Abstract
The EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) assessed the safety of the recycling process Lietpak (EU register number RECYC327), which uses the EREMA MPR technology. The input material is hot caustic washed and dried poly(ethylene terephthalate) (PET) flakes originating from collected post‐consumer PET containers, including no more than 5% PET from non‐food consumer applications. The flakes are heated in a ■■■■■ reactor under vacuum. Having examined the challenge test provided, the Panel concluded that the ■■■■■ decontamination (step 2), for which a challenge test was provided, is critical in determining the decontamination efficiency of the process. The operating parameters to control the performance of this step are temperature, pressure and residence time. It was demonstrated that this recycling process is able to ensure a level of migration of potential unknown contaminants into food below the conservatively modelled migration of 0.1 μg/kg food, derived from the exposure scenario for infants, when such recycled PET is used at up to 100%. Therefore, the Panel concluded that the recycled PET obtained from this process is not of safety concern when used at up to 100% for the manufacture of materials and articles for contact with all types of foodstuffs, including drinking water, for long‐term storage at room temperature or below, with or without hotfill. Articles made of this recycled PET are not intended to be used in microwave or conventional ovens and such uses are not covered by this evaluation.
Keywords: EREMA MPR, food contact materials, Lietpak UAB, plastic, poly(ethylene terephthalate) (PET), recycling process, safety assessment
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
Recycled plastic materials and articles shall only be placed on the market if the recycled plastic is from an authorised recycling process. Before a recycling process is authorised, the European Food Safety Authority (EFSA)'s opinion on its safety is required. This procedure has been established in Article 5 of Regulation (EC) No 282/2008 1 , 2 on recycled plastic materials intended to come into contact with foods and Articles 8 and 9 of Regulation (EC) No 1935/2004 3 on materials and articles intended to come into contact with food.
According to this procedure, the industry submits applications to the competent authorities of Member States, which transmit the applications to EFSA for evaluation.
In this case, EFSA received from the Lithuanian competent authority, an application for evaluation of the recycling process Lietpak, European Union (EU) register No RECYC327. The request has been registered in EFSA's register of received questions under the number EFSA‐Q‐2023‐00278. The dossier was submitted on behalf of Lietpak UAB, Adomo Mickevičiaus g. 165, Čekoniškių k., Zujūnų sen. 14,207 Vilniaus R. Sav., Lithuania (see ‘Documentation provided to EFSA’).
1.2. Terms of Reference
The Lithuanian competent authority requested the safety evaluation of the recycling process Lietpak, in accordance with Regulation (EC) No 282/2008.
1.3. Interpretation of the Terms of Reference
According to Article 5 of Regulation (EC) No 282/2008 on recycled plastic materials intended to come into contact with foods, EFSA is required to carry out risk assessments on the risks originating from the migration of substances from recycled food contact plastic materials and articles into food and deliver a scientific opinion on the recycling process examined.
According to Article 4 of Regulation (EC) No 282/2008, EFSA will evaluate whether it has been demonstrated in a challenge test, or by other appropriate scientific evidence, that the recycling process is able to reduce the contamination of the plastic input to a concentration that does not pose a risk to human health. The poly(ethylene terephthalate) (PET) materials and articles used as input of the process as well as the conditions of use of the recycled PET are part of this evaluation.
2. DATA AND METHODOLOGIES
2.1. Data
The applicant has submitted a dossier following the ‘EFSA guidelines for the submission of an application for the safety evaluation of a recycling process to produce recycled plastics intended to be used for the manufacture of materials and articles in contact with food, prior to its authorisation’ (EFSA, 2008) and the ‘Administrative guidance for the preparation of applications on recycling processes to produce recycled plastics intended to be used for manufacture of materials and articles in contact with food’ (EFSA, 2021). In accordance with Art. 38 of the Commission Regulation (EC) No 178/2002 4 and taking into account the protection of confidential information and of personal data in accordance with Articles 39 to 39e of the same Regulation and of the Decision of the EFSA's Executive Director laying down practical arrangements concerning transparency and confidentiality, 5 the non‐confidential version of the dossier is published on Open.EFSA. 6
According to Art. 32c(2) of Regulation (EC) No 178/2002 and to the Decision of EFSA's Executive Director laying down the practical arrangements on pre‐submission phase and public consultations, 7 EFSA carried out a public consultation on the non‐confidential version of the application from 16 May 2024 to 06 June 2024, for which no comments were received.
Additional information was provided by the applicant during the assessment process in response to a request from EFSA sent on 16 January 2024 (see ‘Documentation provided to EFSA’).
The following information on the recycling process was provided by the applicant and used for the evaluation:
General information:
-
–
general description,
-
–
existing authorisations.
Specific information:
-
–
recycling process,
-
–
characterisation of the input,
-
–
determination of the decontamination efficiency of the recycling process,
-
–
characterisation of the recycled plastic,
-
–
intended application in contact with food,
-
–
compliance with the relevant provisions on food contact materials and articles,
-
–
process analysis and evaluation,
-
–
operating parameters.
2.2. Methodologies
The risks associated with the use of recycled plastic materials and articles in contact with food come from the possible migration of chemicals into the food in amounts that would endanger human health. The quality of the input, the efficiency of the recycling process to remove contaminants as well as the intended use of the recycled plastic are crucial points for the risk assessment (EFSA, 2008).
The criteria for the safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for the manufacture of materials and articles in contact with food are described in the scientific opinion developed by the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (EFSA CEF Panel, 2011). The principle of the evaluation is to apply the decontamination efficiency of a recycling technology or process, obtained from a challenge test with surrogate contaminants, to a reference contamination level for post‐consumer PET, conservatively set at 3 mg/kg PET for contaminants resulting from possible misuse. The resulting residual concentration of each surrogate contaminant in recycled PET (C res) is compared with a modelled concentration of the surrogate contaminants in PET (C mod). This C mod is calculated using generally recognised conservative migration models so that the related migration does not give rise to a dietary exposure exceeding 0.0025 μg/kg body weight (bw) per day (i.e. the human exposure threshold value for chemicals with structural alerts for genotoxicity), below which the risk to human health would be negligible. If the C res is not higher than the C mod, the recycled PET manufactured by such recycling process is not considered to be of safety concern for the defined conditions of use (EFSA CEF Panel, 2011).
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and considering the relevant guidance from the EFSA Scientific Committee.
3. ASSESSMENT
3.1. General information 8
According to the applicant, the recycling process Lietpak is intended to recycle food grade PET containers using the EREMA MPR technology. The recycled PET is intended to be used at up to 100% in direct contact with all kinds of foodstuffs, including bottles for mineral water, soft drink and beer, for long‐term food storage at room temperature or below, with or without hotfill. The final articles are not intended to be used in microwave or conventional ovens.
3.2. Description of the process
3.2.1. General description 9
The recycling process Lietpak produces recycled PET flakes from PET containers from post‐consumer collection systems (kerbside and deposit systems).
It comprises the two steps below.
Input
In step 1, the post‐consumer PET containers are processed into hot caustic washed and dried flakes. This step is performed by third parties.
Decontamination and production of recycled PET material
In step 2, the flakes are crystallised and decontaminated under high temperature and vacuum.
The operating conditions of the process have been provided to EFSA.
3.2.2. Characterisation of the input 10
According to the applicant, the input material for the recycling process Lietpak consists of hot washed and dried flakes obtained from PET containers previously used for food packaging, from post‐consumer collection systems (kerbside and deposit systems). A small fraction may originate from non‐food applications. According to the applicant, the proportion will be no more than 5%.
Technical data on the hot washed and dried flakes are provided, such as on physical properties and residual contents of moisture, poly(vinyl chloride) (PVC), impurities and polyolefins (see Appendix A).
3.3. EREMA Basic technology
3.3.1. Description of the main steps 11
The general scheme of the EREMA MPR technology, as provided by the applicant, is reported in Figure 1. The steps are:
Decontamination in a ■■■■■ reactor (step 2):
FIGURE 1.
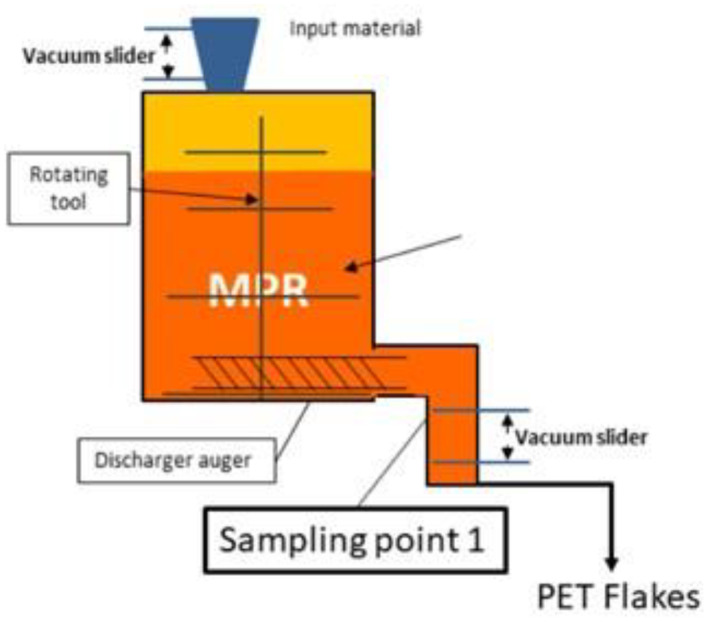
General scheme of the EREMA MPR technology (provided by the applicant).
■■■■■
The process is run under defined operating parameters of temperature, pressure and residence time.
3.3.2. Decontamination efficiency of the recycling process 12
To demonstrate the decontamination efficiency of the recycling process Lietpak, a challenge test on step 2 was submitted to the EFSA.
PET flakes were contaminated with toluene, chlorobenzene, chloroform, methyl salicylate, phenyl cyclohexane, benzophenone and methyl stearate, selected as surrogate contaminants in agreement with the EFSA guidelines (EFSA CEF Panel, 2011) and in accordance with the recommendations of the US Food and Drug Administration (FDA, 2006). The surrogates include different molecular masses and polarities to cover possible chemical classes of contaminants of concern and were demonstrated to be suitable to monitor the behaviour of PET during recycling (EFSA, 2008).
■■■■■ 13 ■■■■■
■■■■■
The Panel noted that decontamination efficiencies, ■■■■■ could be overestimated. In fact, cross‐contamination ■■■■■ is expected to occur (EFSA CEF Panel, 2011).
Therefore, to take into account cross‐contamination, some assumptions and considerations were made by the Panel:
■■■■■
■■■■■
■■■■■
To take into account the cross‐contamination ■■■■■, the evolution of the total residual surrogate content at the outlet of the ■■■■■ reactor (step 2) as a function of residence time was calculated. ■■■■■. Correspondingly corrected concentrations of the surrogates were compared with their initial concentrations ■■■■■ at the inlet of the reactor to derive the decontamination efficiencies (see Table 1).
TABLE 1.
Efficiency of the decontamination of the reactor (step 2) in the challenge test.
| Surrogates | Concentration a of surrogates before step 2 (mg/kg PET) | Concentration b of surrogates after step 2 (mg/kg PET) | Decontamination efficiency c (%) |
|---|---|---|---|
| Toluene | 202 | 0.18 | 99.1 |
| Chlorobenzene | 361 | 0.36 | 99.0 |
| Chloroform | 291 | 0.23 | 99.2 |
| Methyl salicylate | 143 | 0.47 | 96.6 |
| Phenylcyclohexane | 364 | 1.35 | 96.2 |
| Benzophenone | 480 | 2.40 | 94.9 |
| Methyl stearate | 360 | 1.03 | 97.1 |
Abbreviation: PET, poly(ethylene terephthalate).
Initial concentration in the contaminated PET flakes.
Residual concentration derived for green flakes after decontamination.
Decontamination efficiency of step 2 in the challenge test after correction for cross‐contamination (see text).
The decontamination efficiencies presented in Table 1 were calculated for the average residence time in the ■■■■■ reactor (step 2) in the challenge test.
The decontamination efficiency ranged from 94.9% for benzophenone up to 99.2% for chloroform.
3.4. Discussion
Considering the high temperatures used during the process, the possibility of contamination by microorganisms can be discounted. Therefore, this evaluation focuses on the chemical safety of the final product.
Technical data, such as on physical properties and residual contents of PVC and polyolefins, were provided for the input materials (i.e. hot caustic washed and dried flakes, step 1). The flakes are produced from PET containers, e.g. bottles, previously used for food packaging, collected through post‐consumer collection systems. However, a small fraction may originate from non‐food applications, such as bottles for soap, mouth wash or kitchen hygiene agents. According to the applicant, the collection system and the process are managed in such a way that this fraction will be no more than 5% in the input stream, as recommended by the EFSA CEF Panel in its ‘Scientific Opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for manufacture of materials and articles in contact with food’ (EFSA CEF Panel, 2011).
The process is adequately described. The washing and drying of the flakes from the collected PET containers (step 1) is conducted by third parties and, according to the applicant, this step is under control. The EREMA MPR technology comprises the ■■■■■ decontamination (step 2). The operating parameters of temperature, pressure and residence time for these steps have been provided to EFSA.
A challenge test to measure the decontamination efficiency was conducted ■■■■■ on step 2. The reactor was operated under pressure and temperature conditions as well as residence time equivalent to or less severe than those of the commercial process. ■■■■■, the Panel calculated the decontamination efficiencies taking into account also the amount of surrogates possibly transferred ■■■■■ due to cross‐contamination. The Panel considered that this challenge test was performed correctly according to the recommendations of the EFSA guidelines (EFSA, 2008) and concluded that step 2 was critical for the decontamination efficiency of the process. Consequently, temperature, pressure and residence time of step 2 should be controlled to guarantee the performance of the decontamination. These parameters have been provided to EFSA (Appendix C).
The decontamination efficiencies obtained from the challenge test on step 2, ranging from 94.9% to 99.2%, have been used to calculate the residual concentrations of potential unknown contaminants in PET (C res) according to the evaluation procedure described in the ‘Scientific Opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET’ (EFSA CEF Panel, 2011; Appendix B). By applying the decontamination percentages to the reference contamination level of 3 mg/kg PET, the C res for the different surrogates was obtained (Table 2).
TABLE 2.
Decontamination efficiency from the challenge test, residual concentrations of the surrogates (C res) related to the reference contamination level and calculated concentrations of the surrogates in PET (C mod) corresponding to a modelled migration of 0.10 μg/kg food after 1 year at 25°C.
| Surrogates | Decontamination efficiency (%) | C res for 100% rPET (mg/kg PET) | C mod (mg/kg PET); infant scenario |
|---|---|---|---|
| Toluene | 99.1 | 0.03 | 0.09 |
| Chlorobenzene | 99.0 | 0.03 | 0.09 |
| Chloroform | 99.2 | 0.03 | 0.10 |
| Methyl salicylate | 96.6 | 0.10 | 0.13 |
| Phenylcyclohexane | 96.2 | 0.11 | 0.14 |
| Benzophenone | 94.9 | 0.15 | 0.16 |
| Methyl stearate | 97.1 | 0.09 | 0.32 |
Abbreviations: PET, poly(ethylene terephthalate); rPET, recycled poly(ethylene terephthalate).
According to the evaluation principles (EFSA CEF Panel, 2011), the dietary exposure must not exceed 0.0025 μg/kg bw per day, below which the risk to human health is considered negligible. The C res value should not exceed the modelled concentration in PET (C mod) that, after 1 year at 25°C, results in a migration giving rise to a dietary exposure of 0.0025 μg/kg bw per day. A maximum dietary exposure of 0.0025 μg/kg bw per day corresponds to a maximum migration of 0.10 μg/kg of the contaminant into the infant's food and has been used to calculate C mod (EFSA CEF Panel, 2011). The calculated percentages are reported in Table 2. C res reported in Table 2 is calculated for 100% recycled PET, for which the risk to human health is demonstrated to be negligible. The relationship between the key parameters for the evaluation scheme is reported in Appendix B.
On the basis of the provided data from the challenge test and the applied conservative assumptions, the Panel considered that, under the given operating conditions, the recycling process Lietpak using the EREMA Basic technology is able to ensure that the level of migration of unknown contaminants from the recycled PET into food is below the conservatively modelled migration of 0.10 μg/kg food. At this level, the risk to human health is considered negligible when the recycled PET is used at up to 100% to produce materials and articles intended for contact with all types of foodstuffs including drinking water (scenario of infants), for long‐term storage at room temperature or below, with or without hotfill.
4. CONCLUSIONS
The Panel considered that the Lietpak recycling process using the EREMA MPR technology is adequately characterised and that the critical step to decontaminate the PET is identified. Having examined the challenge test provided, the Panel concluded that the temperature, the pressure and the residence time in the ■■■■■ reactor of step 2 are critical for the decontamination efficiency of the process. Therefore, these are the operating parameters to be controlled.
The Panel concluded that the recycling process Lietpak is able to reduce foreseeable accidental contamination of post‐consumer food contact PET to a concentration that does not give rise to concern for a risk to human health if:
it is operated under conditions that are at least as severe as those applied in the challenge test used to measure the decontamination efficiency of the process;
the input material of the process is washed and dried post‐consumer PET flakes originating from materials and articles that have been manufactured in accordance with the EU legislation on food contact materials and contain no more than 5% of PET from non‐food consumer applications;
the recycled PET is used at up to 100% for the manufacture of materials and articles intended for contact with all types of foodstuffs, including drinking water, for long‐term storage at room temperature or below, with or without hotfill.
Articles made of this recycled PET are not intended to be used in microwave and conventional ovens and such uses are not covered by this evaluation.
5. RECOMMENDATION
The Panel recommended periodic verification that the input material to be recycled originates from materials and articles that have been manufactured in accordance with the EU legislation on food contact materials and that the proportion of PET from non‐food consumer applications is no more than 5%. This adheres to good manufacturing practice and the Regulation (EC) No 282/2008, Art. 4b. Critical steps in recycling should be monitored and kept under control. In addition, supporting documentation should be available on how it is ensured that the critical steps are operated under conditions at least as severe as those in the challenge test used to measure the decontamination efficiency of the process.
6. DOCUMENTATION PROVIDED TO EFSA
Dossier ‘Lietpak’. November 2023. Submitted on behalf of Lietpak UAB, Lithuania.
Additional information, April 2024. Submitted on behalf of Lietpak UAB, Lithuania.
ABBREVIATIONS
- bw
body weight
- CEF Panel
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CEP Panel
Panel on Food Contact Materials, Enzymes and Processing Aids
- C mod
modelled concentration in PET
- C res
residual concentration in PET
- PET
poly(ethylene terephthalate)
- PVC
poly(vinyl chloride)
- rPET
recycled poly(ethylene terephthalate)
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
Lithuanian competent authority
QUESTION NUMBER
EFSA‐Q‐2023‐00278
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
José Manuel Barat Baviera, Claudia Bolognesi, Andrew Chesson, Pier Sandro Cocconcelli, Riccardo Crebelli, David Michael Gott, Konrad Grob, Claude Lambré, Evgenia Lampi, Marcel Mengelers, Alicja Mortensen, Gilles Rivière, Vittorio Silano (until 21 December 2020 †), Inger‐Lise Steffensen, Christina Tlustos, Henk Van Loveren, Laurence Vernis, Holger Zorn.
WAIVER
In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to an expert of the Working Group. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the preparation and discussion of the scientific output but was not allowed to take up the role of rapporteur within that time frame. Any competing interests are recorded in the respective minutes of the meetings of the CEP Panel Working Group on Recycling Plastics.
LEGAL NOTICE
Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by EFSA. The full output has been shared with the European Commission, EU Member States (if applicable) and the applicant. The blackening may be subject to review once the decision on the confidentiality requests is adopted by EFSA and in case it rejects some of the confidentiality requests.
APPENDIX A. Technical data of the washed flakes as provided by the applicant 14
A.1.
| Parameter | Value |
|---|---|
| Moisture max. | 1.0% |
| pH value | 7.0–7.4 |
| PVC max. | 50 mg/kg |
| Impurities content max. | 20 mg/kg |
| Polyolefins max. | 25 mg/kg |
APPENDIX B. Relationship between the key parameters for the evaluation scheme (EFSA CEF Panel, 2011)
B.1.
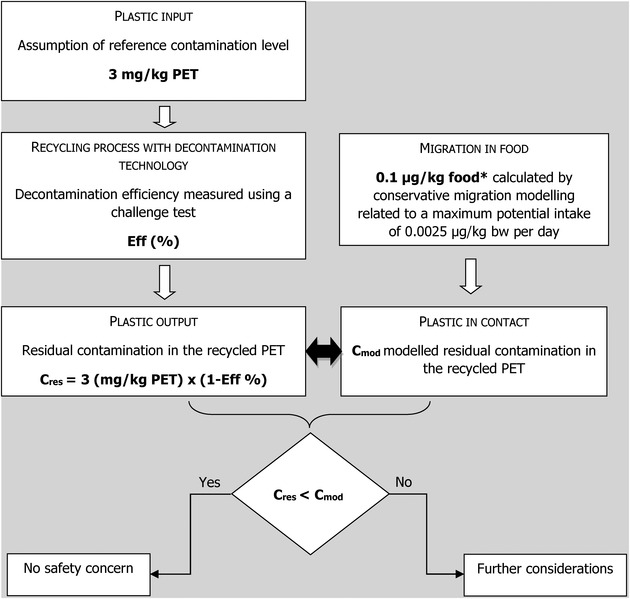
*Default scenario (infant). For adults and toddlers, the migration criterion will be 0.75 and 0.15 μg/kg food, respectively. The figures are derived from the application of the human exposure threshold value of 0.0025 μg/kg bw per day applying a factor of 5 related to the overestimation of modelling.
APPENDIX C. Table of operational parameters 15
C.1.
Although the operational parameters are reported for all the process steps, the critical steps and the corresponding parameters of the challenge test/process, considered for the evaluation of the process and for which it has been concluded that the process is safe, are highlighted in green.
The process should be operated at conditions at least as severe as the ones indicated in green in the table (e.g. lower pressures are more severe than higher, higher temperatures are more severe than lower, longer times are more severe than shorter, higher gas flows generally are more severe than lower).
The official enforcement control shall verify that the recycling plant is operating in a way that complies with its authorisation. Depending on the technology, some of the parameters are inter‐related and changing one parameter to a more severe value may impact another parameter into a less severe value. Therefore, eventual deviations from the values of the parameters indicated as critical (marked in green in the table) should be demonstrated not impacting significantly on the safety assessment. The table does not necessarily report all the tolerances for the operational parameters.
| Process Lietpak (RECYC327) based on the Erema MPR technology | |||
|---|---|---|---|
| ■■■■■ | Step 2 Reactor | ||
| ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | |||
■■■■■
■■■■■
■■■■■
EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids), Lambré, C. , Barat Baviera, J. M. , Bolognesi, C. , Chesson, A. , Cocconcelli, P. S. , Crebelli, R. , Gott, D. M. , Grob, K. , Mengelers, M. , Mortensen, A. , Rivière, G. , Steffensen, I.‐L. , Tlustos, C. , Van Loveren, H. , Vernis, L. , Zorn, H. , Dudler, V. , Milana, M. R. , … Lampi, E. (2024). Safety assessment of the process Lietpak, based on the EREMA MPR technology, used to recycle post‐consumer PET into food contact materials. EFSA Journal, 22 (8), e8914. 10.2903/j.efsa.2024.8914
Adopted: 28 June 2024
Notes
Commission Regulation (EC) No 282/2008 of 27 March 2008 on recycled plastic materials and articles intended to come into contact with foods and amending Regulation (EC) No 2023/2006. OJ L 86, 28.3.2008, pp. 9–18.
Commission Regulation (EC) No 282/2008 was repealed by Commission Regulation (EU) 2022/1616 of 15 September 2022 on recycled plastic materials and articles intended to come into contact with foods, and repealing Regulation (EC) No 282/2008 (OJ L 243 20.9.2022, p. 3) which entered into force on 10 October 2022. Applications submitted to EU Member State competent authorities before the date of entry into force of Commission Regulation (EU) 2022/1616 are evaluated by EFSA in accordance with Commission Regulation (EC) No 282/2008.
Regulation (EC) No 1935/2004 of the European parliament and of the council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. OJ L 338, 13.11.2004, pp. 4–17.
Commission Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp.1–48.
Decision available at https://www.efsa.europa.eu/en/corporate‐pubs/transparency‐regulation‐practical‐arrangements.
The non‐confidential version of the dossier, following EFSA's assessment of the applicant's confidentiality requests, is published on Open.EFSA and is available at the following link: https://open.efsa.europa.eu/dossier/FCM‐2022‐4658.
Decision available at: https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/210111‐PAs‐pre‐submission‐phase‐and‐public‐consultations.pdf.
Technical dossier, section ‘Recycling process’.
Technical dossier, sections ‘Recycling process’, ‘Characterisation of the input’ and ‘Characterisation of the recycled plastic’.
Technical dossier, section ‘Characterisation of the input’.
Technical dossier, sections ‘Recycling process’ and ‘Determination of the decontamination efficiency of the recycling process’.
Technical dossier, section ‘Determination of the decontamination efficiency of the recycling process’.
Conventional recycling commonly includes sorting, grinding, washing and drying steps and produces washed and dried flakes.
Technical dossier, section Characterisation of the input.
Technical report, section ‘Table of operating parameters’.
REFERENCES
- EFSA (European Food Safety Authority) . (2008). Guidelines for the submission of an application for safety evaluation by the EFSA of a recycling process to produce recycled plastics intended to be used for manufacture of materials and articles in contact with food, prior to its authorisation. EFSA Journal, 6(7), 717. 10.2903/j.efsa.2008.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2009). Guidance of the scientific committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part2: General principles. EFSA Journal, 7(5), 1051. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2021). Administrative guidance for the preparation of applications on recycling processes to produce recycled plastics intended to be used for manufacture of materials and articles in contact with food. EFSA supporting publications, 18(3), EN‐6512. 10.2903/sp.efsa.2021.EN-6512 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)) . (2011). Scientific opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for manufacture of materials and articles in contact with food. EFSA Journal, 9(7), 2184. 10.2903/j.efsa.2011.2184 [DOI] [Google Scholar]
- FDA (Food and Drug Administration) . (2006). Guidance for industry: Use of recycled plastics in food packaging: Chemistry considerations . https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/guidance‐industry‐use‐recycled‐plastics‐food‐packaging‐chemistry‐considerations


