Abstract
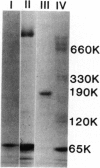
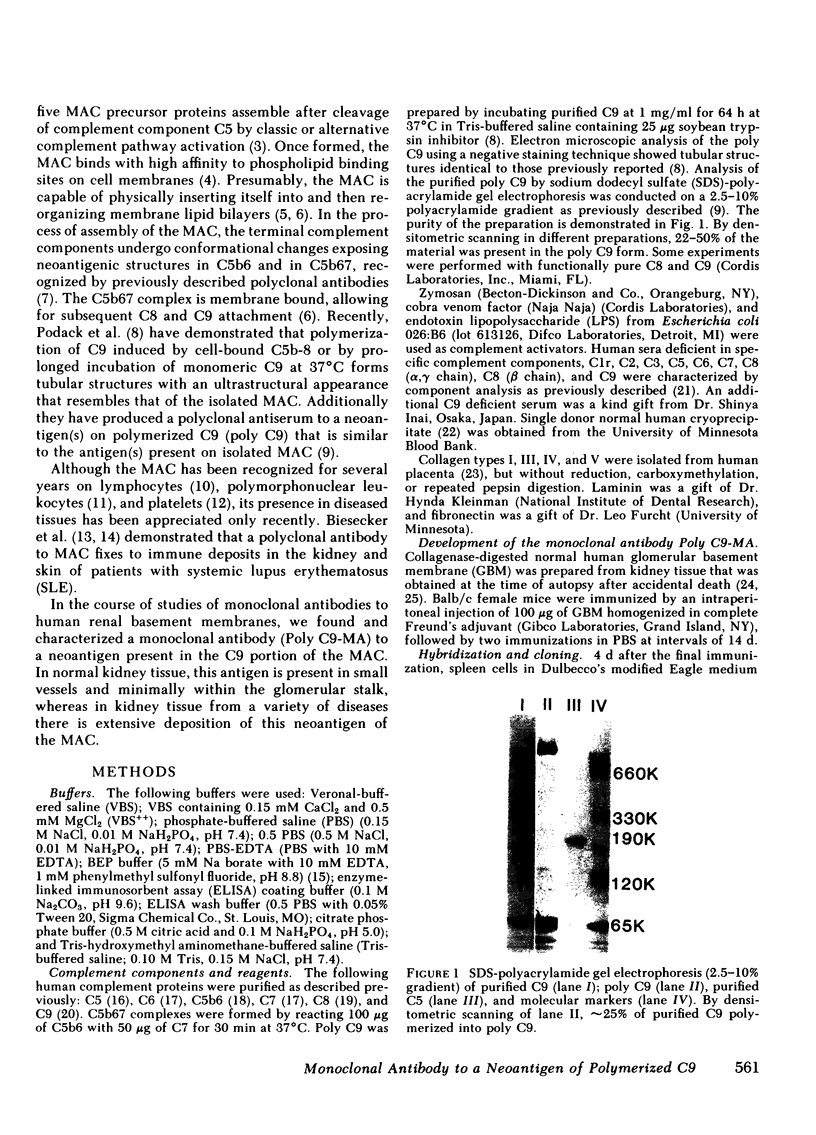
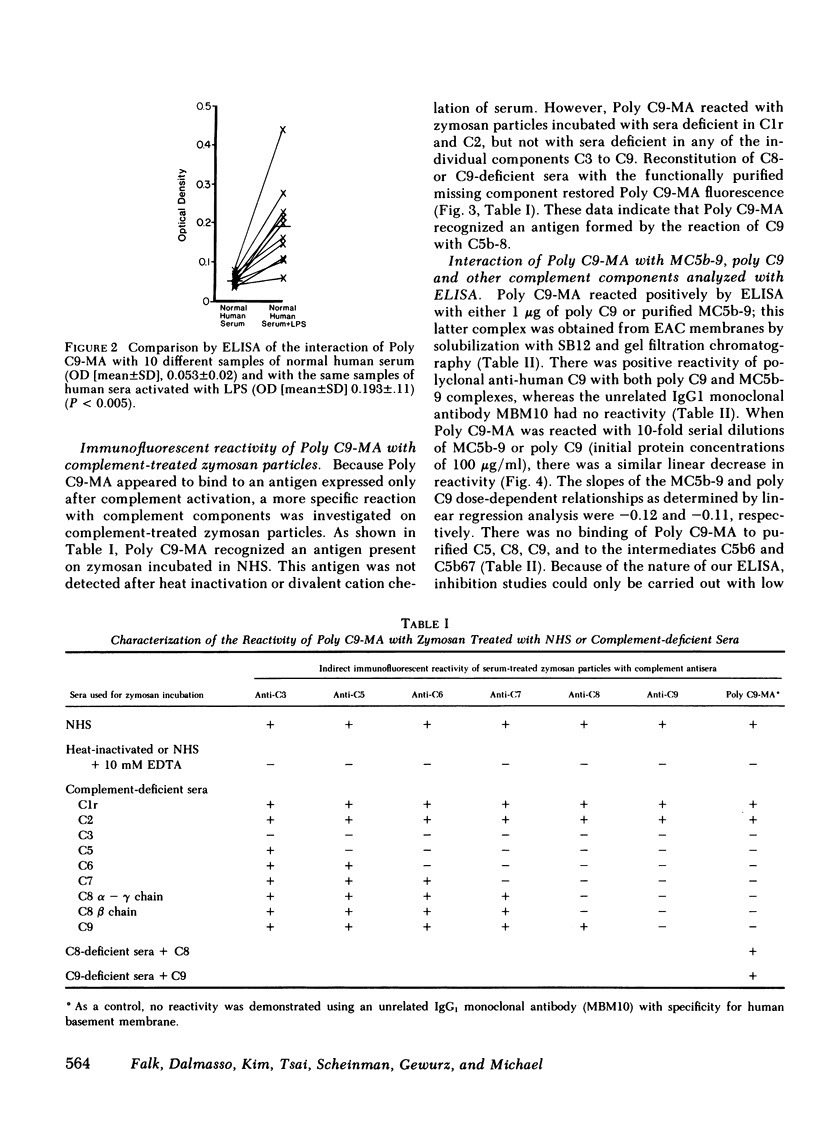
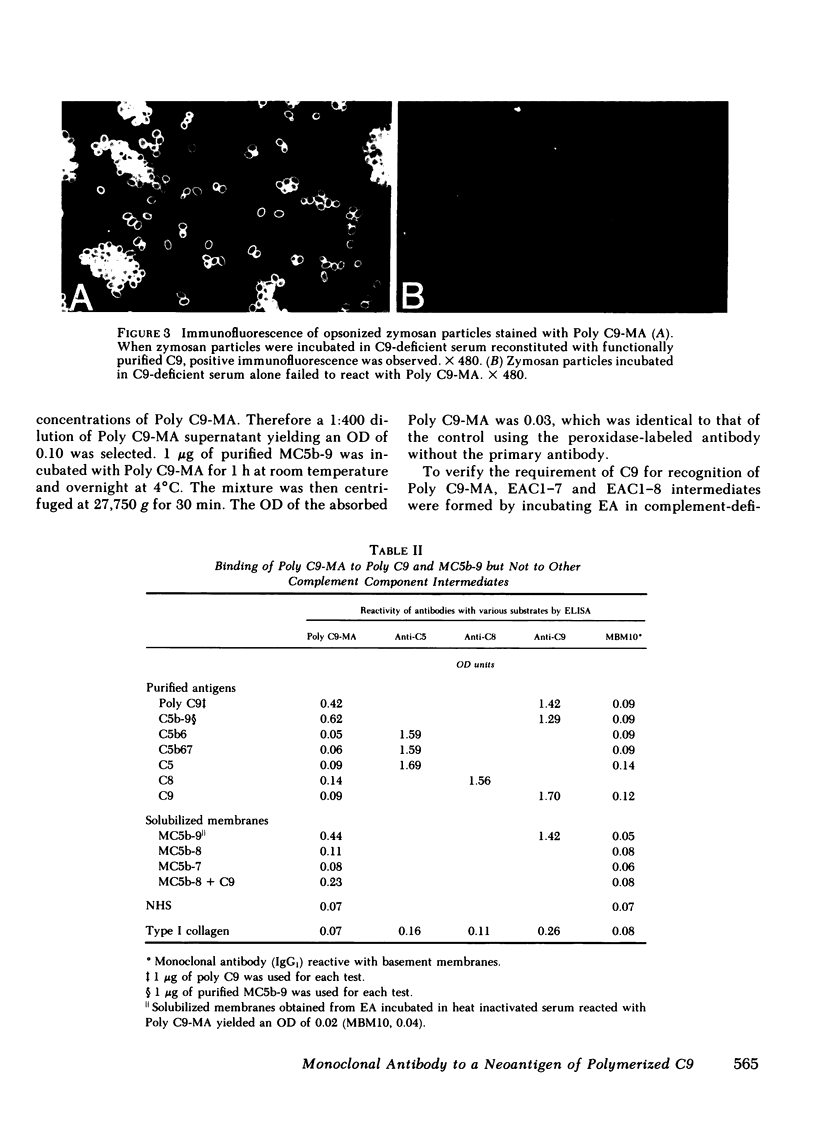
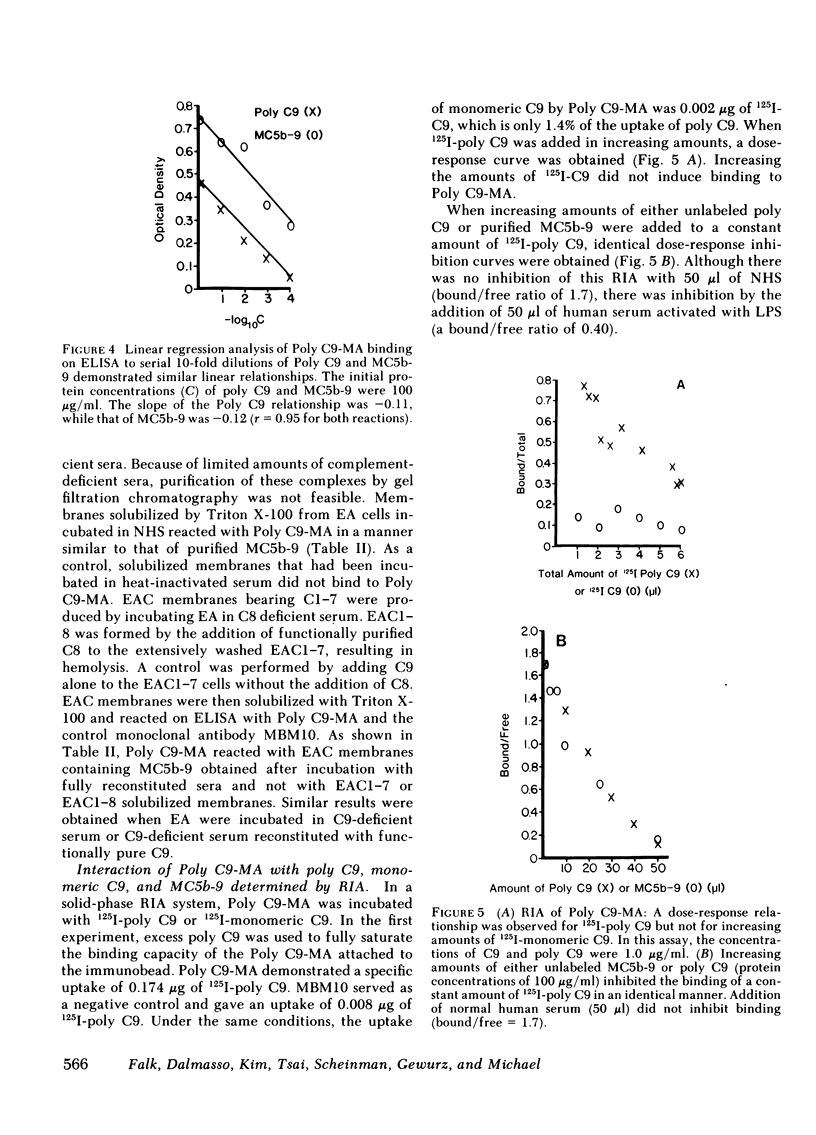
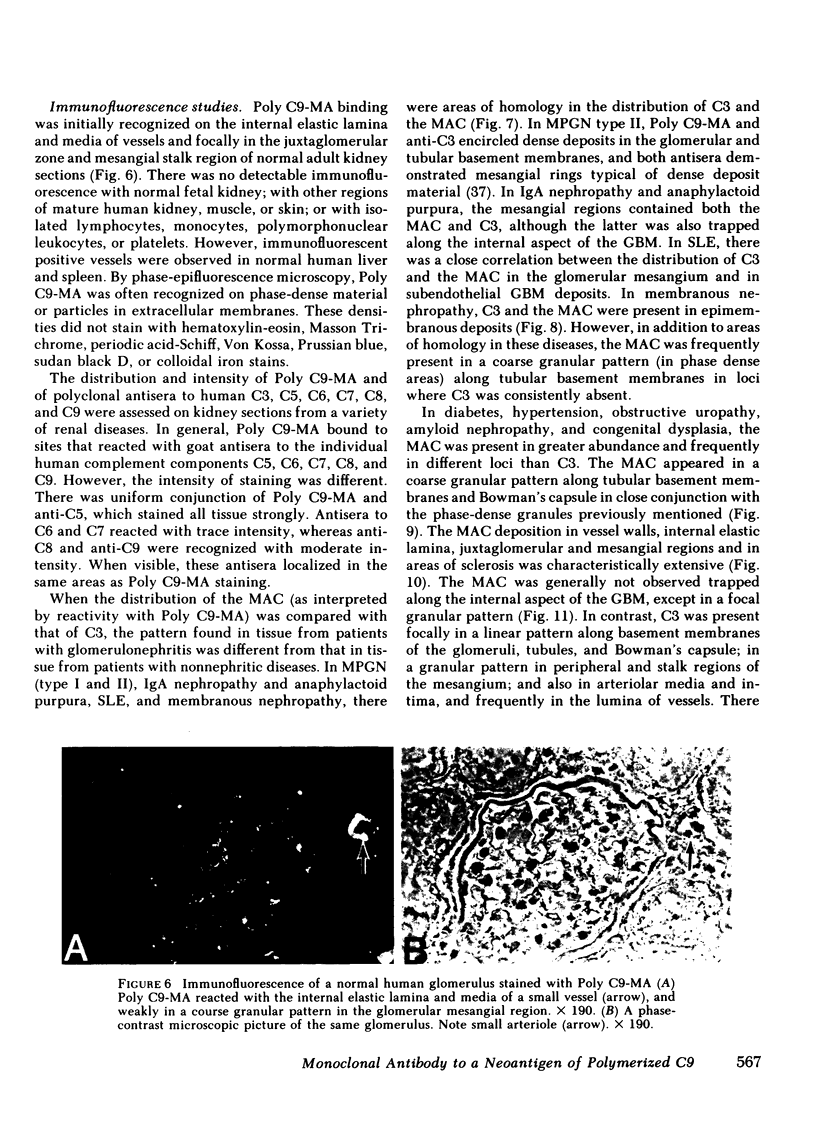
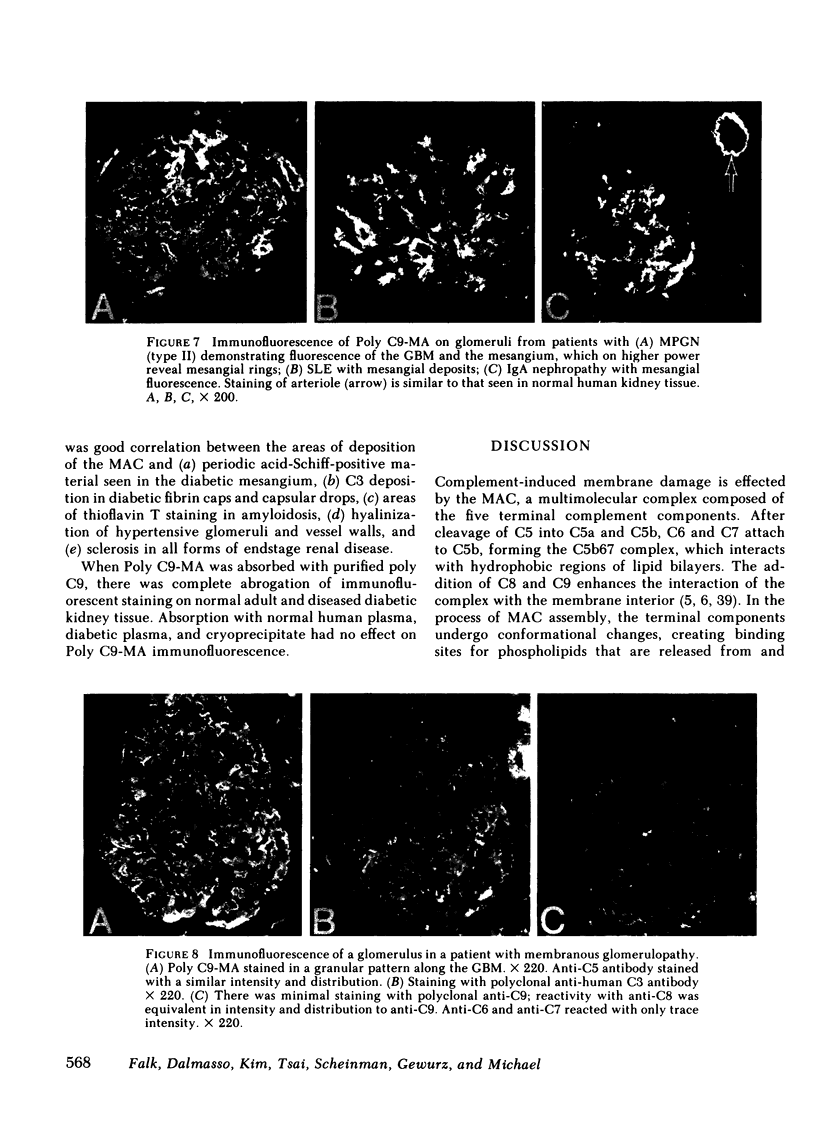
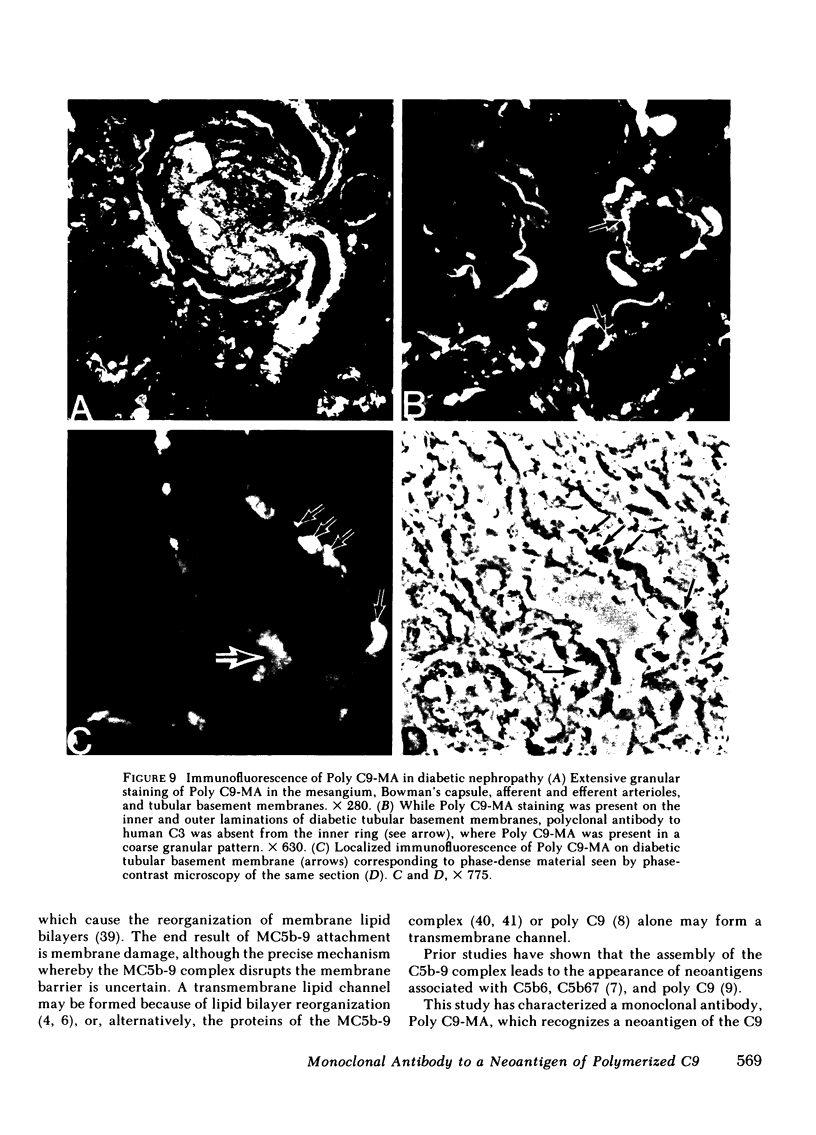
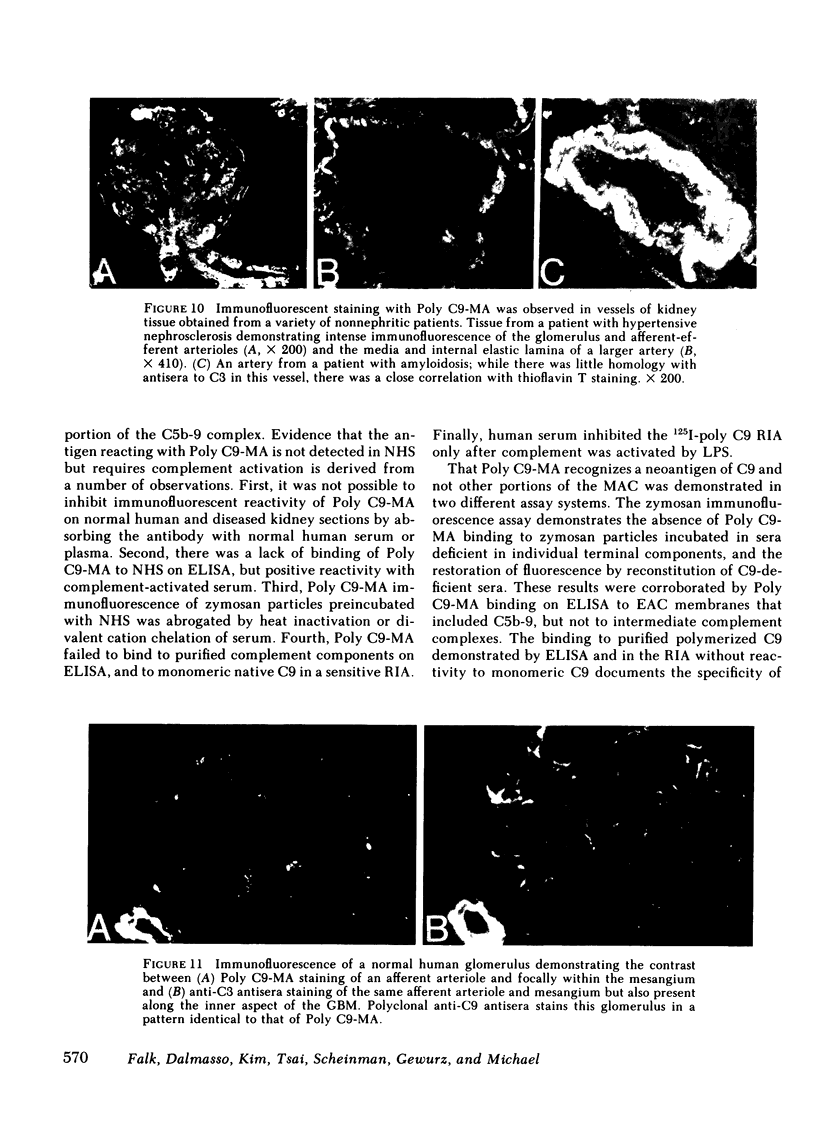
A monoclonal antibody to a neoantigen of the C9 portion of the membrane attack complex (MAC) of human complement has been developed and characterized. The distribution of this neoantigen was assessed by indirect immunofluorescence microscopy in nephritic and nonnephritic renal diseases. The antibody (Poly C9-MA) reacted on enzyme-linked immunosorbent assay (ELISA) with a determinant in complement-activated serum that was undetectable in normal human serum (NHS). Zymosan particles incubated in NHS had positive immunofluorescent staining with Poly C9-MA; however, binding of Poly C9-MA was not observed with zymosan particles incubated in sera deficient in individual complement components C3, C5, C6, C7, C8, or C9. Reconstitution of C9-deficient sera with purified C9 restored the fluorescence with Poly C9-MA. Poly C9-MA reacted positively by ELISA in a dose-dependent manner with purified MC5b-9 solubilized from membranes of antibody-coated sheep erythrocytes treated with NHS but not with intermediate complement complexes. Poly C9-MA also reacted in a dose-dependent manner on ELISA and in a radioimmunoassay with polymerized C9 (37 degrees C, 64 h) (poly C9) but not with monomeric C9. Increasing amounts of either unlabeled poly C9 or purified MC5b-9 inhibited the 125I-poly C9 RIA in an identical manner. These studies demonstrate that Poly C9-MA recognizes a neoantigen of C9 common to both the MAC and to poly C9. By immunofluorescence, Poly C9-MA reacted minimally with normal kidney tissue in juxtaglomerular loci, the mesangial stalk, and vessel walls. Poly C9-MA stained kidney tissue from patients with glomerulonephritis in a pattern similar to that seen with polyclonal anti-human C3. In tissue from patients with nonnephritic renal disease--diabetes, hypertension, and obstructive uropathy--Poly C9-MA was strongly reactive in the mesangial stalk and juxtaglomerular regions, tubular basement membranes, and vascular walls. Poly C9-MA binding was especially prominent in areas of advanced tissue injury. Poly C9-MA frequently stained loci where C3 was either minimally present or absent. These studies provide strong evidence for complement activation not only in nephritic but also in nonnephritic renal diseases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J., Klump O. The terminal membrane C5b-9 complex of human complement. Evidence for the existence of multiple protease-resistant polypeptides that form the trans-membrane complement channel. J Immunol. 1980 May;124(5):2451–2457. [PubMed] [Google Scholar]
- Biesecker G., Katz S., Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med. 1981 Dec 1;154(6):1779–1794. doi: 10.1084/jem.154.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Lavin L., Ziskind M., Koffler D. Cutaneous localization of the membrane attack complex in discoid and systemic lupus erythematosus. N Engl J Med. 1982 Feb 4;306(5):264–270. doi: 10.1056/NEJM198202043060503. [DOI] [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Brade V., Lee G. D., Nicholson A., Shin H. S., Mayer M. M. The reaction of zymosan with the properdin system in normal and C4-deficienct guinea pig serum. Demonstration of C3- and C5-cleaving multi-unit enzymes, both containing factor B, and acceleration of their formation by the classical complement pathway. J Immunol. 1973 Nov;111(5):1389–1400. [PubMed] [Google Scholar]
- Cosio F. G., Ackerman S. K., Douglas S. D., Friend P. S., Michael A. F. Soluble immune complexes binding to human monocytes and polymorphonuclear leucocytes. Immunology. 1981 Dec;44(4):773–780. [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish A. J., Blau E. B., Westberg N. G., Burke B. A., Vernier R. L., Michael A. F. Systemic lupus erythematosus within the first two decades of life. Am J Med. 1977 Jan;62(1):99–117. doi: 10.1016/0002-9343(77)90355-2. [DOI] [PubMed] [Google Scholar]
- Gewurz A. T., Lint T. F., Imherr S. M., Garber S. S., Gewurz H. Detection and analysis of inborn and acquired complement abnormalities. Clin Immunol Immunopathol. 1982 May;23(2):297–311. doi: 10.1016/0090-1229(82)90116-7. [DOI] [PubMed] [Google Scholar]
- Giavedoni E. B., Dalmasso A. P. The indiction by complement of a change in KSCN-dissociable red cell membrane lipids. J Immunol. 1976 Apr;116(4):1163–1169. [PubMed] [Google Scholar]
- Glanville R. W., Rauter A., Fietzek P. P. Isolation and characterization of a native placental basement-membrane collagen and its component alpha chains. Eur J Biochem. 1979 Apr 2;95(2):383–389. doi: 10.1111/j.1432-1033.1979.tb12976.x. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser M. T., Scheinman J. I., Basgen J., Steffes M. W., Michael A. F. Preservation of mesangium and immunohistochemically defined antigens in glomerular basement membrane isolated by detergent extraction. J Clin Invest. 1982 May;69(5):1169–1175. doi: 10.1172/JCI110553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Vernier R. L., Fish A. J., Michael A. F. Immunofluorescence studies of dense deposit disease. The presence of railroad tracks and mesangial rings. Lab Invest. 1979 Apr;40(4):474–480. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lehotay D. C. Studies of normal and nephritic rat glomerular basement membrane. Biochim Biophys Acta. 1975 Jun 25;394(2):193–203. doi: 10.1016/0005-2736(75)90257-6. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Giavedoni E. B., Dalmasso A. P. Complement-induced decrease in membrane mobility: introducing a more sensitive index of spin-label motion. Biochemistry. 1977 Mar 22;16(6):1196–1201. doi: 10.1021/bi00625a026. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Brown D. M. Increased concentration of albumin in kidney basement membranes in diabetes mellitus. Diabetes. 1981 Oct;30(10):843–846. doi: 10.2337/diab.30.10.843. [DOI] [PubMed] [Google Scholar]
- Miller K., Michael A. F. Immunopathology of renal extracellular membranes in diabetes mellitus. Specificity of tubular basement-membrane immunofluorescence. Diabetes. 1976 Aug;25(8):701–708. doi: 10.2337/diab.25.8.701. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Interstitial mononuclear cell populations in renal graft rejection. Identification by monoclonal antibodies in tissue sections. J Exp Med. 1982 Jan 1;155(1):17–30. doi: 10.1084/jem.155.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Muller-Eberhard H. J. The C5b-9 complex: subunit composition of the classical and alternative pathway-generated complex. J Immunol. 1976 May;116(5):1431–1434. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. Purification of the sixth and seventh component of human complement without loss of hemolytic activity. J Immunol. 1976 Feb;116(2):263–269. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977 Dec;119(6):2024–2029. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Nachman R. L. Human complement in thrombin-mediated platelet function: uptake of the C5b-9 complex. J Exp Med. 1979 Sep 19;150(3):633–645. doi: 10.1084/jem.150.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Steckel E. W., York R. G., Monahan J. B., Sodetz J. M. The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem. 1980 Dec 25;255(24):11997–12005. [PubMed] [Google Scholar]
- Sundsmo J. S., Kolb W. P., Müller-Eberhard H. J. Leukocyte complement: neoantigens of the membrane attack complex on the surface of human leukocytes prepared from defibrinated blood. J Immunol. 1978 Mar;120(3):850–854. [PubMed] [Google Scholar]
- Sundsmo J. S., Müller-Eberhard H. J. Neoantigen of the complement membrane attack complex of cytotoxic human peripheral blood lymphocytes. J Immunol. 1979 Jun;122(6):2371–2378. [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Fifth component of human complement: purification from plasma and polypeptide chain structure. Biochemistry. 1979 Apr 17;18(8):1490–1497. doi: 10.1021/bi00575a016. [DOI] [PubMed] [Google Scholar]
- Velosa J., Miller K., Michael A. F. Immunopathology of the end-stage kidney. Immunoglobulin and complement component deposition in nonimmune disease. Am J Pathol. 1976 Jul;84(1):149–162. [PMC free article] [PubMed] [Google Scholar]
- Verroust P. J., Wilson C. B., Cooper N. R., Edgington T. S., Dixon F. J. Glomerular complement components in human glomerulonephritis. J Clin Invest. 1974 Jan;53(1):77–84. doi: 10.1172/JCI107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. F., Wetsel R. A., Kolb W. P. Physicochemical characterization of fluid phase (SC5b-9) and membrane derived (MC5b-9) attack complexes of human complement purified by immunoadsorbent affinity chromatography or selective detergent extraction. Mol Immunol. 1981 Jun;18(6):521–531. doi: 10.1016/0161-5890(81)90130-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]
- Zimmerman T. S., Kolb W. P. Human platelet-initiated formation and uptake of the C5-9 complex of human complement. J Clin Invest. 1976 Jan;57(1):203–211. doi: 10.1172/JCI108261. [DOI] [PMC free article] [PubMed] [Google Scholar]