing that in-person appointments can be challenging for patients to attend after hospitalizations for flares. Care was then handed over to patients’ primary care teams for ongoing management.
Following implementation of this strategy, many aspects of hospital gout care improved: joint aspirations increased; serum urate levels were performed more frequently; use of guideline-recommended flare treatments increased (particularly combination therapy); and gout-specific follow-up was provided to more patients. Rheumatologist input also increased; specialist support for frontline clinicians was felt to be an important facilitator of optimal gout care during our stakeholder consultations [16], supported by previous analyses demonstrating that rheumatology input associates with improvements in care for hospitalized gout patients [19–22].
The biggest change observed following implementation of our strategy was increased initiation of ULT. By 6 months post-discharge, 94% of patients had been prescribed ULT. This is comparable to ULT initiation rates in the Nottingham primary care-based study [5], and substantially better than the 61% of patients who were receiving ULT within 12 months of hospitalization in a recent UK-wide analysis [6]. In particular, there was a 3-fold increase in the proportion of patients initiating and/or up-titrating ULT prior to discharge. There has been extensive debate around the relative benefits and harms of early ULT initiation (vs deferred initiation of ULT after flare resolution), with international guidelines varying widely in this regard [8, 10, 11]. We advocated for early ULT initiation for several reasons. First, hospitalizations provide unique opportunities for clinicians to optimize care for people with long-term conditions, such as gout. Second, accumulating evidence suggests that upfront initiation of ULT does not prolong or worsen intercurrent flares, provided it is initiated alongside flare treatment [23–26]. Third, earlier initiation of ULT leads to more timely reductions in serum urate levels [23–26]. Finally, this approach can help to mitigate a breakdown in communication between secondary care and primary care, whereby post-discharge recommendations to initiate ULT are not acted upon [19].
Despite the marked increase in ULT initiation during flares, we did not observe an increase in hospitalizations for recurrent flares after implementing this strategy. These real-world data support those obtained from trial settings [23–26]. Although not statistically significant, proportionately fewer re-hospitalizations occurred after implementation, relative to before (37.6% relative reduction; 5.6% absolute reduction), suggesting a potential for benefit with this approach. One contributory factor might have been the post-discharge follow-up appointment, which gave patients an opportunity to have any ongoing symptoms reviewed. Advice on flare management was provided within this appointment, empowering patients to self-manage flares. With longer follow-up, there is the potential for more admissions to be prevented with this strategy: observational data show that ULT associates with a significantly reduced risk of recurrent hospitalizations from 12 months after initiation, particularly when urate targets are attained [6]. Future work will help to determine whether primary care workload is also reduced following implementation of better hospital gout care.
Prior to implementation, only 10% of patients achieved a serum urate ≤360 μmol/l within 6 months of discharge. After implementation, urate target attainment more than doubled, to 26.8%. Despite this relative improvement, absolute levels of urate target attainment remained far below those seen in the Nottingham primary care trial (95% attainment by 12 months) [5]. Similarly, target attainment was below that reported in the BSR National Audit of outpatient gout management by UK rheumatologists (45% attainment by 12 months) [27], and only modestly better than what was reported in a UK-wide analysis of post-discharge gout care (1184/7040 [16.8%] patients attaining urate ≤360 μmol/l within 12 months of hospitalization [6]). There are several possible reasons for this. Follow-up in our study was relatively short at only 6 months. Patients in our study were all hospitalized for gout, and therefore are likely to represent a more severe cohort. Perhaps most importantly, in the Nottingham study there were an average of 17 study visits per participant over a 24-month period. In contrast, our gout follow-up clinic was delivered as a single telephone appointment, followed by handover of care to patients’ primary care teams. Indeed, target attainment in our study was comparable to that seen in the usual care group of the Nottingham trial (26.8% vs 26.2%, respectively). Thus, our findings strongly suggest that our intervention, while effective at facilitating ULT initiation, is insufficient for the majority of people hospitalized for gout flares to achieve target urate levels.
There are several ways in which our intervention could be altered to promote urate target attainment. Rheumatologists could take greater ownership of hospitalized gout patients by providing outpatient follow-up until urate targets are achieved. Alternatively, training could be provided for healthcare professionals in primary care (e.g. nurses and/or pharmacists) to deliver optimal treat-to-target ULT, which was shown to be highly effective in the Nottingham study [5], NOR-Gout study [7] and many other studies [28, 29]. Strategies could be modelled on other integrated care services, which proactively identify patients in hospital before transferring them to primary care-based pathways with secondary care support, such as fracture liaison services [30]. Future strategies should also encourage adherence to ULT, which although not assessed in our study, is often sub-optimal and may have contributed to our finding of infrequent urate target attainment despite high levels of ULT initiation [31]; this could incorporate patient education programmes, self-management tools and point-of-care urate testing [30].
A key strength of our study was the close involvement of stakeholders, patients and methodologists when developing our intervention and implementation strategy. Our intervention was based on best practice care from national and international gout management guidelines [8–11], and was modelled on the highly successful intervention used in the Nottingham primary care trial [5]. We adopted a multi-faceted implementation strategy to maximize intervention uptake. This incorporated several implementation strategies recommended in the ERIC guidance [17], including digital enablers, study champions, education sessions and clinician feedback. Adopting a multi-faceted strategy is particularly important when implementing complex interventions in healthcare settings. For example, an implementation strategy involving only educational sessions for clinicians in emergency departments may not succeed, given the challenges of reaching all frontline staff.
Our study also had limitations. Follow-up was only 6 months, which may have been too short to ascertain differences in post-discharge outcomes such as urate target attainment and recurrent hospitalizations. While the numerical reductions in re-hospitalizations we observed following implementation of our strategy might have been clinically meaningful, our study was underpowered to detect significant differences in these relatively rare events. Use of prophylaxis against flares whilst initiating/titrating ULT remained infrequent despite the intervention, particularly when compared with national data [27], and future studies should encourage the use of prophylaxis, given the benefits on flare reduction [8]. Data on comorbidities and other clinical outcomes (e.g. flare frequency or tophus burden) were unavailable. We included all hospitalizations where gout was deemed the likely diagnosis; crystal analysis and rheumatologist input were recommended, but not mandated. These pragmatic inclusion criteria reflect real-world clinical practice, although there remains a potential for diagnostic misclassification. Additionally, we utilized a retrospective comparator, rather than a prospective comparator. This was an a priori decision, to reflect resource availability and the service evaluation remit of this project; however, it is possible that some of the changes observed may represent changes in practice over time, rather than a direct result of the intervention (e.g. changes in service delivery during the COVID-19 pandemic). Our findings should therefore be seen as exploratory, rather than definitive. Finally, as our analyses were conducted at a single UK centre, the findings cannot be assumed to be generalizable to other healthcare settings.
In conclusion, after implementing a strategy designed to optimize care for people hospitalized with gout flares, >90% of patients were initiated on ULT. In the context of a single, nurse-led follow-up appointment, relative improvements in urate target attainment were observed; however, for the majority of hospitalized gout patients to achieve target urate levels, better in-hospital gout care needs to be accompanied by strategies that embed and support optimization of ULT in primary care.
Supplementary Material
Contributor Information
Mark D Russell, Centre for Rheumatic Diseases, King’s College London, London, UK.
Louise Ameyaw-Kyeremeh, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Flora Dell’Accio, Centre for Rheumatic Diseases, King’s College London, London, UK.
Heather Lapham, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Natalie Head, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Christopher Stovin, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Vishit Patel, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Benjamin D Clarke, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Deepak Nagra, Centre for Rheumatic Diseases, King’s College London, London, UK.
Edward Alveyn, Centre for Rheumatic Diseases, King’s College London, London, UK.
Maryam A Adas, Centre for Rheumatic Diseases, King’s College London, London, UK.
Katie Bechman, Centre for Rheumatic Diseases, King’s College London, London, UK.
María A de la Puente, Department of Psychology, Health Psychology Section, Institute of Psychiatry, Psychology, & Neuroscience, King's College London, London, UK.
Benjamin Ellis, Department of Rheumatology, Imperial College Healthcare NHS Foundation Trust, London, UK.
Corrine Byrne, Pharmacy Department, King’s College Hospital NHS Foundation Trust, London, UK.
Rina Patel, Pharmacy Department, King’s College Hospital NHS Foundation Trust, London, UK.
Andrew I Rutherford, Department of Rheumatology, King’s College Hospital NHS Foundation Trust, London, UK.
Fleur Cantle, Department of Emergency Medicine, King’s College Hospital NHS Foundation Trust, London, UK.
Sam Norton, Centre for Rheumatic Diseases, King’s College London, London, UK.
Edward Roddy, School of Medicine, Keele University, Keele, UK.
Joanna Hudson, Department of Psychology, Health Psychology Section, Institute of Psychiatry, Psychology, & Neuroscience, King's College London, London, UK.
Andrew P Cope, Centre for Rheumatic Diseases, King’s College London, London, UK.
James B Galloway, Centre for Rheumatic Diseases, King’s College London, London, UK.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article cannot be shared publicly to ensure the privacy of individuals that participated in the study.
Funding
This work is supported by a National Institute for Health and Care Research (NIHR) Doctoral Fellowship (NIHR300967) awarded to M.D.R. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, Public Health England, or the Department of Health and Social Care. No funding bodies had any role in study design, data collection, analysis or interpretation, manuscript writing, or in the decision to submit the article for publication.
Disclosure statement: M.D.R. has received honoraria from Lilly, Galapagos and Menarini, support for attending conferences from Lilly, Pfizer, Janssen and UCB, and advisory board fees from Biogen. J.B.G. has received honoraria from Abbvie, Biovitrum, Bristol Myers Squib (BMS), Celgene, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB. A.P.C. has received grants from BMS, consulting fees from BMS, AbbVie and GSK/Galvini, speaker fees from BMS and AbbVie, and is on the executive committee of the EULAR research centre. A.I.R. has received fees from Lilly and UCB for attending a conference. K.B. has received grant funding from Versus Arthritis/Pfizer. All other authors declare no competing interests.
References
- 1. Russell MD, Yates M, Bechman K et al. Rising incidence of acute hospital admissions due to gout. J Rheumatol 2020;47:619–23. [DOI] [PubMed] [Google Scholar]
- 2. NHS Digital. Hospital Admitted Patient Care Activity. 2021. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity (6 November 2023, date last accessed).
- 3. Lim SY, Lu N, Oza A et al. Trends in gout and rheumatoid arthritis hospitalizations in the United States, 1993-2011. JAMA 2016;315:2345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rai SK, Avina-Zubieta JA, McCormick N et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada From 2000 to 2011. Arthritis Care Res (Hoboken) 2017;69:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doherty M, Jenkins W, Richardson H et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell MD, Roddy E, Rutherford AI et al. Treat-to-target urate-lowering therapy and hospitalizations for gout: results from a nationwide cohort study in England. Rheumatology 2023;62:2426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uhlig T, Karoliussen LF, Sexton J et al. One- and 2-year flare rates after treat-to-target and tight-control therapy of gout: results from the NOR-Gout study. Arthritis Res Ther 2022;24:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui M, Carr A, Cameron S et al. ; British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology Guideline for the Management of Gout. Rheumatology (Oxford) 2017;56:1246. [DOI] [PubMed] [Google Scholar]
- 9. Richette P, Doherty M, Pascual E et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 10. FitzGerald JD, Dalbeth N, Mikuls T et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken) 2020;72:744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NICE. Gout: diagnosis and management. 2022. https://www.nice.org.uk/guidance/ng219 (6 November 2023, date last accessed).
- 12. Russell MD, Rutherford AI, Ellis B, Norton S et al. Management of gout following 2016/2017 European (EULAR) and British (BSR) guidelines: an interrupted time-series analysis in the United Kingdom. Lancet Regional Heath—Europe 2022;18:100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015;74:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abhishek A, Tata LJ, Mamas M, Avery AJ. Has the gout epidemic peaked in the UK? A nationwide cohort study using data from the Clinical Practice Research Datalink, from 1997 to across the COVID-19 pandemic in 2021. Ann Rheum Dis 2022;81:898–9. [DOI] [PubMed] [Google Scholar]
- 15. Russell MD, Clarke BD, Roddy E, Galloway JB. Improving outcomes for patients hospitalized with gout: a systematic review. Rheumatology (Oxford) 2021;61:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell MD, Nagra D, Clarke BD et al. Hospitalizations for acute gout: process mapping the inpatient journey and identifying predictors of admission. J Rheumatol 2022;49:725–30. [DOI] [PubMed] [Google Scholar]
- 17. Powell BJ, Waltz TJ, Chinman MJ et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NHS. Opt out of sharing your health records. 2023. https://www.nhs.uk/using-the-nhs/about-the-nhs/opt-out-of-sharing-your-health-records/ (6 November 2023, date last accessed).
- 19. Kapadia A, Abhishek A. Inpatient rheumatology consultation for gout flares and advice to initiate urate lowering treatment (ULT) in hospital discharge summary increases ULT prescription in primary care. Joint Bone Spine 2019;86:271–2. [DOI] [PubMed] [Google Scholar]
- 20. Kennedy NJ, Healy PJ, Harrison AA. Inpatient management of gout in a New Zealand hospital: a retrospective audit. Int J Rheum Dis 2016;19:205–10. [DOI] [PubMed] [Google Scholar]
- 21. Sen M. SAT0443 Reconciliation of urate lowering therapies during hospitalization and the impact of rheumatologic consultation on management of inpatient gout flares. Ann Rheum Dis 2019;78:1310–1. [Google Scholar]
- 22. Wright S, Chapman PT, Frampton C et al. Management of Gout in a Hospital Setting: a Lost Opportunity. J Rheumatol 2017;44:1493–8. [DOI] [PubMed] [Google Scholar]
- 23. Hill EM, Sky K, Sit M, Collamer A, Higgs J. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol 2015;21:120–5. [DOI] [PubMed] [Google Scholar]
- 24. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med 2012;125:1126–34 e7. [DOI] [PubMed] [Google Scholar]
- 25. Jia E, Zhang Y, Ma W et al. Initiation of febuxostat for acute gout flare does not prolong the current episode: a randomized clinical trial. Rheumatology 2021;60:4199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satpanich P, Pongsittisak W, Manavathongchai S. Early versus Late Allopurinol Initiation in Acute Gout Flare (ELAG): a randomized controlled trial. Clin Rheumatol 2022;41:213–21. [DOI] [PubMed] [Google Scholar]
- 27. Roddy E, Packham J, Obrenovic K, Rivett A, Ledingham JM. Management of gout by UK rheumatologists: a British Society for Rheumatology national audit. Rheumatology (Oxford) 2018;57:826–30. [DOI] [PubMed] [Google Scholar]
- 28. Goldfien RD, Ng MS, Yip G et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open 2014;4:e003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gill I, Dalbeth N, Ofanoa M, Goodyear-Smith F. Interventions to improve uptake of urate-lowering therapy in patients with gout: a systematic review. BJGP Open 2020;4:bjgpopen20X101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Royal Osteoporosis Society. Fracture Liaison Services. 2023. https://theros.org.uk/healthcare-professionals/clinical-quality-hub/fracture-liaison-services/ (6 November 2023, date last accessed).
- 31. Perez-Ruiz F, Desideri G. Improving adherence to gout therapy: an expert review. Ther Clin Risk Manag 2018;14:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to ensure the privacy of individuals that participated in the study.