Abstract
The increasing prevalence of noncommunicable diseases in the aging population has been correlated with a decline in innate and adaptive immune responses; hence, it is imperative to identify approaches to improve immune function, prevent related disorders, and reduce or treat age-associated health complications. Prebiotic supplementation is a promising approach to modulate the gut microbiome and immune system, offering a potential strategy to maintain the integrity of immune function in older individuals. This review summarizes the current research on prebiotic galacto-oligosaccharide (GOS) immunomodulatory mechanisms mediated by bacterial-derived metabolites, including short-chain fatty acids and secondary bile acids, to maintain immune homeostasis. The potential applications of GOS as immunotherapy for age-related disease prevention in older individuals are also highlighted. This aligns with the global shift toward proactive healthcare and emphasizes the significance of early intervention in directing an individual’s health trajectory.
Keywords: galacto-oligosaccharides, immunosenescence, short-chain fatty acid
Highlights
-
1.
Life expectancy is increasing, and changes in immunity and gut microbiota occur with aging, leading to age-related diseases associated with immunosenescence.
-
2.
GOS are one of the most common and widely known types of nondigestible prebiotics that significantly impact host health, demonstrating promising application efficacy in older individuals.
-
3.
GOS immunomodulates immune homeostasis by modulating the fragile microbiota in older individuals to prevent or alleviate immune-related diseases.
-
4.
SCFAs metabolism and secondary bile acid metabolism activated by GOS-derived bacteria plays a vital role in the immune system.
-
5.
The clinical application of GOS in older individuals requires further study; however, source-specific and structure-specific differences have been noted.
Statement of Significance.
This review provides compelling evidence that galacto-oligosaccharides (GOSs), as a dietary intervention, can significantly enhance gut health and immunomodulation in older adults. Based on these findings, the review urges further research to advance our comprehension of GOSs and their potential to optimize the health of older individuals.
Introduction
As life expectancy increases, the global aging population is expanding at an unprecedented rate. According to the United Nations, in 2020, the global population aged ≥65 y was 800 million. By 2050, this number is projected to double to 1.55 billion, and the aging population will be twice the number of children aged <5 y and almost equivalent to the number of children <12 y. Moreover, the number of people aged ≥80 y are expected to triple between 2020 and 2050, reaching 426 million [1]. With advancing age, multifaceted changes in the immune system emerge, a process termed immunosenescence, which is characterized by a reduction in immune responsiveness, a decrease in mucosal resistance and barrier function, and an increase in susceptibility to infections [2]. Older adults are at risk of a myriad of age-associated diseases, such as chronic respiratory and kidney diseases, diabetes mellitus, and other noncommunicable diseases, which are primarily associated with chronic systemic inflammation (“inflammaging”) [3]. A clear event that highlighted the deterioration of the immune system in older adults was the COVID-19 pandemic, which presented an extraordinary challenge to public health and emphasized the vulnerability of the aging population to emerging diseases [4]. According to the United States Centers for Disease Control and Prevention [5], the hospitalization rate for COVID-19 of individuals aged ≥85 y was 10 times higher than that of 18- to 29-y-olds, while the rate of COVID-19-related deaths for those aged 65 to 74 y was 65 times higher than that for the 18- to 29-y-old group.
A key player in the decline in innate and adaptive immune responses is the gut microbiome, which significantly influences the development and progression of systemic inflammation, exacerbating or mitigating age-related effects on immune function. Therefore, a thorough understanding of the gut–microbiome–immune axis is vital as it represents a critical component of effective strategies to enhance immune function, prevent related disorders, and manage or treat health complications associated with aging.
Prebiotics and probiotics are important dietary supplements that could be effective in promoting health among the elderly by modulating the gut microbiota community and regulating the gut environment, including the immune system [6,7]. A randomized clinical trial showed that prebiotics (soluble corn fiber) combined with probiotics enhanced innate immunity by increasing natural killer (NK) cell activity in the elderly population [8]. Similarly, the prebiotics inulin plus fructo-oligosaccharides (FOSs) have been shown to mitigate frailty syndrome in the elderly [9], and nondigestible polysaccharides have been shown to enhance the immune response and increase the seroprotection rate for virus infection in the elderly [10]. Compared to infants, the study of prebiotics’ effects in adults and older adults is relatively underexplored. As research on prebiotics’ effects on the gut microbiome and the immune system advances toward understanding the effects of pure compounds, the existing literature must be compiled and revised to assist in the experimental design of future clinical trials. GOS has been studied for decades, with emphasis on its beneficial effects as a dietary intervention for infants [11]. However, it is only in the last decade that pure GOS with <10% free sugars (lactose, glucose, galactose) has been evaluated in clinical trials [[12], [13], [14], [15]]. Additionally, research on the effects of GOS on the elderly population is limited, with only 2 clinical trials to date solely focused on the elderly [16,17]. This review aims to bridge this gap by inferring potential effects from existing research on younger populations. We propose that the insights gained from studies on infants and adults can help explore GOS as a promising approach for the elderly. We aim to emphasize the potential benefits of GOS and encourage further research in this direction, adding a new perspective to the existing knowledge on GOS and its applications. In this review, we will discuss the rationale and potential advantages of using GOS as a prebiotic supplement to improve gut health in older adults.
Immune System Alterations Associated with Aging
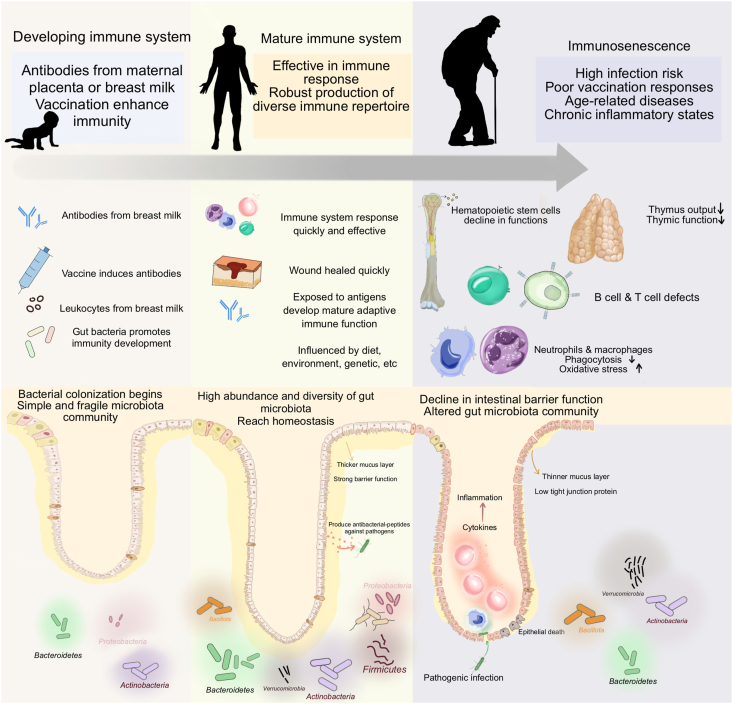
Innate and adaptive immunities become impaired with age (Figure 1). It is well established that, in older adults, there is a decline in the number and function of innate immune cells, leading to impaired pathogen elimination and chronic low-grade inflammation, characterized by increased proinflammatory cytokines [18,19]. The gastrointestinal tract, the largest immune organ, plays a crucial role in immune homeostasis, mediated by intestinal stem cells (ISCs) and intestinal epithelial cells (IECs), which maintain intestinal integrity and facilitate immune responses [20]. Aging adversely affects ISCs, IECs, and the structure of the intestinal crypts and villi, impairing regeneration and immune signaling. Metabolites such as MUFAs, PUFAs, N-acyl ethanolamines, and branched-chain amino acids are linked to longevity and improved immune homeostasis in the elderly [21]. This complex interplay of cells and metabolites underscores the multifaceted nature of immune regulation during aging.
FIGURE 1.
Comparison of the gut microbiota profile and immune function between children, young adults, and older individuals.
Microbial Shifts and Maturation of Immune Function
The evolution of the composition and functionality of the gut microbiota are significantly associated with immune function [[22], [23], [24]]. Establishment of the gut microbiome begins at birth. Assembly of the infant’s gut microbiome is influenced by delivery mode, the environment, and feeding type, as recently reviewed by Korpela et al. [25]. It is widely recognized that the early gut microbiota of vaginal-born, breastfed infants is dominated by Bifidobacterium and Bacteroides species. In contrast, babies born by cesarean section are dominated by Enterobacter with a high abundance of Clostridium in the first week of life [26,27]. Breastmilk delivers maternal soluble factors, such as macromolecules, immunoglobulins, cytokines, and immunologically active milk cells to the infant, which promote the maturation of the immune system [28]. Breastfeeding also introduces the microbes that colonize the infant’s gut and contribute to the development of the microbiota community during the first year, increasing the abundance of Bifidobacterium, Akkermansia, and Bacteroides [29,30]. Prolonged breastfeeding, beyond 6 mo, has been linked to the optimal maturation of the immune system [31] and a reduction of the intestinal dysbiosis associated with diarrhea [32,33]. Just as the infant gut microbiome, which exhibits high variability and low diversity until ∼3 y, the infant’s immune system is also immature and constantly dynamic, undergoing significant changes in the first year of life [25]. In infants, the balance between proinflammatory and anti-inflammatory cytokines is crucial to the appropriate response to pathogens and the development of early immune tolerance [31].
For the adult gut microbiome profile, there is yet to be a consensus on the definition of a healthy gut microbiome, given its susceptibility to be influenced by factors such as geographic location, living conditions, and socioeconomic status. For example, Bacteroides are dominant in people from China (39%), United States (38%), and Spain (23%) compared to those from Peru (1%), Malawi (3%), and Japan (5%) [[35], [36], [37]]. The considerable ecological diversity present in the gut microbiomes of healthy adults makes it exceptionally challenging to define a universal “healthy microbiome” at the population level. However, at the individual level, the characteristics and structure of the gut microbiota tend to remain relatively stable throughout adulthood. Dietary changes or medications can lead to short-term alterations, followed by a rapid restoration to a baseline gut microbiota [[38], [39], [40]]. This resilience of the gut microbiota complements the mature and robust immune system of healthy adults, which is more developed and better equipped to mount an effective immune response compared to that of infants and the elderly [41,42].
With advancing age, it is common to observe shifts in the composition of the gut microbiota and alterations in the immune system [21,43,44]. A detailed discussion of the interactions between specific taxa and the immune system is presented elsewhere; however, a common trend observed in aging is a reduction in the diversity and abundance of beneficial microorganisms, which can lead to the dysregulation of gut homeostasis and the immune system. The older adult gut microbiome is generally dominated by Bacteroidota and Bacillota [26]. Actinomycetota, Pseudomonadota, and Verrucomicrobiota have also been reported in high abundance [26,45,46]. An increase in the relative abundance of Bacteroidota and Pseudomonadota has been observed in subjects aged >70 y compared to the adult population (<60 y) [35], while the relative abundance of Akkermansia and Bifidobacterium has been reported to increase among Super Agers (ages 105–109 y) [47]. However, studies have shown that the diversity within taxa of Bacteroides, Clostridium cluster XIVa, Bifidobacterium, and Faecalibacterium species is lower in older adults (ages 60–80 y) [35,48]. Additionally, Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae showed negative associations with aging, while Eggerthella, Akkermansia, Anaerotruncus, and Bilophila have positive associations with aging [26,35,49]. Over time, compositional changes in the microbiota are reflected in its metabolic function. For example, Clostridium cluster XIVa is associated with short-chain fatty acid (SCFA) production and maintenance of immune homeostasis [50,51]. The abundance of Clostridium cluster XIVa decreases significantly with aging, leading to a decrease in SCFA production [48,52].
A new and exciting possibility for maintaining a youthful and healthy immune system is to modulate the gut microbiome in a controlled, systematic, and personalized manner. This is made possible by the wealth of information provided by high-throughput “omics” technologies. It is well established that antibiotics, diet, and lifestyle influence gut microbial communities [53,54]. The acknowledgment of probiotics as positive modulators of gut health, including the immune system, can be traced back to the early 1900s with the work of Elie Metchnikoff [55]. Similarly, prebiotics have demonstrated effective modulation of the gut microbiota and have shown promise in the elderly demographic.
Galacto-oligosaccharides
Prebiotics including GOSs, inulin, and FOSs, as well as human milk oligosaccharides and starch-derived oligosaccharides such as resistant starch and polydextrose, have been shown to confer multiple health benefits [[56], [57], [58]]. These benefits include enhancing the growth of beneficial bacteria such as Bifidobacterium (bifidogenic effect), improving digestive health, managing blood sugars and lipids, modifying immune markers, and providing anti-inflammatory effects [58,59]. GOS, a well-known type of nondigestible oligosaccharide, particularly benefits gut health by increasing the abundance of Bifidobacterium, Akkermansia, and Bacteroides [60,61]. They have an extensive history of safety and have been used for decades to improve overall host health via modulation of the gut microbiota [62]. GOSs generally contain a mixture of oligosaccharides with different polymerization degrees and linkages and are synthesized using microbial or enzymatic methods [11], often involving β-galactosidases from sources such as Kluyveromyces fragilis, Aspergillus niger, and Lactobacillus reuteri [63,64]. During the conversion from lactose to GOS, a range of mono-, di-, and oligosaccharides is created [64]. Commercial GOSs, such as Vivinal GOS, Bimuno, and Oligomate, vary in composition, impacting study reproducibility and outcome predictability. To tackle this issue, our group has cloned and heterologously expressed the β-hexosyltransferase from Hamamotoa singularis to generate high-purity (>90%) GOS [[64], [65], [66]] and lactosamine-enriched GOS [67].
Selectivity is an essential property of GOS as a prebiotic. Within this context, selectivity pertains to the notion that exclusive beneficial bacterial strains, notably lactobacilli and bifidobacteria, can metabolize GOS as a primary carbon source [56]. Consequently, GOSs increase the abundance of specific primary and secondary degraders, expanding autochthonous beneficial members of the intestinal microbiota [68]. It is well known that GOSs increase the abundance of Bifidobacterium (the “bifidogenic effect”), which is fundamental to the beneficial effect of the prebiotic and can lead to increased concentrations of colonic SCFAs, particularly butyrate [69]. By increasing the abundance of beneficial bacteria, GOSs limit the growth of pathogens, such as inhibition of the growth of Staphylococcus aureus and Pseudomonas aeruginosa in vitro [70] and inhibition of Escherichia coli E2348/69 adherence ex vivo [71]. Another factor contributing to selectivity is the glycosidic linkages and molecular weight of GOS components. It has been proposed that oligosaccharides of higher molecular weight may enhance saccharolytic fermentation in the distal colon to prevent chronic conditions [62].
Although GOS has been shown to beneficially modulate the gut microbiota and immune system, not everyone responds to GOS similarly (responders and nonresponders). Individuals can respond differently to GOS based on their dietary habits, microbiome, and genetics [56,72]. A recent clinical trial using GOS, dextrin, and inulin to investigate the responder mechanism of prebiotics revealed that the baseline gut microbiota profiles and metabolite composition were correlated with the participant response rate [72]. When comparing the initial SCFA concentration in the participants’ fecal samples and their response to prebiotics after 6 wk, those with the highest initial proportional butyrate concentrations had the weakest butyrogenic prebiotic reactions. This finding suggests an inverse relationship between the prebiotic response and baseline fecal SCFA concentration. Older adults with lower butyrate concentrations may have higher GOS response rates than young adults.
Currently, GOSs are approved by the United States Food and Drug Administration for supplementation at a concentration of ≤7.8 g/L or 11 g/serving for use in term infant formula and selected foods and beverages. GOS preparations have 14 Generally Recognized As Safe notifications. However, more research is needed on the recommended supplementation dosage for older adults and the specific type of GOS, its purity level, and its structural characteristics that would impact older individuals’ health.
Immune Modulation of GOS Mediated by Beneficial Bacteria
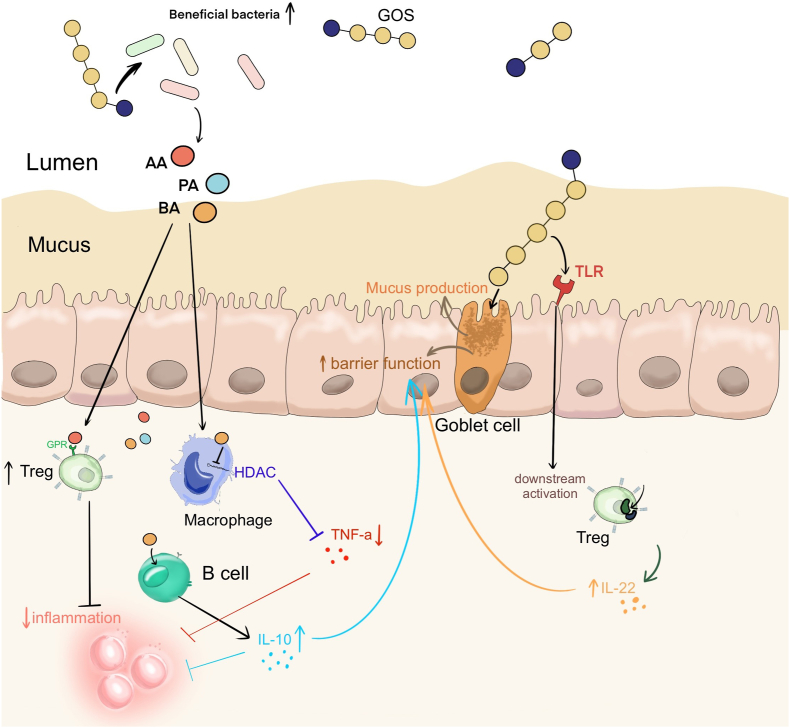
The primary mechanism by which prebiotics modulate immunity is through the enhancement of beneficial bacteria (Figure 2). In adults, GOSs increase the abundance of gut-beneficial taxa, including Bifidobacterium, Lactobacillus, and Akkermansia [12,14]. Likewise, studies on older individuals showed that GOS increased the abundance of Bifidobacterium, Lactobacillus-Enterococcus spp., and Clostridium coccoides–Eubacterium rectale [16,[73], [74], [75]]. The increased abundance of Bifidobacterium has been associated with improved immunological function and protection against inflammatory and infectious diseases, such as irritable bowel syndrome (IBS), pouchitis, and Clostridium difficile infection [76]. Moreover, Bifidobacterium has been shown to enhance the biosynthesis of immunomodulatory molecules such as tryptophan and exopolysaccharides, which stimulate the activity of immune cells, promoting the generation of the cytokine IL-10 [77,78]. Additionally, it has been reported that Bifidobacterium species induce dendritic cell maturation and promote T cell polarization response [79].
FIGURE 2.
Immunomodulatory functions of GOS. AA, acetic acid; BA, butyric acid; GOS, galacto-oligosaccharide; GPR, G-protein-coupled receptor; HDAC, histone deacetylase; IL, interleukin; PA, propionic acid; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cell.
Although the bifidogenic effects of GOS are well established, research regarding other beneficial microbes enhanced by GOS, including Akkermansia muciniphila, is lacking. A. muciniphila, a highly abundant bacterium in the intestinal mucus layer, has attracted significant attention for its beneficial effects against chronic diseases, including inflammatory bowel disease (IBD), cancer, diabetes, and obesity [80]. The abundance of A. muciniphila and acetic acid levels decreases with aging [81]. In mouse models of aging, A. muciniphila induced the adaptive immune response by producing SCFAs, increased mucin production enhancing intestinal barrier integrity [82,83], and reduced levels of proinflammatory cytokines, alleviating lymphocyte infiltration in ileum tissue [84]. SCFAs are the most important and well-studied microbial metabolites, and their beneficial effects have been studied extensively [85,86]. SCFA concentrations show a consistent age-related decline, with the concentration in those aged >80 y being less than half that of younger adults (<50 y), which correlates with the evolution of the gut microbiome as we age [48,87].
Bile acids, also transformed by gut bacteria such as Bacteroides and Lactobacillus, play key roles in host metabolism, cancer progression, and immunity (reviewed in [[88], [89], [90]]). These transformations include deconjugation and dehydroxylation, impacting the signaling properties of bile acids. Evidence suggested a positive correlation between GOS and secondary bile acid metabolism. In a study on rats, administering GOS (800 mg/kg) once a day significantly increased bile salt hydrolase activity in the small intestine, as indicated by serum biochemical parameters [91]. A mouse study showed that GOS modified bile acid metabolism by regulating the intestinal microbiota, translating into a protective effect against Pb toxicity [92]. Notably, for the population aged >100 y, their gut microbiome profile is enriched in microorganisms that can generate unique secondary bile acids [93]. Therefore, the production of secondary bile acids may contribute to healthy aging.
Enhancing the immune system through direct interaction with GOS
Toll-like receptors (TLRs) are essential receptors of the innate immune system that recognize pathogen-associated molecular patterns and initiate immune responses [94]. TLRs, expressed in immune cells, such as antigen-presenting and IECs, activate downstream signaling pathways [95]. TLR4 has been reported to bind to GOS and other oligosaccharides and trigger immune responses that promote the development of a healthy gut microbiome and enhance host immunity [96]. Additionally, Sun et al. [97] reported that GOS reduced LPS-induced inflammation in macrophages through TLR4, exerting anti-inflammatory activity. GOS has also been shown to reduce pathogen bacterial viability via the TLR4/NF-κB pathway and can relieve lung infections and inflammation [98]. TLRs are essential in promoting a network between the gut microbiota, IECs, and the immune system, underpinning the immunomodulatory ability of GOS in humans.
In addition to TLR, GOSs can directly modulate the immune system through additional pathways. In Caco-2 cells, GOS inhibited the NF-κB pathway induced by dextran sulfate sodium salt, thereby counteracting the inflammatory state of the host [99]. In LS174T cells, GOS modulated the function of intestinal goblet cells and improved mucus barrier function by acting directly on the barrier function-related genes MUC2, TFF3, and RETNLB [61,100].
Strengthening the Aging Gut Barrier: the Impact of GOS on Intestinal Permeability
Intestinal barrier function is a significant indicator of gut homeostasis and a factor that drives immunosenescence [101]. With aging, the integrity of the mucus layer deteriorates, leading to a decline in its antimicrobial functionality. This decline can be attributed to various factors, such as changes in mucin production by a reduction in the number of goblet cells, alterations in the composition of the gut microbiota, and an overall diminished efficiency of the immune system due to aging [61,102,103]. These changes can lead to a phenomenon known as “intestinal barrier dysfunction,” where the protective barrier becomes more permeable. The mucus layer loses its antimicrobial function, thus allowing potentially pathogenic bacteria, viruses, and harmful metabolites to pass through the intestinal barrier and into the bloodstream. This increase in permeability is often referred to as “leaky gut” and occurs in older individuals with intestinal inflammation [104]. Under normal conditions, a well-regulated process maintains gut mucosal tolerance by controlling the trafficking of antigens in the body. However, gut dysbiosis disrupts this balance, weakening the gut barrier and allowing harmful substances from the gut to enter the bloodstream, triggering inflammation. This contributes to the development of chronic inflammatory diseases by triggering the release of proinflammatory cytokines, including TNF-α and interferon-γ, which further exacerbate the condition by increasing the permeability of the paracellular pathway for antigen passage, thereby creating a cycle of inflammation [101]. Numerous studies in animal models and humans have established a link between gut permeability, noninfective chronic inflammation, and metabolic changes commonly associated with aging [[105], [106], [107], [108]].
The notion of GOS as a modulator of intestinal barrier function is a recent development. Evidence suggests that GOS can reduce intestinal permeability of the inflamed gut. Studies of aged mice (>60 wk) examining the effects of GOS on the aging gut showed that GOS reduced the age-associated compromised intestinal permeability and increased MUC2 expression and mucus thickness [61,100]. In a study in Caco-2 cells, GOS had a protective effect on the monolayer’s permeability when disrupted by mycotoxin deoxynivalenol [33]. This suggests that GOS could play a significant role in strengthening the aging gut barrier and maintaining gut health in the elderly.
GOS-Induced Immunomodulation in the Elderly
The immunomodulatory effects of GOS are well established in infants and young populations; however, the mechanisms involved remain unclear [11,109,110]. Although less explored in older adults, one study showed that GOS increased phagocytosis and NK cell activity while decreasing the production of proinflammatory cytokines, including IL-6, IL-1β, and TNF-α, and increasing the production of the anti-inflammatory cytokine IL-10 in older individuals (aged 69.3 ± 4.0 y) [17]. In another study, GOS increased the abundance of Bacteroides and Bifidobacterium in elderly individuals (aged 65–80 y). This increase was associated with higher concentrations of lactic acid in fecal samples [16]. This study also revealed enhanced immunity, characterized by elevated IL-10, IL-8, NK cell activity, C-reactive protein levels, and reduced IL-1β levels.
Although most studies on prebiotics and probiotics in older adults have shown positive impacts (Table 1 [9,17,[111], [112], [113], [114], [115], [116], [117]]), certain subgroups, such as those suffering from IBS or IBD, those recovering from viral infections, and specific vaccine cohorts, are underrepresented in this research. It is worth noting that older adults respond differently to interventions. Although some experience positive results, there is a group of individuals who do not respond. This could be due to various factors including the dosage of prebiotics, their initial gut microbiome, lack of adherence to the intervention, and the duration of the intervention itself. All these elements can impact the effectiveness of the intervention. Reported interventions lasted from 3 d [117] to 28 wk [9], and the dosages ranged from 1.3 g/d [113] to 10 g/d [117]. The outcomes of those studies also vary; some showed positive effects on improving exhaustion, lowering potential mortality, reducing inflammation, enhancing SCFA production, and increasing the abundance of beneficial bacteria [17, 111,117, 114], while others showed either placebo effects or no significant changes [113,112–116. There was no obvious association between dosage, duration, or population age and the outcomes. This variability underscores the necessity for further research to understand the underlying mechanisms that influence these differential responses.
TABLE 1.
Summary of health effects of prebiotics in the older population
| Reference | Intervention | Population | Health condition | Study design | Main health outcome | Main findings | Potential implications |
|---|---|---|---|---|---|---|---|
| Walton et al. [111] | 4 g GOS and placebo twice a day for 3 wk, preceded by 3-wk washout period | 39 volunteers aged 50–81 y | Healthy | Randomized, double-blind, placebo-controlled crossover trial |
|
Significant bifidogenic effects. A significant increase in the amount of butyrate. |
GOS can be utilized as a selective ingredient to positively influence the composition of beneficial gut bacteria and mitigate the risk factors related to changes in the colonic microbiota and fermentation that occur with aging. |
| Vulevic et al. [17] | 5.5 g/d GOS for 10 wk, preceded by 4-wk washout period | 44 volunteers aged 69.3 ± 4.0 y | Healthy | Randomized, double-blind, placebo-controlled, crossover study |
|
Significantly increased the numbers of beneficial bacteria, especially bifidobacterial. Significant increases in phagocytosis, NK cell activity, and the production of anti-inflammatory cytokine IL-10. Significant reduction in the production of proinflammatory cytokines. |
B-GOS administration to healthy elderly persons resulted in positive effects on both the microflora composition and the immune response. |
| Bunout et al. [112] | 6 g/d mixture of 70% raftilose and 30% raftiline for 28 wk | 66 volunteers aged ≥70 y | Healthy | RCT |
|
No changes in serum proteins, albumin, Igs, and secretory IgA were observed. Antibodies against influenza B and pneumococcus increased significantly from wk 0 to 8, with no significant differences between groups. Antibodies against influenza A did not increase. No effects of prebiotics on IL-4 and IFN-γ secretion by cultured monocytes were observed. | No immunological effects of the mixture of raftilose and raftiline response to vaccination in the elderly were observed in this study. |
| Schiffrin et al. [113] | 1.3 g/d FOS for 12 wk | 74 volunteers aged 70–99 y | Malnourished or at risk of malnutrition | Prospective, randomized, double-blind, controlled study |
|
Specific mRNA extracted from blood leucocytes showed a different level of proinflammatory gene activation; Serum levels of sCD14, a product shed by activated macrophages, decreased only in the OS group without reaching statistical significance. No significant differences were detected in the fecal gut flora or in the nutritional parameters. | The administration of supplements in older persons at risk of malnutrition may benefit from the addition of prebiotics that can improve the low noise inflammatory process frequently observed in this population. |
| Buigues et al. [9] | Mixture of inulin (3375 mg) and FOS (3488 mg) once daily | 60 volunteers aged 66–90 y | Mobile, non-demented nursing home residents | Randomized, double-blind, placebo-controlled trial |
|
Significantly improved exhaustion and handgrip strength; no significant differences in the serum concentration of TNF-α. | The overall rate of frailty was not significantly modified by prebiotics. |
| Jain et al. [114] | Synbiotics of Lactobacillus acidophilus LA5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, and Lactobacillus bulgaricus with oligofructose, once daily until death or discharge from current hospital | 90 volunteers aged 62–80 y | Patient admitted to an ICU | RCT | Gut permeability and barrier function | After 1 wk of therapy, patients in the synbiotic group had a significantly lower incidence of potentially pathogenic bacteria and multiple organisms in their nasogastric aspirates than controls. There were no significant differences between the groups in terms of intestinal permeability, septic complications, or mortality. | Synbiotics altered the microbial composition of the upper gastrointestinal tract but had no effect on intestinal permeability. |
| Louzada et al. [115] | Synbiotics of Lactobacillus paracasei, Lactobacillus ramnosus, Lactobaciullus acidophilus, Bifidobacterium lactis (108–109 CFU of each) with FOS (6 g), twice daily for 24 wk | 49 volunteers aged 65–90 y | Prefrail individuals | RCT | GDS-15; MMSE; body fat percentage; serum IL-6, TNF-α, and IL-10; serum DAO, IFABP, and LPS | Both placebo and synbiotics groups had reduced body fat percentage, TNF-α, and DAO. IL-10 was significantly increased only in the synbiotics group. |
Synbiotics had weak effect on depressive symptoms and more optimistic effects on cognition in apparently healthy elderly. |
| Neto et al. [116] | Synbiotics of 6 g FOS, 108 to 109 CFU Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus acidophilus, and Bifidobacterium lactis, once daily dose for 3 mo | 17 volunteers aged 60–74 y | Community dwelling older adults fulfilling one of Fried’s frailty criteria | Double-blind, placebo-controlled study | Anthropometric measurements, BIVA, IL-6, and TNF-α | Majority of synbiotics individuals maintained or improved their tissue hydration. | 3 mo of synbiotic supplementation did not promote any significant changes in inflammatory cytokines or body composition but demonstrated a trend toward a preservation of hydration status in apparently healthy elderly individuals. |
| Shimizu et al. [117] | Synbiotics of 3 × 108Bifidobacterium breve strain Yakult, 3 × 108Lactobacillus casei strain Shirota, and 10 g GOS, once daily dose for 3 d | 72 volunteers aged 64–82 y | Septic patients placed on a ventilator within 3 days of ICU admission | Single-blind study |
|
The incidence of enteritis and incidence of VAP was significantly lower in the Synbiotics than the No-Synbiotics group; total organic acid concentration, especially the amounts of acetate, were significantly greater in the Synbiotics group than in the No-Synbiotics group at the first week. | Prophylactic synbiotics could modulate the gut microbiota and environment and may have preventive effects on the incidence of enteritis and VAP in patients with sepsis. |
Abbreviations: BIVA, bioelectric impedance with vectorial analysis; CFU, colony-forming units; DAO, diamine oxidase; FOS, fructo-oligosaccharide; GDS-15, Geriatric Depressive Symptoms Scale-15; GOS, galacto-oligosaccharide; HDL, high density lipoprotein; ICU, intensive care unit; IFABP, intestinal fatty acid binding protein; IFN, interferon; Ig, immunoglobulin; IL, interleukin; LPS, lipopolysaccharide; MMSE, Mini Mental State Examination; NK, natural killer; OS, oligosaccharide; RCT, randomized controlled trial; sCD14, soluble receptor CD14; SCFA, short-chain fatty acid; TNF, tumor necrosis factor; VAP, ventilator-associated pneumonia.
The Role of GOS in Infectious Diseases, Inflammation, and Vaccine Efficacy
GOS has been proven effective in reducing the incidence and severity of infections, including respiratory infections in infants and children, rotavirus (RV)-associated diarrhea in early life, and acute diarrhea in adults, demonstrating its broad efficacy and safety across different age groups [[118], [119], [120]]. Given this broad efficacy, GOS presents a promising option for targeting the immunosenescent population, offering potential benefits in enhancing immune function in the elderly.
IBD is a chronic group of inflammatory conditions primarily affecting the gastrointestinal tract. The exact cause of IBD is not fully understood but is thought to involve genetic, environmental, and immune-related factors. Moreover, several factors, such as polypharmacy, comorbidities, treatment compliance, difficulties in differential diagnosis, and surgical complication risks often confound IBD in older adults [121] leading to their underrepresentation in clinical trials, which often exclude participants aged >65 y [122]. According to the European Crohn’s and Colitis Organization, 25% to 35% of patients with IBD are >60 y old [123]. Fiber consumption or Fermentable, Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) diet is difficult to tolerate for patients with IBD or IBS, as it may exacerbate symptoms such as abdominal pain, discomfort, or diarrhea in specific individuals. However, 4 g of GOS supplementation twice daily did not induce intestinal bloating or abdominal discomfort in the elderly population [111]. In a study of adult mice fed 8.8, 4.4, and 2.2 g GOS/kg body weight showed an improvement in small bowel movements and relieved constipation [124]. Similarly, by increasing the quantity and activity of beneficial bacteria, GOS may benefit the gastrointestinal health of patients with IBD [96,125,126].
RV is a childhood infection that causes acute gastroenteritis. However, the rate of RV infection may be underestimated in the elderly population. In individuals aged ≥65 y, RV was detected in 9% of samples, indicating that RV infections could be more common in this age group than previously thought [127]. In one study, the combination of GOS and FOS induced an intestinal trophic effect and blocked RV infection in rats, thereby ameliorating RV-induced diarrhea [128]. An in vitro assay demonstrated the direct blocking interaction between GOS/FOS and RV, indicating that the virus was less detectable in the presence of the prebiotic mixture [129]. However, additional evidence and research are required to elucidate how GOS inhibits viral infections.
Vaccine efficacy can vary between individuals and is particularly low in older people [130]. Nagafuchi et al. [131] demonstrated that the seroprotective effect of an influenza vaccine was enhanced when fermented milk was administered in conjunction with GOS to H1N1-vaccinated, enterally-fed older individuals. A study showed that GOS improved Th1 responses and B cell activation specific to influenza vaccine in mice [132]. This suggests that the intake of prebiotics could be a practical approach to boost immune responses to the influenza vaccine. Given the immunomodulatory effect of GOS on gut homeostasis, researchers explored the role of GOS in preventing and treating age-related diseases. In a mouse study by Chen et al. [133], GOS reduced kidney inflammation by inhibiting the hypoxia-induced CD44/JNK cascade and cytokine production in renal tubular cells, thereby alleviating acute kidney injury, for which the risk is increased in individuals aged >75 y [134].
Animal and in vitro studies have provided robust evidence of the beneficial effect of GOS on the elderly immune system [124,128,129,132,133]. However, there is a lack of human clinical research in older adults, and most studies administered GOS in combination with other prebiotics (e.g., FOS, inulin) or probiotics, making it difficult to discern the individual effect of each component on the intervention. However, due to the combination of GOS with other prebiotics or probiotics in these trials, the immunomodulatory effects of GOS in the elderly lack robust evidence. Moreover, the effects of GOS supplementation on the immune system in older individuals are impacted by the GOS structures. For example, Maneerat et al. [135] reported no significant immunological or microbial changes in an aging cohort that received GOS that had predominantly Gal(β1–6), Gal(β1–4), and Gal(β1–3) linkages produced with the β-galactosidase from Aspergillus oryzae GC288. In contrast, another study showed that GOS consumption led to substantial increases in Bacteroides and Bifidobacterium, promoted the production of IL-10 and IL-8, and increased NK cell activity and C-reactive protein production in older subjects [17]. The GOS used in that study was produced by Bifidobacterium bifidum NCIMB 41171. These studies suggest that different sources of GOS may exert different immunomodulatory effects, and to better translate GOS into the clinical care of the elderly population and maximize their immunomodulatory effects, the dose, purity, and source of GOS need to be thoroughly investigated.
Limitations of GOS Administration in the Elderly Population
The studies included in this review have reported the benefits of GOS supplementation in the short term (Table 1 [9,17,[111], [112], [113], [114], [115], [116], [117]]), but the long-term effects and sustainability of these benefits are less clear. The safety of GOS in infant formula and adult supplementation has been extensively researched, showing generally positive tolerability profiles with a No Observed Adverse Effect Level established at 5000 mg/kg body weight/d for 90 consecutive days and ≥2000 mg/kg/d [11]. In infants, GOSs are regularly added to formula at a concentration of 5 g/L, which has been shown to effectively enhance the beneficial gut microbiota without adverse effects [11,136]. The most commonly reported side effects among all age groups include bloating and digestive discomfort, which typically increase with higher doses of GOS [137]. For adults, studies have demonstrated that daily supplementation with GOS at doses from 5 to 20 g/d can be tolerated without significant side effects [138,139]. However, given the complicated health conditions in the elderly population (e.g. disease, lifestyle, immunosenescence), potential side effects and tolerability should be further investigated. Moreover, most of the clinical trials have used synbiotics or a mixture of other prebiotics with GOS as interventions in the elderly. The lack of studies administering GOS alone makes it difficult to apply in practice and introduces more variables that could affect the outcome. Also, as discussed earlier, the effectiveness of GOS can vary significantly from individual to individual, making it challenging to predict outcomes or standardize intervention. Although there is growing research on the benefits of GOS, there is still a lack of large-scale, long-term studies specifically focusing on the elderly population. Given these limitations, it is evident that comprehensive studies, including mechanistic and proof-of-concept research, are needed to provide a stronger rationale for utilizing GOS in elderly populations. Such studies would not only help in understanding the underlying mechanisms of how GOS benefits the elderly but also assist in developing standardized guidelines for their use, ensuring maximum efficacy and safety.
Conclusions and Future Perspective
Recent advancements in our understanding of the direct and indirect modulatory effects of GOS have gained significant relevance due to the aging global population and the increasing prevalence of immune-related diseases in older adults. These developments underscore the importance of beneficial interventions in addressing age-related health challenges, with GOS as a potential intervention to prevent or counteract the deterioration of gut homeostasis associated with age. We summarized the health effects of GOS through the modulation of gut microbiota, enhancement of SCFAs and secondary bile acid production, and direct interactions with host receptors. This process contributes to the maintenance of a gut health state that is similar to younger individuals, which, in turn, can lead to an improved immune system and potentially increase longevity.
The positive clinical outcomes associated with GOS suggest that it could be a feasible approach to support immune health and address the health challenges associated with aging. Nevertheless, it is important to recognize that the physiological and immunological alterations unique to the aging process require further targeted research. Such research should focus specifically on the older adult population to ensure that interventions like GOS supplementation are safe, effective, and tailored to meet the unique needs of this demographic.
Author contributions
The authors’ responsibilities were as follows—YH, MAA-P: wrote the manuscript, writing - original draft; MRA: writing - review and editing and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
The authors reported no funding received for this study.
References
- 1.United Nations Department of Economic and Social Affairs . 2020. World Population Ageing 2019. New York. [Google Scholar]
- 2.Teissier T., Boulanger E., Cox L.S. Interconnections between inflammageing and immunosenescence during ageing. Cells. 2022;11(3):359. doi: 10.3390/cells11030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang A.Y., Skirbekk V.F., Tyrovolas S., Kassebaum N.J., Dieleman J.L. Measuring population ageing: an analysis of the global burden of disease study 2017. Lancet Public Health. 2019;4(3):e159–e167. doi: 10.1016/S2468-2667(19)30019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koff W.C., Williams M.A. Covid-19 and immunity in aging populations - a new research agenda. N. Engl. J. Med. 2020;383(9):804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#demographicsovertime [Internet]. [cited Feb 11 2024]. Available from.
- 6.Donati Zeppa S., Agostini D., Ferrini F., Gervasi M., Barbieri E, Bartolacci A, et al. Interventions on gut microbiota for healthy aging. Cells. 2022;12(1):34. doi: 10.3390/cells12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenhuichen C., Cabello-Olmo M., Barajas M., Izquierdo M., Ramírez-Vélez R., Zambom-Ferraresi F., et al. Impact of probiotics and prebiotics in the modulation of the major events of the aging process: a systematic review of randomized controlled trials. Exp. Gerontol. 2022;164 doi: 10.1016/j.exger.2022.111809. [DOI] [PubMed] [Google Scholar]
- 8.Costabile A., Bergillos-Meca T., Rasinkangas P., Korpela K., de Vos W.M., Gibson G.R. Effects of soluble corn fiber alone or in synbiotic combination with Lactobacillus rhamnosus GG and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (Saimes study) Front. Immunol. 2017;8:1443. doi: 10.3389/fimmu.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buigues C., Fernández-Garrido J., Pruimboom L., Hoogland A.J., Navarro-Martínez R., Martínez-Martínez M., et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int. J. Mol. Sci. 2016;17(6):932. doi: 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laue C., Stevens Y., van Erp M., Papazova E., Soeth E., Pannenbeckers A., et al. Adjuvant effect of orally applied preparations containing non-digestible polysaccharides on influenza vaccination in healthy seniors: a double-blind, randomised, controlled pilot trial. Nutrients. 2021;13(8):2683. doi: 10.3390/nu13082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrogi V., Bottacini F., Cao L., Kuipers B., Schoterman M., van Sinderen D. Galacto-oligosaccharides as infant prebiotics: production, application, bioactive activities and future perspectives. Crit. Rev. Food Sci. Nutr. 2023;63(6):753–766. doi: 10.1080/10408398.2021.1953437. [DOI] [PubMed] [Google Scholar]
- 12.Azcarate-Peril M.A., Roach J., Marsh A., Chey W.D., Sandborn W.J., Ritter A.J., et al. A double-blind, 377-subject randomized study identifies Ruminococcus, Coprococcus, Christensenella, and Collinsella as long-term potential key players in the modulation of the gut microbiome of lactose intolerant individuals by galacto-oligosaccharides. Gut Microbes. 2021;13(1) doi: 10.1080/19490976.2021.1957536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chey W., Sandborn W., Ritter A.J., Foyt H., Azcarate-Peril M.A., Savaiano D.A. Galacto-oligosaccharide RP-G28 improves multiple clinical outcomes in lactose-intolerant patients. Nutrients. 2020;12(4):1058. doi: 10.3390/nu12041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azcarate-Peril M.A., Ritter A.J., Savaiano D., Monteagudo-Mera A., Anderson C., Magness S.T., et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. U. S. A. 2017;114(3):E367–E375. doi: 10.1073/pnas.1606722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savaiano D.A., Ritter A.J., Klaenhammer T.R., James G.M., Longcore A.T., Chandler J.R., et al. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial. Nutr. J. 2013;12:160. doi: 10.1186/1475-2891-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulevic J., Drakoularakou A., Yaqoob P., Tzortzis G., Gibson G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008;88(5):1438–1446. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 17.Vulevic J., Juric A., Walton G.E., Claus S.P., Tzortzis G., Toward R.E., et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015;114(4):586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 18.Fulop T., Larbi A., Pawelec G., Khalil A., Cohen A.A., Hirokawa K., et al. Immunology of aging: the birth of inflammaging. Clin. Rev. Allergy Immunol. 2023;64(2):109–122. doi: 10.1007/s12016-021-08899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Li C., Zhang W., Wang Y., Qian P., Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023;8(1):239. doi: 10.1038/s41392-023-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohman L.S., Osborne L.C. A gut-centric view of aging: do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell. 2022;21(9) doi: 10.1111/acel.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y., Hu G., Wang M.C. Host and microbiota metabolic signals in aging and longevity. Nat. Chem. Biol. 2021;17(10):1027–1036. doi: 10.1038/s41589-021-00837-z. [DOI] [PubMed] [Google Scholar]
- 22.Yoo J.Y., Groer M., O Dutra S.V., Sarkar A., McSkimming D.I. Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saini A., Dalal P., Sharma D. Deciphering the interdependent labyrinth between gut microbiota and the immune system. Lett. Appl. Microbiol. 2022;75(5):1122–1135. doi: 10.1111/lam.13775. [DOI] [PubMed] [Google Scholar]
- 24.Asnicar F., Berry S.E., Valdes A.M., Nguyen L.H., Piccinno G., Drew D.A., et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korpela K., de Vos W.M. Infant gut microbiota restoration: state of the art. Gut Microbes. 2022;14(1) doi: 10.1080/19490976.2022.2118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Pace F. Changes in gut microbiota during lifespan. MAMC J. Med. Sci. 2017;3(3):133–139. doi: 10.4103/mamcjms.mamcjms_40_17. [DOI] [Google Scholar]
- 27.Zhou L., Qiu W., Wang J., Zhao A., Zhou C., Sun T., et al. Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of cesarean-born infants: a blinded randomized controlled trial. Cell Host Microbe. 2023;31(7):1232–1247.e5. doi: 10.1016/j.chom.2023.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Molès J.P., Tuaillon E., Kankasa C., Bedin A.S., Nagot N., Marchant A., et al. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant, Pediatr. Allergy Immunol. 2018;29(2):133–143. doi: 10.1111/pai.12841. [DOI] [PubMed] [Google Scholar]
- 29.Li N., Xie Q., Zhao L., Shi J., Evivie S.E., Lv X., et al. Human milk and infant formula modulate the intestinal microbiota and immune systems of human microbiota-associated mice. Food Funct. 2021;12(6):2784–2798. doi: 10.1039/d0fo03004j. [DOI] [PubMed] [Google Scholar]
- 30.Stewart C.J. Breastfeeding promotes bifidobacterial immunomodulatory metabolites. Nat. Microbiol. 2021;6(11):1335–1336. doi: 10.1038/s41564-021-00975-z. [DOI] [PubMed] [Google Scholar]
- 31.Ames S.R., Lotoski L.C., Azad M.B. Comparing early life nutritional sources and human milk feeding practices: personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes. 2023;15(1) doi: 10.1080/19490976.2023.2190305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R.P., Niharika J., Kondepudi K.K., Bishnoi M., Tingirikari J.M.R. Recent understanding of human milk oligosaccharides in establishing infant gut microbiome and roles in immune system. Food Res. Int. 2022;151 doi: 10.1016/j.foodres.2021.110884. [DOI] [PubMed] [Google Scholar]
- 33.Akbari P., Braber S., Alizadeh A., Verheijden K.A.T., Schoterman M.H.C., Kraneveld A.D., et al. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015;145(7):1604–1613. doi: 10.3945/jn.114.209486. [DOI] [PubMed] [Google Scholar]
- 35.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obregon-Tito A.J., Tito R.Y., Metcalf J., Sankaranarayanan K., Clemente J.C., Ursell L.K., et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015;6:6505. doi: 10.1038/ncomms7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta V.K., Paul S., Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuzzo A., Van Horn S., Traini C., Perry C.R., Dumont E.F., Scangarella-Oman N.E., et al. Microbiome recovery in adult females with uncomplicated urinary tract infections in a randomised phase 2A trial of the novel antibiotic gepotidacin (GSK140944) BMC Microbiol. 2021;21(1):181. doi: 10.1186/s12866-021-02245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fragiadakis G.K., Wastyk H.C., Robinson J.L., Sonnenburg E.D., Sonnenburg J.L., Gardner C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020;111(6):1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M., Qian W., Yu L., Tian F., Zhang H., Chen W., et al. Multi-time-point fecal sampling in human and mouse reveals the formation of new homeostasis in gut microbiota after bowel cleansing. Microorganisms. 2022;10(12):2317. doi: 10.3390/microorganisms10122317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19(1):10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 42.Finger C.E., Moreno-Gonzalez I., Gutierrez A., Moruno-Manchon J.F., McCullough L.D. Age-related immune alterations and cerebrovascular inflammation. Mol. Psychiatry. 2022;27(2):803–818. doi: 10.1038/s41380-021-01361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 44.Walrath T., Dyamenahalli K.U., Hulsebus H.J., McCullough R.L., Idrovo J.P., Boe D.M., et al. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021;109(6):1045–1061. doi: 10.1002/JLB.3RI0620-405RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan H., Qin Q., Yan S., Chen J., Yang Y., Li T., et al. Comparison of the gut microbiota in different age groups in China. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.877914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller S., Saunier K., Hanisch C., Norin E., Alm L., Midtvedt T., et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 2006;72(2):1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biagi E., Franceschi C., Rampelli S., Severgnini M., Ostan R., Turroni S., et al. Gut microbiota and extreme longevity. Curr. Biol. 2016;26(11):1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Salazar N., Arboleya S., Fernández-Navarro T., de Los Reyes-Gavilán C.G., Gonzalez S., Gueimonde M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11(8):1765. doi: 10.3390/nu11081765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang S., Chen X., Lu Z., Meng L., Huang Y., Yu X., et al. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat. Aging. 2023;3(4):436–449. doi: 10.1038/s43587-023-00389-y. [DOI] [PubMed] [Google Scholar]
- 50.Murakami M., Iwamoto J., Honda A., Tsuji T., Tamamushi M., Ueda H., et al. Detection of gut dysbiosis due to reduced Clostridium subcluster XIVa using the fecal or serum bile acid profile. Inflamm. Bowel Dis. 2018;24(5):1035–1044. doi: 10.1093/ibd/izy022. [DOI] [PubMed] [Google Scholar]
- 51.Moens F., De Vuyst L. Inulin-type fructan degradation capacity of Clostridium cluster IV and XIVa butyrate-producing colon bacteria and their associated metabolic outcomes. Benef. Microbes. 2017;8(3):473–490. doi: 10.3920/BM2016.0142. [DOI] [PubMed] [Google Scholar]
- 52.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLOS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delcour J.A., Aman P., Courtin C.M., Hamaker B.R., Verbeke K. Prebiotics, fermentable dietary fiber, and health claims. Adv. Nutr. 2016;7(1):1–4. doi: 10.3945/an.115.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunz C. Historical aspects of human milk oligosaccharides. Adv. Nutr. 2012;3(3):430S–439S. doi: 10.3945/an.111.001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufmann S.H.E. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat. Immunol. 2008;9(7):705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 56.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 57.Cunningham M., Azcarate-Peril M.A., Barnard A., Benoit V., Grimaldi R., Guyonnet D., et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021;29(8):667–685. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Pujari R., Banerjee G. Impact of prebiotics on immune response: from the bench to the clinic. Immunol. Cell Biol. 2021;99(3):255–273. doi: 10.1111/imcb.12409. [DOI] [PubMed] [Google Scholar]
- 59.Quigley E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019;17(2):333–344. doi: 10.1016/j.cgh.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 60.Vulevic J., Tzortzis G., Juric A., Gibson G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018;30(11) doi: 10.1111/nmo.13440. [DOI] [PubMed] [Google Scholar]
- 61.Arnold J.W., Roach J., Fabela S., Moorfield E., Ding S., Blue E., et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome. 2021;9(1):31. doi: 10.1186/s40168-020-00980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruno-Barcena J.M., Azcarate-Peril M.A. Galacto-oligosaccharides and colorectal cancer: feeding our intestinal probiome. J. Funct. Foods. 2015;12:92–108. doi: 10.1016/j.jff.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galgano F., Mele M.C., Tolve R., Condelli N., Di Cairano M., Ianiro G., et al. Strategies for producing low FODMAPs foodstuffs: challenges and perspectives. Foods. 2023;12(4):856. doi: 10.3390/foods12040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dagher S.F., Azcarate-Peril M.A., Bruno-Bárcena J.M. Heterologous expression of a bioactive β-hexosyltransferase, an enzyme producer of prebiotics, from Sporobolomyces singularis. Appl. Environ. Microbiol. 2013;79(4):1241–1249. doi: 10.1128/AEM.03491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruno-Bárcena J.M., Dagher S., Azcarate-Peril M.A., North Carolina State University . 2013. Beta-hexosyl transferases and uses thereof.https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/20150307856 Patent PCT/US2013/073870. Filed 9 December. Available from. [Google Scholar]
- 66.Bruno-Bárcena J.M., Dagher S., Azcarate-Peril M.A., North Carolina State University . 2021. Compositions and methods for producing human milk oligosaccharides.https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/20230279368 Patent PCT/US2021/032998. Filed 18 May. Available from. [Google Scholar]
- 67.Arnold J.W., Whittington H.D., Dagher S.F., Roach J., Azcarate-Peril M.A., Bruno-Barcena J.M. Safety and modulatory effects of humanized galacto-oligosaccharides on the gut microbiome. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azcarate-Peril M.A., Altermann E., Goh Y.J., Tallon R., Sanozky-Dawes R.B., Pfeiler E.A., et al. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl. Environ. Microbiol. 2008;74(15):4610–4625. doi: 10.1128/AEM.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander C., Swanson K.S., Fahey G.C., Garleb K.A. Perspective: physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv. Nutr. 2019;10(4):576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortaz E., Nomani M., Adcock I., Folkerts G., Garssen J. Galactooligosaccharides and 2'-fucosyllactose can directly suppress growth of specific pathogenic microbes and affect phagocytosis of neutrophils. Nutrition. 2022;96 doi: 10.1016/j.nut.2022.111601. [DOI] [PubMed] [Google Scholar]
- 71.Shoaf K., Mulvey G.L., Armstrong G.D., Hutkins R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006;74(12):6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmes Z.C., Villa M.M., Durand H.K., Jiang S., Dallow E.P., Petrone B.L., et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome. 2022;10(1):114. doi: 10.1186/s40168-022-01307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran T.T.T., Cousin F.J., Lynch D.B., Menon R., Brulc J., Brown J.R., et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7(1):39. doi: 10.1186/s40168-019-0654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilms E., An R., Smolinska A., Stevens Y., Weseler A.R., Elizalde M., et al. Galacto-oligosaccharides supplementation in prefrail older and healthy adults increased faecal bifidobacteria, but did not impact immune function and oxidative stress. Clin. Nutr. 2021;40(5):3019–3031. doi: 10.1016/j.clnu.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 75.Schoemaker M.H., Hageman J.H.J., Ten Haaf D., Hartog A., Scholtens P.A.M.J., Boekhorst J., et al. Prebiotic galacto-oligosaccharides impact stool frequency and fecal microbiota in self-reported constipated adults: a randomized clinical trial. Nutrients. 2022;14(2):309. doi: 10.3390/nu14020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gavzy S.J., Kensiski A., Lee Z.L., Mongodin E.F., Ma B., Bromberg J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes. 2023;15(2) doi: 10.1080/19490976.2023.2291164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S.H., Cho S.Y., Yoon Y., Park C., Sohn J., Jeong J.J., et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021;6(3):277–288. doi: 10.1038/s41564-020-00831-6. [DOI] [PubMed] [Google Scholar]
- 78.Sun S., Luo L., Liang W., Yin Q., Guo J., Rush A.M., et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. U. S. A. 2020;117(44):27509–27515. doi: 10.1073/pnas.1921223117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz L., Delgado S., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and their molecular communication with the immune system. Front. Microbiol. 2017;8:2345. doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J.S., Kang S.W., Lee J.H., Park S.H., Lee J.S. The evolution and competitive strategies of Akkermansia muciniphila in gut. Gut Microbes. 2022;14(1) doi: 10.1080/19490976.2021.2025017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collado M.C., Derrien M., Isolauri E., de Vos W.M., Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ansaldo E., Slayden L.C., Ching K.L., Koch M.A., Wolf N.K., Plichta D.R., et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–1184. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodogai M., O’Connell J., Kim K., Kim Y., Moritoh K., Chen C., et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 2018;10(467) doi: 10.1126/scitranslmed.aat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma J., Liu Z., Gao X., Bao Y., Hong Y., He X., et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol. Res. 2023;189 doi: 10.1016/j.phrs.2023.106687. [DOI] [PubMed] [Google Scholar]
- 85.Tan J.K., Macia L., Mackay C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023;151(2):361–370. doi: 10.1016/j.jaci.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Kim C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023;20(4):341–350. doi: 10.1038/s41423-023-00987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. U. S. A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi L., Jin L., Huang W. Bile acids, intestinal barrier dysfunction, and related diseases. Cells. 2023;12(14):1888. doi: 10.3390/cells12141888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He P., Yu L., Tian F., Zhang H., Chen W., Zhai Q. Dietary patterns and gut microbiota: the crucial actors in inflammatory bowel disease. Adv. Nutr. 2022;13(5):1628–1651. doi: 10.1093/advances/nmac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim C.S. Roles of diet-associated gut microbial metabolites on brain health: cell-to-cell interactions between gut bacteria and the central nervous system. Adv. Nutr. 2024;15(1) doi: 10.1016/j.advnut.2023.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q., Liu M., Zhang P., Fan S., Huang J., Yu S., et al. Fucoidan and galactooligosaccharides ameliorate high-fat diet-induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition. 2019;65:50–59. doi: 10.1016/j.nut.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Zhai Q., Wang J., Cen S., Zhao J., Zhang H., Tian F., et al. Modulation of the gut microbiota by a galactooligosaccharide protects against heavy metal lead accumulation in mice. Food Funct. 2019;10(6):3768–3781. doi: 10.1039/c9fo00587k. [DOI] [PubMed] [Google Scholar]
- 93.Sato Y., Atarashi K., Plichta D.R., Arai Y., Sasajima S., Kearney S.M., et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599(7885):458–464. doi: 10.1038/s41586-021-03832-5. [DOI] [PubMed] [Google Scholar]
- 94.Minias P., Vinkler M. Selection balancing at innate immune genes: adaptive polymorphism maintenance in toll-like receptors. Mol. Biol. Evol. 2022;39(5) doi: 10.1093/molbev/msac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J., Tang J., Li X., Yan Q., Ma J., Jiang Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-glucan oligosaccharides drive M1 phenotype polarization in murine bone marrow-derived macrophages via activation of MAPKs and NF-κB pathways. Molecules. 2019;24(23):4251. doi: 10.3390/molecules24234251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Fabbro S., Calder P.C., Childs C.E. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc. Nutr. Soc. 2020;79(4):1–11. doi: 10.1017/S0029665120006953. [DOI] [PubMed] [Google Scholar]
- 97.Sun C., Hao B., Pang D., Li Q., Li E., Yang Q., et al. Diverse galactooligosaccharides differentially reduce LPS-induced inflammation in macrophages. Foods. 2022;11(24):3973. doi: 10.3390/foods11243973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Y., van Putten J.P.M., Gilbert M.S., Gerrits W.J.J., Folkerts G., Braber S. Galacto-oligosaccharides as an anti-bacterial and anti-invasive agent in lung infections. Biomaterials. 2022;283 doi: 10.1016/j.biomaterials.2022.121461. [DOI] [PubMed] [Google Scholar]
- 99.Roselli M., Maruszak A., Grimaldi R., Harthoorn L., Finamore A. Galactooligosaccharide treatment alleviates DSS-induced colonic inflammation in Caco-2 cell model. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.862974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Figueroa-Lozano S., Ren C., Yin H., Pham H., Van Leeuwen S., Dijkhuizen L., et al. The impact of oligosaccharide content, glycosidic linkages and lactose content of galacto-oligosaccharides (GOS) on the expression of mucus-related genes in goblet cells. Food Funct. 2020;11(4):3506–3515. doi: 10.1039/d0fo00064g. [DOI] [PubMed] [Google Scholar]
- 101.Jain S., Marotta F., Haghshenas L., Yadav H. Treating leaky syndrome in the over 65s: progress and challenges. Clin. Interv. Aging. 2023;18:1447–1451. doi: 10.2147/CIA.S409801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahmadi S., Razazan A., Nagpal R., Jain S., Wang B., Mishra S.P., et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(7):e9–e21. doi: 10.1093/gerona/glaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rubio C., Lizárraga E., Álvarez-Cilleros D., Pérez-Pardo P., Sanmartín-Salinas P., Toledo-Lobo M.V., et al. Aging in male Wistar rats associates with changes in intestinal microbiota, gut structure, and cholecystokinin-mediated gut-brain axis function. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76(11):1915–1921. doi: 10.1093/gerona/glaa313. [DOI] [PubMed] [Google Scholar]
- 104.Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salazar A.M., Aparicio R., Clark R.I., Rera M., Walker D.W. Intestinal barrier dysfunction: an evolutionarily conserved hallmark of aging. Dis. Model. Mech. 2023;16(4) doi: 10.1242/dmm.049969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J., Jiang Z., Chen C., Yao L., Gao Z., Cheng Z., et al. Age-associated decline in RAB-10 efficacy impairs intestinal barrier integrity. Nat. Aging. 2023;3(9):1107–1127. doi: 10.1038/s43587-023-00475-1. [DOI] [PubMed] [Google Scholar]
- 107.Qi Y., Goel R., Kim S., Richards E.M., Carter C.S., Pepine C.J., et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Dir. Assoc. 2017;18(9):810.e1–810.e4. doi: 10.1016/j.jamda.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mitchell E.L., Davis A.T., Brass K., Dendinger M., Barner R., Gharaibeh R., et al. Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J. Nutr. Health Aging. 2017;21(4):354–361. doi: 10.1007/s12603-016-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guarino M.P.L., Altomare A., Emerenziani S., Di Rosa C., Ribolsi M., Balestrieri P., et al. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients. 2020;12(4):1037. doi: 10.3390/nu12041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lordan C., Thapa D., Ross R.P., Cotter P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11(1):1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walton G.E., van den Heuvel E.G., Kosters M.H., Rastall R.A., Tuohy K.M., Gibson G.R. A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br. J. Nutr. 2012;107(10):1466–1475. doi: 10.1017/S0007114511004697. [DOI] [PubMed] [Google Scholar]
- 112.Bunout D., Hirsch S., Pía de la Maza M., Muñoz C., Haschke F., Steenhout P., et al. Effects of prebiotics on the immune response to vaccination in the elderly. JPEN J. Parenter. Enteral Nutr. 2002;26(6):372–376. doi: 10.1177/0148607102026006372. [DOI] [PubMed] [Google Scholar]
- 113.Schiffrin E.J., Thomas D.R., Kumar V.B., Brown C., Hager C., Van't Hof M.A., et al. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J. Nutr. Health Aging. 2007;11(6):475–479. [PubMed] [Google Scholar]
- 114.Jain P.K., McNaught C.E., Anderson A.D.G., MacFie J., Mitchell C.J. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin. Nutr. 2004;23(4):467–475. doi: 10.1016/j.clnu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Louzada E.R., Ribeiro S.M.L. Synbiotic supplementation, systemic inflammation, and symptoms of brain disorders in elders: a secondary study from a randomized clinical trial. Nutr. Neurosci. 2020;23(2):93–100. doi: 10.1080/1028415X.2018.1477349. [DOI] [PubMed] [Google Scholar]
- 116.Neto J.V., de Melo C.M., Ribeiro S.M. Effects of three-month intake of synbiotic on inflammation and body composition in the elderly: a pilot study. Nutrients. 2013;5(4):1276–1286. doi: 10.3390/nu5041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimizu K., Yamada T., Ogura H., Mohri T., Kiguchi T., Fujimi S., et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit. Care. 2018;22(1):239. doi: 10.1186/s13054-018-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Massot-Cladera M., Rigo-Adrover M.D.M., Herrero L., Franch À., Castell M., Vulevic J., et al. A galactooligosaccharide product decreases the rotavirus infection in suckling rats. Cells. 2022;11(10):1669. doi: 10.3390/cells11101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hasle G., Raastad R., Bjune G., Jenum P.A., Heier L. Can a galacto-oligosaccharide reduce the risk of traveller’s diarrhoea? A placebo-controlled, randomized, double-blind study. J. Travel Med. 2017;24(5) doi: 10.1093/jtm/tax057. [DOI] [PubMed] [Google Scholar]
- 120.Wu M.Z., Sun T.C., Huang Y.W., Wu Y.C., Chen W.J., Chu H.F., et al. Bacillus coagulans BACO-17 alone or in combination with galacto-oligosaccharide ameliorates Salmonella-induced diarrhea and intestinal inflammation. Processes. 2022;10(10):2123. doi: 10.3390/pr10102123. [DOI] [Google Scholar]
- 121.Tran V., Limketkai B.N., Sauk J.S. IBD in the elderly: management challenges and therapeutic considerations. Curr. Gastroenterol. Rep. 2019;21(11):60. doi: 10.1007/s11894-019-0720-7. [DOI] [PubMed] [Google Scholar]
- 122.Taleban S., Colombel J.F., Mohler M.J., Fain M.J. Inflammatory bowel disease and the elderly: a review. J. Crohns Colitis. 2015;9(6):507–515. doi: 10.1093/ecco-jcc/jjv059. [DOI] [PubMed] [Google Scholar]
- 123.Sturm A., Maaser C., Mendall M., Karagiannis D., Karatzas P., Ipenburg N., et al. European Crohn’s and colitis organisation topical review on IBD in the elderly. J. Crohns Colitis. 2017;11(3):263–273. doi: 10.1093/ecco-jcc/jjw188. [DOI] [PubMed] [Google Scholar]
- 124.Wang J., Tsai P.J., Chen P.H., Ye M., Cao H., Guo J., et al. Study on the effect of galacto-oligosaccharide (GOS) in relieving constipation and defecating feces excretion. IOP Conf. Ser. Mater. Sci. Eng. 2020;730(1) doi: 10.1088/1757-899X/730/1/012011. [DOI] [Google Scholar]
- 125.Liu X., Zhang Y., Li W., Yin J., Zhang B., Wang J., et al. Differential responses on gut microbiota and microbial metabolome of 2′-fucosyllactose and galactooligosaccharide against DSS-induced colitis. Food Res. Int. 2022;162(Pt B) doi: 10.1016/j.foodres.2022.112072. [DOI] [PubMed] [Google Scholar]
- 126.Martin-Gallausiaux C., Marinelli L., Blottière H.M., Larraufie P., Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80(1):37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 127.Yandle Z., Coughlan S., Dean J., Hare D., De Gascun C.F. Indirect impact of rotavirus vaccination on viral causes of acute gastroenteritis in the elderly. J. Clin. Virol. 2021;137 doi: 10.1016/j.jcv.2021.104780. [DOI] [PubMed] [Google Scholar]
- 128.Azagra-Boronat I., Massot-Cladera M., Knipping K., Van't Land B., Stahl B., Garssen J., et al. Supplementation with 2'-FL and scGOS/lcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front. Cell. Infect. Microbiol. 2018;8:372. doi: 10.3389/fcimb.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rigo-Adrover M., Saldaña-Ruíz S., van Limpt K., Knipping K., Garssen J., Knol J., et al. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur. J. Nutr. 2017;56(4):1657–1670. doi: 10.1007/s00394-016-1213-1. [DOI] [PubMed] [Google Scholar]
- 130.Lei W.T., Shih P.C., Liu S.J., Lin C.Y., Yeh T.L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9(11):1175. doi: 10.3390/nu9111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagafuchi S., Yamaji T., Kawashima A., Saito Y., Takahashi T., Yamamoto T., et al. Effects of a formula containing two types of prebiotics, bifidogenic growth stimulator and galacto-oligosaccharide, and fermented milk products on intestinal microbiota and antibody response to influenza vaccine in elderly patients: a randomized controlled trial. Pharmaceuticals (Basel) 2015;8(2):351–365. doi: 10.3390/ph8020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao L., Engen P.A., Leusink-Muis T., van Ark I., Stahl B., Overbeek S.A., et al. The combination of 2'-fucosyllactose with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides that enhance influenza vaccine responses is associated with mucosal immune regulation in mice. J. Nutr. 2019;149(5):856–869. doi: 10.1093/jn/nxz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen T.H., Liu C.T., Cheng C.Y., Sue Y.M., Huang N.J., Chen C.H. Oligosaccharides ameliorate acute kidney injury by alleviating cluster of differentiation 44-mediated immune responses in renal tubular cells. Nutrients. 2022;14(4):760. doi: 10.3390/nu14040760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu L., Wu Y., Chen Y., Li R., Wang Z., Li Z., et al. Is acute kidney injury age-dependent in older adults: an observational study in two centers from North China. BMC Geriatr. 2021;21(1):7. doi: 10.1186/s12877-020-01906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maneerat S., Lehtinen M.J., Childs C.E., Forssten S.D., Alhoniemi E., Tiphaine M., et al. Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 2013;2:e44. doi: 10.1017/jns.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fanaro S., Marten B., Bagna R., Vigi V., Fabris C., Peña-Quintana L., et al. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J. Pediatr. Gastroenterol. Nutr. 2009;48(1):82–88. doi: 10.1097/MPG.0b013e31817b6dd2. [DOI] [PubMed] [Google Scholar]
- 137.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fernandes R., do Rosario V.A., Mocellin M.C., Kuntz M.G.F., Trindade E.B.S.M. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: a systematic review. Clin. Nutr. 2017;36(5):1197–1206. doi: 10.1016/j.clnu.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 139.Morel F.B., Dai Q., Ni J., Thomas D., Parnet P., Fança-Berthon P. α-Galacto-oligosaccharides dose-dependently reduce appetite and decrease inflammation in overweight adults. J. Nutr. 2015;145(9):2052–2059. doi: 10.3945/jn.114.204909. [DOI] [PubMed] [Google Scholar]