Highlights
-
•
Early adding radiotherapy to chemo-immunotherapy prolonged PFS to 13.5 months and OS to 31.8 months.
-
•
Receiving radiation dose of over 50 Gy might contribute to superior PFS.
-
•
A low incidence of Grade 3 or above treatment-related adverse events were observed (19%), and no treatment-related death occurred.
Keywords: Esophageal squamous cell carcinoma, Radiotherapy, Immunotherapy, Combination therapy
Abstract
Background
Chemotherapy plus immunotherapy has become the standard first-line treatment of advanced or metastatic esophageal squamous cell carcinoma (ESCC), but median duration of response is only 7.0–8.3 months and progression-free survival (PFS, ∼6 months) is still far from satisfactory. We aim to evaluate whether early involvement of radiotherapy might improve the treatment outcome if objective response to first-line chemo-immunotherapy was observed in locally advanced or metastatic ESCC.
Methods
Patients were retrospectively collected from 3 institutions in China. Patients with histopathologically confirmed diagnoses of locally advanced or metastatic ESCC were identified, who objectively responded to first-line chemo-immunotherapy (complete or partial response, or stable disease) and also received radiotherapy of primary lesions with radiation dose of over 40 Gy, with or without radiotherapy of metastatic lesions before the first disease progression.
Results
A total of 72 eligible patients were identified. With median follow-up duration of 14.6 (range, 7.1–34.8) months, median progression-free survival (PFS) and overall survival (OS) were 13.5 (95 % CI,10.4-NA) months and 31.8 (95 % CI, 23.0-NA) months, respectively. Median duration from initiation of chemo-immunotherapy to radiotherapy was 2.9 (range, 0–15.1) months. Besides lower tumor burden as a significant factor of better treatment outcome, radiation dose ≥ 50 Gy was associated with superior PFS, while OS might be mainly related to tumor response to the induction chemo-immunotherapy. A low incidence of Grade 3 or above treatment-related adverse events were observed (19 %), and no treatment-related death occurred.
Conclusion
Our multi-center retrospective study showed survival benefit brought by early involvement of radiotherapy after first-line chemo-immunotherapy for patients with locally advanced or metastatic ESCC. However, further investigation is warranted in future prospective, controlled trials to assess the value of radio-immunotherapy in advanced or metastatic ESCC.
Introduction
Esophageal cancer is one of the most common malignancies with high cancer-related death rate over the world [1]. Esophageal squamous cell carcinoma (ESCC) accounts for over 90 % of all esophageal cancer cases in China [2]. Immune checkpoint inhibition (ICI) combined with chemotherapy has become the standard first-line therapy in patients with advanced or metastatic ESCC, showing superior overall survival (OS) and progression-free survival (PFS) over chemotherapy alone, but the treatment overcome (PFS ∼ 6 months, OS ∼ 15 months) is still far from satisfactory [3], [4], [5], [6].
Radiotherapy, primarily as a palliative treatment to advanced ESCC which effectively treats esophageal obstruction, may also prolong survival when performed in the presence of well-controlled metastatic disease after initial chemotherapy [7]. Preclinical and clinical evidences in several solid tumor types have indicated the potential of radiotherapy to synergize immunotherapy [8], [9], [10], [11], [12]. Therefore, we presumed that radiotherapy may not simply play a symptom-alleviating role in advanced ESCC in the presence of immunotherapy, but also augment the anti-tumor effect of immunotherapy.
Here, we present a multi-center, retrospective study to evaluate whether early involvement of radiotherapy might improve the treatment outcome if objective response to first-line chemo-immunotherapy was observed in locally advanced or metastatic ESCC.
Material and methods
Study population
Patients were retrospectively collected from 3 institutions (Shanghai Chest Hospital, Ren Ji Hospital, The First Affiliated Hospital of University of Science and Technology of China) in China between January 2019 and June 2022. Patients with histopathologically confirmed diagnoses of locally advanced or metastatic ESCC were identified, who objectively responded to first-line chemo-immunotherapy (complete or partial response, or stable disease) and also received radiotherapy of primary lesions with radiation dose of over 40 Gy, with or without radiotherapy of metastatic lesions before the first disease progression. Patients with early involvement of radiotherapy, that is, who received radiotherapy shortly after completion of first-line chemoimmunotherapy or during maintenance immunotherapy, as long as they received radiotherapy prior to disease progression, were permitted to be included. We excluded patients with other malignancy history within 5 years, and esophageal adenocarcinoma or small cell carcinoma. Additionally, patients who underwent resection of esophageal carcinoma before disease progression were also excluded. This study was ethically approved by the Institutional Committee of Shanghai Chest Hospital.
Radiation therapy
All patients received radiotherapy of primary lesions. Irradiation of metastatic lesions were decided by the treating physicians according to the symptoms or potential risk. Planning CT scans were performed at 3–5 mm per slice with intravenous contrast. Gross tumor volume (GTV) was defined as the primary tumor and involved lymph nodes, according to available resources, including diagnostic CT, 18FDG PET/CT, barium esophagram and endoscopic reports. Clinical target volume was coincident with GTV and no prophylactic irradiation to regional lymph nodes was performed. Planning target volume (PTV) was CTV plus a patient- specific margin to compensate for variation in treatment set-up and internal organ motion. The target volume was treated using Intensity modulated radiotherapy (IMRT) with 6-MV photons.
Evaluation
The clinical evaluation at initial diagnosis included a complete medical history, physical examination, laboratory tests, gastroesophageal endoscopy, chest computed tomography (CT) and/or positron emission tomography (PET)-CT. Oligometastatic esophageal carcinoma was defined as a maximum of 5 metastases and 3 organs involved at initial diagnosis, excluding the presence of diffuse serosal metastases or bone marrow involvement [13]. Efficacy of chemo-immunotherapy was assessed with Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) [3], [4]. A maximum of five lesions total (and a maximum of two lesions per organ) were identified as targe lesions and were recorded and measured at baseline. The largest lesions which could be reproducibly measured would be selected as target lesions. Usually, lymph nodes with a short axis of 15 mm by CT scan would be selected as target lesions. And esophageal primary lesion would be identified as non-target lesion due to its unmeasurable nature and be recorded at baseline. Treatment-related outcomes were analyzed, including PFS and OS. Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Statistical analysis
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. PFS was defined as the time from the first cycle chemo-immunotherapy to the date of development of new lesions, progression of existing lesions, or death. OS was measured as the time from the first cycle chemo-immunotherapy to the date of death from any cause. The PFS and OS were estimated with Kaplan-Meier method, combined with univariate and multivariate cox proportional hazards regression models to identify significant prognostic factors of PFS and OS. The log-rank test was used to assess the difference in PFS and OS among different groups. Statistical analyses were performed with R (Version 4.1.3).
Results
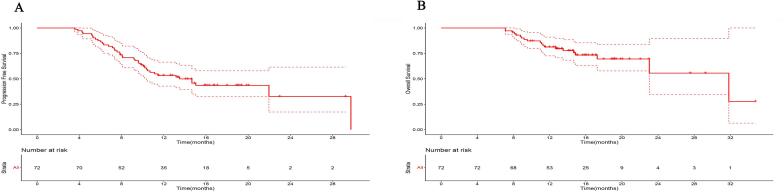
A total of 72 eligible patients were identified (20 oligometastatic and 10 multimetastatic at initial diagnosis), with tumor and treatment-related characteristics shown in Table 1. Median duration from initiation of chemo-immunotherapy to radiotherapy was 2.9 (range, 0–15.1) months, equivalent of receiving 4 cycles of chemo-immunotherapy. Approximately 60 % of patients developed CR or PR to initial chemo-immunotherapy and 66.7 % received radiotherapy to both primary tumor and metastatic lesions. Median duration of total exposure to immunotherapy before disease progression was 3.5 (range, 0.7–18.9) months and the median dose of radiotherapy was 5040 cGy. After median follow-up of 14.6 (range, 7.1–34.8) months, median PFS (Fig. 1A) and OS (Fig. 1B) were 13.5 (95 % CI, 10.4-NA) months and 31.8 (95 % CI, 23.0-NA) months, respectively.
Table 1.
Patient Characteristics (n = 72).
| Characteristics | No. of patients (%) |
|---|---|
| Total patients | 72 (1 0 0) |
| Age, years | |
| Median (range) | 66 (43–82) |
| Gender | |
| Female | 4 (5.6) |
| Male | 68 (94.4) |
| Single/multiple primary lesions | |
| Single | 65 (90.3) |
| Multiple | 7 (9.7) |
| Location of primary tumor | |
| Cervical | 5 (6.9) |
| Upper | 16 (22.2) |
| Middle | 21 (29.2) |
| Lower | 23 (31.9) |
| Multiple primary | 7 (9.7) |
| Tumor length(cm) | |
| Median (range) | 6 (2–22) |
| T stage (AJCC 8th) | |
| T1-T2 | 16 (22.2) |
| T3-T4 | 56 (77.8) |
| N stage (AJCC 8th) | |
| N1 | 12 (16.7) |
| N2 | 38 (52.8) |
| N3 | 22 (30.6) |
| M stage (AJCC 8th) | |
| M0 | 42 (58.3) |
| M1 | 30 (41.7) |
| Tumor burden | |
| Advanced disease* (IVA) | 42 (58.3) |
| Oligometastases | 20 (27.8) |
| Multiple metastases | 10 (13.9) |
| Induction chemo-immunotherapy duration (months) | |
| Median (range) | 2.85 (0–15.1) |
| Response to initial chemo-immunotherapy | |
| Complete response | 1 (1.4) |
| Partial response | 42 (58.3) |
| Stable disease | 29 (40.3) |
| Radiation site | |
| Primary tumour | 24 (33.3) |
| Primary tumour and metastasis | 48 (66.7) |
| Radiation dose to primary tumor | |
| 40–49 Gy | 6 (8.3) |
| 50–59 Gy | 44 (61.1) |
| 60–69 Gy | 22 (30.6) |
*Advanced disease includes unresectable T4 and N2-3 without distant metastases (extra-regional lymph node or visceral metastases).
Fig. 1.
Progression-free survival (PFS) and Overall survival (OS) curves. A. PFS curve for all patients. B. OS curve for all patients. Dash lines showed 95% confidence interval.
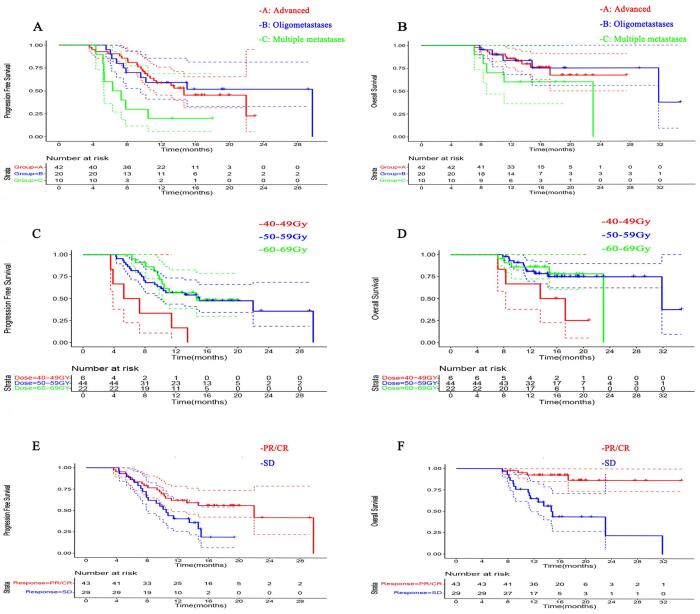
Tumor length < 7 cm (P = 0.028), advanced diseases (P = 0.011), radiation dose ≥ 50 Gy (50–60 Gy, P = 0.011 and ≥ 60 Gy, P = 0.001), and induction chemo-immunotherapy duration ≥ 4 months (P = 0.002) were associated with superior PFS, after adjusting for multiple covariates (Table 2, Fig. 2). However, superior OS seemed mainly associated with lower tumor burden, i.e. tumor length < 7 cm (P = 0.036), and response (CR/PR) to the induction chemo-immunotherapy (P = 0.003), but not influenced by other treatment-related factors (Table 3, Fig. 2).
Table 2.
Univariate and multivariable analysis for factors associated with PFS.
| Variable | No. of patients (%) | mPFS (months) | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|---|
| HR(95 %CI) | p-value | HR(95 %CI) | p-value | |||
| Age, years | ||||||
| <67 | 38(52.8) | 10.5 | Ref. | Ref. | ||
| ≥67 | 34(47.2) | 29.7 | 0.46(0.24–0.91) | 0.024 | 0.50(0.22–1.13) | 0.094 |
| Single/multiple primary lesions | ||||||
| Single | 65(90.3) | 14.7 | Ref. | |||
| Multiple | 7(9.7) | 10.7 | 1.24(0.44–3.50) | 0.684 | ||
| Location of primary tumor | ||||||
| Cervical/Upper thoracic | 24(33.3) | 29.7 | Ref. | |||
| Middle/Lower thoracic | 48(66.7) | 11.4 | 1.64(0.80–3.38) | 0.176 | ||
| Tumor length(cm) | ||||||
| <7 | 41(56.9) | 22.0 | Ref. | Ref. | ||
| ≥7 | 31(43.1) | 9.6 | 2.45(1.28–4.66) | 0.007 | 2.15(1.09–4.25) | 0.028 |
| T stage | ||||||
| T1-2 | 16(22.2) | 14.7 | Ref. | |||
| T3-4 | 56(77.8) | 13.2 | 1.08(0.51–2.29) | 0.837 | ||
| N stage | ||||||
| N1-2 | 50(69.4) | 14.7 | Ref. | |||
| N3 | 22(30.6) | 13.5 | 0.89(0.44–1.79) | 0.738 | ||
| M stage | ||||||
| M0 | 42(58.3) | 14.7 | Ref. | |||
| M1 | 30(41.7) | 10.2 | 1.31(0.69–2.47) | 0.409 | ||
| Tumor burden | ||||||
| Advanced | 42(58.3) | 14.7 | Ref. | |||
| Oligometastases | 20(27.8) | 29.7 | 0.87(0.4–1.89) | 0.724 | 0.85(0.36–2.01) | 0.717 |
| Multiple metastases | 10(13.9) | 6.8 | 3.01(1.33–6.85) | 0.008 | 3.49(1.33–9.13) | 0.011 |
| Induction chemo-immunotherapy duration(months) | ||||||
| <4 | 57(79.2) | 10.5 | Ref. | Ref. | ||
| ≥4 | 15(20.8) | 22.0 | 0.35(0.14–0.92) | 0.033 | 0.17(0.05–0.53) | 0.002 |
| Response to initial chemo-immunotherapy | ||||||
| PR/CR | 43(59.7) | 22.0 | Ref. | Ref. | ||
| SD | 29(40.3) | 10.5 | 2.03(1.07–3.87) | 0.031 | 1.77(0.86–3.65) | 0.122 |
| Radiation site | ||||||
| Primary tumour | 24(33.7) | 13.2 | Ref. | |||
| Primary tumour /metastasis | 48(66.7) | 14.7 | 1.08(0.55–2.14) | 0.819 | ||
| Radiation dose to primary tumor | ||||||
| 40–49 Gy | 6(8.3) | 6.3 | Ref. | |||
| 50–59 Gy | 44(61.1) | 15.0 | 0.25(0.1–0.63) | 0.003 | 0.22(0.07–0.71) | 0.011 |
| 60–69 Gy | 22(30.6) | 14.7 | 0.22(0.08–0.6) | 0.003 | 0.12(0.03–0.43) | 0.001 |
Abbreviations: PR = partial response; CR = complete response; SD = stable disease; HR = hazard ratio; CI = confidence interval.
Fig. 2.
PFS and OS curves for subgroups. A. PFS curves for patients with advanced, oligometastatic and multi-metastatic diseases. B. OS curves for patients with advanced, oligometastatic and multi-metastatic diseases. C. PFS curves for patients receiving different radiation doses to primary tumor. D. OS curves for patients receiving different radiation doses to primary tumor. E. PFS curves for patients developing different responses to initial chemo-immunotherapy. F. OS curves for patients developing different responses to initial chemo-immunotherapy. Dash lines showed 95% confidence interval.
Table 3.
Univariate and multivariable analysis for factors associated with OS.
| Variable | No. of patients (%) | mOS (months) | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|---|
| HR(95 %CI) | p-value | HR(95 %CI) | p-value | |||
| Age, years | ||||||
| <67 | 38(52.8) | 23.0 | Ref. | Ref. | ||
| ≥67 | 34(47.2) | NA | 0.27(0.09–0.81) | 0.019 | 0.79(0.22–2.78) | 0.708 |
| Single/multiple primary lesions | ||||||
| Single | 65(90.3) | 31.8 | Ref. | |||
| Multiple | 7(9.7) | NA | 1.37(0.31–5.99) | 0.680 | ||
| Location of primary tumor | ||||||
| Cervical/Upper thoracic | 24(33.3) | 23.0 | Ref. | |||
| Middle/Lower thoracic | 48(66.7) | 31.8 | 1.23(0.47–3.21) | 0.673 | ||
| Tumor length(cm) | ||||||
| <7 | 41(56.9) | 31.8 | Ref. | |||
| ≥7 | 31(43.1) | NA | 3.11(1.15–8.39) | 0.025 | 3.04(1.07–8.63) | 0.036 |
| T stage | ||||||
| T1-2 | 16(22.2) | 31.8 | Ref. | |||
| T3-4 | 56(77.8) | 23.0 | 0.95(0.33–2.67) | 0.916 | ||
| N stage | ||||||
| N1-2 | 50(69.4) | 31.8 | Ref. | |||
| N3 | 22(30.6) | 23.0 | 0.95(0.36–2.5) | 0.917 | ||
| M stage | ||||||
| M0 | 42(58.3) | NA | Ref. | |||
| M1 | 30(41.7) | 31.8 | 1.26(0.5–3.15) | 0.622 | ||
| Tumor burden | ||||||
| Advanced | 42(58.3) | NA | Ref. | |||
| Oligometastases | 20(27.8) | 31.8 | 0.80(0.25–2.6) | 0.714 | ||
| Multiple metastases | 10(13.9) | 23.0 | 2.27(0.77–6.71) | 0.138 | ||
| Induction chemo-immunotherapy duration(months) | ||||||
| <4 | 57(79.2) | 23.0 | Ref. | |||
| ≥4 | 15(20.8) | NA | 0.51(0.15–1.75) | 0.281 | ||
| Response to initial chemo-immunotherapy | ||||||
| PR/CR | 43(59.7) | NA | Ref. | Ref. | ||
| SD | 29(40.3) | 14.9 | 7.52(2.49–22.69) | 0.000 | 6.54(1.86–23.00) | 0.003 |
| Radiation site | ||||||
| Primary tumour | 24(33.7) | NA | Ref. | |||
| Primary tumour /metastasis | 48(66.7) | 31.8 | 0.90(0.36–2.28) | 0.830 | ||
| Radiation dose to primary tumor | ||||||
| 40–49 Gy | 6(8.3) | 15.4 | Ref. | |||
| 50–59 Gy | 44(61.1) | 31.8 | 0.28(0.09–0.9) | 0.032 | 0.42(0.13–1.37) | 0.149 |
| 60–69 Gy | 22(30.6) | 23.0 | 0.31(0.08–1.15) | 0.079 | 0.34(0.09–1.29) | 0.113 |
Abbreviations: PR = partial response; CR = complete response; SD = stable disease; HR = hazard ratio; CI = confidence interval.
Treatment-related adverse events were summarized in Table 4. Grade 3–4 treatment-related adverse events occurred in 14 of 72 (19 %) patients. The most frequent Grade 3–4 treatment-related adverse events were Neutropenia (9.7 %, 7/72), leukopenia (8.3 %, 6/72), esophagitis (5.6 %, 4/72), pneumonitis (1.4 %, 1/72), thrombocytopenia (1.4 %, 1/72) and hypothyroidism (1.4 %, 1/72). No treatment-related death was reported by the date of publication.
Table 4.
Grade 3–4 treatment-related adverse events occurred during and/or after RT.
| Adverse event | Grade 3 | Grade 4 |
|---|---|---|
| N (%) | ||
| Adverse events related to treatment | 11(15.3) | 3(4.2) |
| Leukopenia | 6(8.3) | 0 |
| Neutropenia | 5(6.9) | 2(2.8) |
| Esophagitis | 3(4.2) | 1(1.4) |
| Pneumonia | 1(1.4) | 0 |
| Thrombocytopenia | 1(1.4) | 0 |
| Hypothyroidism | 1(1.4) | 0 |
Abbreviation: RT = radiation therapy.
Discussion
To our knowledge, this is the first multi-center retrospective study investigating the value of early involvement of radiotherapy after 1st-line chemo-immunotherapy for locally advanced or metastatic ESCC. In the present study, irradiation of over 40 Gy to the primary tumor, delivered shortly after objective response was achieved with initial chemo-immunotherapy, brought a promising survival outcomes in advanced or metastatic ESCC.
In previous phase III randomized trials of comparing ICI combined with chemotherapy versus chemotherapy in ESCC, PFS was 5.8–7.3 months and OS was 12.6–17.2 months for anti–PD-1–based regimens [3], [4], [5], [6], [14], [15]. In particular, patients who got a CR/PR to 1st-line chemo-immunotherapy without receiving radiotherapy before disease progression had a median duration of response (DOR) of only 7.0–8.3 months [ESCORT-1st, Keynote 590]. Comparing to the above historical control, early adding radiotherapy to chemo-immunotherapy prolonged PFS to 13.5 months and OS to 31.8 months in our results. And the survival benefit was more evident in oligometastatic patients (median PFS and OS, 29.7 and 31.8 months). Receiving definitive radiation dose (≥50GY) showed significant benefit in PFS, which however was unable to translate into a significant OS benefit.
In the era of chemotherapy as 1st-line standard treatment for metastatic ESCC, radiotherapy was regarded as a palliative treatment to relieve esophageal obstruction. But retrospective evidence showed that radiotherapy to the primary tumor, even with a palliative dose (20–45 Gy in 5–25 fractions), might also prolong survival if metastatic disease was well controlled after initial chemotherapy, compared to chemotherapy alone (23.3 vs 14 months) [7]. Radiotherapy to oligometastatic disease after initial treatment, especially with biological effective dose (BED10) ≥ 60 Gy, was also proved to benefit in extending survival [16]. Moreover, in a phase II study for patients with oligometastatic ESCC, stereotactic body radiotherapy (SBRT), preferring 48 Gy in 6 fractions, delivered to all oligometastatic lesions after at least 4 cycles of chemotherapy, achieved similar efficacy to radical therapy for locally advanced ESCC (median PFS and OS, 13.3 and 24.6 months) [17]. This efficacy was further confirmed in the recently published phase II ESO-Shanghai13 trial: systemic therapy combined with local treatment (including radiotherapy, surgery, or ablation) of all oligometastatic lesions versus systemic therapy only (chemotherapy, PD-1 monoclonal antibody, or a combination of the two) reached a statistically significant difference in the primary study endpoint of PFS (15.3 and 6.4 months, HR = 0.26 [95 %CI 0.16–0.42]; P < 0.0001) [18]. However, more than half of the patients enrolled in this study received only chemotherapy as their systemic treatment. Therefore, the value of local radiotherapy in the background of immunotherapy in locally advanced or metastatic esophageal cancer should be reevaluated in the era of immunotherapy.
We demonstrated in this multi-center retrospective study that radiotherapy applied with anti-PD-1 regimens exhibited great potential in improving survival of metastatic ESCC, with median OS of 31.8 months. And it might be attributed to the synergistic antitumor effect of radiotherapy combined with ICI, which is quite different from the mechanism of chemo-radiotherapy combination. As several preclinical studies reported, radiotherapy generates an immune response with increased tumor antigen release and presentation, and T-cell infiltration in irradiated tumors [19], [20]. In mouse models, radiotherapy with PD-L1 inhibition synergistically increased antitumor immunity by promoting CD8 + T cell infiltration and reduced the accumulation of tumor-infiltrating myeloid-derived suppressor cells [11], [12]. Additionally, the potential synergy between radiotherapy and PD-1/PD-L1 blockage was also demonstrated in several metastatic tumor types [21], [22], [23], [24]. However, the survival benefit of radiotherapy at the background of 1st-line chemo-immunotherapy in metastatic ESCC merits further validation in future prospective randomized trials.
The optimal radiation dose remains controversial for metastatic ESCC. Guttmann et al conducted an observational cohort study of 12,683 patients with metastatic esophageal cancer using the National Cancer Data Base [25]. Compared with chemotherapy alone, chemotherapy plus definitive dose radiotherapy (5040 cGy) was associated with improved OS (8.3 vs 11.3 months, HR = 0.72, 95 % CI: 0.70–0.74, p ≤ 0.001)), whereas chemotherapy plus palliative dose radiotherapy (<5040 cGy) was associated with slightly inferior outcomes (8.3 vs 7.5 months, HR = 1.10, 95 % CI: 1.07–1.13, p ≤ 0.001). Definitive radiation dose seems more important to those with limited metastatic burden who have a relative better prognosis and can potentially be cured by adding local treatment. As shown by the study of Li et al, compared with BED10 < 60 Gy, BED10 ≥ 60 Gy significantly prolonged median OS (16 vs 10 months, P = 0.033) for metachronous oligometastatic esophageal cancer patients [16]. In the current study, we are the first to find that when combined with immunotherapy, radiation dose ≥ 50 Gy was significantly associated with improved PFS (Fig. 2C) compared to radiation dose < 50 Gy (15.0 vs 6.3 months, p < 0.01), though only a clear trend of benefit instead of significance was observed in OS (Fig. 2D). Since both the potential synergistic rationale and the possible increased toxicities might be accompanied with the combination of radiotherapy and ICIs in ESCC, the optimal radiation dose still deserves further investigation in future large-sample, prospectively-designed studies.
Toxicities would be another concern about the combination treatment. In a phase Ib trial of radiotherapy (60 Gy in 30 fractions) plus camrelizumab as first-line treatment for locally advanced ESCC, 10 (53 %) of 19 patients experienced grade 3–4 adverse events [26]. In EC-CRT-001 trial, a phase II study investigating toripalimab combined with definitive chemoradiotherapy (50.4 Gy in 28 fractions) in locally advanced ESCC, 36 (86 %) of 42 patients developed grade 3–4 adverse events, among which 17 (41 %) experienced non-haematological adverse events, and one (2 %) patient died from treatment-related pneumonitis [27]. Generally, triple combination might correlate with relative higher incidence of toxicities. However, in the present study of advanced or metastatic ESCC, only a low incidence of Grade 3 or above treatment-related adverse events were observed (19 %), and no treatment-related death occurred. In another retrospective study, chemoimmunotherapy combined with radiation therapy was also found to be safe and well tolerated in the treatment of recurrent or metastatic ESCC, with no increased toxicity with the addition of radiation therapy, except for some hematologic complications, which is similar to our findings [28]. But this study focused on the value of radiotherapy combined with immunotherapy in the backline treatment. In contrast, our study focused on the value of radiotherapy before disease progression after first-line use of chemoimmunotherapy in patients with locally advanced or metastatic ESCC. Retrospective collection of toxicity profile with limited sample size might lead to underestimation in some degree and the true toxicity of (chemo)radiotherapy combined with immunotherapy should be evaluated in larger-sample prospective studies.
The limitations of this study include its retrospective nature and small sample size. The possibility of selection bias exists when there were no predefined criteria for receiving radiotherapy which depend on treating physicians. And definitive radiation dose is more likely to be prescribed to limited metastatic burden while palliative dose is usually given with the aim of symptom relief to patients with expectedly poor prognosis. Therefore, efficacy and safety of combination therapy should be validated only in randomized trials that also investigate the optimal timing of combination and appropriate radiation dose. A phase II, multi-center, randomized study will be carried out in our institution to address the above concerns (SCR-ESCC-01 study).
Conclusions
Our multi-center retrospective study showed that early involvement of radiotherapy after first-line chemo-immunotherapy brought encouraging survival benefit for patients with locally advanced or metastatic ESCC. However, further investigation is warranted in future prospective, randomized trials to assess the value of radio-immunotherapy.
CRediT authorship contribution statement
Hui-Hui Hu: Data curation, Investigation, Methodology, Software, Validation, Formal analysis, Writing – original draft. Xin Xu: Data curation, Investigation, Software, Validation, Formal analysis, Resources. Xiao-yang Li: Data curation, Investigation, Software, Resources. Ya Zeng: Data curation, Investigation, Software, Resources. Yue Li: Data curation, Investigation, Software, Resources. Xin-yun Song: Data curation, Investigation, Software, Resources. Xiao-long Fu: Conceptualization, Methodology, Supervision. Xiu-mei Ma: Conceptualization, Methodology, Visualization, Resources, Supervision. Wen Yu: Conceptualization, Methodology, Validation, Visualization, Supervision, Writing – review & editing, Funding acquisition.
Acknowledgements
This work was supported by Shanghai Chest Hospital Project of Collaborative Innovation (YJXT20190202Z).
Declaration
All procedures were performed in compliance with relevant laws and institutional guidelines and have been approved by the appropriate institutional committee(s). The clinical trial registration number is NCT05978193.
Contributor Information
Xiu-Mei Ma, Email: sallyma1203@163.com.
Wen Yu, Email: yuwen@sjtu.edu.cn.
References
- 1.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 4.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic Esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doki Y., Ajani J.A., Kato K., et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 6.Lu Z., Wang J., Shu Y., et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. doi: 10.1136/bmj-2021-068714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hingorani M., Dixit S., Johnson M., et al. Palliative radiotherapy in the presence of well-controlled metastatic disease after initial chemotherapy may prolong survival in patients with metastatic esophageal and gastric cancer. Cancer Res Treat. 2015;47(4):706–717. doi: 10.4143/crt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theelen W., Chen D., Verma V., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 9.Ho A.Y., Barker C.A., Arnold B.B., et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–860. doi: 10.1002/cncr.32599. [DOI] [PubMed] [Google Scholar]
- 10.Arina A., Gutiontov S.I., Weichselbaum R.R. Radiotherapy and immunotherapy for cancer: from “Systemic” to “Multisite”. Clin Cancer Res. 2020;26(12):2777–2782. doi: 10.1158/1078-0432.CCR-19-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong X., Li X., Jiang T., et al. Combined radiotherapy and Anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–1097. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Deng L., Liang H., Burnette B., et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingemans A.C., Hendriks L.E.L., Berghmans T., et al. Definition of synchronous oligometastatic non-small cell lung cancer-a consensus report. J Thorac Oncol. 2019;14(12):2109–2119. doi: 10.1016/j.jtho.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Xu J., Kato K., Raymond E., et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24(5):483–495. doi: 10.1016/S1470-2045(23)00108-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z.X., Cui C., Yao J., et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40(3):277–88 e3. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Wen Y., Xiang Z., et al. Radical radiotherapy for metachronous oligometastasis after initial treatment of esophageal cancer. Radiother Oncol. 2021;154:201–206. doi: 10.1016/j.radonc.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Zhu Z., Chen Y., et al. Phase 2 study of stereotactic body radiation therapy for patients with oligometastatic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;108(3):707–715. doi: 10.1016/j.ijrobp.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q., Chen J., Lin Y., et al. Systemic therapy with or without local intervention for oligometastatic oesophageal squamous cell carcinoma (ESO-Shanghai 13): an open-label, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2024;9(1):45–55. doi: 10.1016/S2468-1253(23)00316-3. [DOI] [PubMed] [Google Scholar]
- 19.Kamrava M., Bernstein M.B., Camphausen K., Hodge J.W. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the three musketeers or just another quixotic combination? Mol Biosyst. 2009;5(11):1262–1270. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demaria S., Coleman C.N., Formenti S.C. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2(6):286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D., Barsoumian H.B., Yang L., et al. SHP-2 and PD-L1 inhibition combined with radiotherapy enhances systemic antitumor Effects in an Anti-PD-1-resistant model of non-small cell lung cancer. Cancer Immunol Res. 2020;8(7):883–894. doi: 10.1158/2326-6066.CIR-19-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z.I., Ho A.Y., McArthur H.L. Combined radiation therapy and immune checkpoint blockade therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2017;99(1):153–164. doi: 10.1016/j.ijrobp.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld J.D., Giobbie-Hurder A., Ranasinghe S., et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022;23(2):279–291. doi: 10.1016/S1470-2045(21)00658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh A.R., Szabolcs A., Allen J.N., et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2(11):1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guttmann D.M., Mitra N., Bekelman J., et al. Improved overall survival with aggressive primary tumor radiotherapy for patients with metastatic esophageal cancer. J Thorac Oncol. 2017;12(7):1131–1142. doi: 10.1016/j.jtho.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W., Yan C., Gao X., et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. 2021;26(7):e1110–e1124. doi: 10.1002/onco.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y., Wen J., Li Q., et al. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): a single-arm, phase 2 trial. Lancet Oncol. 2023;24(4):371–382. doi: 10.1016/S1470-2045(23)00060-8. [DOI] [PubMed] [Google Scholar]
- 28.Wu X., Li Y., Zhang K., et al. Immunotherapy with or without radiotherapy for metastatic or recurrent esophageal squamous cell carcinoma: a real-world study. Clin Transl Radiat Oncol. 2023;38:130–137. doi: 10.1016/j.ctro.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]