Abstract
Background and Objective
Although more frequent in the adult population, rectal prolapse is a common anorectal condition that can occur in children and adolescents. While many cases spontaneously resolve without the need for intervention, the advent of newer minimally invasive procedures and operations have provided options for pediatric patients. Here, we review the pathophysiology, etiology, presentation, diagnosis and principles of management of rectal prolapse in the pediatric population as it has evolved over the past several decades.
Methods
The literature was queried from free databases available to the public including the National Institute of Health National Library of Medicine MEDLINE and PubMed for manuscripts published from January 1, 1975 to December 1, 2023. Manuscripts without an accompanying English translation or those written entirely in foreign languages were excluded.
Key Content and Findings
Numerous conditions contribute to rectal prolapse in children, including constipation, gastrointestinal infectious and non-infectious etiologies, cystic fibrosis, malnutrition, neurogenic, anatomic, lead points, and abuse. Initial management of rectal prolapse is medical management, addressing the underlying condition associated with rectal prolapse along with attempted manual reduction. For patients with recurrent rectal prolapse, a variety of noninvasive and procedural management options are available including injection sclerotherapy and anal encirclement in addition to surgical rectopexy by open and newer minimally invasive methods.
Conclusions
Despite significant advances in the evaluation, procedural and surgical management of pediatric anorectal conditions in the last few decades, there continues to be substantial variation in clinicians’ and surgeons’ practice for the treatment of rectal prolapse in children and adolescents. Much remains to be studied in the future to improve clinical outcomes for this patient population.
Keywords: Rectal prolapse, pediatric, anorectal disease
Introduction
Rectal prolapse was described as far back as Hippocrates in the 400s before christ (BC) who recognized the condition and offered risk-factors and descriptions of herbal medication for diarrhea and pain control as well as manual reduction (1). Among children, rectal prolapse was initially described first by Frederick Salmon in 1831 with an intention to treat by both medical and procedural management (2). In many ways, although the diagnosis and management of rectal prolapse have evolved, there continues to be variations in practice and management.
Although often self-limited in nature and responsive to non-operative management, pediatric rectal prolapse presents as a uniquely distressing problem for both parents and children alike (3-6). Rectal prolapse in children can be attributed to a series of different underlying disorders, and the approach to management of the prolapse differs based on etiology. Surgical management in particular has been widely debated, and significant variation in practice patterns among surgeons exists. While previous literature reviews have focused on diagnostic criteria and presentation of rectal prolapse, few have attempted to give significant detail to the interventional and surgical options available for the treatment of rectal prolapse including newer minimally invasive options and their currently studied outcomes. In the following manuscript, we review the pathophysiology, etiology, presentation, diagnosis and principles of management of rectal prolapse in the pediatric population as it has evolved over the past several decades. We present this article in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-128/rc).
Methods
The search query was performed of free databases available to the public including the National Institute of Health National Library of Medicine MEDLINE and PubMed for manuscripts published from January 1, 1975 to December 1, 2023 (Table 1). Manuscripts without an accompanying English translation or those written entirely in foreign languages were excluded.
Table 1. Search strategy summary.
| Items | Specification |
|---|---|
| Date of search | December 1, 2023 |
| Databases and other sources searched | National Institute of Health National Library of Medicine PubMed and MEDLINE |
| Search terms used | (“children” OR “pediatric”) AND “rectal prolapse” (MeSH Term), “prolapse, rectal” (MeSH Term), “anorectal prolapse”, “prolapse of rectum” |
| Timeframe | January 1, 1975 through December 1, 2023 |
| Inclusion and exclusion criteria | Inclusion: reports and literature reviews of patients <18 years old with rectal prolapse |
| Exclusion: patients ≥18 years old, studies written in languages other than English without an accompanying translation | |
| Selection process | All authors conducted the selection |
Discussion
Anatomy and pathophysiology
The rectum of a child, particularly under the age of three to four years old, is shorter and straighter compared to adult patients. Anatomic factors that predispose children to extrusion of rectal mucosa through the external anal sphincter include a more mobile sigmoid colon, connective tissue attachments with greater laxity between the rectum and sacrum, diminished ischiorectal fat, poor levator ani support and a low-lying rectum. In addition, the valves of Houston are not present in 75% of infants under the age of one (3). Rectal prolapse, at times described as a circumferential intussusception phenomenon, presents as a result of the aforementioned anatomic features (7).
The condition is divided into two subtypes with a further breakdown of the second subtype. Type I is considered a false rectal prolapse, otherwise considered to be mucosal extrusion through the external anal sphincter without full thickness prolapse. This presentation can be distinguished by radial folds of the rectal mucosa, usually less than 2 cm. Type II refers to a true rectal prolapse with a full-thickness prolapse of the rectal wall. Within type II prolapse, there is further subdivision: first degree (including the mucocutaneous junction), less than 5 cm; second degree (without mucocutaneous junction involvement), between 2–5 cm; and third degree, occult internal prolapse with no passage through the anal verge (8).
In early stages of prolapse, the rectum protrudes from the anus after defecation and retracts spontaneously after completion of a bowel movement. As time progresses, an increased frequency of rectal procidentia occurs both upon straining and spontaneously, at times requiring manual reduction. Complications associated with rectal prolapse include edema, bleeding, ulceration, and thrombosis (9).
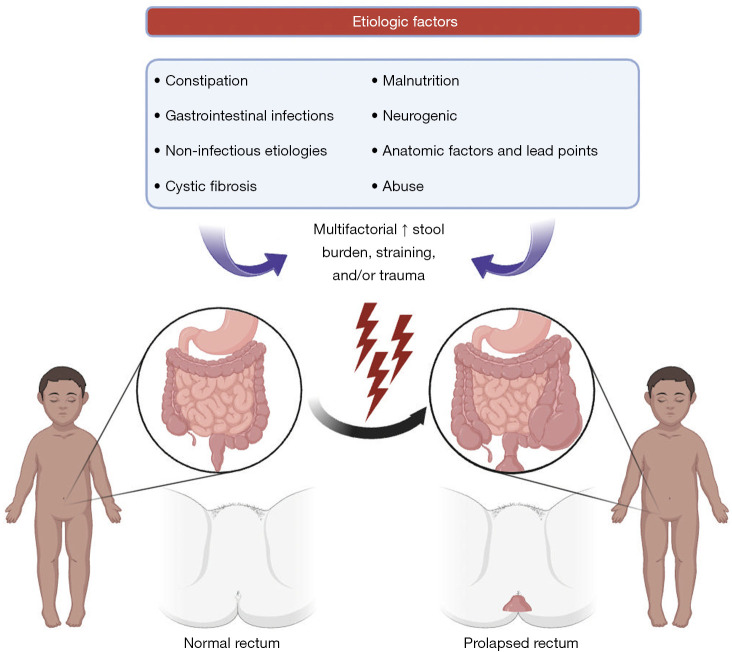
Etiologic factors
Various conditions contribute to rectal prolapse and including constipation, gastrointestinal infectious and non-infectious etiologies, cystic fibrosis (CF), malnutrition, neurogenic and anatomic factors and lead points, and abuse (Figure 1). The most common cause of rectal prolapse in developed nations is chronic constipation (3). The physical straining associated with constipation secondary to increased abdominal pressure can contribute to rectal prolapse. Bowel regimens with laxative use and high fiber diet are among first-line options in the medical management of rectal prolapse (10).
Figure 1.
Pathophysiology and etiologic factors of rectal prolapse in the pediatric population.
A variety of infectious gastrointestinal protozoan including parasitic and bacterial agents as well as viral illnesses can contribute to rectal prolapse (11). Bhandari and colleagues in India report the following organisms have contributed to the incidence of pediatric rectal prolapse, particularly in tropical regions of the world: bacillary dysentery, giardia, amoebic, trichuriasis, bilharziasis (12). Other organisms associated with diarrhea include Salmonella, Shigella, E. coli 01557:H7, cytomegalovirus (CMV), Clostridium diffcile (3,13). Non-infectious etiologies associated with disordered bowel motility include inflammatory and autoimmune conditions such as ulcerative colitis and celiac disease, and short bowel syndromes (14,15). In such cases, treatment of rectal prolapse is focused on treating the underlying disorder.
The evaluation of CF screening and its relationship to rectal prolapse management has similarly evolved over time (16,17). Historically, rectal prolapse would prompt initiation of a sweat chloride test to rule out CF. CF, with intractable cough and increased intra-abdominal pressure, copious diarrheal stools, and malnutrition was felt to contribute to rectal prolapse. In the past two decades, with the advent of newborn CF screening programs, the incidence of rectal prolapse associated with CF has decreased significantly (13).
Malnutrition can contribute to rectal prolapse secondary to the loss of the ischiorectal fat pad and decreased rectal support. The mechanism of malnutrition is two-fold. The low-protein state can lead to mucosal edema and an immunosuppressed state may predispose to gastrointestinal infections (8). Of note, a randomized clinical trial by Mazumder in Bangladesh found implementation of a high-energy dense diet for 75 children resulted in a higher resolution of rectal prolapse versus the control milk-cereal group (26% in the energy-dense diet group vs. 8%, in the control arm after 5 days; 13% in the energy-dense diet group vs. 6% in the control arm after 10 days) after acute shigellosis (18).
Neurologic conditions related to rectal prolapse include myelomeningocele, tethered cord, neurogenic bladder, spinal cord injury and spina bifida. Pelvic floor weakness, levator ani paralysis, and decreased sphincter tone contribute to the development of rectal prolapse (6). Other rare congenital conditions associated with rectal prolapse include Ehlers-Danlos syndrome, Hirschsprung’s disease, and protein allergy (13,19). Lead points in the form of polyps, lymphoid hyperplasia, and pseudopolyps in ulcerative colitis can also increase risk for intussusception and subsequent rectal prolapse (13). Prolapsed polyps, while rare in childhood, can present with bleeding or anal prolapse and should remain within the differential diagnosis when evaluating prolapse in children (20). Rare congenital anatomic lead points such as rectal duplication cysts have also been documented as causative factors for prolapse in the pediatric population (21).
Behavioral disorders have been associated with increased rectal prolapse incidence. Shah et al. describe children with behavioral or developmental delays who are at higher risk of defecation-related disorders (22). The pathology includes straining against a closed sphincter mechanism which may increase the risk of rectal prolapse; this pattern may become cyclic and obsessive (13,22). Hill and colleagues describe the under-recognized population of rectal prolapse for children with behavioral and psychiatric disorders (BPD) who have higher risk for complication and recurrence (23). Recent literature has highlighted multi-disciplinary therapy with behavioral and physical therapy, including biofeedback therapy, in addition to medical therapy, to be critical in optimization of rectal prolapse outcomes in children with BPD (23).
Finally, it is critical all pediatric healthcare providers consider anal penetration as a causative factor associated with pediatric rectal prolapse; sexual abuse and nonaccidental trauma should be considered and ruled out in the management of children presenting with rectal prolapse (24).
Clinical presentation and diagnosis
Rectal prolapse is usually brought to the attention of healthcare providers by way of a parent or caregiver. A painless red bulge appearing at the anus is present upon defecation, usually with associated straining. Although benign, the condition can be disturbing to parents and as the authors in a review of rectal prolapse surgical management write, “it is worthwhile to notice that all parents were distressed and at their wits’ end” when medical management was not successfully achieved (25). A thorough history and physical examination can be sufficient to make the diagnosis. A photograph or description shared by caregivers is also useful in confirming the diagnosis. Akkoyun and colleagues also write of their success for their pediatric patients and parents in Turkey using digital photos to demonstrate anal pathology to a dedicated pediatric surgeon (26). In an increasingly digital era, the use of “telemedicine” to provide improved differential diagnoses is a viable solution to a distressing issue for parents. At times, it is difficult to elicit the prolapse in the clinic; having patients perform a Valsalva maneuver or squat may help (13,26). Other symptoms include bleeding, diarrhea, constipation, and abdominal pain (27). Here it is again critical to distinguish mucosal prolapse as compared to full thickness wall prolapse. Rectal prolapse etiology varies by age. Patients under the age of four usually present with rectal prolapse secondary to anatomic factors including a mobile sigmoid colon, lack of Houston valves, and a low, straight rectum. Children over the age of four typically present with rectal prolapse secondary to a predisposing condition as described above (13,27). In patients with persistent or recurrent rectal prolapse, further evaluation with barium contrast and a flexible sigmoidoscopy is warranted to rule out solitary rectal ulcer syndrome and determine if there is a prolapse lead point (28-30). Defecography has emerged as a valuable method of diagnosing pelvic floor disorders particularly in the adult population as an adjunct to help identify rectal prolapse; however, this modality continues to have limited applicability in the pediatric population and has been understudied in these patients (31).
Medical management
Attempts at reduction can be performed in-office and taping of the buttocks can be utilized post-procedure if rectal prolapse immediately recurs (13). The recommended procedure typically includes placing the patient prone; well lubricated gloves are used to apply pressure to the prolapsed mucosa and a finger can be used to guide the rectal wall reduction; digital rectal examination is performed after reduction and a pressure dressing or tape can be used if the prolapse recurs immediately. If the prolapse is difficult to reduce, classically, sugar can also be applied to decrease the edema and allow for manual reduction (32,33); the use of hyperosmolar solutions such as 50% glucose solution have also been reported to aid with reduction (34,35). Parents and patients can also be taught to reduce the prolapse (9).
Initial management of rectal prolapse is medical management and addressing the underlying condition associated with rectal prolapse (i.e., constipation, malnutrition, CF, etc.). Scott and colleagues in their single-center review extensively discuss their bowel management program and success with rectal prolapse resolution (10). Their program included a contrast enema to evaluate for lead points and ruling out CF. Their bowel management program involved good toileting habits (less than 5 minutes on the toilet), bulking agents, and high dose stimulant laxatives (2 mg/kg/day) if there was prior evidence of constipation. Follow up also included daily abdominal radiographs and phone calls by the clinic nurse staff to adjust laxative dosing with families. Patients were followed and re-assessed at the 6-month mark and expectations and management goals were reviewed with families; recurrent prolapse without complication was described as “not dangerous” and providers recommended continuing with their bowel management program in order to decrease the need for surgery. In their retrospective review of their institution between 2011 and 2020, 47 children with rectal prolapse were identified. Median time to resolution was 9 months (interquartile range, 4–13 months) and nearly 94% of patients avoided surgery. Children over the age of four had a longer median time to spontaneous resolution by 7 months (10).
The discussion of age as a feature to consider in treatment of rectal prolapse is nuanced. Children over the age of four, as described above, are more likely to have rectal prolapse because of an underlying condition. Some authors recommend earlier referral for surgical management in patients who are likely to have recurrent rectal prolapse (8,22).
Procedural management
The difficulty in choosing the appropriate procedural management for pediatric rectal prolapse is highlighted by the over 130 surgical procedures that have been described for adults and over 40 procedures for children (3,25,36,37). Surgical options are utilized when patients have recurrent episodes of rectal prolapse, ulceration associated with bleeding, frequent hospital visits, reductions, prolapses, failure of medical management and/or patient or family hesitation to continue with medical management (38). Below, we review a variety of commonly discussed surgical techniques. A summary of the medical and procedural options available for pediatric rectal prolapse management is provided in Table 2.
Table 2. Summary of medical and procedural management strategies for pediatric rectal prolapse.
| Management | Technique and notes | Resolution vs. recurrence | Complications and considerations |
|---|---|---|---|
| Manual reduction (medical) | Patient placed prone, lubrication applied, digital rectal exam performed and manual reduction of rectal prolapse is assisted with hyperosmolar agent | May require multiple reductions, can be performed outpatient and by parents | If unable to reduce, may require inpatient hospitalization |
| Bowel regimen (medical) | Use of laxatives, good toileting habits, high fiber diet and constipation management after ruling out other underlying conditions | Up to 94% success rate with non-operative management | Children over the age of 4 may have longer time to resolution or are more likely to fail this management option |
| Injection sclerotherapy (procedural) | Sclerosing agents injected above the dentate line to induce inflammation. Agents include: ethyl alcohol, 5% phenol in almond oil, 50% dextrose, 15% saline, and sodium tetradecyl | First line treatment; resolution rates are 55–96% (varies widely) | May require general anesthesia and multiple procedural interventions; complications: perianal fistula, rectovaginal fistula, temporary limping, and abscess formation |
| Thiersch stitch/anal cerclage (procedural) | A circumferential absorbable suture placed between skin and anal mucosa and tied down over Hager dilators | 90% resolution with 1–2 Thiersch procedures. Also useful for patients who have recurrent rectal prolapse | May require multiple procedures prior to successful resolution |
| Ekehorn’s rectopexy (procedural) | U-shaped suture inserted through the rectal ampulla and tied externally at sacrococcygeal junction, tying down the suture over a piece of iodine-soaked gauze | 100% resolution in 3 studies | Requires general anesthesia; occasional localized infection requiring antibiotics |
| Suture rectopexy (procedural) | Intra-abdominal technique with rectal tissue sutured to pre-sacral space to promote adherence. Approach can be laparoscopic or robotic. Resection rectopexy is controversial | 0–40% recurrence rates (varies widely) | Use of general anesthesia; complications: urinary retention, constipation |
Injection sclerotherapy often serves as a first-line treatment for recurrent rectal prolapse with variable efficacy ranging from 55% to 96% (28,39,40). Sclerosing agents including ethyl alcohol, 5% phenol in almond oil, 50% dextrose, 15% saline, and sodium tetradecyl sulfate have been reported for use with varying efficacy (40-43). The procedure relies on principles of inflammation, adhesion and fibrosis, that are utilized to adhere the rectal wall to perirectal tissue to prevent procidentia relapse. The sclerosant is injected above the dentate line and initiates an inflammatory reaction; the technique may require multiple injections and requires general anesthesia (3). Complications can include perianal fistula, rectovaginal fistula, temporary limping, and abscess formation (3,28). Injection sclerotherapy nevertheless is felt to be a reasonable first-line option given the low-risk nature of the procedure. In continuing with inflammatory attempts to induce adhesions similar to injection sclerotherapy, the use of pre-sacral packing or the Lockhart Mummery procedure has also been described as a low-risk procedure. Gauze packing is used in the retrorectal space and removed in order to induce inflammation with an 87% to 100% success rate reported in some case series (3,33,44).
Frequently associated and performed with injection sclerotherapy is the Thiersch stitch or anal cerclage. The technique includes using electrocautery to create a defect at the border of the skin and anal mucosa and placing a circumferential absorbable suture, tying down the knot over one or two Hager dilators. Linear cauterization can also be used as a modification (45). The procedure has good success, although it may require repeat procedures (45,46).
The use of Ekehorn’s rectopexy has been described as early as 1909 with good results. Schepens and Verhelst describe their experience with Ekehorn’s rectopexy in 22 children between 1976 and 1991 with 100% resolution. In brief, the technique is described as a U-shaped suture, inserted through the rectal ampulla with the suture tied externally at the sacrococcygeal junction (38). Under general anesthesia, a digital rectal exam is performed and the prolapse is reduced with digital reduction. The rectal mucosa is lifted upwards with the left forefinger and the sacrococcygeal junction is identified internally with the left forefinger and externally with the left thumb. A 0 braided silk is passed from the posterior rectal wall through the lowest part of the sacrum, passing through the rectal wall, perirectal fat, rectal and rectosacral and presacral fascia, bone, subcutaneous fat and skin in a blind fashion. The other strand of suture is passed again in the same way but on the opposite side, around 2.5 cm apart horizontally and 2 cm above the puborectalis sling. The suture is tied in a knot over a piece of gauze soaked in iodine solution. Patients healed well with occasional localized infection that at times were treated with antibiotics. The gauze was removed on the fifth post-operative day (25). Lasheen offers an extended version of Ekehorn’s rectopexy in the form of closed rectosacropexy, using five postanal stab incisions with a similar “U’ stich with non-absorbable monofilament thread around the endorectum that also successfully treated 42 children in Egypt (47).
Rectopexy can also be performed with an intrabdominal technique via laparoscopic suture rectopexy (LSR). The rectal tissue is sutured to the presacral area to promote adherence. Pandey and collogues discuss a classic approach to a LSR with rectal dissection in the mesorectal plane and use of bilateral non-absorbable suture placement, fixing the rectum to the presacral fascia, as well as a modified technique that only utilized rectopexy on the left side of the rectum, both with recurrence rates under 5% at 3 months (48). Overall recurrence rates for LSR vary greatly between 0 to 40% (3). Complications include urinary retention and most significantly, constipation. Resection rectopexy for children remains controversial and may be recommended for patients with history of persistent constipation and delayed colonic transit (3). Newer minimally invasive surgical options include robotic technology that has continued to evolve for many pediatric surgical conditions. Hiller et al. demonstrated the efficacy of robotic rectopexy in a case series of four pediatric patients at a single institution. Of these, one patient required conversion to an open procedure and short-term resolution up to 1 year of follow-up in these pediatric patients was observed (49). As robotic-assisted approaches continue to be more widely implemented and refined, more prospective series with larger patient cohorts will be needed to assess its long-term efficacy for rectal prolapse in the pediatric population.
Finally, Flum and colleagues shared their experience managing recurrent rectal prolapse after initial surgical treatment. The majority of their patients first had a Thiersch procedure and noted prolapse resolution after 1–2 modified Thiersch procedures. For children who fail multiple modified Thiersch procedures, the authors recommend a modified Altemeier procedure. Their modified Thiersch procedure involves everting the rectal mucosa and proceeding with five radial linear cauterizations int the rectal mucosa and submucosa, after which sclerotherapy is injected and a Thiersch stitch is placed over 2 Hagar dilators with a #1 PDS suture. Their modified Altemeier again involves exteriorization of the rectal wall with redundant mucosa and submucosa excised to 1.5 cm above the dentate line and subsequent rectal wall re-approximation. Of the 29 patients studied over a 14-year period, 90% had success with 1–2 modified Thiersch procedures and three cases required a modified Altemeier procedure (45).
Challenges in the management of pediatric rectal prolapse are highlighted by the 2019 American Pediatric Surgical Association survey conducted to review practice patterns. Difficulties associated with pediatric rectal prolapse is also a reflection of the limited frequency with which patients present. Seventy-one percent of participants had seen 1–5 patients with rectal prolapse in the prior 2 years and only 5% had seen more than 10 patients. Again 71% of participants performed 0–1 procedure for rectal prolapse in the last 2 years and less than 1% of participants performed over 5 procedures in the past year. As with any surgical procedure, volume is critically associated with outcomes. Fifty-nine percent participants stated that they would treat a 6-year-old differently than a 2-year-old and would be more likely to offer surgery early (50). Overall, surgeons tended to perform techniques and surgeries they are most comfortable with.
Conclusions
The management of pediatric rectal prolapse is closely related to its multifactorial etiology. Principles of management include good toileting habits and prevention of constipation, a common cause of rectal prolapse in young children. Local therapy should be reserved for patients after failure of medical management and should reflect procedures the surgeon is most comfortable with. Challenges associated with management include the relative infrequency with which patients are seen by pediatric surgeons, and the extended period of time required for successful non-operative management. Additional prospective research and data are necessary to evaluate the long-term outcomes associated with different procedural and surgical management styles in order to optimize therapy for rectal prolapse in the pediatric population in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Biorender.com for providing the platform to create science figures.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-128/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-128/coif). E.A.P. serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from January 2022 to December 2024. The other authors have no conflicts of interest to declare.
References
- 1.Tsoucalas G, Papaioannou TG, Papatheodoridis GV, et al. Hippocratic views in the treatment of rectal prolapse. Acta Gastroenterol Belg 2017;80:411-5. [PubMed] [Google Scholar]
- 2.Tsoucalas G. British Surgeon Frederick Salmon (1796-1868) and His "Trans-Fixing Pins and Excision" Surgical Procedure for the "Rectum Prolapsus". Surg Innov 2018;25:88-9. 10.1177/1553350617731384 [DOI] [PubMed] [Google Scholar]
- 3.Rentea RM, St Peter SD. Pediatric Rectal Prolapse. Clin Colon Rectal Surg 2018;31:108-16. 10.1055/s-0037-1609025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laituri CA, Garey CL, Fraser JD, et al. 15-Year experience in the treatment of rectal prolapse in children. J Pediatr Surg 2010;45:1607-9. 10.1016/j.jpedsurg.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Rincon-Cruz L, Staffa SJ, Dickie B, et al. Influence of Initial Treatment Strategy on Outcomes for Children With Rectal Prolapse. J Pediatr Gastroenterol Nutr 2023;77:603-9. 10.1097/MPG.0000000000003924 [DOI] [PubMed] [Google Scholar]
- 6.Freeman NV. Rectal prolapse in children. J R Soc Med 1984;77 Suppl 3:9-12. [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Amano S, Matsumoto K, et al. Anorectal motility in children with complete rectal prolapse. Prog Pediatr Surg 1989;24:105-14. 10.1007/978-3-642-74493-8_12 [DOI] [PubMed] [Google Scholar]
- 8.Siafakas C, Vottler TP, Andersen JM. Rectal prolapse in pediatrics. Clin Pediatr (Phila) 1999;38:63-72. 10.1177/000992289903800201 [DOI] [PubMed] [Google Scholar]
- 9.Emeka CK, Patrick AL, Chikaodili ET. Rectal Prolapse in Children: How Effective is Non-Operative Treatment? J Paediatr Med Sur 2022;6:181. [Google Scholar]
- 10.Short SS, Wynne EK, Zobell S, et al. Most children experience resolution of idiopathic pediatric rectal prolapse with bowel management alone. J Pediatr Surg 2022;57:354-8. 10.1016/j.jpedsurg.2021.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Eriksen CA, Hadley GP. Rectal prolapse in childhood--the role of infections and infestations. S Afr Med J 1985;68:790-1. [PubMed] [Google Scholar]
- 12.Bhandari B, Mandowara SL. Rectal prolapse in childhood. Indian J Pediatr 1984;51:89-94. 10.1007/BF02753532 [DOI] [PubMed] [Google Scholar]
- 13.Cares K, El-Baba M. Rectal Prolapse in Children: Significance and Management. Curr Gastroenterol Rep 2016;18:22. 10.1007/s11894-016-0496-y [DOI] [PubMed] [Google Scholar]
- 14.Cares K, Klein M, Thomas R, et al. Rectal Prolapse in Children: An Update to Causes, Clinical Presentation, and Management. J Pediatr Gastroenterol Nutr 2020;70:243-6. 10.1097/MPG.0000000000002546 [DOI] [PubMed] [Google Scholar]
- 15.Martini N, Kara Tahhan N, Aldarwish MS, et al. Rectal prolapse as a manifestation of inflammatory bowel disease with celiac disease in a 2-year-old male: a rare case report. Ann Med Surg (Lond) 2023;85:1235-9. 10.1097/MS9.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern RC, Izant RJ, Jr, Boat TF, et al. Treatment and prognosis of rectal prolapse in cystic fibrosis. Gastroenterology 1982;82:707-10. 10.1016/0016-5085(82)90315-8 [DOI] [PubMed] [Google Scholar]
- 17.El-Chammas KI, Rumman N, Goh VL, et al. Rectal prolapse and cystic fibrosis. J Pediatr Gastroenterol Nutr 2015;60:110-2. 10.1097/MPG.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 18.Mazumder RN, Ashraf H, Hoque SS, et al. Effect of an energy-dense diet on the clinical course of acute shigellosis in undernourished children. Br J Nutr 2000;84:775-9. 10.1017/S0007114500002142 [DOI] [PubMed] [Google Scholar]
- 19.Traisman E, Conlon D, Sherman JO, et al. Rectal prolapse in two neonates with Hirschsprung's disease. Am J Dis Child 1983;137:1126-7. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric rectosigmoid atypical juvenile polyps presenting with anal prolapse and acute bleeding: a case report and a comprehensive review of the literature. 2023 [cited 2024 Feb 19]. Available online: https://www.researchsquare.com [DOI] [PubMed]
- 21.Khushbakht S, ul Haq A. Rectal Duplication Cyst: A Rare Cause of Rectal Prolapse in a Toddler. J Coll Physicians Surg Pak 2015;25:909-10. [PubMed] [Google Scholar]
- 22.Shah A, Parikh D, Jawaheer G, et al. Persistent rectal prolapse in children: sclerotherapy and surgical management. Pediatr Surg Int 2005;21:270-3. 10.1007/s00383-005-1384-y [DOI] [PubMed] [Google Scholar]
- 23.Hill SR, Ehrlich PF, Felt B, et al. Rectal prolapse in older children associated with behavioral and psychiatric disorders. Pediatr Surg Int 2015;31:719-24. 10.1007/s00383-015-3733-9 [DOI] [PubMed] [Google Scholar]
- 24.Loredo-Abdalá A, Trejo-Hernández J, Monroy-Villafuerte A, et al. Rectal prolapse in pediatrics. Clin Pediatr (Phila) 2000;39:131-2. [PubMed] [Google Scholar]
- 25.Schepens MA, Verhelst AA. Reappraisal of Ekehorn's rectopexy in the management of rectal prolapse in children. J Pediatr Surg 1993;28:1494-7. 10.1016/0022-3468(93)90439-R [DOI] [PubMed] [Google Scholar]
- 26.Akkoyun I, Akbiyik F, Soylu SG. The use of digital photos and video images taken by a parent in the diagnosis of anal swelling and anal protrusions in children with normal physical examination. J Pediatr Surg 2011;46:2132-4. 10.1016/j.jpedsurg.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 27.Corman ML. Rectal prolapse in children. Dis Colon Rectum 1985;28:535-9. 10.1007/BF02554107 [DOI] [PubMed] [Google Scholar]
- 28.Morrison ZD, LaPlant M, Hess D, et al. A systematic review of management options in pediatric rectal prolapse. J Pediatr Surg 2019;54:1782-7. 10.1016/j.jpedsurg.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 29.Alexander-Williams J. Solitary-ulcer syndrome of the rectum. Its association with occult rectal prolapse. Lancet 1977;1:170-1. [DOI] [PubMed] [Google Scholar]
- 30.Godbole P, Botterill I, Newell SJ, et al. Solitary rectal ulcer syndrome in children. J R Coll Surg Edinb 2000;45:411-4. [PubMed] [Google Scholar]
- 31.Zhang SC, Wang WL, Liu X. Defecography used as a screening entry for identifying evacuatory pelvic floor disorders in childhood constipation. Clin Imaging 2014;38:115-21. 10.1016/j.clinimag.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 32.Coburn WM, 3rd, Russell MA, Hofstetter WL. Sucrose as an aid to manual reduction of incarcerated rectal prolapse. Ann Emerg Med 1997;30:347-9. 10.1016/S0196-0644(97)70174-4 [DOI] [PubMed] [Google Scholar]
- 33.Qvist N, Rasmussen L, Klaaborg KE, et al. Rectal prolapse in infancy: conservative versus operative treatment. J Pediatr Surg 1986;21:887-8. 10.1016/S0022-3468(86)80015-X [DOI] [PubMed] [Google Scholar]
- 34.Bordeianou L, Paquette I, Johnson E, et al. Clinical Practice Guidelines for the Treatment of Rectal Prolapse. Dis Colon Rectum 2017;60:1121-31. 10.1097/DCR.0000000000000889 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Kohyama A, Suzuki H, et al. Slug Method: A Technique for Stoma Prolapse Reduction Using High Osmolality of the 50% Glucose Solution. Dis Colon Rectum 2020;63:e565. 10.1097/DCR.0000000000001798 [DOI] [PubMed] [Google Scholar]
- 36.Chino ES, Thomas CG, Jr. Transsacral approach to repair of rectal prolapse in children. Am Surg 1984;50:70-5. [PubMed] [Google Scholar]
- 37.Zhou W, Shi Y, Zhang M, et al. The Remission Effects of First Injection of Sclerotherapy for Pediatric Rectal Prolapse: A Systematic Review and Meta-Analysis. Front Surg 2022;9:835235. 10.3389/fsurg.2022.835235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sander S, Vural O, Unal M. Management of rectal prolapse in children: Ekehorn's rectosacropexy. Pediatr Surg Int 1999;15:111-4. 10.1007/s003830050528 [DOI] [PubMed] [Google Scholar]
- 39.Dutta BN, Das AK. Treatment of prolapse rectum in children with injections of sclerosing agents. J Indian Med Assoc 1977;69:275-6. [PubMed] [Google Scholar]
- 40.Chan WK, Kay SM, Laberge JM, et al. Injection sclerotherapy in the treatment of rectal prolapse in infants and children. J Pediatr Surg 1998;33:255-8. 10.1016/S0022-3468(98)90441-9 [DOI] [PubMed] [Google Scholar]
- 41.Gysler R, Morger R. Sclerosing treatment with ethoxysclerol in anal prolapse in children. Z Kinderchir 1989;44:304-5. [DOI] [PubMed] [Google Scholar]
- 42.Bahador A, Foroutan HR, Hosseini SM, et al. Effect of submucosal alcohol injection on prolonged rectal prolapse in infants and children. J Indian Assoc Pediatr Surg 2008;13:11-3. 10.4103/0971-9261.42566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kay NR, Zachary RB. The treatment of rectal prolapse in children with injections of 30 per cent saline solutions. J Pediatr Surg 1970;5:334-7. 10.1016/0022-3468(70)90190-9 [DOI] [PubMed] [Google Scholar]
- 44.Balde I, Mbumbe-King A, Vinand P. The Lockhart-Mummery technique in the treatment of the total rectal prolapse among children. Concerning 25 cases (author's transl). Chir Pediatr 1979;20:375-7. [PubMed] [Google Scholar]
- 45.Flum AS, Golladay ES, Teitelbaum DH. Recurrent rectal prolapse following primary surgical treatment. Pediatr Surg Int 2010;26:427-31. 10.1007/s00383-010-2565-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antao B, Bradley V, Roberts JP, et al. Management of rectal prolapse in children. Dis Colon Rectum 2005;48:1620-5. 10.1007/s10350-005-0074-0 [DOI] [PubMed] [Google Scholar]
- 47.Lasheen AE. Closed rectosacropexy for rectal prolapse in children. Surg Today 2003;33:642-4. 10.1007/s00595-003-2548-9 [DOI] [PubMed] [Google Scholar]
- 48.Pandey V, Khanna AK, Srivastava V, et al. Simplified Laparoscopic Suture Rectopexy for Idiopathic Rectal Prolapse In Children: Technique and Results. J Pediatr Surg 2020;55:972-6. 10.1016/j.jpedsurg.2019.10.049 [DOI] [PubMed] [Google Scholar]
- 49.Hiller DJ, Bohl JL, Zeller KA. Robotic Rectopexy for Rectal Prolapse in Pediatric Patients. Am Surg 2017;83:1386-9. 10.1177/000313481708301223 [DOI] [PubMed] [Google Scholar]
- 50.Trappey AF, 3rd, Galganski L, Saadai P, et al. Surgical management of pediatric rectal prolapse: A survey of the American Pediatric Surgical Association (APSA). J Pediatr Surg 2019;54:2149-54. 10.1016/j.jpedsurg.2019.02.017 [DOI] [PubMed] [Google Scholar]