Abstract
Objective:
The aim of this study was to identify independent predictors of hospital readmission for patients undergoing lobectomy for lung cancer.
Summary Background Data:
Hospital readmission after lobectomy is associated with increased mortality. Greater than 80% of the variability associated with readmission after surgery is at the patient level. This underscores the importance of using a data source that includes detailed clinical information.
Methods:
Using the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD), we conducted a retrospective cohort study of patients undergoing elective lobectomy for lung cancer. Three separate multivariable logistic regression models were generated: the first included preoperative variables, the second added intraoperative variables, and the third added postoperative variables. The c statistic was calculated for each model.
Results:
There were 39,734 patients from 277 centers. The 30-day readmission rate was 8.2% (n = 3237). In the final model, postoperative complications had the greatest effect on readmission. Pulmonary embolus {odds ratio [OR] 12.34 [95% confidence interval (CI),7.94–19.18]} and empyema, [OR 11.66 (95% CI, 7.31–18.63)] were associated with the greatest odds of readmission, followed by pleural effusion [OR 7.52 (95% CI, 6.01–9.41)], pneumothorax [OR 5.08 (95% CI, 4.16–6.20)], central neurologic event [OR 3.67 (95% CI, 2.23–6.04)], pneumonia [OR 3.13 (95% CI, 2.43–4.05)], and myocardial infarction [OR 3.16 (95% CI, 1.71–5.82)]. The c statistic for the final model was 0.736.
Conclusions:
Complications are the main driver of readmission after lobectomy for lung cancer. The highest risk was related to postoperative events requiring a procedure or medical therapy necessitating inpatient care.
Keywords: Lobectomy, readmission, thoracic surgery
During the last several years, reducing hospital readmissions has been a priority of national health policy. To reduce preventable readmissions, the Centers for Medicare and Medicaid Services (CMS) began the Hospital Readmissions Reduction Program (HRRP) which decreases reimbursement if a hospital has a readmission rate above the average risk-adjusted rate.1 The 30-day risk standardized readmission measure includes all-cause unplanned readmissions that occur within 30 days of discharge from the index hospitalization; the readmission may be to the same hospital or another acute care hospital.
Although pulmonary lobectomy is not yet included in the HRRP, hospital readmission is an active area of research because it has been identified as a marker for hospital quality.2,3 In addition, hospital readmission after lobectomy is associated with increased mortality,4,5 with 1 study reporting a 90-day mortality rate 6 times higher among Medicare patients readmitted within 30 days of discharge.6 Therefore, readmissions have important implications for both hospitals and patients, even though the relationship between mortality and what occurs before or after readmission is unclear.
One of the challenges with analyzing hospital readmissions after surgery is that there are several levels of factors that may contribute to an increased risk of readmission including patient, surgeon, and hospital-level predictors. Most previous studies of readmission after lobectomy have developed prediction models using primarily patient factors. However, some have used administrative databases4,6 or clinical registries such as the National Cancer Database (NCDB) that do not capture detailed perioperative data.5,7 Others have used the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database that defines readmission as unplanned and within 30 days of surgery, whereas CMS defines it as all-cause and within 30 days of discharge.8–10
Gani et al determined the proportion of 30-day readmission attributable to patient, surgeon, and surgical subspecialty in a large cohort of surgical patients from a single institution and found that >80% of the variability associated with the likelihood of readmission after surgery is at the patient level.11 Therefore, when predicting readmission at the patient level, it is important to use a database that includes detailed clinical information. Ideally, a unified definition of the outcome measure, hospital readmission, according to the CMS HRRP, permits a precise comparison among studies derived from different databases.
Using the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD), our objective was to identify independent predictors of hospital readmission within 30 days of discharge for patients undergoing lobectomy for lung cancer. We sought to determine whether preoperative, intraoperative, or postoperative factors were most predictive of readmission. We tested our hypothesis that postoperative complications would have the greatest effect on the risk of readmission after lobectomy.
METHODS
STS GTSD
The STS GTSD is the largest clinical thoracic surgical database in North America comprising >506,000 general thoracic surgery procedure records and >950 participating surgeons. Participating institutions receive data analysis reports after each semiannual data harvest comparing their institution to the STS and Nationwide Inpatient Sample (NIS) benchmarks. The data collection form and training manual can be found on the STS website.12
Patient Cohort
This is a retrospective cohort study of all patients undergoing elective lobectomy for lung cancer between January 2, 2012 and June 30, 2017 (data collection form versions 2.2 and 2.3). As noted in the consort diagram, we excluded those who died during the index hospitalization and those that died before having a readmission as these patients were at zero to minimal risk of readmission (Fig. 1). We also excluded those whose discharge disposition was coded as either outpatient or observation, and patients with missing data for death, readmission, or forced expiratory volume in 1 second (FEV1). Lastly, we excluded patients with a Zubrod score >3 or American Society of Anesthesiologists (ASA) class >III (Fig. 1), as our assumption is that their weakened preoperative state as defined by poor performance score placed them at excessive risk for readmission.
FIGURE 1.
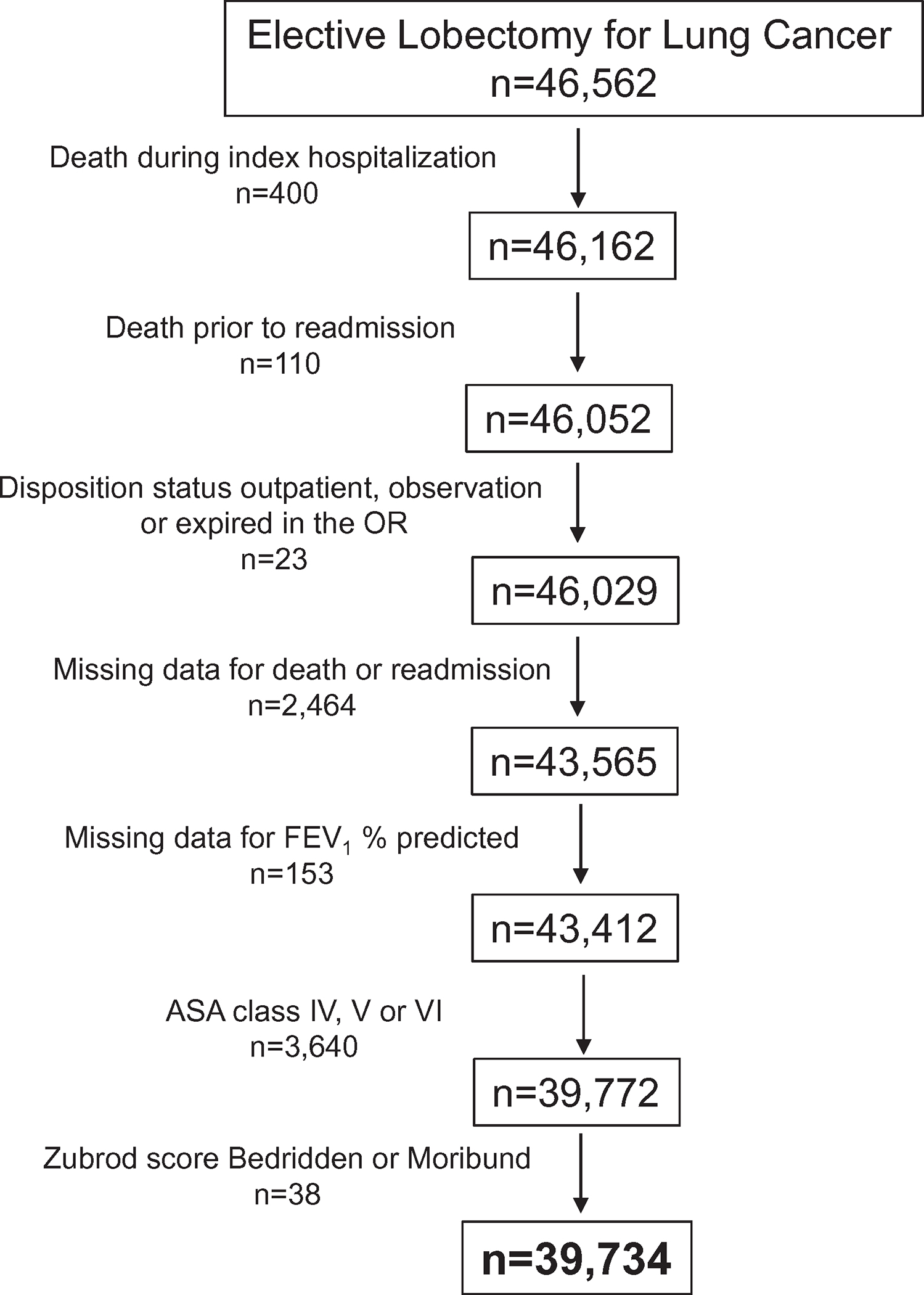
Patient cohort.
Primary Outcome and Predictor Variables
The primary outcome was readmission within 30 days of discharge from the index hospitalization for elective lobectomy for lung cancer. The association between FEV1 and readmission was modeled using 2 linear splines: FEV1 80% and FEV1 >80%. Furthermore, the scale of the splines was set to be interpreted at 10% increments in FEV1. Steroid use was defined as taking oral or intravenous steroids within 24 hours of surgery, excluding a 1-time prophylactic dose. Past smokers quit before 1 month before surgery and current smokers continued to smoke within 1 month of surgery. Reoperation was defined as any previous cardiothoracic operation that affected the operative field of the index operation. The administration of cephalosporin antibiotic was coded as “yes” if it was ordered, if it was documented that it was not indicated or there was an allergy or another reason for substitution. Renal failure was defined as either an increase in serum creatinine level 3 times greater than baseline or ≥4mg/dL; acute rise was at least 0.5 mg/dL or a new requirement for dialysis. Urinary complication was a composite predictor variable including urinary tract infection, urinary retention requiring catheterization, and/or patient was discharged home with urinary catheter.
Statistical Analyses
Descriptive results were compared by readmission status using Wilcoxon tests and chi-square tests for continuous and categorical variables, respectively. Multivariable logistic regression was used to determine independent predictors of readmission. Three models were generated: the first with preoperative variables, the second with preoperative and intraoperative variables, and the third with preoperative, intraoperative, and postoperative variables. The c statistic was calculated for each model. The first model using only preoperative variables included the same variables as the STS database risk models for major morbidity and mortality after lung cancer resection.13 We used generalized estimating equations (GEE) with an independent working correlation structure to account for the clustering of patients within hospitals. This allows for robust variance estimates for the estimated adjusted odds of readmission ratios for each variable of interest. Each model fit was investigated by plotting the observed and expected values into 10 homogeneous groups defined by deciles of expected values. Additionally, each variable was checked for collinearity with a variance inflation factor (VIF) determined for each variable.
Missing values of postoperative complications were imputed to absence of a complication. In the STS GTSD, “postoperative events” is a parent variable. If this variable is coded as “no”, then none of the corresponding individual complications are coded. For our analyses, there were 4 patients (0.01%) with missing data for “postoperative events” and we imputed this variable and the corresponding individual complications to be “no.” In addition, if “postoperative events” was coded as “no,” we imputed the corresponding individual complications to be “no.” For the remainder of the variables, complete case analyses were considered for the models as missingness was rare and unlikely to be explained by known variables. For each variable, there were <4.0% missing data: 9 variables with <1% missingness, 9 with 1% to 2%, and maximum missingness for FEV1 (3.3%). All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). This study was exempted from review by the University of California, Davis Institutional Review Board.
RESULTS
There were 46,562 patients undergoing elective lobectomy for lung cancer at 280 centers. After exclusion criteria were applied, the final cohort consisted of 39,734 patients from 277 centers (Fig. 1). The 30-day readmission rate was 8.2%, (n = 3237). Predictor variables included in the risk models by readmission are listed in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | Not Readmitted (n = 36,497) | Readmitted Within 30 Days of Discharge (n = 3237) | P |
|---|---|---|---|
|
| |||
| Preoperative characteristics | |||
| Age, median (IQR) | 68 (61–74) | 69 (61–75) | <0.0001 |
| Sex | <0.0001 | ||
| Male | 15,585 (42.7%) | 1594 (49.2%) | |
| Female | 20,911 (57.3%) | 1643 (50.8%) | |
| Race | 0.001 | ||
| White | 30,312 (83.1%) | 2739 (84.6%) | |
| Black | 3195 (8.8%) | 288 (8.9%) | |
| Asian | 1125 (3.1%) | 60 (1.9%) | |
| Hispanic | 948 (2.6%) | 83 (2.6%) | |
| Other | 553 (1.5%) | 45 (1.4%) | |
| Insurance status | <0.0001 | ||
| Medicare/other | 34,089 (93.4%) | 3005 (92.8%) | |
| Medicaid | 1805 (5.0%) | 179 (5.5%) | |
| None/self-pay | 603 (1.7%) | 53 (1.6%) | |
| Lung cancer clinical stage | <0.0001 | ||
| Stage I | 26,330 (72.1%) | 2242 (69.3%) | |
| Stage II | 5068 (13.9%) | 538 (16.6%) | |
| Stage III | 3611 (9.9%) | 339 (10.5%) | |
| Stage IV | 518 (1.4%) | 57 (1.8%) | |
| BMI kg/m2, median (IQR) | 26.9 (23.5–30.8) | 26.3 (23.0–30.4) | <0.0001 |
| FEV1 (%) predicted, median (IQR) | 85 (72–98) | 81 (67–95) | <0.0001 |
| Cigarette smoking | <0.001 | ||
| Past smoker | 22182 (60.8%) | 1999 (61.8%) | |
| Current smoker | 8282 (22.7%) | 845 (26.1%) | |
| Interstitial fibrosis | 392 (1.1%) | 64 (2.0%) | <0.0001 |
| Hypertension | 21899 (60.0%) | 2047 (63.2%) | 0.0007 |
| Steroids | 1015 (2.8%) | 138 (4.3%) | <0.0001 |
| Congestive heart failure | 731 (2.0%) | 90 (2.8%) | 0.003 |
| Coronary artery disease | 6683 (18.3%) | 774 (23.9%) | <0.0001 |
| Peripheral vascular disease | 2800 (7.7%) | 362 (11.2%) | <0.0001 |
| Cerebrovascular disease | <0.0001 | ||
| Transient ischemic attack | 1429 (3.9%) | 163 (5.0%) | |
| Cerebrovascular accident | 1100 (3.0%) | 151 (4.7%) | |
| Diabetes | 6457 (17.7%) | 647 (20.0%) | 0.002 |
| Induction chemotherapy alone | 2761 (7.6%) | 249 (7.7%) | 0.8 |
| Induction chemoradiation therapy | 1532 (4.2%) | 155 (4.8%) | 0.1 |
| Creatinine ≥2 | 401 (1.1%) | 54 (1.7%) | 0.004 |
| Preoperative dialysis | 145 (0.4%) | 23 (0.7%) | 0.01 |
| Zubrod Score | <0.0001 | ||
| 0 | 16,897 (46.3%) | 1276 (39.4%) | |
| 1 | 18,370 (50.3%) | 1799 (55.6%) | |
| 2 or 3 | 1224 (3.4%) | 160 (4.9%) | |
| American Society of Anesthesiologists | |||
| Risk class | <0.001 | ||
| I or II | 6256 (17.1%) | 394 (12.2%) | |
| III | 30,227 (82.8%) | 2842 (87.8%) | |
| Intraoperative characteristics | |||
| Open (vs minimally invasive) | 11,976 (32.8%) | 1230 (38.0%) | <0.0001 |
| Reoperation | 1911 (5.2%) | 195 (6.0%) | 0.06 |
| Lung cancer location | <0.0001 | ||
| Upper lobe | 21,507 (58.9%) | 1896 (58.6%) | |
| Middle lobe | 2682 (7.4%) | 170 (5.3%) | |
| Lower lobe | 11,788 (32.3%) | 1117 (34.5%) | |
| Unspecified | 520 (1.4%) | 54 (1.7%) | |
| Intraoperative packed red blood cells | 643 (1.8%) | 95 (2.9%) | <0.0001 |
| IV antibiotics given within 1 h | 34,522 (94.6%) | 3107 (96.0%) | 0.3 |
| DVT prophylaxis measures employed | 34,744 (95.2%) | 3135 (96.9%) | 0.9 |
| Cephalosporin antibiotic ordered | 34,402 (94.3%) | 3105 (95.9%) | 0.006 |
| Postoperative characteristics | |||
| Unexpected reoperation | 935 (2.6%) | 247 (7.6%) | <0.0001 |
| Air leak with duration >5 days | 3728 (10.2%) | 694 (21.4%) | <0.0001 |
| Pneumonia | 911 (2.5%) | 390 (12.1%) | <0.0001 |
| Bronchopleural fistula | 73 (0.2%) | 41 (1.3%) | <0.0001 |
| Atelectasis requiring bronchoscopy | 1,132 (3.1%) | 184 (5.7%) | <0.0001 |
| Pulmonary embolus | 75 (0.2%) | 96 (3.0%) | <0.0001 |
| Pleural effusion requiring drainage | 315 (0.9%) | 290 (9.0%) | <0.0001 |
| Pneumothorax requiring chest tube | 872 (2.4%) | 457 (14.1%) | <0.0001 |
| Atrial arrhythmia requiring treatment | 3694 (10.1%) | 521 (16.1%) | <0.0001 |
| Myocardial infarction | 69 (0.2%) | 30 (0.9%) | <0.0001 |
| Deep venous thrombosis | 108 (0.3%) | 63 (2.0%) | <0.0001 |
| Urinary complication | 3084 (8.5%) | 472 (14.6%) | <0.0001 |
| Empyema | 61 (0.2%) | 126 (3.9%) | <0.0001 |
| Sepsis | 95 (0.3%) | 73 (2.3%) | <0.001 |
| Central neurologic event | 94 (0.3%) | 50 (1.5%) | <0.0001 |
| Delirium | 734 (2.0%) | 146 (4.5%) | <0.0001 |
| Renal failure | 123 (0.3%) | 42 (1.3%) | <0.0001 |
| Chylothorax requiring | |||
| Drainage/medical treatment | 171 (0.5%) | 28 (0.9%) | 0.002 |
| Postoperative packed red blood cells transfusion | 1125 (3.1%) | 238 (7.4%) | <0.0001 |
| Unexpected admission to ICU | 918 (2.5%) | 232 (7.2%) | <0.0001 |
| Discharge location other than home | 2013 (5.5%) | 335 (10.3%) | <0.0001 |
ICU indicates intensive care unit; IQR, interquartile range.
The first multivariable analysis included only preoperative characteristics (Table 2). There were several independent predictors of readmission. Interstitial fibrosis was the predictor with the largest effect {odds ratio [OR] 1.69, [95% confidence interval (CI), 1.31–2.19], P < 0.0001}. Steroid use [OR 1.42 (95% CI, 1.18–1.72), P = 0.0002], cerebrovascular accident [OR 1.36 (95% CI, 1.13–1.63), P = 0.001], and Zubrod score 2 or 3 [OR 1.35 (95% CI, 1.12–1.62), P = 0.001] were the next 3 independent predictors in terms of effect. The c statistic for this model was 0.600.
TABLE 2.
Multivariable Logistic Regression Model: Preoperative Characteristics c statistic: 0.600
| Patient Characteristics | Adjusted OR | 95% CI | P |
|---|---|---|---|
|
| |||
| Preoperative characteristics | |||
| Age (per 10-y increase) | 1.09 | (1.04–1.15) | 0.0003 |
| Male | 1.19 | (1.10–1.28) | <0.0001 |
| Race (reference: white) | |||
| Black | 1.03 | (0.91–1.18) | 0.6 |
| Asian | 0.65 | (0.49–0.85) | 0.002 |
| Hispanic | 1.03 | (0.77–1.38) | 0.9 |
| Other | 1.02 | (0.76–1.38) | 0.9 |
| Insurance status (reference: medicare/other) | |||
| Medicaid | 1.22 | (1.02–1.45) | 0.03 |
| None/self-pay | 1.03 | (0.76–1.40) | 0.9 |
| Clinical stage (reference: Stage I) | |||
| Stage II | 1.17 | (1.06–1.30) | 0.002 |
| Stage III | 1.08 | (0.93–1.26) | 0.3 |
| Stage IV | 1.28 | (0.95–1.71) | 0.1 |
| BMI | 0.99 | (0.98–0.99) | 0.0002 |
| FEV1% ≤80% predicted | 0.90 | (0.86–0.94) | <0.0001 |
| FEV1% >80% predicted | 0.98 | (0.95–1.01) | 0.2 |
| Cigarette smoking (reference: never smoker) | |||
| Former smoker | 1.09 | (0.96–1.24) | 0.2 |
| Current smoker | 1.20 | (1.04–1.39) | 0.01 |
| Interstitial fibrosis | 1.69 | (1.31–2.19) | <0.0001 |
| Hypertension | 0.98 | (0.90–1.07) | 0.7 |
| Steroids | 1.42 | (1.18–1.72) | 0.0002 |
| Congestive heart failure | 1.03 | (0.79–1.32) | 0.8 |
| Coronary artery disease | 1.15 | (1.04–1.27) | 0.009 |
| Peripheral vascular disease | 1.20 | (1.06–1.37) | 0.004 |
| Cerebrovascular disease | |||
| Transient ischemic attack | 1.12 | (0.94–1.33) | 0.2 |
| Cerebrovascular accident | 1.36 | (1.13–1.63) | 0.001 |
| Diabetes | 1.09 | (0.99–1.19) | 0.07 |
| Induction chemotherapy alone | 0.84 | (0.64–1.10) | 0.2 |
| Induction chemoradiation therapy | 1.32 | (0.97–1.81) | 0.08 |
| Creatinine ≥2 | 1.10 | (0.80–1.50) | 0.6 |
| Preoperative dialysis | 1.26 | (0.74–2.13) | 0.4 |
| Zubrod score (reference: 0) | |||
| 1 | 1.16 | (1.06–1.27) | 0.001 |
| 2 or 3 | 1.35 | (1.12–1.62) | 0.001 |
| ASA class (reference: I or II) | |||
| III | 1.23 | (1.07–1.40) | 0.003 |
The second multivariable model included preoperative and intraoperative characteristics (Table 3). Every independent preoperative predictor in the first model remained statistically significant in the second model with added intraoperative characteristics. Again, interstitial fibrosis had the greatest effect [OR 1.66 (95% CI, 1.28–2.14), P = 0.0001]. The intraoperative characteristics independently predictive of readmission were surgical approach, intraoperative packed red blood cell transfusion, and the location of lobe. Those undergoing open lobectomy had a 17% increased risk of readmission compared to lobectomy via a minimally invasive approach. Patients undergoing middle lobectomy were 25% less likely to be readmitted and those undergoing lower lobectomy 15% more likely than after upper lobectomy. Not associated with readmission were several operative process measures including IV antibiotic administration within 1 hour of incision, whether the antibiotic was a cephalosporin and deep vein thrombosis (DVT) prophylaxis. The c statistic for this model was 0.604.
TABLE 3.
Multivariable Logistic Regression Model: Preoperative and Intraoperative Characteristics C-statistic: 0.604
| Patient Characteristics | Adjusted OR | 95% CI | P |
|---|---|---|---|
|
| |||
| Preoperative characteristics | |||
| Age (per 10-y increase) | 1.09 | (1.04–1.15) | 0.0003 |
| Male | 1.18 | (1.09–1.28) | <0.0001 |
| Race (reference: white) | |||
| Black | 1.04 | (0.91–1.18) | 0.6 |
| Asian | 0.63 | (0.47–0.83) | 0.001 |
| Hispanic | 1.01 | (0.76–1.34) | 0.9 |
| Other | 1.04 | (0.77–1.41) | 0.8 |
| Insurance status (reference: medicare/other) | |||
| Medicaid | 1.22 | (1.02–1.45) | 0.03 |
| None/self-pay | 1.01 | (0.74–1.38) | 0.9 |
| Clinical Stage (reference: Stage I) | |||
| Stage II | 1.12 | (1.01–1.24) | 0.03 |
| Stage III | 1.06 | (0.91– 1.23) | 0.5 |
| Stage IV | 1.26 | (0.94–1.70) | 0.1 |
| BMI | 0.99 | (0.98–0.99) | 0.0002 |
| FEV1% ≤80% predicted | 0.90 | (0.86–0.94) | <0.0001 |
| FEV1% >80% predicted | 0.98 | (0.95–1.02) | 0.4 |
| Cigarette smoking (reference: never smoker) | |||
| Former smoker | 1.10 | (0.96–1.25) | 0.2 |
| Current smoker | 1.22 | (1.05–1.41) | 0.009 |
| Interstitial fibrosis | 1.66 | (1.28–2.14) | 0.0001 |
| Hypertension | 0.98 | (0.90–1.07) | 0.6 |
| Steroids | 1.43 | (1.19–1.73) | 0.0002 |
| Congestive heart failure | 1.01 | (0.78–1.31) | 0.9 |
| Coronary artery disease | 1.14 | (1.03–1.27) | 0.01 |
| Peripheral vascular disease | 1.21 | (1.06–1.37) | 0.004 |
| Cerebrovascular disease | |||
| Transient ischemic attack | 1.10 | (0.92–1.31) | 0.3 |
| Cerebrovascular accident | 1.38 | (1.15–1.66) | 0.0007 |
| Diabetes | 1.08 | (0.98–1.19) | 0.1 |
| Induction chemotherapy alone | 0.82 | (0.63–1.07) | 0.1 |
| Induction chemoradiation therapy | 1.31 | (0.95–1.80) | 0.1 |
| Creatinine ≥2 | 1.10 | (0.80–1.51) | 0.5 |
| Preoperative dialysis | 1.24 | (0.73–2.10) | 0.4 |
| Zubrod score (reference: 0) | |||
| 1 | 1.14 | (1.04–1.24) | 0.003 |
| 2 or 3 | 1.32 | (1.10–1.57) | 0.003 |
| ASA class (reference: I or II) | |||
| III | 1.19 | (1.06–1.35) | 0.005 |
| Intraoperative characteristics | |||
| Open (reference: minimally invasive) | 1.17 | (1.07–1.27) | 0.0007 |
| Reoperation | 1.01 | (0.88–1.15) | 0.9 |
| Lobe (reference: upper lobe) | |||
| Middle lobe | 0.75 | (0.64–0.89) | 0.001 |
| Lower lobe | 1.15 | (1.05–1.25) | 0.002 |
| Unspecified* | 1.28 | (0.93–1.77) | 0.1 |
| Intraoperative PRBCs | 1.31 | (1.01–1.69) | 0.04 |
| IV antibiotics given within 1 h of incision | 0.86 | (0.64–1.16) | 0.3 |
| DVT prophylaxis | 0.96 | (0.54–1.71) | 0.9 |
| Cephalosporin antibiotic ordered | 0.96 | (0.75–1.24) | 0.8 |
ICU indicates intensive care unit; IV, intravenous; PRBC, packed red blood cells.
Missing data for n = 574 patients (1.4%).
The third multivariable model included the addition of postoperative to preoperative and intraoperative characteristics (Table 4). Postoperative complications had the greatest effect on readmission risk. Pulmonary embolus (PE) [OR 12.34 (95% CI, 7.94–19.18), P < 0.0001] and empyema [OR 11.66 (95% CI, 7.31–18.63), P < 0.0001] were the 2 complications with the greatest impact on readmission. In descending order of effect, the next predictors were pleural effusion requiring drainage, pneumothorax requiring chest tube, central neurologic event, myocardial infarction (MI), and pneumonia. Of 15 preoperative independent predictors of readmission in the first model, only 10 remained independent predictors in the third model. And only 1 intraoperative characteristic was associated with readmission: lower lobectomy was associated with an 18% increased risk of readmission. The c statistic for this model was 0.736. The VIF was <5 for each variable; the highest VIF was 2.75.
TABLE 4.
Multivariable Logistic Regression Model: Preoperative, Intraoperative, and Postoperative Characteristics c statistic: 0.736
| Patient Characteristics | Adjusted OR | 95% CI | P |
|---|---|---|---|
|
| |||
| Preoperative characteristics | |||
| Age (per 10-y increase) | 1.06 | (1.00–1.12) | 0.03 |
| Male | 1.07 | (0.98–1.16) | 0.1 |
| Race (reference: white) | |||
| Black | 1.08 | (0.94–1.25) | 0.3 |
| Asian | 0.68 | (0.51–0.91) | 0.009 |
| Hispanic | 1.04 | (0.75–1.44) | 0.8 |
| Other | 1.05 | (0.77–1.42) | 0.8 |
| Insurance status (reference: medicare/other) | |||
| Medicaid | 1.20 | (0.99–1.45) | 0.07 |
| None/self-pay | 1.05 | (0.72–1.53) | 0.8 |
| Clinical Stage (reference: Stage I) | |||
| Stage II | 1.14 | (1.02–1.28) | 0.02 |
| Stage III | 1.02 | (0.87–1.19) | 0.8 |
| Stage IV | 1.33 | (0.96–1.84) | 0.09 |
| BMI | 1.00 | (0.99–1.00) | 0.2 |
| FEV1% <80% predicted | 0.95 | (0.90–0.99) | 0.03 |
| FEV1% >80% predicted | 0.99 | (0.96–1.03) | 0.7 |
| Cigarette smoking (reference: Never smoker) | |||
| Former smoker | 1.06 | (0.93–1.22) | 0.4 |
| Current smoker | 1.09 | (0.93–1.28) | 0.3 |
| Interstitial fibrosis | 1.48 | (1.11–1.97) | 0.007 |
| Hypertension | 0.99 | (0.90–1.09) | 0.8 |
| Steroids | 1.41 | (1.15–1.72) | 0.0009 |
| Congestive heart failure | 1.01 | (0.75–1.35) | 0.9 |
| Coronary artery disease | 1.14 | (1.02–1.27) | 0.02 |
| Peripheral vascular disease | 1.10 | (0.96–1.27) | 0.2 |
| Cerebrovascular disease | |||
| Transient ischemic attack | 1.10 | (0.91–1.33) | 0.3 |
| Cerebrovascular accident | 1.38 | (1.15–1.66) | 0.0004 |
| Diabetes | 1.10 | (1.00–1.22) | 0.05 |
| Induction chemotherapy alone | 0.79 | (0.60–1.04) | 0.09 |
| Induction chemoradiation therapy | 1.34 | (0.95–1.87) | 0.09 |
| Creatinine ≥2 | 1.01 | (0.71–1.43) | 0.9 |
| Preoperative dialysis | 1.47 | (0.87–2.50) | 0.2 |
| Zubrod score (reference: 0) | |||
| 1 | 1.12 | (1.02–1.23) | 0.01 |
| 2 or 3 | 1.30 | (1.05–1.61) | 0.01 |
| ASA Class (reference: I or II) | |||
| III | 1.18 | (1.04–1.35) | 0.01 |
| Intraoperative characteristics | |||
| Open (reference: minimally invasive) | 1.08 | (0.98–1.20) | 0.1 |
| Reoperation | 0.95 | (0.81–1.11) | 0.5 |
| Lobe (reference: upper lobe) | |||
| Middle lobe | 0.86 | (0.73–1.02) | 0.09 |
| Lower lobe | 1.18 | (1.07–1.30) | 0.0008 |
| Unspecified* | 1.14 | (0.81–1.63) | 0.5 |
| Intraoperative PRBCs | 1.09 | (0.81–1.45) | 0.6 |
| IV antibiotics given within 1 h of incision | 0.75 | (0.55–1.03) | 0.07 |
| DVT prophylaxis | 1.02 | (0.53–1.96) | 0.9 |
| Cephalosporin antibiotic ordered | 1.04 | (0.71–1.52) | 0.8 |
| Postoperative characteristics | |||
| Unexpected reoperation | 1.35 | (1.04–1.76) | 0.02 |
| Prolonged air leak | 1.63 | (1.45–1.84) | <0.0001 |
| Pneumonia | 3.13 | (2.43–4.05) | <0.0001 |
| Bronchopleural fistula | 1.55 | (0.80–3.01) | 0.2 |
| Atelectasis requiring bronchoscopy | 0.63 | (0.49–0.80) | 0.0002 |
| Pulmonary embolus | 12.34 | (7.94–19.18) | <0.0001 |
| Pleural effusion requiring drainage | 7.52 | (6.01–9.41) | <0.0001 |
| Pneumothorax requiring chest tube | 5.08 | (4.16–6.20) | <0.0001 |
| Atrial arrhythmia requiring treatment | 1.21 | (1.07–1.38) | 0.003 |
| Myocardial infarction | 3.16 | (1.71–5.82) | 0.0002 |
| Deep venous thrombosis | 1.77 | (1.00–3.13) | 0.05 |
| Urinary complication | 1.21 | (1.04–1.40) | 0.01 |
| Empyema | 11.66 | (7.31–18.63) | <0.0001 |
| Sepsis | 1.68 | (0.93–3.04) | 0.09 |
| Central neurologic event | 3.67 | (2.23 –6.04) | <0.0001 |
| Delirium | 1.00 | (0.77–1.31) | 0.9 |
| Renal failure | 1.08 | (0.61–1.89) | 0.8 |
| Chylothorax requiring treatment | 1.52 | (0.90–2.59) | 0.1 |
| Postoperative PRBC transfusion | 1.06 | (0.82–1.36) | 0.7 |
| Unexpected Admission to ICU | 0.83 | (0.63–1.09) | 0.2 |
| Discharge to location other than home | 0.98 | (0.82–1.16) | 0.8 |
ICU indicates intensive care unit; IV, intravenous; PRBC, packed red blood cells.
Missing data for n = 574 patients (1.4%).
DISCUSSION
In our cohort of patients, the readmission rate was 8.2%; corresponding to 1 in every 12 patients undergoing lobectomy for lung cancer. Our rate is similar to previous studies wherein it ranged from 4.3% to 12.8%.4–6,9,10 CMS defines 30-day readmission as an unplanned readmission for any reason to any hospital. Although the definition of readmission is important, and the STS definition of 30-day readmission varies by including planned or unplanned readmission to any hospital, only a small proportion, 7.7%, of readmissions after general, vascular, and thoracic surgery are planned.8 Studies using NCDB underestimate this rate as only readmission to the hospital where the operation was done are considered 30-day readmissions in that database.5 It is estimated that up to one-third of readmissions are to hospitals other than where the index operation was done.4,6 ACS-NSQIP includes only unplanned readmissions to any hospital, but defines readmission as 30 days from the date of surgery rather than the date of discharge making comparisons between studies using other data sources difficult.8–10 Our results reflect most closely a report of readmission after lobectomy using the State Inpatient Databases (SID) Healthcare Cost and Utilization Project (HCUP) that included all readmissions to any hospital; the rate of 30-day readmission in that study was 11.5%.4
Our study illustrates the dominating force of postoperative events over predictors that precede them in time, but are diminished in their effect by the time postoperative events occur. Although preoperative and intraoperative variables matter for the general prediction of operative risk, our findings suggest that readmission may be a consequence of incomplete care that was originally rendered with the intent to reverse the effect of specific complications. The order of modeling we selected for this study realistically recapitulates the temporal sequence in which first preoperative, then intraoperative, and finally postoperative factors impart their effect on readmission. No other order of modeling would more appropriately describe and expose the dominance of postoperative events on readmission as a potential measure of failure in their care.
Postoperative complications had the greatest effect on the risk of readmission, especially complications requiring a procedure or inpatient medical treatment. Our study adds to the existing literature by including detailed postoperative complication data in the multivariable analysis. Pulmonary complications account for approximately 25% to 55% of readmissions after lung resection.4,6,9,10 Bhagat et al reported that pulmonary complications accounted for 55.7% of readmissions after VATS lobectomy and 51.8% after open lobectomy.9 In that study, pneumothorax was the most common admission diagnosis after VATS lobectomy and pneumonia after open lobectomy.9 Importantly, that study included 10 complications in the composite outcome pulmonary complication.9 As the STS GTSD focuses solely on thoracic surgery patients and includes detailed 30-day morbidity data, we included individual complications in the multivariable analysis model, rather than composite complication outcomes. We found that certain complications such as pulmonary embolus and empyema had a much greater effect on the risk of readmission compared to others such as atrial arrhythmia and prolonged air leak. Pulmonary embolus, empyema, pleural effusion requiring drainage, and pneumothorax requiring chest tube all increased the odds of readmission at least 5-fold. It may be possible to decrease the readmission rate for those with pleural effusion or pneumothorax if systems allow for outpatient or 23-hour observation status to treat the problem. The next 3 complications in order of risk of readmission were central neurologic event, MI, and pneumonia, each increasing the odds of readmission about 3-fold; these three are likely to require admission for medical therapy. Interestingly, atelectasis requiring bronchoscopy was associated with a 37% decreased risk of readmission indicating that it is important to clear mucus plugs before discharge. Bronchopleural fistula and unexpected admission to an intensive care unit were not predictive of readmission and this may be because they are associated with other complications, for example, bronchopleural fistula and empyema.
The accuracy of models for risk prediction can be evaluated to determine how well they discriminate between those who experience the outcome and those who do not; the c statistic is a measure of discrimination.14 The c statistic ranges from 0.5, for which the model discriminates no better than a coin flip, to 1.0 where the scores for all of those with the outcome are greater than those without.14 Our multivariable model including predictors from all 3 phases of care had a c statistic of 0.736 which is higher than all others ranging from 0.6 to 0.7 for similar cohorts.5,6,8 Our model has precedent as the preoperative factors have been used in the STS GTSD risk model for predictors of mortality and major morbidity for lung cancer resection.13,15 Glance et al demonstrated that by predicting postoperative complications, the risk of readmission can be forecasted.16 Therefore, we used preoperative factors known to increase the risk of complications in patients undergoing lung cancer resection. Moreover, our model performed well because despite complications being the main driver of readmissions, there were several preoperative factors that retained their significance in the final model. Pulmonary specific variables such as FEV1 and interstitial fibrosis were important in this cohort of lobectomy patients. In addition, and similar to other studies,8,16 well established metrics such as Zubrod score (performance status) and ASA class (physical status) performed well in the final model. Not surprising, those undergoing lower lobectomy were at increased risk compared to those undergoing upper lobectomy. The lower lobes are typically larger, particularly in comparison to a right upper lobe and receive more perfusion than ventilation thereby contributing more to gas exchange. Moreover, COPD tends to be heterogeneous, affecting the upper lobes more than the lower lobes and therefore patients undergoing lower lobectomy typically lose the largest proportion of functional lung. Undergoing middle lobectomy was protective compared to upper lobectomy, likely because this lobe is the smallest and accounts for approximately 5% of lung function. None of the intraoperative process measures were independently predictive of readmission in the final model. DVT prophylaxis, another process measure, was not significant. This is likely because it is the outcome, DVT or PE, rather than the process measure DVT prophylaxis, that is more important in terms of risk of readmission. Surgical approach, VATS, and thoracotomy are commonly compared in the thoracic surgery literature. We and others4,9,10,17 have shown that surgical approach is not predictive of readmission after lung resection.
Our study has several limitations. Data on the timing of both complications and readmission are not available in the STS GTSD database. Both are important to better characterize hospital readmissions so that interventions to effectively prevent them can be implemented. A strength of the ACS NSQIP database is that this information is available. Glance et al16 reported that the 30-day readmission rate after noncardiac surgery was 78.3% for patients with a complication identified either in-hospital or post-discharge, compared to 12.3% for those with an in-hospital complication. A large study of VA patients also showed that patients with post-discharge complications had the highest odds of readmission.18 Bhagat et al9 showed that for patients undergoing lung resection, most complications occurred as an inpatient. However, for the patients who had a hospital readmission, most complications occurred after discharge.9 It is this subset of patients that warrants further study as they account for a large proportion of readmissions after lung resection.
Although the STS GTSD includes detailed data on comorbidities, intraoperative factors and 30-day outcomes, socioeconomic factors are lacking. In an NCDB study of patients undergoing lobectomy for lung cancer, lower median household income, metropolitan (vs urban or rural) residence and lack of private insurance were associated with readmission.7 However, this study did not have detailed data on comorbidities or complications and it is unclear whether these socioeconomic factors would continue to be significant in a more comprehensive model. Ideally, a complete model would include these factors as well as the ones we have included. We included insurance status and Medicaid was a significant predictor of readmission in the first 2 models but lost significance in the final model. Beyond insurance status, the STS GTSD does not collect data on income nor education level. Moreover, most data sources do not include information on social support or caregiver availability among other social determinants that are important at the time of discharge and may contribute not only to readmission but increased health care utilization post-discharge after major surgery. Furthermore, emerging data in general surgery cohorts indicate that preoperative opioid use and postoperative pain trajectories predict readmission.19–21 Patients undergoing lung resection experience some of the most intense postoperative pain.22,23 Accordingly, there is a need to study the effect of preoperative chronic pain syndromes and opioid use as well as postoperative pain control practices to determine their effect on readmission after thoracic surgery.
Previous work included length of stay (LOS) in readmission analyses and reported that the odds of readmission are 3.5 times greater if there was a prolonged LOS.8 We did not include LOS in our model for 2 conceptual reasons. First, prolonged LOS is a surrogate for postoperative complications and the driver of readmission is the complication, not the LOS. Second, there are a variety of factors affecting LOS that are not associated with the patient’s clinical course including social determinants as well as health care factors such as surgeon preferences and hospital discharge planning. On the other end of the spectrum, early discharge after lung cancer surgery does not increase readmissions.24
Strategies to reduce readmissions after lobectomy will be most effective if they target patients at greatest risk. Using the independent predictors of readmission we have identified, surgeons performing lobectomy may be better able to determine which patients are most likely to be readmitted. This is advantageous because interventions can prevent readmissions.25 In a meta-analysis of 42 clinical trials, the interventions that were most effective were those that increased patient capacity for self-care and those that were complex, including at least 5 unique components.25 There are few studies of interventions to address thoracic surgery patients’ needs around the time of discharge. One study contacted all patients undergoing lung resection via telephone after discharge and found that one-third had problems requiring counseling via telephone, whereas 12% required escalation of care.26 For those who required escalation of care, most problems were resolved via clinic appointment or additional telephone counseling and only 6.5% were referred to the ED.26 An integrated comprehensive care program consisting of an in-depth needs assessment of each patient undergoing major thoracic surgery and then meeting those needs as the patient transitions to home showed a trend toward fewer ED visits and readmissions.27 In 2 thoracic surgery studies, readmission most often occurred within 2 weeks of discharge; therefore, short-term follow-up, especially in those who had a complication, may reduce the risk of readmission.9,10
In conclusion, postoperative factors, specifically, complications are the main driver of readmission within 30 days of discharge after lobectomy for lung cancer. Among all complications, those requiring a procedure or medical therapy necessitating inpatient care are associated with the highest risk of readmission. Our findings may guide surgeons performing lobectomy in identifying patients at high risk of readmission. Further work is needed to explore other factors that may be associated with readmission such as sociodemographic and pain status. Once all relevant factors have been considered, interventions aimed toward reducing readmissions in high-risk patients need to be tested.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors report no conflict of interests.
REFERENCES
- 1.Centers for Medicare and Medicaid Services Readmissions Reduction Program. [Google Scholar]
- 2.Krumholz HM, Wang K, Lin Z, et al. Hospital-readmission risk—isolating hospital effects from patient effects. NEJM. 2017;377:1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai TC, Joynt KE, Orav EJ, et al. Variation in surgical readmissions and relationship to quality of hospital care. NEJM. 2013;369:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiles BM, Poon A, Giambrone GP, et al. Incidence and factors associated with hospital readmission after pulmonary lobectomy. Ann Thorac Surg. 2016;101:434–443. [DOI] [PubMed] [Google Scholar]
- 5.Puri V, Patel AP, Crabtree TD, et al. Unexpected readmission after lung cancer surgery: a benign event? J Thorac Cardiovasc Surg. 2015;150:1496–1505. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, McMurry TL, Isbell JM, et al. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg. 2014;148:2261–2267. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medbery RL, Gillespie TW, Liu Y, et al. Socioeconomic factors are associated with readmission after lobectomy for early stage lung cancer. Ann Thorac Surg. 2016;102:1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas DJ, Haider A, Haut E, et al. Assessing readmission after general, vascular, and thoracic surgery using ACS-NSQIP. Ann Surg. 2013;258:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagat R, Bronsert MR, Ward AN, et al. National analysis of unplanned readmissions after thoracoscopic versus open lung cancer resection. Ann Thorac Surg. 2017;104:1782–1790. [DOI] [PubMed] [Google Scholar]
- 10.Rajaram R, Ju MH, Bilimoria KY, et al. National evaluation of hospital readmission after pulmonary resection. J Thorac Cardiovasc Surg. 2015;150:1508–1514. [DOI] [PubMed] [Google Scholar]
- 11.Gani F, Lucas DJ, Kim Y, et al. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150:1042–1049. [DOI] [PubMed] [Google Scholar]
- 12.The Society of Thoracic Surgeons. Available at: https://www.sts.org.
- 13.Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: Predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–881. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez FG, Kosinki AS, Burfeind W, et al. The society of thoracic surgeons lung cancer resection risk model: higher quality data and superior outcomes. Ann Thorac Surg. 2016;102:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glance LG, Kellerman AL, Osler TM, et al. Hospital readmission after noncardiac surgery: the role of major complications. JAMA Surg. 2014;149:439–445. [DOI] [PubMed] [Google Scholar]
- 17.Assi R, Wong DJ, Boffa DJ, et al. Hospital readmission after pulmonary lobectomy is not affected by surgical approach. Ann Thorac Surg. 2015;99:393–398. [DOI] [PubMed] [Google Scholar]
- 18.Morris MS, Deierhoi RJ, Richman JS, et al. The relationship between timing of surgical complications and hospital readmission. JAMA Surg. 2014;149:348–354. [DOI] [PubMed] [Google Scholar]
- 19.Cron DC, Englesbe MJ, Bolton CJ, et al. Preoperative opioid use is independently associated with increased costs and worse outcomes after major abdominal surgery. Ann Surg. 2017;265:695–701. [DOI] [PubMed] [Google Scholar]
- 20.Waljee JF, Cron DC, Steiger RM, et al. Effect of preoperative opioid exposure on healthcare utilization and expenditures following elective abdominal surgery. Ann Surg. 2017;265:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Boussard T, Graham LA, Desai K, et al. The fifth vital sign: postoperative pain predicts 30-day readmissions and subsequent emergency department visits. Ann Surg. 2017;266:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochroch EA, Gottschalk A, Troxel AB, et al. Women suffer more short and long-term pain than men after major thoracotomy. Clin J Pain. 2006;22:491–498. [DOI] [PubMed] [Google Scholar]
- 23.Doan LV, Augustus J, Androphy R, et al. Mitigating the impact of acute and chronic post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2014;28:1048–1056. [DOI] [PubMed] [Google Scholar]
- 24.Rosen JE, Salazar MC, Dharmarajan K, et al. Length of stay from the hospital perspective. Ann Surg. 2017;266:383–388. [DOI] [PubMed] [Google Scholar]
- 25.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonoff MB, Ragalie W, Correa AM, et al. Results of postdischarge nursing telephone assessments: persistent symptoms common among pulmonary resection patients. Ann Thorac Surg. 2016;102:276–281. [DOI] [PubMed] [Google Scholar]
- 27.Shargall Y, Hanna WC, Schneider L, et al. The integrated comprehensive care program: a novel home care initiative after major thoracic surgery. Semin Thorac Cardiovasc Surg. 2016;28:574–582. [DOI] [PubMed] [Google Scholar]


