Abstract
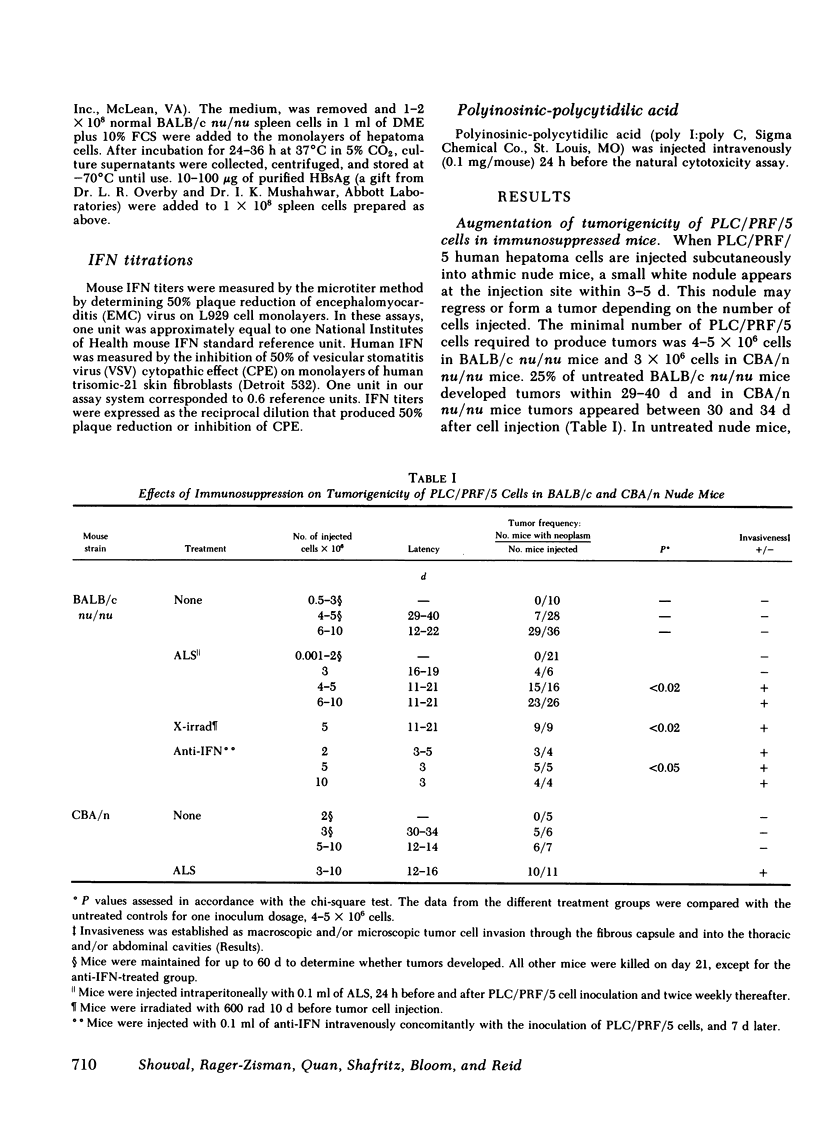
The human hepatoma cell line, PLC/PRF/5, which is persistently infected with hepatitis B virus (HBV), has integrated HBV-DNA, secretes HBV surface antigen (HBsAg), and does not grow readily in congenitally athymic (nu/nu) mice. The present investigation was undertaken to ascertain whether the low tumorigenicity of this cell line was governed by a host immune response and/or was related to expression of HBsAg. Subcutaneous injection of 4-5 X 106 cells into BALB/c nude mice produced localized encapsulated tumors with morphologic features of primary hepatocellular carcinoma in 25% of the animals within 29-40 d. No tumor growth was observed at lower cell inocula. In contrast, SK-HEP-1, an HBV-negative human hepatoma cell line, produced tumors at 1-5 X 106 cells inocula in 66% of the animals. Immunosuppression of mice with antilymphocyte serum (ALS) or irradiation increased tumor incidence in mice inoculated with 1 X 106 PLC/PRF/5 cells to almost 100% and produced local invasiveness. Immunosuppression also reduced the latency, i.e., time to tumor appearance, and increased mean tumor weight. These results suggest that tumorigenicity was limited by the host immune response.
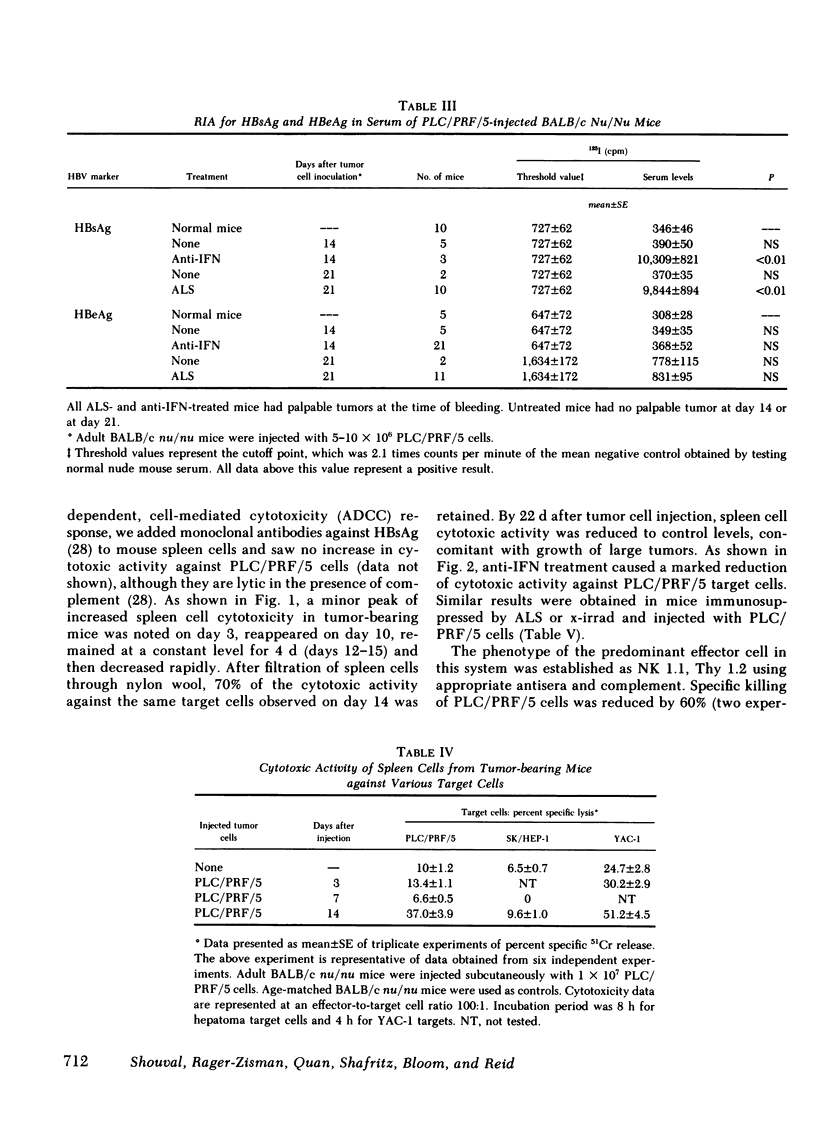
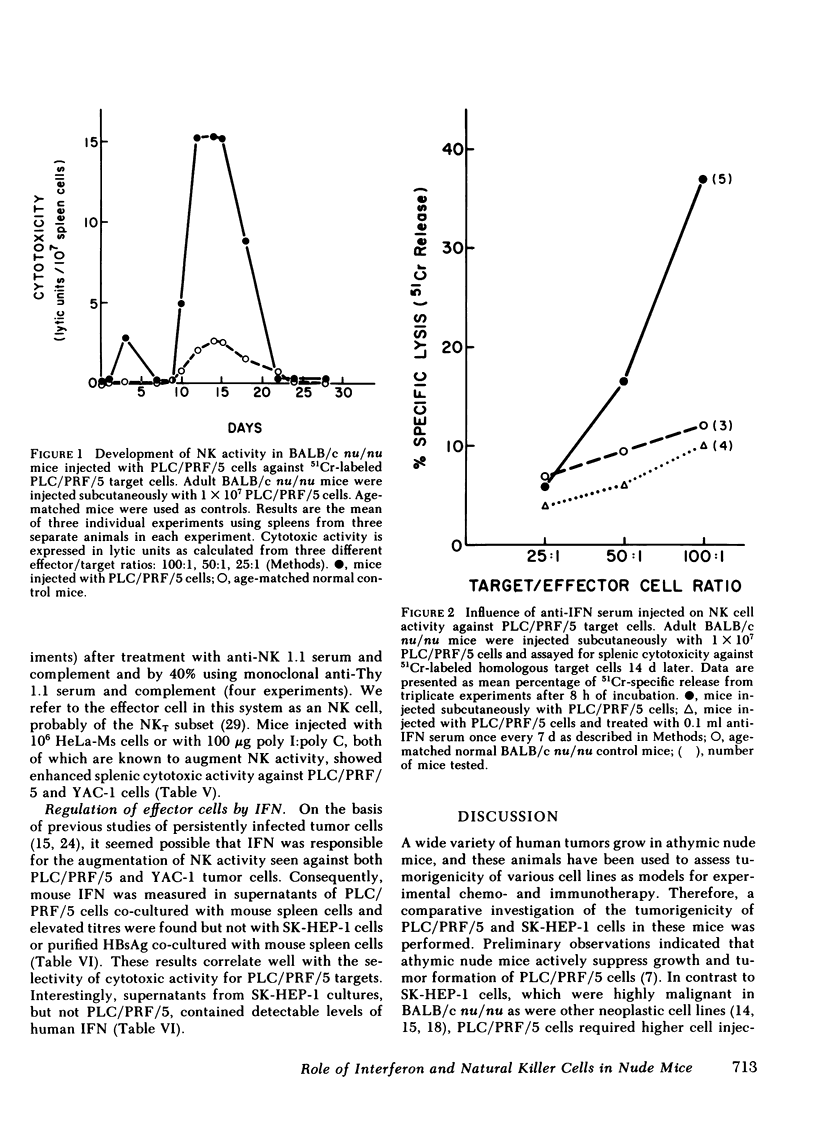
The nature of the response was delineated by treating nude mice challenged with tumor cells with sheep anti-mouse interferon globulin (anti-IFN). When 2 X 106 cells were injected, tumor growth occurred in 75% of anti-IFN-treated mice, whereas controls injected with the same number of cells, but not receiving anti-IFN, failed to develop tumors. The tumors in the anti-IFN-treated mice were highly invasive and the latency period until tumor appearance was reduced to 3-5 d. An inverse correlation was found between susceptibility of the hepatoma cells to natural killer (NK) activity in vitro and resistance to tumor growth in vivo. In vitro cytotoxicity for PLC/PRF/5 cells was eliminated by anti-NK 1.1 and complement, establishing the effector cell as an NK cell. NK cell activity 14 d after inoculation of mice with PLC/PRF/5 cells was augmented against PLC/PRF/5 target cells but not against SK-HEP-1 cells. Treatment of mice with ALS, irradiation, or anti-IFN abolished NK activity against PLC/PRF/5 cells. Co-cultivation of nude mouse spleen cells with PLC/PRF/5 but not with HBsAg or SK-HEP-1 cells induced secretion of murine IFNα. These results suggest that the IFN/NK cell system may play a role in limiting tumorigenicity and invasiveness of HBV-infected human hepatocellular carcinoma cells by a mechanism similar to that found for other cells persistently infected with viruses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassendine M. F., Arborgh B. A., Shipton U., Monjardino J., Aranguibel F., Thomas H. C., Sherlock S. Hepatitis B surface antigen and alpha-fetoprotein secreting human primary liver cell cancer in athymic mice. Gastroenterology. 1980 Sep;79(3):528–532. [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Casali P., Sissons J. G., Buchmeier M. J., Oldstone M. B. In vitro generation of human cytotoxic lymphocytes by virus. Viral glycoproteins induce nonspecific cell-mediated cytotoxicity without release of interferon. J Exp Med. 1981 Sep 1;154(3):840–855. doi: 10.1084/jem.154.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. R., Ruiz-Opazo N., Shouval D., Shafritz D. A. Identification of integrated hepatitis B virus DNA and expression of viral RNA in an HBsAg-producing human hepatocellular carcinoma cell line. Nature. 1980 Jul 31;286(5772):531–533. doi: 10.1038/286531a0. [DOI] [PubMed] [Google Scholar]
- Chin T. W., Hollinger F. B., Rich R. R., Troisi C. L., Dreesman G. R., Melnick J. L. Cytotoxicity by NK-like cells from hepatitis B-immune patients to a human hepatoma cell line secreting HBsAg. J Immunol. 1983 Jan;130(1):173–180. [PubMed] [Google Scholar]
- Chisari F. V., Bieber M. S., Josepho C. A., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. II. Cytotoxic effector cell killing of targets that naturally express hepatitis B surface antigen and liver-specific lipoprotein. J Immunol. 1981 Jan;126(1):45–49. [PubMed] [Google Scholar]
- Dienstag J. L., Bhan A. K. Enhanced in vitro cell-mediated cytotoxicity in chronic hepatitis B virus infection: absence of specificity for virus-expressed antigen on target cell membranes. J Immunol. 1980 Nov;125(5):2269–2276. [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Garfinkel E., Hirschman S. Z., Thung S. N., Panagiotatos T. Immune and enzyme histochemical studies of a human hepatocellular carcinoma cell line producing hepatitis B surface antigen. J Immunol. 1981 Mar;126(3):1085–1089. [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gresser I. On the varied biologic effects of interferon. Cell Immunol. 1977 Dec;34(2):406–415. doi: 10.1016/0008-8749(77)90262-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Salazar F. H., Alexander J. J., Robinson W. S. State of hepatitis B viral DNA in a human hepatoma cell line. J Virol. 1980 Feb;33(2):795–806. doi: 10.1128/jvi.33.2.795-806.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant B., Snippe H., Lizzio E. F., Inman J. K. X-linked genetic control of hapten-polysaccharide-mediated specific immune unresponsiveness in CBA/N mice. J Immunol. 1978 Apr;120(4):1362–1368. [PubMed] [Google Scholar]
- Minato N., Bloom B. R., Jones C., Holland J., Reid L. M. Mechanism of rejection of virus persistently infected tumor cells by athymic nude mice. J Exp Med. 1979 May 1;149(5):1117–1133. doi: 10.1084/jem.149.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Reid L., Cantor H., Lengyel P., Bloom B. R. Mode of regulation of natural killer cell activity by interferon. J Exp Med. 1980 Jul 1;152(1):124–137. doi: 10.1084/jem.152.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S. B., Tam M. R., Nowinski R. C., Emmons S. L. Presence of T cell-associated surface antigens on murine NK cells. J Immunol. 1979 Oct;123(4):1818–1821. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Jones C. L., Holland J. Virus carrier state suppresses tumorigenicity of tumor cells in athymic (nude) mice. J Gen Virol. 1979 Mar;42(3):609–614. doi: 10.1099/0022-1317-42-3-609. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Minato N., Gresser I., Holland J., Kadish A., Bloom B. R. Influence of anti-mouse interferon serum on the growth and metastasis of tumor cells persistently infected with virus and of human prostatic tumors in athymic nude mice. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1171–1175. doi: 10.1073/pnas.78.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I., Frantz M. M., Steinberg A. D. The genetics of the immune response to a synthetic double-stranded RNA in a mutant CBA mouse strain. J Immunol. 1973 May;110(5):1396–1401. [PubMed] [Google Scholar]
- Scullard G. H., Andres L. L., Greenberg H. B., Smith J. L., Sawhney V. K., Neal E. A., Mahal A. S., Popper H., Merigan T. C., Robinson W. S. Antiviral treatment of chronic hepatitis B virus infection: improvement in liver disease with interferon and adenine arabinoside. Hepatology. 1981 May-Jun;1(3):228–232. doi: 10.1002/hep.1840010306. [DOI] [PubMed] [Google Scholar]
- Shouval D., Reid L. M., Chakraborty P. R., Ruiz-Opazo N., Morecki R., Gerber M. A., Thung S. N., Shafritz D. A. Tumorigenicity in nude mice of a human hepatoma cell line containing hepatitis B virus DNA. Cancer Res. 1981 Apr;41(4):1342–1350. [PubMed] [Google Scholar]
- Skelly J., Copeland J. A., Howard C. R., Zuckerman A. J. Hepatitis B surface antigen produced by a human hepatoma cell line. Nature. 1979 Dec 6;282(5739):617–618. doi: 10.1038/282617a0. [DOI] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Szmuness W. Recent advances in the study of the epidemiology of hepatitis B. Am J Pathol. 1975 Dec;81(3):629–650. [PMC free article] [PubMed] [Google Scholar]



