Abstract
Objective
There is growing interest in the early identification of patients with axial PsA (axPsA). We aimed to evaluate whether a dermatology-based screening strategy could help to identify axPsA patients.
Methods
The dermatologist-centred screening (DCS) questionnaire was administrated by dermatologists to consecutive patients fulfilling the inclusion criteria [(i) age ≥18 years and (ii) clinical diagnosis of psoriasis made by a dermatologist] to identify patients eligible (affirmative answers 1–3c of the DCS) for rheumatological evaluation. Clinical, laboratory, genetic and imaging data were collected from all referred patients.
Results
Among the 365 patients screened, 265 fulfilled the inclusion criteria and 124/265 (46.8%) were eligible for rheumatological referral. Diagnosis of axPsA, with or without peripheral PsA (pPsA), was made in 36/124 (29.0%) patients; pPsA without axial involvement was found in 21/124 (16.9%) patients. Back pain at screening was recorded in 174 (66%) patients, with 158 (60%) reporting a back pain duration longer than 3 months and 140 (53%) reporting back pain onset before the age of 45 years. Active inflammatory and/or structural post-inflammatory changes in the sacroiliac joints and/or spine were observed in all axPsA patients. Patients with PsA showed a numerically longer duration of back pain and higher CRP levels in comparison with patients with psoriasis without PsA.
Conclusion
The DCS tool proved to be a valuable screening strategy for detecting and characterizing patients with axPsA in a real-life cohort of psoriasis patients in a dermatological setting and helped to identify a substantial number of patients affected by undiagnosed pPsA.
Keywords: psoriatic arthritis, axial, screening, early diagnosis, clinical characterization, dermatologist
Rheumatology key messages.
Axial PsA (axPsA) is still a disease to be characterized and classified among SpA.
The dermatologist-centred screening tool was confirmed to be valuable for the detection of axPsA.
Our study reports novel data on epidemiological, clinical and instrumental features of axPsA patients.
Introduction
PsA is a chronic systemic inflammatory musculoskeletal disease that occurs in up to 30% of patients with psoriasis [1–3] and is classified among seronegative SpA.
PsA is characterized by different disease domains including axial and peripheral joint involvement, enthesitis, dactylitis, and skin and nail disease [4]. Axial PsA (axPsA) is clinically characterized by chronic back pain, which reflects active inflammation of the sacroiliac joints and/or spine, leading to structural damage in the long term [5, 6].
Skin psoriasis represents the cutaneous domain and is the most common extra-articular manifestation of PsA, usually preceding peripheral and/or axial involvement [7, 8]. Typical psoriatic nail lesions (e.g. psoriatic onychopathy), including matrix and/or nail bed alterations, are frequently associated with an increased risk of developing PsA and are described to correlate with PsA disease severity [9–11].
The diagnostic delay is a relevant problem in PsA, particularly in the case of axPsA, leading to irreversible and invalidating joint damage [12–14], and early diagnosis—particularly in the dermatologic context—and rapid administration of targeted treatment are essential in terms of morbidity and cost-effectiveness [4, 15].
Indeed, in the absence of reliable serological and/or radiological markers for PsA diagnosis [16], several screening/referral tools are emerging [17, 18], and recently the dermatologist-centred screening tool (DCS) has been validated for the detection of axPsA [19].
The DCS tool has been designed to facilitate the early identification of axPsA in patients with psoriasis, and it could play a crucial role in clinical practice and research, enabling dermatologists to assess and refer patients with psoriasis who may be affected by axial inflammation (axPsA) rapidly and efficiently. With a focus on a few specific criteria, the DCS tool could streamline the process of pinpointing potential axPsA cases, ensuring timely intervention and improving patient outcomes.
In this study, the DCS has been translated and applied in an Italian dermatological referral centre for its validation and to improve the early diagnosis of axPsA in patients with psoriasis.
Methods
Study design and outcomes
This is a cross-sectional monocentric study conducted at ‘Azienda Ospedaliero-Universitaria delle Marche’ in Ancona (Italy), in cooperation between the Medical Clinic and the Dermatology Clinic, from February 2022 to February 2023.
The acronym ATTRACT (Axial psoriaTic arThritis scReening AnCona iTaly) was coined for the study, which aims to evaluate the performance of the DCS tool in a Dermo-Rheumatologic clinic [20], focusing on the early identification of axPsA in a population of patients affected by psoriasis.
The primary aim of the study was to evaluate whether the DCS tool can identify axPsA patients among a cohort of patients with psoriasis. The secondary aims were the classification and characterization of axial involvement, including radiologic features both on X-ray and on MRI.
Patients, screening and referral strategy
Consecutive patients with psoriasis who consented to participate in the study were screened in the Dermatology Clinic to determine their eligibility for rheumatological referral.
Inclusion criteria, screening and referral strategies have been elsewhere described and are schematically represented in Fig. 1. A detailed description of patients, screening and referral strategy is available in Supplementary Materials and Methods, and Supplementary Fig. S1 (available at Rheumatology online).
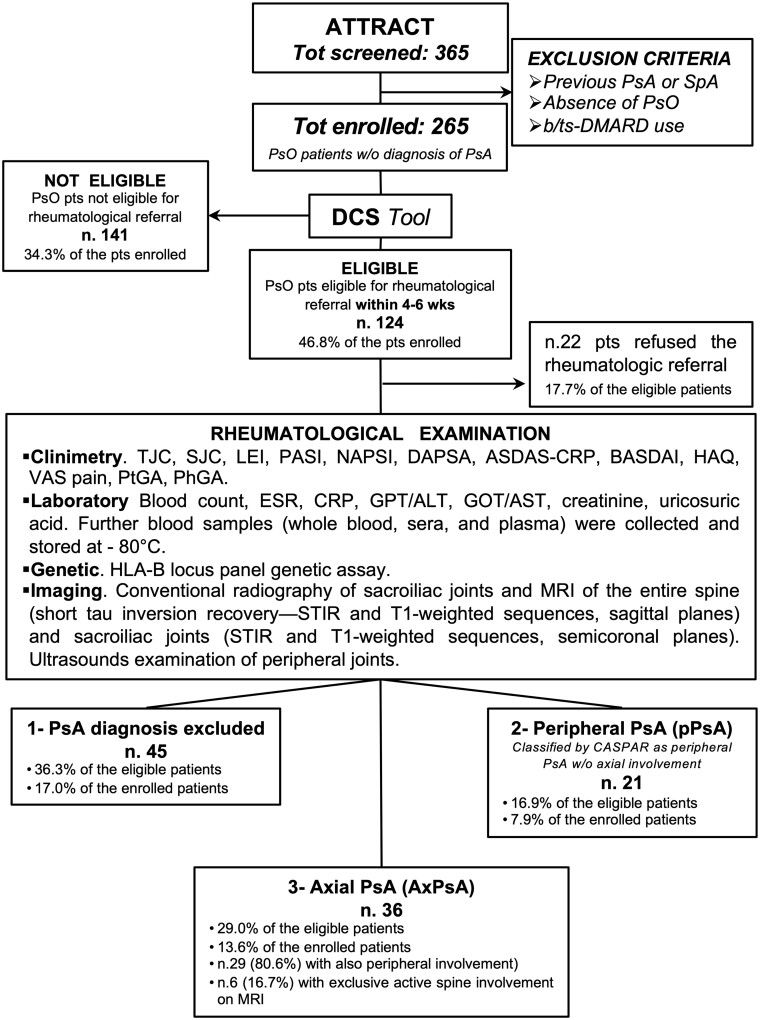
Figure 1.
Schematic design of the ATTRACT study. The figure shows the design of the ATTRACT (Axial psoriaTic arThritis scReening AnCona iTaly) study. N = 365 consecutive patients with psoriasis, who consented to participate in the study, were screened in the Dermatology Clinic to determine their eligibility for rheumatological referral and then evaluated in the rheumatologic outpatients’ clinic as shown in the box. Pso: psoriasis; b/tsDMARDs: biologic/targeted synthetic DMARDs; DCS: dermatologist-centred screening; TJC: tender joint count; SJC: swollen joint count; LEI: Leeds Enthesitis Index; PASI: Psoriasis Area Severity Index; NAPSI: Nail Psoriasis Severity Index; DAPSA: Disease Activity Index For Psoriatic Arthritis: ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score With C-Reactive Protein; VAS: Visual Analogue Scale for pain; PtGA and PhGA: Patient and Physician Global Assessment, respectively; STIR: short tau inversion recovery
Patients considered eligible at screening underwent rheumatological evaluation within a short time (2–4 weeks).
A complete rheumatological examination, including clinimetric, laboratory and genetic data, as well as radiological imaging of the entire spine and pelvis, and US examinations of the peripheral musculoskeletal system (see section ‘Imaging’), was conducted on all referred patients to classify them into the following categories: (i) PsA excluded; (ii) peripheral PsA involvement only; and (iii) confirmed axPsA, with or without peripheral involvement. The presence of PsA was determined by a team of rheumatologists and radiologists through the assessment of clinical, laboratory and imaging data, following the Assessment of SpondyloArthritis international Society (ASAS) and Classification Criteria for Psoriatic Arthritis (CASPAR) [21, 22].
This study included human participants and received approval from the ‘Comitato Etico Regione Marche’ (CERM) (protocol number 2411, CERM 2). The study was conducted according to the principles outlined in the Declaration of Helsinki. Participants provided informed consent before participation. Patients and/or the public were not involved in this research’s design, conduct, reporting or dissemination plans.
Clinical evaluation and laboratory tests
Screened patients
Demographic and clinical data were collected in all the psoriatic patients who filled out the DCS, including gender, age, BMI, back and peripheral pain duration, psoriasis duration, inflammatory back pain characteristics, previous DMARD use, smoking history, and a full list of comorbidities including cardiovascular, metabolic, infective, neoplastic and psychological (Table 1).
Table 1.
Characteristics of the ATTRACT study screened population
| Patients enrolled, total number (%) | All patients, 265 (100) | Referral-eligible, 124 (46.8) | Not eligible, 141 (53.2) | P |
|---|---|---|---|---|
| Gender (F/M), n (%) | 131 (49.4)/134 (50.6) | 75 (60.5)/49 (39.5) | 56 (39.7)/85 (60.3) | <0.001 |
| Age, years, mean ± s.d. | 52.1 ± 17.1 | 49.2 ± 15.5 | 54.7 ± 18.1 | 0.006 |
| BMI, kg/m2, mean ± s.d. | 26.3 ± 5.1 | 26.2 ± 5.1 | 26.5 ± 5.0 | 0.278 |
| Smoker, never, n (%) | 109 (41.1) | 57 (46.0) | 52 (36.9) | 0.090 |
| Smoker, previous, n (%) | 66 (24.9) | 24 (19.4) | 42 (29.1) | 0.034 |
| Smoker, active, n (%) | 90 (34.0) | 43 (34.7) | 47 (33.3) | 0.859 |
| Psoriasis duration, months, median (IQR) | 122 (253) | 75 (254) | 146 (262) | 0.013 |
| PASI, mean ± s.d. | 3.17 ± 5.55 | 3.24 ± 6.26 | 3.09 ± 4.76 | 0.408 |
| Onichopathy, n (%) | 139 (52.5) | 83 (66.9) | 57 (40.4) | 0.010 |
| NAPSI (per nail), mean ± s.d. | 1.81 ± 1.46 | 1.90 ± 1.48 | 1.70 ± 1.43 | 0.769 |
| Comorbidities, n (%) | 173 (65.2) | 83 (66.9) | 91 (64.5) | 0.684 |
| Cardiovascular diseasesa | 91 (34.3) | 36 (29.0) | 55 (39.0) | 0.080 |
| Metabolic diseasesb | 108 (40.8) | 51 (41.1) | 57 (40.4) | 0.944 |
| Chronic infective diseasesc | 4 (1.5) | 3 (2.4) | 1 (0.7) | 0.259 |
| Neurologic/psychiatricd | 14 (5.3) | 7 (5.7) | 7 (5.0) | 0.818 |
| Neoplasiae | 16 (6.0) | 8 (6.5) | 8 (5.7) | 0.805 |
| Otherf | 59 (22.2) | 36 (29.0) | 23 (16.3) | 0.022 |
| Back pain features | ||||
| Active back pain at screening (DCS question 3), n (%) | 174 (65.7) | 124 (100) | 50 (35.5) | <0.001 |
| Back pain duration >3 months (DCS question 3a), n (%) | 158 (59.6) | 124 (100) | 34 (24.1) | <0.001 |
| Back pain started before the age of 45 years (DCS question 3b), n (%) | 140 (52.8) | 124 (100) | 16 (11.4) | <0.001 |
| IBP, ASAS criteria, n (%) | 106 (40.0) | 95 (76.6) | 11 (7.8) | <0.001 |
| IBP, Berlin criteria, n (%) | 122 (46.0) | 98 (79.0) | 24 (17.0) | <0.001 |
Demographic and clinical characteristics of the patients with psoriasis who met the inclusion criteria and were screened with the DCS tool.
Includes ischaemic cardiomyopathy, heart failure, hypertension, cardiac arrhythmias (any), valvular disease (any), venous thromboembolism, pericardial disease and other cardiomyopathies.
Including diabetes type 1 or type 2, dyslipidaemia, obesity, osteoporosis and anaemias (any).
Includes chronic infection from HBV, HCV, VZV, HIV and Mycobacterium tuberculosis.
Includes stroke, transient ischaemic attack, migraine, demyelinating diseases (any), Parkinson’s disease and Parkinsonism, depressive and/or anxiety disorders, psychotic disorders and other psychiatric disorders.
Includes solid cancer, haematological cancer and non-melanoma skin cancer.
Includes any other disease not listed above. P-values are shown in bold if <0.05, indicating significant comparisons between eligible and not eligible patients. Statistical analysis was carried out by Pearson’s chi-squared test and Student's t-test. ATTRACT: Axial psoriaTic arThritis scReening AnCona iTaly; F: female; M: male; PASI: Psoriasis Area Severity Index; NAPSI: Nail Psoriasis Severity Index; DCS: dermatologist-centred screening tool (see Supplementary Fig. 1, available at Rheumatology online); IBP: inflammatory back pain; IQR: interquartile range.
Referred patients
A detailed clinical evaluation of each PsA domain, including axial, peripheral, enthesitis, dactylitis, skin, nail, uveitis and IBD, was performed in each eligible patient who consented to the rheumatological examination. PsA clinimetric tests were collected in all referred patients, recording tender joint count, swollen joint count, Leeds Enthesitis Index, Psoriasis Area Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Disease Activity Index For Psoriatic Arthritis (DAPSA), BASDAI, Ankylosing Spondylitis Disease Activity Score With C-Reactive Protein (ASDAS-CRP), HAQ, Visual Analogue Scale for pain, and Patient and Physician Global Assessment (Table 2).
Table 2.
Characteristics of the ATTRACT study referred population
| Patients, total number (%) | Patients referred, 102 (82.3) | Group 1 (Pso), 45 (36.3) | Group 2 (pPsA), 21 (16.9) | Group 3 (axPsA), 36 (29.0) | P | P, 1 vs 2 | P, 1 vs 3 | P, 2 vs 3 |
|---|---|---|---|---|---|---|---|---|
| Gender (F/M), n (%) | 60 (58.8)/42 (41.2) | 21 (46.7)/24 (53.3) | 14 (66.7)/7 (33.3) | 25 (69.4)/11 (30.6) | 0.084 | |||
| Age, years, mean ± s.d. | 49.6 ± 15.0 | 46.2 ± 15.5 | 51.2 ± 10.6 | 52.9 ± 16.0 | 0.118 | 0.618 | 0.136 | 1.000 |
| BMI, kg/m2, mean ± s.d. | 26.1 ± 5.3 | 25.7 ± 5.5 | 26.8 ± 5.0 | 26.2 ± 5.3 | 0.904 | 1.000 | 1.000 | 1.000 |
| Smoker, never, n (%) | 47 (46.1) | 20 (44.4) | 8 (38.1) | 19 (52.8) | 0.539 | |||
| Smoker, previous, n (%) | 23 (22.5) | 12 (26.7) | 6 (28.6) | 5 (13.9) | 0.891 | |||
| Smoker, active, n (%) | 32 (31.4) | 13 (28.9) | 7 (33.3) | 12 (33.3) | 0.298 | |||
| Psoriasis duration, months, median (IQR) | 74 (261) | 110 (237) | 88 (305) | 17 (144) | 0.565 | 1.000 | 1.000 | 0.596 |
| Back pain duration, months median (IQR) | 134 (206) | 74 (203) | 128 (229) | 195 (267) | 0.373 | 1.000 | 0.056 | 0.547 |
| Peripheral pain duration, months, median (IQR) | 63 (109) | 65 (107) | 39 (53) | 87 (176) | 0.044 | 1.000 | 0.227 | 0.053 |
| Back pain features | ||||||||
| Back pain at screening (DCS question 3), n (%) | 102 (100) | 45 (100) | 21 (100) | 36 (100) | ||||
| Back pain duration >3 months (DCS question 3a), n (%) | 102 (100) | 45 (100) | 21 (100) | 36 (100) | ||||
| Back pain started before age of 45 years (DCS question 3b), n (%) | 102 (100) | 45 (100) | 21 (100) | 36 (100) | ||||
| IBP, ASAS criteria, n (%) | 75 (73.5) | 33 (73.3) | 12 (57.1) | 30 (83.3) | 0.097 | |||
| IBP, Berlin criteria, n (%) | 79 (77.5) | 31 (68.9) | 15 (71.4) | 33 (91.6) | 0.039 | |||
| PsA domains involvement | ||||||||
| Axial disease, n (%) | 36 (35.3) | 0 (0.0) | 0 (0.0) | 36 (100) | <0.001 | |||
| Peripheral disease, n (%)a | 50 (49.0) | 0 (0.0) | 21 (100) | 29 (80.6) | <0.001 | |||
| Peripheral arthritis, n (%) | 39 (38.2) | 0 (0.0) | 19 (90.5) | 20 (55.6) | <0.001 | |||
| Enthesitis, n (%) | 38 (37.3) | 0 (0.0) | 16 (76.2) | 22 (61.1) | <0.001 | |||
| Dactylitis, n (%) | 10 (9.8) | 0 (0.0) | 5 (23.8) | 5 (13.9) | 0.006 | |||
| Onichopathy, n (%) | 70 (68.6) | 29 (64.4) | 12 (57.1) | 29 (80.6) | 0.167 | |||
| IBD, any, n (%) | 8 (7.8) | 1 (2.2) | 3 (14.3) | 4 (11.1) | 0.157 | |||
| Uveitis, ever, n (%) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (2.8) | 0.396 | |||
| Clinimetric scores and laboratory | ||||||||
| TJC, mean ± s.d. | 4.04 ± 7.64 | 1.33 ± 2.38 | 7.57 ± 12.59 | 5.40 ± 7.21 | <0.001 | 0.005 | 0.045 | 0.847 |
| SJC, mean ± s.d. | 1.08 ± 3.42 | 0.04 ± 0.21 | 1.10 ± 1.51 | 2.40 ± 5.45 | <0.001 | 0.687 | 0.006 | 0.460 |
| LEI, mean ± s.d. | 0.78 ± 3.42 | 0.49 ± 0.82 | 1.05 ± 1.47 | 1.00 ± 1.55 | <0.001 | 0.285 | 0.221 | 1.000 |
| PASI, mean ± s.d. | 3.31 ± 6.24 | 3.89 ± 7.70 | 2.33 ± 3.06 | 3.13 ± 5.51 | <0.001 | 1.000 | 1.000 | 1.000 |
| NAPSI (per nail), mean ± s.d. | 2.04 ± 1.57 | 1.83 ± 1.40 | 2.44 ± 1.74 | 2.12 ± 1.73 | 0.615 | 0.977 | 1.000 | 1.000 |
| DAPSA, mean ± s.d. | 14.5 ± 13.3 | / | 19.67 ± 16.61 | 19.00 ± 14.68 | 1.000 | / | / | 1.000 |
| ASDAS-CRP, mean ± s.d. | 1.72 ± 0.87 | / | 1.93 ± 0.83 | 2.06 ± 0.84 | 1.000 | / | / | 1.000 |
| BASDAI, mean ± s.d. | 4.18 ± 2.37 | / | 4.83 ± 2.32 | 4.91 ± 2.33 | 1.000 | / | / | 1.000 |
| HLA-B27 positive, n (%) | 9 (8.8) | 0 (0.0) | 2 (9.5) | 7 (19.4) | 0.009 | |||
| CRP (mg/l), mean ± s.d. | 0.33 ± 0.32 | 0.22 ± 0.16 | 0.39 ± 0.33 | 0.43 ± 0.42 | <0.001 | 0.108 | 0.010 | 1.000 |
| Elevated CRP (>0.5 mg/dl), n (%) | 17 (16.7) | 1 (2.2) | 7 (33.3) | 9 (25.0) | 0.002 | |||
| Imaging features | ||||||||
| Radiographic sacroiliitis (mNY criteria), bilateral, n (%) | 6 (5.9) | 0 (0.0) | 0 (0) | 6 (16.8) | <0.001 | |||
| Radiographic sacroiliitis (mNY criteria), monolateral, n (%) | 4 (3.9) | 0 (0.0) | 0 (0) | 4 (11.1) | <0.001 | |||
| Active inflammation, sacroiliac joint (MRI), n (%) | 25 (24.5) | 0 (0.0) | 0 (0( | 25 (69.4) | <0.001 | |||
| Structural post-inflammatory changes, sacroiliac joint (MRI), n (%) | 27 (26.5) | 1 (2.2) | 1 (4.8) | 25 (69.4) | <0.001 | |||
| Active inflammation, spine (MRI), n (%) | 11 (10.8) | 0 (0.0) | 0 (0( | 11 (30.6) | <0.001 | |||
| Structural post-inflammatory changes, spine (MRI), n (%) | 17 (16.7) | 2 (4.4) | 1 (4.8) | 14 (38.9) | <0.001 |
Demographic and clinical characteristics of the eligible patients who underwent a complete rheumatological evaluation after screening with the DCS tool.
Peripheral disease includes patients with polyarticular or monoarticular arthritis, enthesitis and/or dactylitis. Pso: patients with a diagnosis of psoriasis only; pPsA: patients with a diagnosis of psoriatic arthritis with peripheral involvement only; axPsA: patients with a diagnosis of psoriatic arthritis with axial involvement (with or without peripheral involvement).
Statistical analyses were carried out using Pearson’s chi-squared test and ANOVA with Bonferroni post-hoc test. P-values are shown in bold if <0.05, indicating significance.
ATTRACT: Axial psoriaTic arThritis scReening AnCona iTaly; F: female; M: male; IBP: inflammatory back pain; SJC: swollen joint count; TJC: tender joint count; LEI: Leeds Enthesitis Index; PASI: Psoriasis Area Severity Index; NAPSI: Nail Psoriasis Severity Index; DAPSA: Disease Activity in Psoriatic Arthritis; ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score with CRP; mNY criteria: modified New York criteria; DCS: dermatologist-centred screening tool (see Supplementary Fig. 1, available at Rheumatology online); IQR: interquartile range
Laboratory panel comprehensive of complete blood count, hepatic and renal function test, uricosuric acid, ESR and CRP, in addition to the assessment of HLA B27 genetic assay, was performed in all referred patients. Further blood samples (whole blood, sera and plasma) were collected and stored in the Marche Biobank facility (Ancona, Italy) for ancillary analyses.
Imaging
All referred patients underwent imaging with conventional radiography of the pelvis and MRI of sacroiliac joints and of the entire spine. MRI was carried out with short tau inversion recovery (STIR) and T1-weighted sequences, sagittal planes of the spine and STIR and T1-weighted sequences, and semicoronal planes of sacroiliac joints. Images were evaluated by a panel comprising at least two rheumatologists and a musculoskeletal radiologist. The presence or absence of radiographic sacroiliitis and the sacroiliitis grade on radiographs according to the modified New York (mNY) criteria [23] was recorded by consensus. The panel also assessed the presence or absence of active inflammatory and structural changes on MRI that were compatible with axial involvement of PsA and fulfilled the ASAS/OMERACT definitions for a positive MRI [24–26].
A musculoskeletal US examination of the tender peripheral joints and tendons, and all the entheses according to the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index [27], was performed in all patients with a diagnosis of PsA to confirm or exclude the peripheral involvement by a sonograph ‘MyLab X8’ (Esaote Biomedica Genoa, Italy). The joints, tendons and entheses were assessed according to OMERACT recommendations and updated definitions [28, 29].
Data management and analysis
Study data were collected and managed using the REDCap software (www.project-redcap.org) [30].
For descriptive analyses, categorical variables were reported as absolute numbers and frequencies, continuous variables as mean and s.d., or median and interquartile range (IQR) depending on their distribution. The proportion of patients with psoriasis diagnosed with axPsA or peripheral PsA (pPsA) fulfilling the ASAS classification criteria for axial SpA (axSpA) and the CASPAR classification criteria for PsA was calculated out of the total number of psoriasis patients referred and seen at the rheumatology clinic.
Statistically significant differences between patients diagnosed with axPsA and those with psoriasis without axial or peripheral PsA were determined using the Student’s t test or the analysis of variance (ANOVA) for continuous variables and the χ2 test for categorical variables.
A backward stepwise logistic regression was used to identify possible predictors of the outcomes (axPsA or pPsA diagnosis) out of the following candidate variables: gender, age, BMI, active smoking habit, PASI, duration of skin psoriasis, presence of nail disease and previous biologic use. At each step, variables were deleted based on P-values, and a P-value threshold of 0.01 was used to set a limit on the total number of variables included in the final model.
All statistical analyses were performed using the STATA software. The GraphPad Prism software and R statistical software were used to generate the figures. A P-value <0.05 was considered significant.
Results
Diagnosis of axPsA and pPsA
From 15 February 2022 to 28 February 2023, a total of 365 patients were screened, of whom 100 met the exclusion criteria. Among the remaining cohort of 265 patients, 124 (46.8%) were eligible for rheumatological referral, and out of those 102 (82.3%) underwent the rheumatological examination. The remaining 22 (17.7%) eligible patients, with clinical and demographic characteristics similar to the referred ones (data not shown), declined the rheumatologic examination.
Diagnosis of axPsA was made in 36/124 (29.0%) patients and, among them, 29/36 (80.6%) presented also peripheral involvement.
Diagnosis of pPsA (without axial involvement) was made in 21/124 (16.9%) patients. Finally, 45/124 (36.3%) patients were classified as neither axPsA nor pPsA (Fig. 1).
The ASAS classification criteria for axSpA [22] were fulfilled in 30/36 (83.3%) of patients diagnosed with axPsA (28/30 fulfilled the imaging branch, 2/30 the clinical branch). The CASPAR classification criteria for PsA [21] were fulfilled in all but one patient diagnosed with axPsA (35/36) and 21/21 with pPsA (Fig. 1).
Clinical characteristics of the screened patients
The demographic and clinical characteristics of the 265 patients with psoriasis screened are fully shown in Table 1.
The female gender proportion was 49% (n = 131), the mean ± s.d. age was 52 ± 17 years, BMI 26.3 ± 5.1 and PASI 3.2 ± 5.6, and the median (IQR) duration of psoriasis 122 (253) months. More than half of the patients (n = 139, 52%) showed nail involvement with a mean NAPSI (per nail) of 1.8 ± 1.5.
Most patients had relevant comorbidities (n = 173, 65%), with 91 presenting cardiovascular diseases and 108 having metabolic disorders. Additionally, 90 patients reported an active smoking habit, while 66 reported former tobacco use.
The presence of active back pain at the screening was recorded in 174 (66%) patients; 158 (60%) had a back pain duration longer than 3 months and 140 (53%) reported the onset of back pain before the age of 45 years.
Among all the screened patients, the proportion of inflammatory back pain (IBP), defined according to ASAS [31] and Berlin criteria [32], was higher in eligible patients (76.6% and 79.0%, respectively) than in non-eligible ones (7.8% and 17.2%, respectively, P < 0.001).
Clinical characteristics of the referred patients
Demographic and clinical characteristics of the patients are detailed in Table 2.
The mean age was similar between patients diagnosed with pPsA (51.2 ± 10.6 years) and patients with axPsA (52.9 ± 16.0 years), while patients without PsA were slightly younger (46.2 ± 15.5 years).
The proportion of female patients was higher among patients diagnosed with pPsA (14/21, 66.7%) and axPsA (25/36, 69.4%), and lower in patients without PsA (21/45, 46.7%).
Patients with axPsA had a lower median (IQR) psoriasis duration [17 (144) months] and a higher median (IQR) back pain duration [195 (267) months], whereas patients without PsA showed the highest median (IQR) psoriasis duration [110 (237) months] and the lowest median (IQR) back pain duration [74 (203) months]. Nevertheless, those differences did not reach statistical significance.
The comparison between axPsA and pPsA patients shows similar disease activity, assessed according to DAPSA (19.0 ± 14.7 vs 19.7 ± 16.6, respectively, Δ0.7), ASDAS-CRP (2.06 ± 0.84 vs 1.93 ± 0.83, respectively, Δ–0.13) and BASDAI (4.91 ± 2.33 vs 4.83 ± 2.32, respectively, Δ–0.08) scores.
However, axPsA patients presented a numerically higher proportion of onychopathy than those with pPsA.
Among patients evaluated by a rheumatologist, the proportion of IBP, defined according to ASAS [31] and Berlin criteria [32], was higher in axPsA patients (83.3% and 91.7%, respectively) compared with patients with pPsA (57.1% and 71.4%, respectively) and those without PsA (73.3% and 68.9%, respectively). The difference was statistically significant for IBP according to Berlin criteria (P = 0.039), but not for IBP according to ASAS criteria (P = 0.097).
The results of the multivariate regression analysis showed that PsA and pPsA diagnosis was associated with female gender [odds ratio (OR) 2.06, 95% CI 1.00–4.22 and OR 2.28, 95% CI 1.07–4.87, respectively] and inversely associated with previous biologic (bDMARDs) and/or targeted synthetic (tsDMARDs) use (OR 0.25, 95% CI 0.10–0.65 and OR 0.34, 95% CI 0.13–0.89, respectively). AxPsA diagnosis was significantly associated only with nail disease (OR 3.02, 95% CI 1.15–7.91). The results were consistent in the sensitivity analysis excluding patients who did not undergo rheumatologic evaluation (Table 3).
Table 3.
Multivariable logistic regression
| (A) Factors associated with PsA diagnosis | ||||||
|---|---|---|---|---|---|---|
| Analysis including ‘drop-out’ patientsa |
Analysis NOT including ‘drop-out’ patientsb |
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender | 2.023 | 0.999–4.091 | 0.050 | 2.065 | 1.009–4.228 | 0.047 |
| Female vs male | ||||||
| Previous b/tsDMARDs use | 0.264 | 0.104–0.674 | 0.005 | 0.256 | 0.100–0.659 | 0.005 |
| Present vs absent | ||||||
| (B) Factors associated with axPsA final diagnosis | ||||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P| | OR | 95% CI | P | |
| Gender | 2.030 | 0.880–4.682 | 0.097 | 2.034 | 0.875–4.728 | 0.099 |
| Female vs male | ||||||
| Onychopathy | 2.966 | 1.139–7.723 | 0.026 | 3.024 | 1.156–7.912 | 0.024 |
| Present vs absent | ||||||
| Pso duration (years) | 0.971 | 0.943–1.002 | 0.068 | 0.973 | 0.943–1.003 | 0.078 |
| (C) Factors associated with pPsA final diagnosis | ||||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender | 2.236 | 1.059–4.722 | 0.035 | 2.286 | 1.072–4.876 | 0.035 |
| Female vs male | ||||||
| Previous b/tsDMARDs use | 0.349 | 0.135–0.901 | 0.030 | 0.342 | 0.132–0.891 | 0.028 |
| Present vs absent | ||||||
A backward stepwise logistic regression was conducted to identify potential predictors of the outcomes from a poll of candidate variables, including gender, age, BMI, active smoking habit, PASI, duration of skin psoriasis, presence of nail disease and previous biologic use. At each step, variables were removed based on P-values, with a threshold of 0.01 used to determine the final set of variables in the model. The table displays the results of two logistic regression analyses:
one including eligible patients who declined the rheumatological evaluation (on the left), and
one excluding this group (on the right). In the first analysis, the ‘drop-out’ patients were classified as having psoriasis without a diagnosis of PsA. Results are shown as follows: (A) probability of being diagnosed with PsA, (B) probability of being diagnosed with axPsA and (C) probability of being diagnosed with pPsA, for the patients enrolled in the ATTRACT (Axial psoriaTic arThritis scReening AnCona iTaly) study. The P-value was considered significant if <0.05, and significant P-values are shown in bold. Statistical analyses were conducted using ‘Stata’ software. OR: odds ratio; axPsA: axial PsA; pPsA: peripheral PsA; Pso: psoriasis; b/tsDMARDs: biologic and/or targeted synthetic DMARDs.
Laboratory and imaging features of the referred patients
Laboratory and imaging characteristics of all the referred patients are shown in Table 2, Supplementary Figs S2 and S3 (available at Rheumatology online).
The patients diagnosed with axPsA showed significantly higher CRP levels than patients without PsA (0.43 ± 0.42 vs 0.22 ± 0.16, respectively; Δ–0.21); furthermore, the proportion of patients with elevated CRP above the upper normal limit (i.e. CRP >0.5 mg/dl) was significantly higher in axPsA (25%) and pPsA (33%) patients than in those without PsA (2%).
In our group of patients, we found a low overall incidence of HLA-B27 positivity: in the axPsA group, 7 (19.4%) patients were HLA-B27 positive, while only 2 (9.5%) were found in the pPsA group. No HLA-B27 positivity was found in the patients with psoriasis and without a PsA diagnosis.
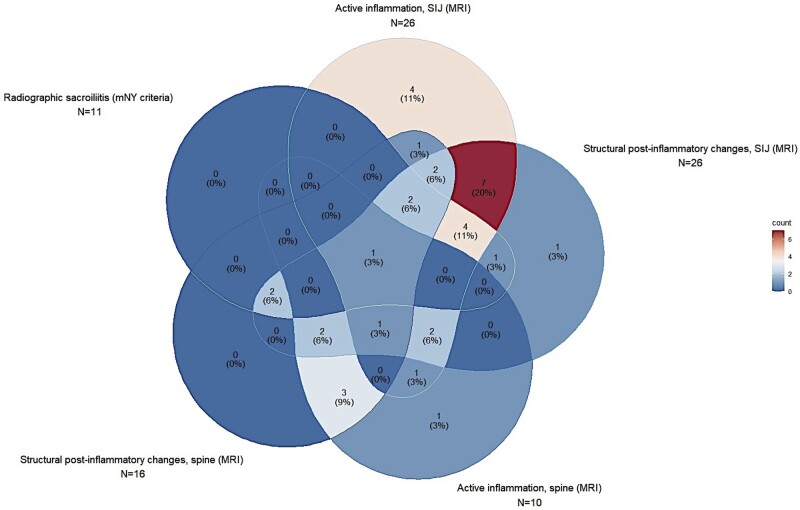
All the patients with axPsA had active inflammatory and/or structural post-inflammatory changes in the sacroiliac joints and/or spine on imaging (Table 2, Fig. 2, and Supplementary Fig. S2, available at Rheumatology online).
Figure 2.
Imaging features of axial involvement in patients with psoriasis diagnosed with axPsA. This five-entry Venn diagram represents the distribution of radiological features on axial imaging in the ATTRACT (Axial psoriaTic arThritis scReening AnCona iTaly) patients diagnosed with axPsA. Every piriform figure represents one of the five main features of axPsA imaging: radiographic sacroiliitis as per mNY criteria at the upper left corner, active inflammation on MRI of SIJ at the top, structural post-inflammatory changes on MRI of SIJ at the upper right corner, active inflammation on MRI of the spine at the bottom right and structural post-inflammatory changes on MRI of the spine at the bottom left. The radiological overlap of the five features is represented by the overlap of each figure with the others, in every possible combination. In every section of each figure (overlapping or non-overlapping with the others) is shown the number (%) of patients affected by that feature(s). The percentage of patients is also scaled according to the colour legend on the right (blue for the lowest, red for the highest). It could be observed that the highest percentage of patients present both active and structural post-inflammatory changes in the SIJ, as represented by the red overlapping section between the top and upper right corner figures. A consistent proportion of patients presented spine involvement (bottom figures) without an active SIJ inflammation on MRI or a radiographic sacroiliitis; such patients did not fulfill the ASAS classification criteria for axSpA. The patient with only structural post-inflammatory changes on MRI without any other radiological feature (top right corner) was diagnosed with axPsA because of HLA-B27 positivity (‘clinical branch’). axPsA: PsA with axial involvement; mNY: modified New York; SIJ: sacroiliac joint
Radiographic sacroiliitis, defined according to mNY criteria [23], was found in 10 (27.8%) patients: 6/10 showed sacroiliac joint involvement grade ≥2 bilaterally, and 4/10 showed sacroiliac joint involvement grade ≥3 unilaterally (Fig. 2 and Supplementary Fig. S2, available at Rheumatology online).
Active sacroiliac joint inflammation and active spinal inflammation on MRI, both defined according to ASAS definitions [24–26], were found respectively in 25 (69.4%) and 11 (30.6%) patients, whereas structural post-inflammatory sacroiliac and spinal changes were found respectively in 25 (69.4%) and 14 (38.9%) patients (Fig. 2 and Supplementary Fig. S2, available at Rheumatology online).
In 4 (11.1%) patients, MRI changes indicative of axial involvement were found only in the spine, and another 2 (5.6%) patients showed active spine inflammation associated with structural post-inflammatory changes in the sacroiliac joints (Fig. 2). Such patients did not fulfill the ASAS classification criteria for axSpA, also in the clinical arm.
Among the patients diagnosed with pPsA or not diagnosed with PsA, we found 5 (7.6%) patients with structural changes of the sacroiliac joints and/or spine on MRI suspected of being post-inflammatory, without any active lesions.
Previous and current treatments
A relevant proportion of screened patients (25.7%) had a history of previous bDMARDs use for psoriasis. However, no patient was taking bDMARDs in the 12 weeks before the screening, as per exclusion criteria.
The most common systemic psoriasis therapy ongoing was MTX (in 6.4% of the screened patients). Common topical psoriasis therapies included steroids, tacrolimus and vitamin D analogues (in 26% of the patients). All patients were not taking opioid analgesics in the 5 days before the screening.
Discussion
Diagnostic delay in PsA is a well-known problem, though recent studies have shown no improvement in recent years, with a median diagnostic delay exceeding 2 years [33], and in the case of axSpA the delay increases to >6 years [12].
In this scenario, our study is the first to apply a translated version of the DCS tool [19] in a population of Italian patients with psoriasis.
In our opinion, the early identification of axial inflammation in patients with psoriasis holds significant importance for several reasons: (i) diagnostic delay in detecting axial inflammation is consistently longer than for peripheral clinical phenotype [12]; (ii) prolonged diagnostic delay in axSpA is associated with significant spinal bone damage, which can lead to severe physical impairment and a relevant economic and humanistic burden [34, 35]; and (iii) differently from pPsA, in patients with axPsA therapy with conventional synthetic DMARDs is not recommended [36, 37], while an early initiation of bDMARDs is strongly recommended since it is efficacious and could prevent spinal bone damage, as shown in axSpA [38, 39].
Considering this scenario, the DCS tool may prove valuable in the screening of early axPsA and, as shown in our study, in some cases pPsA as well.
In our study, we found that 46% of patients referred to a rheumatologist (22% of the total screened patients with psoriasis) were diagnosed with PsA (21/124 with pPsA and 36/124 with axPsA) and among this group, we found axial involvement in 63% of the patients (related to the screening methodology focusing on axial symptoms). These data are consistent with a recent meta-analysis reporting a prevalence of PsA among adult patients with psoriasis of 21.6% [40] and with previous studies suggesting that 25–70% of patients with PsA may present axial involvement [41, 42].
Interestingly, we also identified 37% of PsA patients with exclusive peripheral involvement, confirmed by musculoskeletal US analysis, and reporting chronic back pain. These findings highlight the importance of early PsA diagnosis in the dermatologic setting and the potential for identifying a substantial number of undiagnosed pPsA patients, in this scenario.
Another important issue concerning PsA is the need for updated classification criteria for axPsA, taking into consideration the difference between axPsA and axSpA [43, 44].
In our study, 28% of the axPsA patients demonstrated radiographic sacroiliitis according to mNY criteria (17% bilateral and 11% unilateral). Investigating the overlap between X-ray and MRI findings in the sacroiliac joints, we observed that all patients with radiographic sacroiliitis also exhibited MRI changes, but 21 patients with inflammatory and/or structural post-inflammatory changes in the sacroiliac joints on MRI did not fulfill X-ray mNY criteria of sacroiliitis.
Furthermore, we identified exclusive involvement of the spine on MRI, with inflammatory and/or structural post-inflammatory changes, in four patients, while another two presented active spine lesions and only structural post-inflammatory sacroiliac changes on MRI (without radiographic sacroiliitis on X-ray); accordingly, such patients did not meet the ASAS classification criteria for axSpA.
Additionally, we found structural changes in the sacroiliac joints and/or spine in five patients who reported back pain with inflammatory features but who were not diagnosed with axPsA due to the absence of active inflammation on MRI and radiographic sacroiliitis on X-ray. Considering the features of their ongoing back pain, further exploration is needed to understand why these patients continue to experience pain without active lesions.
These findings emphasize the importance of MRI in detecting axial involvement in the absence of definitive radiographic changes in the sacroiliac joints and underscore the significance of recognizing exclusive spine involvement in the classification of axPsA.
Our study also provides valuable clinical and laboratory insights into axPsA.
Consistent with previous studies [45], we observed a higher proportion of females with axial involvement, and onychopathy was strongly associated with axPsA diagnosis. Moreover, axPsA presented a longer duration of symptoms, suggesting that axial involvement is more common in long-standing PsA [46].
Regarding laboratory findings, axPsA patients showed higher levels of CRP, with a higher proportion of patients in both the axial (25%) and peripheral PsA (33%) populations showing CRP levels above the upper normal limit in comparison with psoriasis patients, thus supporting the reported association between CRP levels and the incidence of PsA [47–49] or axial involvement [50]. In our study, elevated CRP levels could potentially indicate the presence of active inflammatory changes in the MRI scans of most patients classified as axPsA. Finally, in our study, the incidence of HLA-B27 positivity in the axPsA group was lower than in a previous study [19]. This discrepancy could be explained by the wide range of HLA-B27 positivity observed in other studies [50]. Additionally, it may be attributed to sampling bias in the studied population (considering the monocentric nature of the study) or, intriguingly, to a peculiar genetic background of the patients, considering that in our study we did not find any HLA-B27-positive patients among the psoriasis patients without arthritis.
This study has several strengths. First, it confirms the effectiveness of the DCS tool in identifying undiagnosed axPsA in a different population of the previous study [19]. Secondly, the prospective design allowed for the high-quality collection of data, enhancing previous reports on the epidemiologic, clinical and imaging features of axPsA.
The study has limitations as well. First, being a monocentric study conducted in a single tertiary referral centre, the study’s sample may not fully represent the broader population of patients with psoriasis. Secondly, a notable limitation of our study is the absence of a comprehensive evaluation of the DCS’s specificity and negative predictive value since patients not eligible for the referral strategy were not evaluated. While the DCS successfully served its primary purpose as a screening tool within the dermatological setting, it did not undergo validation beyond this context. Future research endeavours should prioritize this validation process to assess the tool’s true accuracy and reliability.
Thirdly, an inability to perform X-rays of the whole spine on all patients in our study could limit a comprehensive assessment of structural damage. This limitation arose from ethical considerations about limiting ionizing radiation exposure in patients screened for early axial manifestations, but it has to be acknowledged.
Fourthly, a notable proportion of patients declined rheumatological evaluation; however, the clinical and demographic characteristics of these patients do not seem to differ significantly from those referred.
In conclusion, our study confirms that the application of the DCS tool in a dermatological setting is a fast, efficient and valuable method for detecting axPsA in patients with psoriasis. Notably, we found a substantial number of undiagnosed pPsA patients in the dermatologic setting, emphasizing the importance of early diagnosis for both pPsA and axPsA.
Supplementary Material
Contributor Information
Michele Maria Luchetti Gentiloni, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Valentino Paci, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Ilaria Cimaroli, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Alice Agostinelli, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Melania Giannoni, Dermatology Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Anna Campanati, Dermatology Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Federico Diotallevi, Dermatology Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Marina Carotti, Radiology Clinic, Department of Radiological Sciences, Polytechnic University of Marche, Ancona, Italy.
Francesco Sessa, Neuroradiology Clinic, Department of Radiological Sciences, Polytechnic University of Marche, Ancona, Italy.
Raffaella Sordillo, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Cristina Macchini, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Federico Fiorini, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Leonardo Massaccesi, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Monia Ciferri, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Marco Gigli, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Valentina Marconi, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Lucia Perini, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy; Internal Medicine Residency Programme, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Andrea Marani, Dermatology Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Andrea Giovagnoni, Radiology Clinic, Department of Radiological Sciences, Polytechnic University of Marche, Ancona, Italy.
Gabriele Polonara, Neuroradiology Clinic, Department of Radiological Sciences, Polytechnic University of Marche, Ancona, Italy.
Anna Maria Offidani, Dermatology Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Devis Benfaremo, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Fabian Proft, Department of Gastroenterology, Infectiology and Rheumatology (including Nutrition Medicine), Charité-Universitätsmedizin, Berlin, Germany.
Denis Poddubnyy, Department of Gastroenterology, Infectiology and Rheumatology (including Nutrition Medicine), Charité-Universitätsmedizin, Berlin, Germany.
Gianluca Moroncini, Medical Clinic, Department of Clinical and Molecular Sciences, Marche Polytechnic University, Ancona, Italy.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data are available upon reasonable request.
Contribution statement
All authors contributed to the study design, interpretation of results and development of this manuscript. M.M.L.G. is the guarantor for this study.
Funding
Medical writing was funded through a donation by AbbVie Srl.
Disclosure statement: M.M.L.G. reports research support from AbbVie; consulting fees from AbbVie, Janssen, Novartis and Pfizer; speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis and Pfizer. A.C. reports speaker and consultancy fees from Abbvie, Almiral, Amgen, Eli Lilly, Janssen, Leofarma, Novartis, Pfizer, Sanofi and UCB. A.M.O. reports speaker and consultancy fees from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi and UCB. F.P. reports grants and personal fees from Novartis, Eli Lilly and UCB; personal fees from AbbVie, AMGEN, BMS, Celgene, Eli Lilly, Janssen, Hexal, Medscape, MSD, Pfizer and Roche, all outside the presented work. D.P. reports research support from AbbVie, Eli Lilly, MSD, Novartis and Pfizer; consulting fees from AbbVie, Biocad, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, MSD, Moonlake, Novartis, Pfizer, Samsung Bioepis and UCB; speaker fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, MSD, Medscape, Novartis, Peervoice, Pfize, and UCB. G.M. reports consulting fees from AbbVie, Janssen and Pfizer; speaker fees from AbbVie, Janssen, Novartis and Pfizer.
References
- 1. Gelfand JM, Gladman DD, Mease PJ et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005;53:573.e1–13. [DOI] [PubMed] [Google Scholar]
- 2. Eder L, Haddad A, Rosen CF et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol 2016;68:915–23. [DOI] [PubMed] [Google Scholar]
- 3. Zabotti A, De Lucia O, Sakellariou G et al. Predictors, risk factors, and incidence rates of psoriatic arthritis development in psoriasis patients: a systematic literature review and meta-analysis. Rheumatol Ther 2021;8:1519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coates LC, Soriano ER, Corp N et al. ; GRAPPA Treatment Recommendations Domain Subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poddubnyy D, Callhoff J, Spiller I et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD Open 2018;4:e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisman MH. Inflammatory back pain. Rheum Dis Clin North Am 2012;38:501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottlieb AB, Merola JF. Axial psoriatic arthritis: an update for dermatologists. J Am Acad Dermatol 2021;84:92–101. [DOI] [PubMed] [Google Scholar]
- 8. Wilson FC, Icen M, Crowson CS et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum 2009;61:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raposo I, Torres T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermosifiliogr 2015;106:452–7. [DOI] [PubMed] [Google Scholar]
- 10. Zabotti A, De Marco G, Gossec L et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis 2023;82:1162–70. [DOI] [PubMed] [Google Scholar]
- 11. De Marco G, Zabotti A, Baraliakos X et al. Characterisation of prodromal and very early psoriatic arthritis: a systematic literature review informing a EULAR taskforce. RMD Open 2023;9:e003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao SS, Pittam B, Harrison NL et al. Diagnostic delay in axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2021;60:1620–8. [DOI] [PubMed] [Google Scholar]
- 13. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015;74:1045–50. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ, Palmer JB, Liu M et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol 2018;45:1389–96. [DOI] [PubMed] [Google Scholar]
- 15. Ramiro S, Nikiphorou E, Sepriano A et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34. [DOI] [PubMed] [Google Scholar]
- 16. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clinical Immunol 2020;214:108390. [DOI] [PubMed] [Google Scholar]
- 17. Mishra S, Kancharla H, Dogra S, Sharma A. Comparison of four validated psoriatic arthritis screening tools in diagnosing psoriatic arthritis in patients with psoriasis (COMPAQ Study). Br J Dermatol 2017;176:765–70. [DOI] [PubMed] [Google Scholar]
- 18. Mease PJ, Palmer JB, Hur P et al. Utilization of the validated Psoriasis Epidemiology Screening Tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: a cross-sectional analysis from the US-based Corrona Psoriasis Registry. J Eur Acad Dermatol Venereol 2019;33:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Proft F, Lüders S, Hunter T et al. Early identification of axial psoriatic arthritis among patients with psoriasis: a prospective multicentre study. Ann Rheum Dis 2022;81:1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luchetti MM, Benfaremo D, Campanati A et al. Clinical outcomes and feasibility of the multidisciplinary management of patients with psoriatic arthritis: two-year clinical experience of a dermo-rheumatologic clinic. Clin Rheumatol 2018;37:2741–9. [DOI] [PubMed] [Google Scholar]
- 21. Taylor W, Gladman D, Helliwell P et al. ; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 22. Rudwaleit M, Van Der Heijde D, Landewé R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 23. Van Der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 24. Sieper J, Rudwaleit M, Baraliakos X et al. The assessment of spondyloarthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68(Suppl 2):ii1–44. [DOI] [PubMed] [Google Scholar]
- 25. Lambert RGW, Bakker PAC, Van Der Heijde D et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 26. Baraliakos X, Østergaard M, Lambert RGW et al. MRI lesions of the spine in patients with axial spondyloarthritis: an update of lesion definitions and validation by the ASAS MRI working group. Ann Rheum Dis 2022;81:1243–51. [DOI] [PubMed] [Google Scholar]
- 27. Maksymowych WP, Mallon C, Morrow S et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 28. D'Agostino M-A, Terslev L, Aegerter P et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce—Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 2017;3:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruyn GA, Iagnocco A, Naredo E et al. ; OMERACT Ultrasound Working Group. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J Rheumatol 2019;46:1388–93. [DOI] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sieper J, Van Der Heijde D, Landewé R et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 32. Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. [DOI] [PubMed] [Google Scholar]
- 33. Karmacharya P, Wright K, Achenbach SJ et al. Diagnostic Delay in Psoriatic Arthritis: a Population-based Study. J Rheumatol 2021;48:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martindale J, Goodacre L. The journey to diagnosis in AS/axial SpA: the impact of delay. Musculoskelet Care 2014;12:221–31. [DOI] [PubMed] [Google Scholar]
- 35. Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther 2020;7:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coates LC, Corp N, van der Windt DA et al. GRAPPA treatment recommendations: 2021 update. J Rheumatol 2022;49:52–4. [DOI] [PubMed] [Google Scholar]
- 37. Gossec L, Baraliakos X, Kerschbaumer A et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torgutalp M, Rios Rodriguez V, Dilbaryan A et al. Treatment with tumour necrosis factor inhibitors is associated with a time-shifted retardation of radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 2022;81:1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun J, Baraliakos X, Deodhar A et al. ; MEASURE 1 Study Group. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017;76:1070–7. [DOI] [PubMed] [Google Scholar]
- 40. Alinaghi F, Calov M, Kristensen LE et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–65.e19. [DOI] [PubMed] [Google Scholar]
- 41. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA) - an analysis of 220 patients. Q J Med 1987;62:127–41. [PubMed] [Google Scholar]
- 42. Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep 2007;9:455–60. [DOI] [PubMed] [Google Scholar]
- 43. Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol 2018;14:363–71. [DOI] [PubMed] [Google Scholar]
- 44. Michelena X, López-Medina C, Erra A et al. Original research: characterising the axial phenotype of psoriatic arthritis: a study comparing axial psoriatic arthritis and ankylosing spondylitis with psoriasis from the REGISPONSER registry. RMD Open 2022;8:2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nas K, Kiliç E, Tekeoğlu İ et al. The effect of gender on disease activity and clinical characteristics in patients with axial psoriatic arthritis. Mod Rheumatol 2021;31:869–74. [DOI] [PubMed] [Google Scholar]
- 46. Giovannini I, Zabotti A, Cicciò C et al. Axial psoriatic disease: clinical and imaging assessment of an underdiagnosed condition. J Clin Med 2021;10:2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mulder MLM, van Hal TW, Wenink MH et al. Clinical, laboratory, and genetic markers for the development or presence of psoriatic arthritis in psoriasis patients: a systematic review. Arthritis Res Ther 2021;23:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loo YP, Loo CH, Lim AL et al. Prevalence and risk factors associated with psoriatic arthritis among patients with psoriasis. Int J Rheum Dis 2023;26:1788–98. [DOI] [PubMed] [Google Scholar]
- 49. Braga MV, de Oliveira SC, Vasconcelos AHC et al. Prevalence of sacroiliitis and acute and structural changes on MRI in patients with psoriatic arthritis. Sci Rep 2020;10:11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benavent D, Plasencia C, Poddubnyy D et al. Unveiling axial involvement in psoriatic arthritis: an ancillary analysis of the ASAS-perSpA study. Semin Arthritis Rheum 2021;51:766–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.