ABSTRACT
Background
Chronic kidney disease (CKD) and gout are risk factors for renal cancer. We analysed the effects of comorbid diabetic kidney disease and gout on renal cancer.
Methods
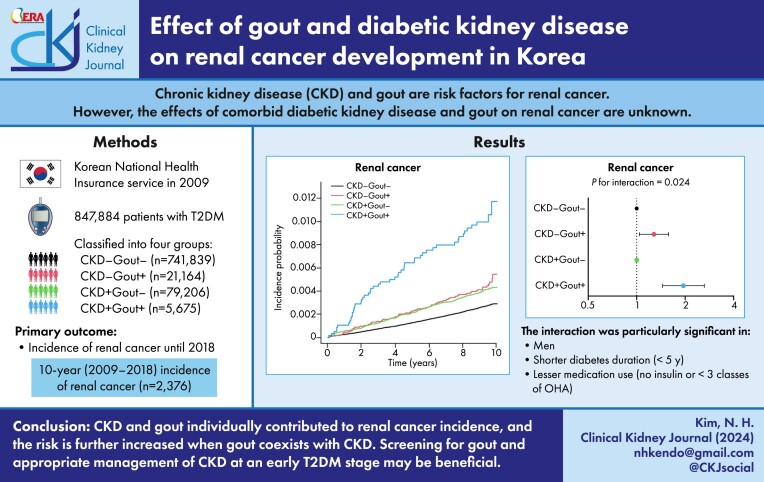
This retrospective cohort study enrolled 847 884 patients with type 2 diabetes mellitus (T2DM) who underwent health assessments provided by the Korean National Health Insurance Service in 2009. Based on CKD occurrence (glomerular filtration rate <60 ml/min/1.73 m2) and gout (two outpatient visits or one hospitalization within 5 years), patients were classified into four groups: CKD−Gout− (87.5%), CKD−Gout+ (2.5%), CKD+Gout− (9.3%) and CKD+Gout+ (0.7%). Patients with incident renal cancer (International Classification of Diseases code C64) were followed up until December 2018.
Results
Renal cancer was diagnosed in 2376 patients (0.3%). Renal cancer incidence increased in sequential order of CKD−Gout− [0.29/1000 person-years (PY), CKD+Gout− and CKD−Gout+ (0.44 and 0.48/1000 PY, respectively) and CKD+Gout+ (1.14/1000 PY). Comorbid gout increased renal cancer risk depending on CKD occurrence {hazard ratio [HR] 1.28 [95% confidence interval (CI) 1.04–1.58 among those without CKD; HR 1.95 [95% CI 1.45–2.63] among those with CKD; P-value for interaction = 0.024}. The interaction was significant, particularly in men and patients with a shorter diabetes duration (<5 years) and lesser medication use (no insulin or fewer than three classes of oral hypoglycaemic agents).
Conclusions
CKD and gout individually contributed to renal cancer incidence, and the risk is further increased when gout coexists with CKD. Screening for gout and appropriate management of CKD at an early T2DM stage may be beneficial.
Keywords: chronic, diabetes mellitus, gout, kidney neoplasms, renal insufficiency, type 2
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Diabetes is one of the leading causes of chronic kidney disease (CKD).
CKD is a modifiable risk factor for renal cancer.
Gout is associated with a decline in renal function.
This study adds:
Gout and diabetic kidney disease (DKD) individually and in combination increase the risk of renal cancer.
Gout has a greater impact on renal cancer in the presence of DKD.
The interaction is valid in men, patients with shorter diabetes duration and those on fewer medications.
Potential impact:
Screening for gout and management of CKD at an early diabetes stage is warranted.
INTRODUCTION
Diabetes is one of the leading causes of chronic kidney disease (CKD), accounting for 30–50% of CKD cases [1]. Prolonged hyperglycaemia induces inflammation and oxidative stress, damaging blood vessels and glomeruli in the kidney and resulting in the accumulation of uraemic toxins [2]. Interestingly, CKD has been identified as a modifiable risk factor for renal cancer [3, 4], which is the 14th most common malignancy globally, with an estimated 431 288 new cases reported in 2020 [5]. The underlying mechanism is unclear, however, insulin resistance, chronic inflammation and DNA damage in the kidneys of patients with type 2 diabetes mellitus (T2DM) may induce renal carcinogenesis [6].
Gout is associated with DM and a decline in renal function [7, 8]. However, the importance of evaluating and managing gout has been less emphasized in patients with diabetic kidney disease (DKD). Recent studies have suggested a possible link between gout and renal cancer [9–11], and its impact may be greater if CKD is accompanied by gout in patients with T2DM.
In this study, we aimed to analyse the risk of renal cancer according to CKD and gout occurrence in patients with T2DM over a 10-year period based on the National Health Insurance Database (NHID) from 2009 to 2018 in Korea.
MATERIALS AND METHODS
Data source and study population
This retrospective cohort study was conducted using data from the NHID of the National Health Insurance Service (NHIS) of Korea. The NHIS operates a mandatory public insurance program for all citizens and supports public health policies and research activities by developing and maintaining the NHID [12]. Before commencement of the study, approval was obtained from the Institutional Review Board of Soongsil University Hospital (SSU-202003-HR-201-01) and the requirement for informed consent was waived.
A total of 927 234 Korean individuals with T2DM [International Classification of Diseases, Tenth Revision (ICD-10) codes E11–E14 with prescription of antidiabetic drugs or fasting blood glucose ≥126 mg/dl) who underwent at least one general health checkup between 1 January 2009 and 31 December 2009 were enrolled in this study (Fig. 1). The following individuals were excluded from the study: those <20 years of age (n = 85), with missing data of a general health checkup (n = 41 250), previously diagnosed with cancer [ICD-10 codes C00–C97 with cancer benefit coverage (V193) before 2009; n = 22 696) and who did not have gout or had only one outpatient visit for gout (ICD-10 code M10) within 5 years (n = 15 319). A total of 847 884 participants were included in the final analysis. The participants were classified according to the presence of CKD and gout and were followed up until the date of renal cancer diagnosis or 31 December 2018.
Figure 1:
Flowchart for participant inclusion.
Definition of CKD and gout
CKD was defined when a participants estimated glomerular filtration rate (eGFR, using the Modification of Diet in Renal Disease formula: 186 × [creatinine] − 1.154 × [age] − 0.203 × [0.742 if female] × [1.210 if black] was <60 ml/min/1.73 m2 [13]. Gout was defined when a participant had two outpatient visits or one hospitalization due to an ICD-10 code of M10 within 5 years prior to the health check-up. Based on the presence of CKD and gout, the participants were classified into four groups: no CKD or gout (CKD−Gout−), gout without CKD (CKD−Gout+), CKD without gout (CKD+Gout−) and CKD and gout (CKD+Gout+).
Demographic factors and variables
Data on sex, age, income, smoking status, drinking, exercise, presence of hypertension and dyslipidaemia, duration of diabetes, use of insulin and oral hypoglycaemic agents (OHAs), anthropometrics and laboratory parameters at the time of enrolment were collected.
Income was based on percentiles and classified into <25th and >25th percentile. The smoking status was classified as non-smoker, ex-smoker or current smoker. Drinking status was classified as non-, mild or heavy drinker (those consuming alcohol ≥30 g/day). Regular exercise was defined as vigorous exercise >3 days/week or moderate-intensity activity >5 days/week. Hypertension was defined by an ICD-10 code for hypertension (I10–I13, I15) with antihypertensive medications or systolic blood pressure (BP) ≥140 mmHg and/or diastolic BP ≥90 mmHg at a general health check-up. Dyslipidaemia was defined using the ICD-10 code for dyslipidaemia (E78) with lipid-lowering agents or total cholesterol levels of ≥240 mg/dl at a general health check-up. The duration of diabetes was measured in years. Data on the use of insulin and OHAs were also collected. Information on whether three or more classes of OHAs were used and the types of OHAs used was collected in detail, including metformin, sulfonylurea (SU), meglitinides, thiazolidinedione (TZD), dipeptidyl peptidase-4 inhibitor (DPP4i) and α-glucosidase inhibitor (AGI). Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared (kg/m2). BP was measured twice in the sitting position and the mean value was calculated. Venous samples were collected after an overnight fast. Serum glucose, eGFR, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglyceride (TG) were measured.
Outcomes
The primary outcome of this study was the incidence of renal cell carcinoma. Renal cancer was defined as participants who were registered with cancer benefit coverage with an ICD-10 code of C64 until 31 December 2018.
Statistical analysis
Differences in demographics according to the presence of CKD and gout (CKD−Gout−, CKD−Gout+, CKD+Gout− and CKD+Gout+) were evaluated using an analysis of variance test for continuous variables and the χ2 test for categorical variables. The cumulative incidence was calculated using the Kaplan–Meier curve and the logrank test was performed to examine whether the risk of renal cancer significantly differed according to the presence of CKD and gout. The incidence of renal cancer was expressed as the number of events per 1000 person-years. Follow-up duration was calculated based on the time at which renal cancer was diagnosed. The hazard ratios (HRs) of incident renal cancer were analysed using a Cox proportional hazards model. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA) and a P-value of .05 was considered significant.
RESULTS
Baseline characteristics
Participant characteristics according to the presence of CKD and gout are presented in Table 1. The prevalence of CKD−Gout−, CKD−Gout+, CKD+Gout− and CKD+Gout+ was 87.5% (n = 741 839), 2.5% (n = 21 164), 9.3% (n = 79 206) and 0.7% (n = 5675), respectively. Participants with CKD+Gout+ tended to be older, predominantly male and had a lower income (<25%). The proportions of current smokers, heavy drinkers and regular exercisers were the highest among the CKD−Gout+ participants. The prevalence of hypertension and dyslipidaemia, the proportion of participants with a diabetes duration >5 years and those using insulin increased in sequential order of CKD−Gout−, CKD−Gout+, CKD+Gout− and CKD+Gout+. The proportion of participants using three or more classes of OHAs was the highest in the CKD+Gout− group. Glucose, eGFR and total cholesterol were highest in CKD−Gout− group and lowest in CKD+Gout+ group.
Table 1:
Baseline characteristics of the participants.
| Characteristics | CKD−Gout− | CKD−Gout+ | CKD+Gout− | CKD+Gout+ | P-value |
|---|---|---|---|---|---|
| Patients, n (%) | 741 839 (87.5) | 21 164 (2.5) | 79 206 (9.3) | 5675 (0.7) | |
| Age (years), mean ± SD | 56.2 ± 11.8 | 57.7 ± 10.7 | 65.4 ± 11.6 | 65.5 ± 10.0 | <.001 |
| Male, n (%) | 464 267 (62.6) | 17 117 (80.9) | 38 841 (49.0) | 4362 (76.9) | <.001 |
| Income (<25%), n (%) | 155 135 (20.9) | 4127 (19.5) | 14 577 (18.4) | 1044 (18.4) | <.001 |
| Smoker | <.001 | ||||
| Non- | 402 664 (54.3) | 9406 (44.4) | 52 765 (66.6) | 3102 (54. 7) | |
| Ex- | 134 154 (18.1) | 5537 (26.2) | 13 495 (17.0) | 1575 (27.8) | |
| Current | 205 021 (27.6) | 6221 (29.4) | 12 946 (16.3) | 998 (17.6) | |
| Drinker | <.001 | ||||
| Non- | 408 254 (55.0) | 9640 (45.6) | 57 082 (72.1) | 3785 (66.7) | |
| Mild | 255 111 (34.4) | 8289 (39.2) | 18 234 (23.0) | 1501 (26.5) | |
| Heavy | 78 474 (10.6) | 3235 (15.3) | 3890 (4.9) | 389 (6.9) | |
| Regular exercise, n (%) | 160 150 (21.6) | 5041 (23.8) | 15 892 (20.1) | 1280 (22.6) | <.001 |
| Hypertension, n (%) | 405 796 (54.7) | 14 342 (67.8) | 58 732 (74.2) | 4850 (85.5) | <.001 |
| Dyslipidaemia, n (%) | 292 271 (39.4) | 10 420 (49.2) | 38 312 (48.4) | 3217 (56.7) | <.001 |
| Diabetes duration ≥ 5 years, n (%) | 231 594 (31.2) | 6997 (33.1) | 37 575 (47.4) | 2871 (50.6) | <.001 |
| Insulin use, n (%) | 55 447 (7.5) | 1878 (8.9) | 12 694 (16.0) | 1276 (22.5) | <.001 |
| OHAs ≥3 classes, n (%) | 112 220 (15.1) | 3430 (16.2) | 16 222 (20.5) | 1030 (18.2) | <.001 |
| Metformin | 322 288 (43.4) | 10 217 (48.3) | 39 552 (49.9) | 2454 (43.2) | <.001 |
| SU | 348131 (46.9) | 10 732 (50.7) | 45 859 (57.9) | 3311 (58.3) | <.001 |
| Meglitinides | 13 683 (1.8) | 457 (2.2) | 2908 (3.7) | 349 (6.2) | <.001 |
| TZD | 57 821 (7.8) | 1908 (9.0) | 6869 (8.7) | 547 (9.6) | <.001 |
| DPP4i | 23 045 (3.1) | 782 (3.7) | 2367 (3.0) | 176 (3.1) | <.001 |
| AGI | 100028 (13.5) | 3125 (14.8) | 16 563 (20.9) | 1274 (22.5) | <.001 |
| SBP (mmHg), mean ± SD | 129.0 ± 15.8 | 130.3 ± 15.6 | 130.8 ± 16.9 | 131.3 ± 17.2 | <.001 |
| DBP (mmHg), mean ± SD | 79.3 ± 10.2 | 80.2 ± 10.3 | 78.4 ± 10.5 | 78.6 ± 11.1 | <.001 |
| BMI (kg/m2), mean ± SD | 25.0 ± 3.3 | 25.7 ± 3.3 | 24.9 ± 3.3 | 25.3 ± 3.2 | <.001 |
| Glucose (mg/dl), mean ± SD | 147.7 ± 49.5 | 140.4 ± 46.1 | 142.2 ± 53.2 | 133.1 ± 48.9 | <.001 |
| eGFR (ml/min/1.73 m2), mean ± SD | 89.8 ± 15.4 | 86.1 ± 15.4 | 41.3 ± 19.7 | 40.7 ± 17.4 | <.001 |
| Total cholesterol (mg/dl), mean ± SD | 197.9 ± 42.3 | 194.3 ± 44.3 | 195.7 ± 45.0 | 187.4 ± 45.5 | <.001 |
| HDL (mg/dl), mean ± SD | 52.3 ± 25.3 | 50.7 ± 22.4 | 55.3 ± 52.7 | 49.4 ± 36.7 | <.001 |
| LDL (mg/dl), mean ± SD | 111.1 ± 43.2 | 103.2 ± 42.3 | 111.0 ± 42.6 | 102.5 ± 42.8 | <.001 |
| TG (mg/dl), mean (95% CI) | 149.01 (148.81–149.21) | 169.42 (168.04–170.82) | 151.36 (150.78–151.93) | 164.04 (161.66–166.46) | <.001 |
DBP: diastolic blood pressure; SBP: systolic blood pressure; SD: standard deviation.
Renal cancer risk according to the presence of CKD and gout
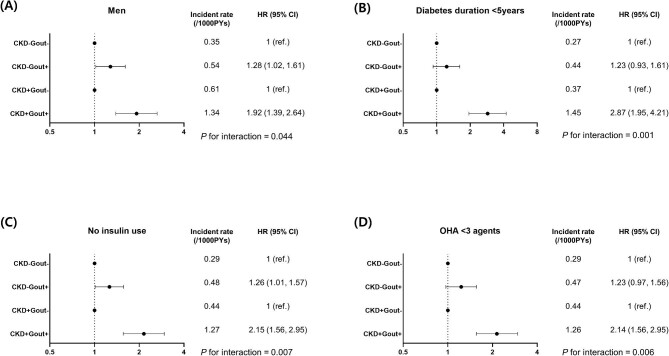
During the study period (1 January 2009–31 December 2018), 2376 (0.3%) patients were diagnosed with renal cancer. The cumulative incidence of renal cancer was highest in participants with CKD+Gout+, similarly high in those with CKD−Gout+ and CKD+Gout− and lowest in those with CKD−Gout− (logrank test P < .001) (Fig. 2). The incidence rates of renal cancer were 0.29, 0.48, 0.44 and 1.14 per 1000 person-years in the CKD−Gout−, CKD−Gout+, CKD+Gout− and CKD+Gout+ groups, respectively (Table 2). The Cox proportional hazards model was adjusted as follows: model 1, unadjusted; model 2, adjusted for age, sex, income, hypertension, dyslipidaemia, smoking, drinking, regular exercise and BMI; and model 3, model 2 plus diabetes duration, insulin use, OHA use and serum glucose levels. In model 3, compared with the CKD−Gout− group, the risk of incident renal cancer increased significantly in the CKD−Gout+ {HR 1.28 [95% confidence interval (CI) 1.04–1.58], CKD+Gout− [HR 1.20 (95% CI 1.06–1.37)] and CKD+Gout+ [HR 2.35 (95% CI 1.77–3.11)]. Compared with those without gout, comorbid gout significantly increased the risk of incidental renal cancer either among those without CKD [HR 1.28 (95% CI 1.04–1.58)] or with CKD [HR 1.95 (95% CI 1.45–2.63)]. The P-value for the interaction was .024, indicating that gout affects the risk of renal cancer depending on the presence of CKD.
Figure 2:
Kaplan–Meier curve of renal cancer according to the presence of CKD and gout. Logrank test P-value <.001.
Table 2:
Incidence rate and risk of renal cancer according to the presence of CKD and gout.
| Groups | Renal cancer events/total | Incidence rate/1000 person-years | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|---|
| CKD−Gout− | 1943/741 839 | 0.29 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| CKD−Gout+ | 91/21 164 | 0.48 | 1.67 (1.35, 2.05) | 1.30 (1.05, 1.60) | 1.28 (1.04, 1.58) | 1.67 (1.35, 2.05) | 1.30 (1.05, 1.60) | 1.28 (1.04, 1.58) |
| CKD+Gout− | 291/79 206 | 0.44 | 1.52 (1.35, 1.72) | 1.21 (1.06, 1.37) | 1.20 (1.06, 1.37) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| CKD+Gout+ | 51/5675 | 1.14 | 3.97 (3.00, 5.23) | 2.39 (1.81, 3.17) | 2.35 (1.77, 3.11) | 2.60 (1.93, 3.51) | 1.98 (1.47, 2.67) | 1.95 (1.45, 2.63) |
| P for interaction | 0.016 | 0.022 | 0.024 |
Model 1: unadjusted; model 2: adjusted for age, sex, income, hypertension, dyslipidaemia, smoking, drinking, regular exercise and BMI; model 3: adjusted for the variables in model 2 plus diabetes duration, insulin, OHA and glucose.
Subgroup analysis
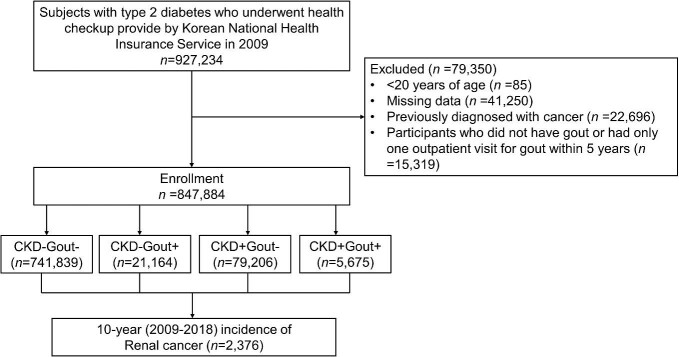
To determine in which subgroups comorbid CKD and gout increases the risk of renal cancer, subgroup analysis was performed according to age (<65 or ≥65 years), sex, diabetes duration (<5 or ≥5 years), insulin use (yes or no) and OHA use (≥3 or <3 classes) (Supplementary Table 1). After adjustments, compared with CKD−Gout−, the risk of incidental renal cancer increased in a similar range in CKD−Gout+ and CKD+Gout− and significantly increased in CKD+Gout+ among those <65 and ≥65 years of age, men, diabetes duration <5 and ≥5 years and those not using insulin or using fewer than three OHAs. Compared with those without gout, comorbid gout increased the risk of incidental renal cancer more prominently among those with CKD, particularly in men, participants with a diabetes duration <5 years, those who did not use insulin and those using fewer than three OHAs (P for interaction <.05, all) (Fig. 3).
Figure 3:
Subgroup analyses: (A) men, (B) diabetes duration <5 years, (C) no insulin users and (D) patients using less than three OHAs.
DISCUSSION
This study analysed the prevalence of CKD and gout among patients with T2DM and their effects on incident renal cancer using Korean NHIS data. The incidence rate and the risk of renal cancer increased in sequential order of CKD−Gout−, CKD+Gout−, CKD−Gout+ and CKD+Gout+. Gout has a greater impact on renal cancer in the presence of CKD, particularly in men, patients with a shorter duration of diabetes (<5 years) and those on fewer medications (no insulin or fewer than three OHAs).
The presence of DKD (eGFR <60 ml/min/1.73 m2), but not gout, increased the risk of renal cancer by 1.2 times in those without CKD and gout. CKD and renal cancer share common risk factors: T2DM, obesity, hypertension and smoking [3, 14, 15]. It is well known that CKD, even with only moderate loss of kidney function, influences the development of renal cancer. According to the meta-analysis of six prospective studies, compared with those with eGFR ≥75 ml/min/1.73 m2, those with eGFR <45 ml/min/1.73 m2 or on dialysis had 1.6 to 2.3 times higher risk of urinary tract cancer (ICD codes C64–C68) [16]. Among elderly people (>65 years), the presence of end-stage renal disease (ESRD) increases the risk of renal cancer by a factor of 2.4 [17]. The incidence of renal cancer is 5.4 times and 5.0 times higher among patients with ESRD on dialysis and after kidney transplantation, respectively [18]. The epidemiological relationship between CKD and renal cancer is well known; however, the detailed mechanism is unclear. The induction of acute kidney injury in mice promotes the development of renal papillary tumours in an adenoma–carcinoma sequence over 36 weeks [19]. Tissue injury causes DNA damage and increases the likelihood of gene mutations and cancerous growth, which may contribute to an increased risk of renal cancer [19].
The presence of gout, but not CKD, in patients with T2DM increased the risk of renal cancer by 1.28 times compared with those without gout and CKD. According to Korean NHIS data, the risk of cancer overall was higher in patients with gout than in controls. In particular, the HR for renal cancer among patients with gout is 1.28 [11]. In a meta-analysis of 24 studies, increments of 1 mg/dl of serum uric acid increased the risk of urinary system cancer by a factor of 1.04 [10]. In addition, a review of three prospective studies revealed that gout is a significant risk factor for cancer, particularly urological, digestive and lung cancers [9]. The components of insulin resistance (T2DM, obesity and hypertension), fructose- and purine-rich diets and high alcohol consumption contribute to hyperuricaemia [20]. Uric acid may act as a chemoattractant in a dose-dependent manner, promoting immunosuppressive lymphocytes, reducing tumour surveillance [21] and downregulating intracellular xanthine oxidoreductase expression, resulting in tumour growth and proliferation [22]. Colchicine downregulates pro-inflammatory pathways and increases levels of anti-inflammatory mediators [23], and interestingly, its use within 3 years after gout showed a marginal reduction in renal cancer of 0.62 times, although this reduction was not significant [24]. Further studies investigating the preventive role of gout treatment in incident renal cancer are warranted.
There was an interaction between gout and CKD; the hazardous effect of gout on renal cancer was additive and the risk increased by 1.95 times with the coexistence of CKD than in those without. We hypothesized that the coexistence of CKD and gout may maximize the risk of renal cancer through chronic uraemia and hyperuricaemia-induced inflammation and immunosuppression. Long-term kidney damage due to metabolic risk factors results in chronic inflammation and oxidative stress, which not only increase the incidence of atherosclerosis and cardiovascular disease [25], but also increase the incidence of renal cancer [1, 15]. In addition, the insulin resistance of kidney impairs the excretion of urinary ammonium, lowers urinary pH and may accelerate hyperuricaemia [26, 27]. In contrast, hyperuricaemia causes renovascular endothelial dysfunction by reducing nitric oxide bioavailability, stimulating oxidative stress and activating the renin–angiotensin system [28]. Therefore, the coexistence of CKD and gout in patients with T2DM increases the risk of renal cancer in a cyclic manner. In addition to the management of DKD with optimal glycaemic and BP control [13], screening for gout in patients with T2DM is warranted to lower the risk of renal cancer.
The male-specific interaction between CKD and gout in incident renal cancer may be due to the disproportionate burden of gout in men [29]. Indeed, men accounted for 75–80% of the CKD−Gout+ and CKD+Gout+ populations in this study. In addition, male sex is a non-modifiable risk factor for renal cancer [3]. However, the interaction between CKD and gout for incident renal cancer among participants with a shorter duration of diabetes (<5 years) and participants with lesser medication (no insulin, fewer than three OHA agents) suggests that preventing CKD and gout at the early stage of T2DM is crucial for preventing renal cancer.
The main strength of this study is that, to the best of our knowledge, this is the first study to show the additive effect of CKD and gout on incident renal cancer in patients with T2DM. This study was based on the most representative high-quality health data sources in Korea. We investigated the extent to which the risk of renal cancer is augmented when gout and CKD are currently present. The severity of diabetes was reflected by insulin administration and the number of OHA medications taken. However, our study has some limitations. First, the presence of gout was dependent on the ICD-10 code; therefore, the proportion of patients classified as having gout may vary depending on the operational definition. Second, data on gout medications were not collected. Third, the CKD stages were not distinguished. Fourth, the renal cancer subtypes were unclear, and urothelial cancer was not included as an outcome. Finally, this study was conducted in the Korean population, thus the results cannot be generalized to other ethnic groups.
In conclusion, CKD and gout are individually and in combination associated with an increased risk of renal cancer in patients with T2DM. Additionally, for appropriate management of DKD, screening for gout, especially in the early stages of T2DM, is warranted to reduce the risk of renal cancer.
Supplementary Material
Contributor Information
Seung Min Chung, Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
Inha Jung, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
Da Young Lee, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
So Young Park, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
Ji Hee Yu, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
Jun Sung Moon, Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
Ji A Seo, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
Kyungdo Han, Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea.
Nan Hee Kim, Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
FUNDING
This work was supported by a National Research Foundation of Korea grant funded (NRF-2023R1A2C2003479) and the Bio & Medical Technology Development Program of the NRF funded by the Ministry of Science and ICT (NRF-2019M3E5D3073102). It was also supported by a National IT Industry Promotion Agency grant funded by the Ministry of Science and ICT [S0252-21-1001; Development of AI Precision Medical Solution (Doctor Answer 2.0)] and by a Korea Health Industry Development Institute grant funded by the Ministry of Science and ICT (HI23C0679; Development and practice of main counselling doctor and patient support technology for diabetes digital healthcare).
AUTHORS’ CONTRIBUTIONS
S.M.C. and I.J. were responsible for the methodology and writing the original draft. D.Y.L. and J.S.M. were responsible for the methodology and review and editing of the manuscript. S.Y.P., J.H.Y. and J.A.S. were responsible for review and editing of the manuscript. K.H. was responsible for conceptualization and formal analysis. N.H.K. was responsible for conceptualization, funding acquisition, supervision and review and editing of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the NHIS of Korea, but restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. Data are available from the authors upon reasonable request and with permission of the NHIS of Korea.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Webster AC, Nagler EV, Morton RL et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102:S1–127. 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 3. Bukavina L, Bensalah K, Bray F et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol 2022;82:529–42. 10.1016/j.eururo.2022.08.019 [DOI] [PubMed] [Google Scholar]
- 4. Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol 2018;36:3574. 10.1200/JCO.2018.79.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 6. Rysz J, Franczyk B, Lawinski J et al. The role of metabolic factors in renal cancers. Int J Mol Sci 2020;21:7246. 10.3390/ijms21197246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tung YC, Lee SS, Tsai WC et al. Association between gout and incident type 2 diabetes mellitus: a retrospective cohort study. Am J Med 2016;129:1219.e17–25. 10.1016/j.amjmed.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 8. Desai RJ, Franklin JM, Spoendlin-Allen J et al. An evaluation of longitudinal changes in serum uric acid levels and associated risk of cardio-metabolic events and renal function decline in gout. PLoS One 2018;13:e0193622. 10.1371/journal.pone.0193622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang W, Xu D, Wang B et al. Increased risk of cancer in relation to gout: a review of three prospective cohort studies with 50,358 subjects. Mediators Inflamm 2015;2015:680853. 10.1155/2015/680853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie Y, Xu P, Liu K et al. Hyperuricemia and gout are associated with cancer incidence and mortality: a meta-analysis based on cohort studies. J Cell Physiol 2019;234:14364–76. 10.1002/jcp.28138 [DOI] [PubMed] [Google Scholar]
- 11. Oh YJ, Lee YJ, Lee E et al. Cancer risk in Korean patients with gout. Korean J Intern Med 2022;37:460–7. 10.3904/kjim.2020.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheol Seong S, Kim YY, Khang YH et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol 2017;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi JH, Lee KA, Moon JH et al. 2023 clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J 2023;47:575–94. 10.4093/dmj.2023.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weikert S, Boeing H, Pischon T et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol 2008;167:438–46. 10.1093/aje/kwm321 [DOI] [PubMed] [Google Scholar]
- 15. Saly DL, Eswarappa MS, Street SE et al. Renal cell cancer and chronic kidney disease. Adv Chronic Kidney Dis 2021;28:460–8.e1. 10.1053/j.ackd.2021.10.008 [DOI] [PubMed] [Google Scholar]
- 16. Wong G, Staplin N, Emberson J et al. Chronic kidney disease and the risk of cancer: an individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016;16:488. 10.1186/s12885-016-2532-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shebl FM, Warren JL, Eggers PW et al. Cancer risk among elderly persons with end-stage renal disease: a population-based case–control study. BMC Nephrol 2012;13:65. 10.1186/1471-2369-13-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart JH, Vajdic CM, van Leeuwen MT et al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant 2009;24:3225–31. 10.1093/ndt/gfp331 [DOI] [PubMed] [Google Scholar]
- 19. Peired AJ, Antonelli G, Angelotti ML et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci Transl Med 2020;12:eaaw6003. 10.1126/scitranslmed.aaw6003 [DOI] [PubMed] [Google Scholar]
- 20. McCormick N, O'Connor MJ, Yokose C et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheumatol 2021;73:2096–104. 10.1002/art.41779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eisenbacher JL, Schrezenmeier H, Jahrsdorfer B et al. S100A4 and uric acid promote mesenchymal stromal cell induction of IL-10+/IDO+ lymphocytes. J Immunol 2014;192:6102–10. 10.4049/jimmunol.1303144 [DOI] [PubMed] [Google Scholar]
- 22. Fini MA, Elias A, Johnson RJ et al. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med 2012;1:e16. 10.1186/2001-1326-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther 2014;36:1465–79. 10.1016/j.clinthera.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 24. Kuo M-C, Chang S-J, Hsieh M-C. Colchicine significantly reduces incident cancer in gout male patients. Medicine (Baltimore) 2015;94:e1570. 10.1097/MD.0000000000001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato S, Chmielewski M, Honda H et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3:1526–33. 10.2215/CJN.00950208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spatola L, Ferraro PM, Gambaro G et al. Metabolic syndrome and uric acid nephrolithiasis: insulin resistance in focus. Metabolism 2018;83:225–33. 10.1016/j.metabol.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 27. Rendina D, De Filippo G, D'Elia L et al. Metabolic syndrome and nephrolithiasis: a systematic review and meta-analysis of the scientific evidence. J Nephrol 2014;27:371–6. [DOI] [PubMed] [Google Scholar]
- 28. Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis 2017;70:422–39. 10.1053/j.ajkd.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum 2020;50(3 Suppl):S11–6. 10.1016/j.semarthrit.2020.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the NHIS of Korea, but restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. Data are available from the authors upon reasonable request and with permission of the NHIS of Korea.