Abstract
In this review article, we explore the interplay between obstructive sleep apnea (OSA) and type 2 diabetes mellitus (T2DM), highlighting a significant yet often overlooked comorbidity. We delve into the pathophysiological links between OSA and diabetes, specifically how OSA exacerbates insulin resistance and disrupts glucose metabolism. The research examines the prevalence of OSA in diabetic patients and its role in worsening diabetes-related complications. Emphasizing the importance of comprehensive management, including weight control and positive airway pressure therapy, the study advocates integrated approaches to improve outcomes for patients with T2DM and OSA. This review underscores the necessity of recognizing and addressing OSA in diabetes care to ensure more effective treatment and better patient outcomes.
Keywords: Obstructive sleep apnea, Type 2 diabetes mellitus, Macrovascular, Mic-rovascular, Weight loss, Positive airway pressure
Core Tip: A comprehensive management strategy for type 2 diabetes mellitus should include the recognition and treatment of obstructive sleep apnea (OSA), one of the most prevalent comorbidities in these patients. Addressing OSA is crucial for optimizing overall diabetes care and patient outcomes.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder, and manifests as a reduction or complete cessation of airflow despite sustained efforts to breathe. During sleep, individuals with OSA experience muscle relaxation, leading to the collapse of soft tissues at the rear of the throat and subsequent obstruction of the upper airway. This sequence of events results in both partial reductions and complete pauses in breathing[1,2].
Diagnosing OSA is a multifaceted process, as outlined by the American Academy of Sleep Medicine (AASM)[1,2]. A conclusive diagnosis requires the presence of signs and symptoms, along with an evaluation of associated medical or psychiatric disorders. Additionally, a crucial criterion for diagnosis is the observation of five or more respiratory events, predominantly obstructive, per hour of sleep during polysomnography (PSG). Alternatively, a diagnosis of OSA can be established when there are ≥ 15 obstructive respiratory events per hour[1,2].
The severity of OSA is stratified by the AASM into three categories: Mild, moderate, and severe. These classifications are based on the respiratory disturbance index (RDI), which measures the number of events per hour. Mild OSA is defined by an RDI of ≥ 5 events/hour, moderate OSA by 5-15 events/hour, and severe OSA by an RDI of ≥ 30 events/hour[1,2].
PATHOPHYSIOLOGY OF OSA AND ITS IMPACT ON TYPE 2 DIABETES MELLITUS
An independent link between OSA severity and insulin resistance in individuals without type 2 diabetes mellitus (T2DM) has been shown by multiple studies[3].
When healthy volunteers were subjected to five hours of intermittent hypoxia (IH) during wakefulness, a significant 17% reduction in insulin sensitivity was observed, notably without a compensatory increase in insulin secretion. In a similar fashion, another experiment demonstrated that while insulin secretion remained unchanged, healthy volunteers exposed to three hours of IH, resulting in an average of 25 desaturations per hour, experienced a notable increase in plasma glucose levels[3]. This suggests a potential threshold for the intensity or duration of hypoxemia that may negatively impact insulin sensitivity.
Sleep fragmentation's role in glucose metabolism has been demonstrated in various human experiments, with stimuli disrupting non-rapid eye movement slow-wave sleep reducing insulin sensitivity by 20% to 25%.
The precise causal mechanisms underlying the association between OSA and glucose metabolism dysregulation remain to be fully elucidated, but it is evident that a complex interplay of multiple pathways is involved. Direct measurements of muscle sympathetic nerve activity in patients with OSA have revealed heightened sympathetic nervous system activity, which persists throughout the day and is attenuated by continuous positive airway pressure (CPAP) therapy. This elevated sympathetic tone adversely impacts the function of endocrine organs, thereby disrupting the hormonal regulation crucial for glucose homeostasis. This includes the inhibition of insulin secretion by the pancreas, increased hepatic glucose production, and altered adipocyte functions in energy balance regulation. These findings underscore the intricate relationship between sleep-disordered breathing and metabolic health, highlighting the need for further research to unravel these complex interactions[4,5].
The sympathetic hyperactivity and parasympathetic withdrawal associated with OSA are likely key mediators of its negative impact on glucose tolerance, with intestinal factors influencing insulin response also linked to this altered autonomic nervous system activity. Measurement of norepinephrine levels can assess systemic sympathetic nervous system activity. Rodent studies indicate Beta-cell dysfunction or death after IH exposure, with partial recovery observed upon cessation[6].
Further research is needed to determine if poorly controlled diabetes worsens OSA and hypoxemia by impacting respiratory control or airway reflexes.
PREVALENCE
The prevalence of OSA has witnessed a surge, aligning with the escalating obesity epidemic. Previous studies, employing outdated diagnostic criteria, indicated a prevalence of moderate to severe OSA ranging from 4% to 7% in middle-aged women and 9% to 14% in middle-aged males[7-11]. However, recent investigations utilizing contemporary diagnostic criteria have unveiled significantly higher rates. In women, the frequency of moderate to severe OSA is now observed at 23%, while in males, it stands at 49%. This shift in prevalence is attributed to the combined impact of elevated obesity rates and the application of advanced polysomnographic techniques and scoring standards.
Furthermore, OSA has been established as a comorbidity of type 2 diabetes, exhibiting higher prevalence among diabetes patients compared to non-diabetics. The Sleep Heart Health Study comprehensively analyzed various United States-based population cohorts, revealing a 33.9% occurrence of mild OSA in individuals with diabetes. Concurrently, the prevalence of moderate-to-severe OSA in the same group was 23.8%. In contrast, among individuals without diabetes, the rates of mild and moderate-to-severe OSA were 27.0% and 15.6%, respectively. Subsequent investigations corroborate that OSA is more prevalent in individuals with diabetes, exceeding an overall prevalence of 50%. Notably, more pronounced OSA significantly increases the likelihood of having diabetes, underscoring a robust association between OSA and diabetes, independent of age and weight. Recent scoring standards emphasize strong correlations between OSA and related health conditions, including T2DM, metabolic syndrome, hypertension, cardiovascular disease, and depression[12].
EFFECT OF OSA ON GLUCOSE CONTROL
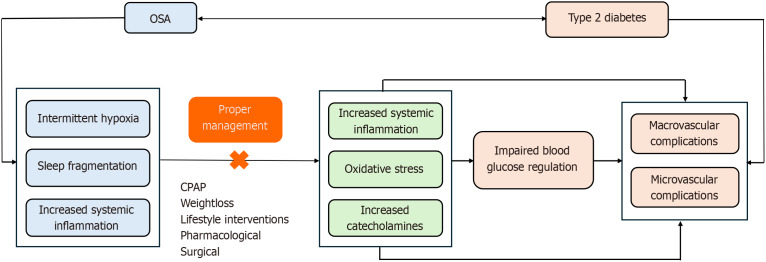
OSA has been consistently linked to disruptions in blood sugar regulation, leading to an increased risk of insulin resistance and type 2 diabetes, as reported in numerous studies. Individuals affected by OSA face a 1.35%-fold increased likelihood of developing T2DM[13]. While the exact pathophysiological and causal connections between OSA and glucose metabolism dysregulation remain elusive, it is likely that multiple mechanistic pathways contribute to this association, as illustrated in Figure 1. The primary mechanisms through which OSA induces metabolic disorders and heightens the risk of T2DM involve IH and sleep fragmentation[3]. These factors elevate sympathetic tone, exerting a detrimental effect on overall body metabolism.
Figure 1.
The relationship between obstructive sleep apnea and type 2 diabetes mellitus. OSA: Obstructive sleep apnea; CPAP: Continuous positive airway pressure.
IH
Prolonged conditions of OSA create an environment of IH, which has been reported to directly harm pancreatic beta cells, hepatocytes, and adipose tissue. These cells play a crucial role in the normal regulation of blood glucose in the body and contribute to reduced insulin sensitivity in body tissues[3,14,15]. The impact of IH includes heightened sympathetic stimulation, increased oxidative stress, and chronic inflammation[14]. Research on experimental animals further supports these findings, indicating that IH leads to elevated levels of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-4, and IL-13. This rise in systemic inflammation exacerbates insulin resistance[15].
Sleep fragmentation
Previous studies indicated that sleep fragmentation reduces insulin sensitivity by 20% to 25%. Sleep fragmentation not only diminishes sensitivity but also elevates the body's sympathetic tone, thereby contributing to impaired blood glucose regulation[14]. Additionally, sleep fragmentation has been linked to heightened systemic inflammatory conditions, leading to increased cytokines such as IL-6 and tumour necrosis factor-α, which further worsen insulin resistance[16].
Sympathetic activation
Individuals with OSA have documented elevated sympathetic activity, and this hyperexcitable sympathetic environment persists in untreated OSA patients[3]. In this state of heightened sympathetic activity, there is an increase in the release of epinephrine and norepinephrine (catecholamines). Catecholamines contribute to an elevation in hepatic glucose production and a reduction in insulin sensitivity, affecting glucose absorption mediated by insulin. Furthermore, sympathetic activity induces lipolysis, raising non-esterified fatty acid levels, thereby exacerbating insulin sensitivity and glucose tolerance[15].
OSA and homeostatic model assessment for insulin resistance
Another assessment of impaired blood glucose regulation is homeostatic model assessment for insulin resistance (HOMA-IR). This is a quantitative method used to assess and to predict the degree of insulin resistance by calculating the values of fasting glucose and insulin concentration [fasting glucose (mg/dL) × fasting insulin (μU/mL)/405][17]. The baseline defining insulin resistance is a HOMA-IR value of ≥ 2.5[18]. However, the HOMA-IR baseline cut-off can be different and varies between ethnic groups[19]. A large multi-center prospective cohort study in the United States revealed a significant association between HOMA-IR and OSA. This study demonstrated an increased incidence of OSA in subjects with higher fasting insulin levels and elevated HOMA-IR[20]. Another large-scale study in China also reported similar results where OSA was associated with HOMA-IR. This study revealed that the administration of CPAP as the primary treatment for OSA led to improvements in insulin resistance conditions[21]. These results suggest that OSA is independently associated with insulin resistance, and that insulin resistance may play a more significant role than hyperglycemia in the pathogenesis of OSA development.
OSA and hemoglobin A1c level
In the European Sleep Apnea Cohort study that involved 6616 participants, Kent et al[22] showed that the prevalence of OSA increased significantly corresponding to the degree of OSA severity. Furthermore, patients with T2DM with severe OSA had higher hemoglobin A1c (HbA1c) levels compared to those without sleep disordered breathing (adjusted mean HbA1c levels 0.72% higher in the former group)[22]. In the restoring insulin secretion study, Mokhlesi et al[23] showed that higher apnea hypopnea index (AHI) was associated with higher HbA1c levels. Finally, analysis from the United Kingdom Biobank study showed that T2DM patients with a high risk of OSA were associated with higher HbA1c levels (high vs low risk for OSA: +0.07% (95%CI: 0.02 to 0.11)[24].
OSA AND MACROVASCULAR COMPLICATIONS
A growing body of evidence indicates a bidirectional relationship between OSA and T2DM[4]. OSA has been demon-strated to induce glycemic excursions in individuals with diabetes, leading to poorer outcomes. Conversely, diabetes is associated with an increased severity of OSA. Our discussion of pertinent studies underscores this complex interplay.
One illustrative study conducted by Alshehri et al[25] revealed that the presence of diabetes mellitus (DM) with abnormal ankle-brachial index was linked to more severe OSA (β = 3.25, P = 0.001) in a cross-sectional investigation involving 3779 participants. It is noteworthy that OSA can exacerbate peripheral arterial disease (PAD) through sympathetic neural activity triggered by hypoxic events associated with OSA. Additionally, the attenuation of vagal function observed in OSA patients may contribute to atherosclerosis by limiting the production of anti-inflammatory cytokines[25].
However, it is essential to acknowledge certain limitations in the study by Alshehri et al[25]. The findings were specific to the Hispanic/Latino population, and the study design posed constraints on drawing causal inferences for the broader population.
Conversely, OSA emerges as an independent risk factor for lower extremity artery disease (LEAD) in T2DM patients. Zhao et al[26] conducted a study revealing that, even after adjusting for risk factors, the mild, moderate, and severe OSA groups exhibited associated risks of 6.83, 27, and 28.07, respectively, for developing LEAD. The observed incremental increase in LEAD risks corresponding to the severity of OSA underscores the imperative for proactive case identification in T2DM patients[26].
Nevertheless, it is crucial to acknowledge several critical weaknesses in the study. The inclusion of only hospitalized subjects, a small sample size, and the cross-sectional nature of the investigation collectively limit the robustness of the conclusions drawn.
Numerous studies have consistently demonstrated that the coexistence of OSA and T2DM is linked to unfavorable atherogenic indices and inflammatory profiles even before the manifestation of micro and macrovascular complications.
In a cross-sectional study involving 529 patients with T2DM, Protasiewicz Timofticiuc et al[27] revealed that the OSA severity, assessed using the STOP-Bang questionnaire with a score > 5 categorized as severe, was associated with a heightened 10-year risk of fatal and non-fatal coronary heart disease. It is noteworthy, however, that being a cross-sectional study, the findings underscore the need for further prospective investigations. Additionally, despite STOP-Bang's 100% sensitivity for AHI ≥ 30, its non-specificity (37%) necessitates the inclusion of more precise methods such as PSG or home sleep apnea testing. Furthermore, it is crucial to recognize that the primary outcome, coronary heart disease risk, serves as a surrogate marker of the actual outcome.
In another cross-sectional study by Hermans et al[28], which involved 305 participants, OSA was found to be associated with adverse atherogenic indices and inflammatory markers. This association translated into a notable difference in the risk of coronary artery disease (CAD) as estimated by the UKPDS Risk Engine's 10-year absolute event predictions, with a 6% increase in the absolute risk of CAD.
Drawing from the collective insights of these studies, it becomes evident that intervening in the prepathogenesis phase, prior to the emergence of visible micro or macrovascular diseases, holds paramount importance. These findings underscore the significance of early identification and targeted interventions to mitigate the long-term cardiovascular risks associated with the coexistence of OSA and DM.
Regarding the risk of macrovascular complications in patients with T2DM and OSA, studies consistently reveal an elevated risk compared to T2DM alone, with a particularly pronounced impact in older adults.
In a large cohort utilizing a general practice database, Adderley et al[29] enrolled 3667 T2DM patients who developed incident OSA during follow-up, matching them with 10450 T2DM patients without OSA. Incident OSA was associated with increased risks of macrovascular complications, including ischemic heart disease [adjusted hazard ratio (aHR): 1.55, 95%CI: 1.26-1.90], stroke/transient ischemic attack (aHR: 1.57, 95%CI: 1.27-1.94), and heart failure (HF) (aHR: 1.67, 95%CI: 1.35-2.06), but not PAD. Additionally, incident OSA was linked to a higher mortality risk (aHR: 1.24, 95%CI: 1.10-1.40). Notably, in T2DM patients with prevalent OSA, the associations with macrovascular complications were attenuated, suggesting potential benefits from better vascular prevention in this group.
In a 25-year follow-up cohort involving over 35000 subjects, Strausz et al[30] found comparable risks of coronary heart disease in patients without OSA and with T2DM (HR: 1.36, 95%CI: 1.12-1.64) compared to those without T2DM. However, the risk was higher in females (HR: 2.01, 95%CI: 1.31-3.07). The association between OSA without T2DM and all-cause mortality was non-significant, while it was significant in OSA patients with T2DM (HR: 1.35, 95%CI: 1.06-1.71).
A prospective cohort study by Labarca et al[31] involving 1476 Chilean patients from the Santiago OSA cohort demonstrated that the coexistence of OSA and DM increased the risk of stroke [odds ratio (OR): 3.44, 95%CI: 1.73-5.59] and incident cardiovascular mortality (HR: 2.37, 95%CI: 1.16-4.82) compared to a reference group without OSA or DM.
In the older adult population, Su et al[32] conducted a multicenter prospective cohort study involving 1113 individuals without a history of cardiovascular disease. They found that OSA and DM increased the risk of major adverse cardiovascular events (MACE) by 64% and hospitalization for unstable angina by 111%. In individuals aged 70 and above, the risks were further heightened (aHR: 1.95) and female gender was associated with a higher risk of MACE (aHR: 2.46), underscoring the importance of recognizing atypical presentations of cardiovascular disease in women. The findings suggest the need for targeted interventions and close monitoring in older adults, particularly those with coexisting OSA and DM.
The impact of OSA in DM patients is also important in acute disease such as acute coronary syndrome (ACS).
In a prospective cohort study involving 804 Chinese patients, Wang et al[33] demonstrated that the concomitant presence of OSA and T2DM synergistically heightened the risk of major adverse cardiovascular and cerebrovascular events (MACCE) in comparison to patients with T2DM alone. Specifically, there was a notable 1.5-fold increase in the risk of MACCE for individuals with ACS [aHR: 2.49 (1.16, 5.35), P = 0.02]. Notably, this elevated risk of MACCE was observed primarily in patients with baseline glucose levels (≥ 5.9 mmol/L) or HbA1c (≥ 6.0%) levels above the median.
Conversely, the study revealed no heightened risk of MACCE in individuals with OSA but without T2DM[33]. These findings underscore the distinctive impact of the coexistence of OSA and T2DM on cardiovascular outcomes, particularly in those with elevated baseline glucose or HbA1c levels.
In addition to exacerbating microvascular and macrovascular complications associated with T2DM, evidence indicates that OSA amplifies complications following percutaneous coronary intervention procedures. A comprehensive meta-analysis, involving 1168 participants, of whom 614 had coexisting OSA, revealed a substantial 118% increased risk of MACE and a 95% increased risk of all-cause mortality post-procedure[34]. Notably, no significant differences in risks were observed regarding MACCEs, hospitalization for HF, reinfarction, stroke, target vessel revascularization, and target lesion revascularization. These findings underscore the importance of recognizing and addressing the impact of OSA in the post-percutaneous coronary intervention setting to optimize patient outcomes (Table 1).
Table 1.
The impact of obstructive sleep apnea on macrovascular complications in type 2 diabetes mellitus
|
Ref.
|
Summary of findings
|
| Aurora and Punjabi[4] | Evidence of a bidirectional relationship between OSA and T2DM; OSA exacerbates glycemic control, and T2DM increases OSA severity |
| Alshehri et al[25] | Presence of DM with abnormal ABI linked to severe OSA; OSA may worsen PAD through hypoxia-induced sympathetic activity |
| Zhao et al[26] | OSA identified as an independent risk factor for LEAD in T2DM patients, with risk levels increasing alongside OSA severity |
| Protasiewicz Timofticiuc et al[27] | OSA severity in T2DM patients associated with increased 10-year risk of coronary heart disease; highlights need for prospective studies and precise diagnostic methods |
| Hermans et al[28] | OSA associated with adverse atherogenic indices and inflammatory markers, indicating a higher risk of CAD |
| Adderley et al[29] | Incident OSA in T2DM patients linked to increased macrovascular complications and mortality, but not PAD |
| Strausz et al[30] | Comparable risks of CHD in T2DM patients with and without OSA; higher risk in females |
| Labarca et al[31] | Coexistence of OSA and DM in the SantOSA cohort showed increased risks of stroke and cardiovascular mortality |
| Su et al[32] | In older adults, coexistence of OSA and DM increased the risk of MACE and hospitalization for unstable angina, especially in females over 70 years |
| Wang et al[33] | Concurrent OSA and T2DM in ACS patients increased the risk of MACCE, particularly with elevated baseline glucose or HbA1c levels |
| Wang et al[34] | OSA in DM patients post-PCI linked to increased risk of MACEs and all-cause mortality |
OSA: Obstructive sleep apnea; T2DM: Type 2 diabetes mellitus; ABI: Ankle-brachial index; PAD: Peripheral arterial disease; LEAD: Lower extremity artery disease; CAD: Coronary artery disease; CHD: Coronary heart disease; MACE: Major adverse cardiovascular events; ACS: Acute coronary syndrome; MACCE: Major adverse cardiovascular and cerebrovascular events; PCI: Percutaneous coronary intervention.
OSA AND MICROVASCULAR COMPLICATIONS
Microvascular complications
Diabetic microvascular complications encompass diabetic peripheral neuropathy (DPN), diabetic nephropathy (DN), and diabetic retinopathy (DR). Cross-sectional studies have indicated a higher prevalence of diabetes related DPN, DN, and DR in patients with both OSA and T2DM compared to those with T2DM alone, even after adjusting for a diverse range of variables[35].
Furthermore, it is associated with various pathophysiological deficits frequently observed in diabetes[36]. There is a direct correlation between sleep-disturbed breathing and both glucose intolerance and insulin resistance[37]. Individuals with OSA have a 35% higher likelihood of developing T2DM (Table 2).
Table 2.
The impact of obstructive sleep apnea on microvascular complications in type 2 diabetes mellitus
|
Ref.
|
Summary of findings
|
| Antza et al[35] | Three months treatment with CPAP significantly increased the eGFR and decreased the serum creatinine levels in patients with OSA. Patients with T2D and OSA who were compliant with CPAP had reduced development of proliferative retinopathy over 5 years and lower decline in eGFR over 2.5 years compared to those non-compliant with CPAP or with mild OSA |
| Tahrani et al[38] | Neuropathy prevalence was higher in patients with OSA than those without (60% vs 27%, P < 0.001) |
| Zhang et al[40] | Parameters of nocturnal hypoxemia are associated with DN and renal function of T2DM patients. The associated parameters increased from two (the average SPO2 and CT90%) to three (ODI, the lowest SPO2, and CT85%) when the severity of DN increased from microalbuminuria to renal insufficiency. The eGFR was independently correlated with ODI (β = -0.172, P = 0.029) and the lowest SPO2 (β = 0.354, P = 0.004) after adjustments |
| Leong et al[41] | Studies that performed multi-variable analysis demonstrated significant associations between OSA (assessed using either AHI or ODI) and DKD in T2DM. This was confirmed by meta-analysis (pooled OR 1.73, 95%CI: 1.13-2.64). There was some evidence to suggest that %TST < 90 may have an association with DKD |
| Tahrani et al[42] | OSA and DN prevalence was 64.3 and 40.2, respectively. DN prevalence was higher in patients with OSA (OSA+) compared with those without OSA (OSA-) (49.3% vs 23.8%, P < 0.001). After adjustment, OSA [OR: 2.64 (95%CI: 1.13-6.16), P = 0.02] remained independently associated with DN. After an average follow-up of 2.5 (0.7) years, eGFR decline was greater in OSA+ compared with OSA- patients [median -6.8% (interquartile range -16.1 to 2.2) vs -1.6% (-7.7 to 5.3%), P = 0.002] |
| Furukawa et al[43] | Microalbuminuria (model 1: OR, 3.41; 95%CI: 1.85-6.40; model 2: OR, 3.69; 95%CI: 1.85-7.59 and model 3: OR, 3.12; 95%CI: 1.45-6.95) and nephropathy (model 1: OR, 4.51; 95%CI: 1.58-15.1; model 2: OR, 7.31; 95%CI: 2.11-31.6 and model 3: OR, 5.23; 95%CI: 1.45-23.8) were derived as factors from all three statistical models and constantly associated with nocturnal intermittent hypoxia only in women |
| Shiba et al[44] | 4% ODI and CT90% in the PDR group were significantly higher than in the NPDR (4% ODI, 7.8 vs 4.9; P = 0.007; CT90%, 2.2 vs 0.8; P = 0.0006). Lowest SPO2 was significantly lower in the PDR group than in the nonproliferative diabetic retinopathy groups (82.4 vs 87.0; P = 0.0006). Logistic regression analysis identified being younger, having a lower value for the lowest SPO2, and a high HbA1c value to be risk factors for PDR (age: odds ratio, 0.90; 95%CI: -0.86 to −0.94; P < .0001; lowest SPO2: OR, 0.93; 95%CI: 0.88 to 0.99; P = 0.02; hemoglobin A1c: odds ratio, 1.00 to 1.69; P = 0.047) |
| Abbas et al[45] | OSA was found to be independently associated with both advanced DR [preproliferative (R2) or proliferative (R3)] (OR = 6.29; 95%CI: 1.08-6.65; P = 0.04) and maculopathy (OR = 12.92; 95%CI: 3.97-4.79; P < 0.001). Moreover, OSA severity was directly related to DR grade (r = 0.5, P < 0.001) |
| Chew et al[46] | Higher AHI (OR 1.04; 95%CI: 1.00, 1.07) and short sleep duration (OR 3.22; 95%CI: 1.18-8.79) were associated with moderate DR. VTDR was associated with moderate OSA (OR 4.73; 95%CI: 1.46-15.31), higher AHI (OR 1.06; 95%CI: 1.02-1.10) and lower minimum SaO2 (OR 0.89; 95%CI: 0.83-0.96). High risk for insomnia was associated with DME (OR 4.01; 95%CI: 1.09-14.73) |
| Chang et al[47] | An association was seen between DR and severe OSA (OR: 2.18, 95%CI: 1.14-4.18, P = 0.019). Proliferative DR was associated with severe OSA versus no DR (OR: 2.40, 95%CI: 1.12-5.14, P = 0.024) and mild nonproliferative DR (OR: 2.87, 95%CI: 1.26-6.55, P = 0.012). Comparing all nonproliferative DR with proliferative DR, proliferative DR and severe OSA were associated (OR: 2.20, 95%CI: 1.03-4.70, P = 0.043), as well as diabetic macular edema and severe OSA (OR: 2.89, 95%CI: 1.58-5.27, P = 0.001) |
| Altaf et al[48] | STDR and OSA prevalence rates were 36.1% and 63.9%, respectively. STDR prevalence was higher in patients with OSA than in those without OSA (42.9% vs 24.1%; P = 0.004). After adjustment for confounders, OSA remained independently associated with STDR (OR, 2.3; 95%CI: 1.1-4.9; P = 0.04). After a median follow-up of 43.0 months, patients with OSA were more likely than patients without OSA to develop preproliferative/proliferative DR (18.4% vs 6.1%; P = 0.02). After adjustment for confounders, OSA remained an independent predictor of progression to preproliferative/PDR (OR, 5.2; 95%CI: 1.2-23.0; P = 0.03). Patients who received CPAP treatment were significantly less likely to develop preproliferative/PDR |
| Kaba et al[49] | OSA prevalence was significantly higher in the DME+ group (70.7%) than DME- group (42.4%, P < 0.05). A significantly lower average minimum SaO2 was noted in OSA+DME+ (81.74%) than OSA+DME- eyes (88.23%, P < 0.05) |
OSA: Obstructive sleep apnea; T2D: Type 2 diabetes; eGFR: Estimated glomerular filtration rate; CPAP: Continuous positive airway pressure; DN: Diabetic neuropathy; DKD: Diabetic kidney disease; DR: Diabetic retinopathy; PDR: Proliferative diabetic retinopathy; DME: Diabetic macular edema; STDR: Sight-threatening diabetic retinopathy; ODI: Oxygen desaturation index; AHI: Apnea-hypopnea index; VTDR: Vision-threatening diabetic retinopathy.
OSA and diabetic polyneuropathy
In a landmark study by Tahrani et al[38], several key findings regarding the association between OSA and DPN were observed. Firstly, even mild OSA was linked to DPN in T2DM patients, and this association persisted after adjusting for traditional risk factors. Additionally, OSA showed associations with AHI and nadir nocturnal oxygen saturations. Thirdly, nitrosative and oxidative stress were found to correlate with nocturnal hypoxemia, contributing to DPN by reducing nerve perfusion, impairing vascular reactivity of epineural arterioles, and damaging peripheral nervous system structures. Lastly, abnormal microvascular blood flow regulation was demonstrated in T2DM patients, with reduced responses to nitric oxide, particularly evident upon stimulation with sodium nitroprusside and acetylcholine.
The same research group conducted a cross-sectional study involving T2DM patients from a United Kingdom secondary care hospital, revealing an association between elevated AHI, Poly ADP ribose Polymerase activation (a key factor in the pathogenesis of DPN), and a reduced intraepidermal nerve fibers density[39]. Additionally, OSA was linked to an increased risk of diabetic foot ulcers (OR 3.34, 95%CI: 1.19-9.38, P = 0.022). Interestingly, no significant difference in diabetic foot ulcer prevalence was observed between mild and moderate-to-severe OSA.
In contrast, Zhang et al[40] did not identify a significant association between OSA and DPN in T2DM patients. Parameters of OSA, including AHI, oxygen desaturation index (ODI), lowest and average peripheral oxygen saturation, and cumulative time spent at SPO2 of < 90% and < 85% (CT90% and CT85%), showed no association with DPN. Thus, conflicting evidence regarding OSA and DPN highlights a gap in the evidence for patient stratification, specifically identifying those most vulnerable to DPN among T2DM patients with OSA.
OSA and DN
OSA is anticipated to impact diabetic kidney disease (DKD) development and progression through multiple pathways. It induces IH, elevating oxidative stress and activating inflammatory pathways, leading to endothelial dysfunction. Disrupted sleep in OSA results in sporadic changes in kidney blood flow due to sympathetic nervous system activation, causing insufficient blood supply and subsequent kidney damage. Case studies, including one reporting complete remission of proteinuria with bi-level positive airway pressure (PAP) therapy, suggest OSA's association with secondary focal glomerulosclerosis[41]. Additionally, renin angiotensin aldosterone system activation in OSA patients may contribute to the onset and progression of DN[42].
In a prospective cohort study with a 2.5-year follow-up, individuals with T2DM and OSA experienced a greater decline in estimated glomerular filtration rate (eGFR), compared to those with T2DM alone[42]. Baseline OSA and AHI were independent predictors of study-end eGFR after adjusting important confounders. Importantly, even mild OSA was associated with DN and worsening eGFR.
The study by Furukawa et al showed that Japanese women with T2DM and five or more episodes of 3% ODI per hour had an increased risk of developing microalbuminuria, even after adjusting for traditional confounders[43]. ODI levels were assessed using a pulse oximeter. Another cross-sectional study in 880 patients with T2DM found that CT90%, representing the cumulative time of oxygen saturation (SPO2) below 90%, was linked to DN, microalbuminuria, and macroalbuminuria[40]. Additionally, average SPO2 and CT90% were strongly associated with microalbuminuria. While there was no correlation between AHI and microalbuminuria, a significant association was observed between AHI and macroalbuminuria, as well as renal insufficiency.
OSA and DR
Cross-sectional studies consistently link OSA with DR, despite variations in research populations. These investigations consistently demonstrate a correlation between OSA, hypoxemia, and the occurrence of DR. Multiple cross-sectional studies underscore this association.
In Japanese patients undergoing vitreous surgery, those with proliferative DR (PDR) exhibited elevated ODI compared to those without PDR. Moreover, higher levels of oxygen saturation were observed to offer protection against the development of PDR, even after adjusting for factors such as age, HbA1c levels, and hypertension[44].
In a cross-sectional study with 110 patients diagnosed with T2DM, including 66 with OSA, there was a six-fold increased risk for advanced DR [preproliferative (R2) and proliferative (R3)] in OSA+ patients (OR 6.29; 95%CI: 1.08-6.65; P = 0.04)[45]. Additionally, they were also at an elevated risk for maculopathy (OR 12.92; 95%CI: 3.97-4.79; P < 0.001).
Another cross-sectional study, which recruited 92 diabetic patients from retinal clinics, found that a higher AHI was associated with moderate DR (OR 1.04; 95%CI: 1.00-1.07). Furthermore, moderate OSA (OR 4.73; 95%CI: 1.46-15.31) and a higher AHI (OR 1.06; 95%CI: 1.02-1.10) were associated with vision threatening DR[46].
In a retrospective chart review involving 317 diabetes patients, of which 95% were T2DM patients, OSA was linked to an increased risk for both PDR (OR: 2.20, 95%CI: 1.03-4.70, P = 0.043) and diabetic macular edema (DME) (OR: 2.89, 95%CI: 1.58-5.27, P = 0.001)[47].
Consistent findings have also emerged in prospective studies. In a study involving 230 patients recruited from diabetes clinics in two United Kingdom hospitals with a median follow-up of 42 months, it was observed that OSA patients were more than five times more likely to develop pre-/PDR (OR 5.2, 95%CI: 1.2-23.0, P = 0.03) and 2.5 times more likely to develop diabetic maculopathy (OR 2.6, 95%CI: 1.2-5.8, P = 0.01)[48]. Fortunately, CPAP therapy may prevent the progression of DR. The significance of CPAP is further highlighted by a prospective study demonstrating that adherence to CPAP in OSA patients with DME prevented the progression of DR[49]. This study provides valuable insights into the importance of combining anti-vascular endothelial growth factor treatment with CPAP in OSA patients with DME.
MANAGEMENT
The management of OSA and T2DM is summarized in Table 3.
Table 3.
The impact of obstructive sleep apnea management on type 2 diabetes mellitus control
|
Ref./study
|
Summary of findings
|
| The Sleep AHEAD study[50] | Regular exercise on its own helps improve OSA, and the more someone exercises, the lower the chances of having moderate to severe OSA-odds decrease with 1-2 hours per week (0.62), 3-6 hours per week (0.39), and at least 7 hours per week (0.31) compared to those who do not exercise vigorously |
| Shechter et al[51] | For individuals with T2DM and OSA, weight loss from a lifestyle intervention is more crucial for improving glycemic control than reductions in OSA severity |
| MIMOSA[52] | The MLG group exhibited improved insulin and HOMA–IR profiles compared to the SCG counterpart |
| The Interdisciplinary Weight Loss and Lifestyle Intervention for OSA (INTERAPNEA)[53] | Mediterranean lifestyle group demonstrated the potential benefits of interdisciplinary weight reduction and lifestyle therapies in improving the severity, symptoms, and cardiometabolic profiles, including T2DM, of individuals with OSA, including improvements in blood pressure and in glucose and lipid metabolism. In the intervention group, there were lower glucose and insulin levels, and lower HOMA-IR |
| The SCALE sleep apnea[55] | 3.0 mg liraglutide reduced AHI, bodyweight, HbA1c, and SBP significantly compared to the placebo group |
| Herth et al[63] | The beneficial impact of CPAP on HbA1c levels was influenced by the duration of CPAP use per night, while it was not affected by baseline AHI, BMI, or diabetes duration |
| Zamarrón et al[65] | Participants in the CPAP treatment arm, with good adherence, exhibited a greater reduction in UACR [MD, -10.56% (95%CI: -19.06 to -2.06); P = 0.015], along with improvements in glycemic control and insulin resistance |
| Ding et al[59] | Surgical weight loss improves T2DM and OSA control. Mini GBP is associated with highest remission rate while BPD is associated with sustained long term remission |
OSA: Obstructive sleep apnea; T2DM: Type 2 diabetes mellitus; MLG: Mediterranean lifestyle group; HOMA-IR: Homeostatic model assessment for insulin resistance; AHI: Apnea hypopnea index; RDI: Respiratory disturbance index; CPAP: Continuous positive airway pressure; SBP: Systolic blood pressure; UACR: Urine albumin creatinine ratio; GBP: Gastric bypass; BPD: Biliopancreatic diversion without duodenal switch.
Weight management
Weight loss is crucial in the management of both T2DM and OSA, given the prevalence of overweight and obesity in T2DM patients. This dual approach can significantly enhance the status of both conditions. Lifestyle interventions, weight-loss medications, and bariatric surgery are outlined here as beneficial strategies for effectively managing T2DM and OSA.
The Sleep AHEAD study, focusing on patients with overweight or obesity, T2DM, and OSA, demonstrated that a lifestyle intervention resulted in significant weight loss and a notable reduction in AHI at the 1-year follow-up[50]. Notably, exercise independently improves OSA, and there is a dose-dependent correlation between exercise and a lower prevalence of OSA. Compared to non-vigorous exercisers, the likelihood of moderate to severe OSA decreased with increasing exercise: Adjusted ORs were 0.62 for 1 to 2 hours per week, 0.39 for 3 to 6 hours per week, and 0.31 for at least 7 hours per week. The Sleep AHEAD study also found that implementing a rigorous lifestyle modification program decreased the AHI and caused a more significant decrease in HbA1c levels compared to receiving routine diabetic support and education[50].
Shechter et al[51] conducted a study to assess the impact of an intensive lifestyle intervention (ILI) on OSA severity during rapid-eye movement (REM) sleep and its association with changes in glycemic control over time in individuals with T2DM and OSA. The randomized controlled trial (RCT) involved 264 overweight or obese adults with T2DM and OSA, comparing an ILI focused on weight loss with a diabetes support and education (DSE) control group. The ILI significantly reduced OSA severity during both REM and non-REM sleep compared to the DSE group. However, changes in glycemic control were strongly associated with weight changes rather than OSA severity during REM or non-REM sleep. The findings suggest that, for individuals with T2D and OSA, weight loss from a lifestyle intervention is more crucial for improving glycemic control than reductions in OSA severity[51].
In the MIMOSA study, a 12-month, single-center, single-blind, parallel-group (1:1), randomized, controlled clinical trial, Georgoulis et al[52] compared a standard care group (SCG) with a Mediterranean diet group (MDG) and a Mediterranean lifestyle group (MLG). While both MDG and MLG groups focused on dietary intervention, the latter group also aimed to increase physical activity and achieve an optimal sleep duration and quality. While not specific to T2DM patients, the MLG group exhibited improved insulin and HOMA-IR profiles compared to the SCG counterpart.
Another landmark lifestyle intervention study, The Interdisciplinary Weight Loss and Lifestyle Intervention for OSA by Carneiro-Barrera et al[53] demonstrated the potential benefits of interdisciplinary weight reduction and lifestyle therapies in improving the severity, symptoms, and cardiometabolic profiles, including T2DM, of individuals with OSA. Importantly, they noted significant improvements in blood pressure and in glucose and lipid metabolism. Specifically, in the intervention group, there were lower glucose and insulin levels, and lower HOMA-IR.
Weight-reducing medications used in the treatment of diabetes, such as GLP1 receptor agonists (GLP1-RA) such as liraglutide and semaglutide, along with newer agents like tirzepatide, have demonstrated a superior weight loss effect and potentially improving OSA status in T2DM patients[54]. The SCALE sleep apnea RCT which included 276 (134 liraglutide vs 142 placebo) obese patients with moderate to severe OSA but without T2DM showed that 3.0 mg liraglutide reduced AHI, bodyweight, HbA1c, and systolic blood pressure significantly compared to the placebo group[55]. Future clinical trials will answer the potential utility of tirzepatide in treating OSA, as well as assessing the benefits of liraglutide for patients with both OSA and T2DM[56,57]. Bariatric surgery has been recognized as a successful intervention for treating diabetes and OSA, resulting in substantial enhancements in OSA and T2DM[3].
A meta-analysis conducted by Oweidat et al[58], encompassing 2310 patients from 32 studies, revealed that bariatric surgery resulting in significant weight loss led to a high OSA remission rate (65%, 95%CI: 0.54, 0.76) and improvement in OSA parameters, including AHI and RDI. Despite these promising outcomes, the presence of considerable heterogeneity and potential publication bias introduces uncertainty to their findings.
In a network meta-analysis of RCTs by Ding et al[59], investigating the most effective bariatric surgeries for obese T2DM patients, it was found that, compared to non-surgical treatment, all types of surgical weight loss interventions were associated with a higher T2DM remission rate. However, the mini gastric bypass demonstrated the highest remission rate, while biliopancreatic diversion without duodenal switch was linked to sustained long-term T2DM remission. Taken together, we can conclude that surgical weight loss improves T2DM and OSA control.
PAP
PAP serves as the primary therapy for symptomatic OSA at any severity level[60,61]. PAP devices, worn over the nose or both the nose and mouth, deliver pressure to the airway, preventing collapse during inspiration. PAP achieves normalization of the AHI in over 90% of patients while in use. The degree of benefit depends on adherence, with greater hours of nightly use correlating with improved symptom relief and blood pressure reduction. Adequate adherence, often defined as using the device for at least 4 hours per night for at least 5 nights per week, is a common criterion for continued reimbursement, according to the Center for Medicare and Medicaid Services. A 2019 report on over 2.6 million patients initiating PAP therapy between 2014 and 2017 revealed that 75% achieved this level of adherence within the first 90 days, with an overall usage rate of 93% of nights and a mean duration of 6.0 (2.0) hours per night[62].
In a recent systematic review and meta-analysis conducted by Herth et al[63], the benefits of CPAP on glucose metabolism in OSA patients with T2DM were investigated. The analysis included 11 RCTs with a total of 964 patients. While CPAP treatment did not show improvement in fasting blood glucose, fructosamine, or HOMA-IR, it demonstrated positive effects on HbA1c levels (MD: -0.24%, 95%CI: -0.43, -0.06%, P = 0.001) and 24 hours plasma glucose (MD -0.60 mmol·L−1, 95%CI: -0.72, -0.47 mmol·L−1, P = 0.001), compared to inactive controls. Notably, the beneficial impact of CPAP on HbA1c levels was influenced by the duration of CPAP use per night, while it was not affected by baseline AHI, body mass index, or diabetes duration.
Recent clinical trial results on this topic by Aurora et al[64] have been disappointing. The trial included 184 T2DM patients with moderate to severe OSA, randomized to either 3 months of PAP therapy with lifestyle counseling or counseling alone. Glucose variability, monitored through continuous glucose monitoring and self-monitoring of blood glucose, did not show significant differences in primary and secondary endpoints between the two groups overall. However, PAP therapy exhibited a noticeable effect on the standard deviation of glucose levels in female participants. Additionally, PAP treatment led to lower post-dinner glucose levels by 20.1 mg/dL (P < 0.01) and bedtime glucose levels by 34.6 mg/dL (P < 0.01).
In a RCT investigating the influence of CPAP on urine albumin-to-creatinine ratio (UACR) in individuals with DKD and OSA, Zamarrón et al[65] observed that participants in the CPAP treatment arm, with good adherence, exhibited a greater reduction in UACR [MD, -10.56% (95%CI, -19.06 to -2.06); P = 0.015], along with improvements in glycemic control and insulin resistance.
Given the conflicting evidence, further studies are warranted to identify which OSA patients with T2DM will derive significant benefits from PAP therapy.
FUTURE DIRECTIONS
In the advancing era of artificial intelligence in healthcare, machine learning is revolutionizing disease phenotyping[66,67]. Through disease phenotyping, we can appreciate the heterogeneity of clinical presentations in OSA patients. This is particularly impactful in OSA management, where it enables patient-specific treatment strategies, especially in T2DM. However, further research is essential to understand OSA's relationship with other diabetes forms, including type 1 diabetes mellitus, latent autoimmune diabetes in adults, and maturity-onset diabetes of the young. The summary of our review article is depicted in the Figure 1.
CONCLUSION
This minireview highlights the significant yet often overlooked link between OSA and T2DM, emphasizing the importance of integrated management strategies. Recognizing and treating OSA in diabetes patients is crucial, as it can significantly impact insulin resistance and diabetes outcomes. Effective management should include routine OSA screening and tailored interventions such as weight management and PAP therapy. Future research is essential to optimize treatment protocols and improve quality of life for these patients.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors who contributed their efforts in this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: Indonesia
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade C
Novelty: Grade B
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Romani A, United States S-Editor: Qu XL L-Editor: Webster JR P-Editor: Che XX
Contributor Information
Eric D Tenda, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia; Head of Research Group Artificial Intelligence and Digital Health, Indonesian Medical Education and Research Institute, Faculty of Medicine University of Indonesia, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
Joshua Henrina, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia. joshuahenrina@gmail.com.
Jin H Cha, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
Muhammad R Triono, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
Ersananda A Putri, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
Dahliana J Aristy, Division of Respirology and Critical Care, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
Dicky L Tahapary, Division of Endocrinology, Metabolism and Diabetes, Department of Internal Medicine, Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital, DKI Jakarta, Jakarta Pusat 10430, Indonesia.
References
- 1.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 2.Troester MM, Quan SF, Berry RB, Plante DT, Abreu AR, Alzoubaidi M, Bandyopadhyay A, DelRosso L, Ebben M, Kwon Y, Mao MM, Munir SS, Pressman MR, Rodriguez AJ, Ryals S, So JY, Vaughn BV, Thomas SM (eds) American Academy of Sleep Medicine - The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications. Available from: https://www.aasm.org/
- 3.Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017;152:1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–338. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 5.Greco C, Spallone V. Obstructive Sleep Apnoea Syndrome and Diabetes. Fortuitous Association or Interaction? Curr Diabetes Rev. 2015;12:129–155. doi: 10.2174/1573399811666150319112611. [DOI] [PubMed] [Google Scholar]
- 6.Fang Y, Zhang Q, Tan J, Li L, An X, Lei P. Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and protective effects of antioxidant intervention. Nutr Diabetes. 2014;4:e131. doi: 10.1038/nutd.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 12.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig. 2018;9:991–997. doi: 10.1111/jdi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reutrakul S, Mokhlesi B. Can Long-term Treatment of Obstructive Sleep Apnea With CPAP Improve Glycemia and Prevent Type 2 Diabetes? Diabetes Care. 2020;43:1681–1683. doi: 10.2337/dci20-0014. [DOI] [PubMed] [Google Scholar]
- 14.Mok Y, Tan CW, Wong HS, How CH, Tan KL, Hsu PP. Obstructive sleep apnoea and Type 2 diabetes mellitus: are they connected? Singapore Med J. 2017;58:179–183. doi: 10.11622/smedj.2017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song SO, He K, Narla RR, Kang HG, Ryu HU, Boyko EJ. Metabolic Consequences of Obstructive Sleep Apnea Especially Pertaining to Diabetes Mellitus and Insulin Sensitivity. Diabetes Metab J. 2019;43:144–155. doi: 10.4093/dmj.2018.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis. 2015;7:1343–1357. doi: 10.3978/j.issn.2072-1439.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son DH, Ha HS, Park HM, Kim HY, Lee YJ. New markers in metabolic syndrome. Adv Clin Chem. 2022;110:37–71. doi: 10.1016/bs.acc.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 18.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–464. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 19.Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16:102581. doi: 10.1016/j.dsx.2022.102581. [DOI] [PubMed] [Google Scholar]
- 20.Huang T, Sands SA, Stampfer MJ, Tworoger SS, Hu FB, Redline S. Insulin Resistance, Hyperglycemia, and Risk of Developing Obstructive Sleep Apnea in Men and Women in the United States. Ann Am Thorac Soc. 2022;19:1740–1749. doi: 10.1513/AnnalsATS.202111-1260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Liang C, Zou J, Yi H, Guan J, Gu M, Feng Y, Yin S. Interaction between obstructive sleep apnea and short sleep duration on insulin resistance: a large-scale study : OSA, short sleep duration and insulin resistance. Respir Res. 2020;21:151. doi: 10.1186/s12931-020-01416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent BD, Grote L, Ryan S, Pépin JL, Bonsignore MR, Tkacova R, Saaresranta T, Verbraecken J, Lévy P, Hedner J, McNicholas WT. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146:982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 23.Mokhlesi B, Tjaden AH, Temple KA, Edelstein SL, Sam S, Nadeau KJ, Hannon TS, Manchanda S, Mather KJ, Kahn SE, Ehrmann DA, Van Cauter E RISE Consortium. Obstructive Sleep Apnea, Glucose Tolerance, and β-Cell Function in Adults With Prediabetes or Untreated Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care. 2021;44:993–1001. doi: 10.2337/dc20-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan X, Benedict C. Sleep characteristics and HbA1c in patients with type 2 diabetes on glucose-lowering medication. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshehri MM, Alqahtani AS, Alenazi AM, Aldhahi M, Alothman S, Gray C, Alqahtani B, Khunti K, Kluding P. Associations between ankle-brachial index, diabetes, and sleep apnea in the Hispanic community health study/study of Latinos (HCHS/SOL) database. BMC Cardiovasc Disord. 2020;20:118. doi: 10.1186/s12872-020-01402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Yu X, Xin S, Zhang W, Zhang X, Ji L. Correlation between OSAHS and Early Peripheral Atherosclerosis Indices in Patients with Type 2 Diabetes Mellitus in China: A Cross-Sectional Inpatient Study. J Diabetes Res. 2021;2021:6630020. doi: 10.1155/2021/6630020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protasiewicz Timofticiuc DC, Vladu IM, Ștefan AG, Clenciu D, Mitrea A, Pădureanu V, Efrem IC, Diaconu ID, Turcu A, Țenea-Cojan TȘ, Hâncu AM, Forțofoiu M, Mirea Munteanu O, Moța M. Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. J Clin Med. 2022;11 doi: 10.3390/jcm11154403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermans MP, Ahn SA, Mahadeb YP, Rousseau MF. Sleep apnoea syndrome and 10-year cardiovascular risk in females with type 2 diabetes: relationship with insulin secretion and insulin resistance. Diabetes Metab Res Rev. 2013;29:227–234. doi: 10.1002/dmrr.2387. [DOI] [PubMed] [Google Scholar]
- 29.Adderley NJ, Subramanian A, Toulis K, Gokhale K, Taverner T, Hanif W, Haroon S, Thomas GN, Sainsbury C, Tahrani AA, Nirantharakumar K. Obstructive Sleep Apnea, a Risk Factor for Cardiovascular and Microvascular Disease in Patients With Type 2 Diabetes: Findings From a Population-Based Cohort Study. Diabetes Care. 2020;43:1868–1877. doi: 10.2337/dc19-2116. [DOI] [PubMed] [Google Scholar]
- 30.Strausz S, Havulinna AS, Tuomi T, Bachour A, Groop L, Mäkitie A, Koskinen S, Salomaa V, Palotie A, Ripatti S, Palotie T. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open. 2018;8:e022752. doi: 10.1136/bmjopen-2018-022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labarca G, Dreyse J, Salas C, Schmidt A, Rivera F, Letelier F, Jorquera J. Risk of mortality among patients with moderate to severe obstructive sleep apnea and diabetes mellitus: results from the SantOSA cohort. Sleep Breath. 2021;25:1467–1475. doi: 10.1007/s11325-020-02283-y. [DOI] [PubMed] [Google Scholar]
- 32.Su X, Li JH, Gao Y, Chen K, Gao Y, Guo JJ, Shi M, Zou X, Xu W, Zhao LB, Wang H, Wang Y, Liu J, Xu H, Kong X, Lin J, Qian X, Han J, Liu L. Impact of obstructive sleep apnea complicated with type 2 diabetes on long-term cardiovascular risks and all-cause mortality in elderly patients. BMC Geriatr. 2021;21:508. doi: 10.1186/s12877-021-02461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Fan J, Du Y, Ma C, Ma X, Nie S, Wei Y. Clinical significance of obstructive sleep apnea in patients with acute coronary syndrome in relation to diabetes status. BMJ Open Diabetes Res Care. 2019;7:e000737. doi: 10.1136/bmjdrc-2019-000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Li X, Tang Z, Gong G. Cardiovascular Outcomes Post Percutaneous Coronary Intervention in Patients with Obstructive Sleep Apnea and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2020;11:1795–1806. doi: 10.1007/s13300-020-00870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antza C, Ottridge R, Patel S, Slinn G, Tearne S, Nicholls M, Cooper B, Ali A, Tahrani AA. The impact of sleep disorders on microvascular complications in patients with type 2 diabetes (SLEEP T2D): the protocol of a cohort study and feasibility randomised control trial. Pilot Feasibility Stud. 2021;7:80. doi: 10.1186/s40814-021-00817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qureshi UA, Sami A, Altaf U, Ahmad K, Iqbal J, Wani NA, Mir Z, Ali I. Thiamine responsive acute life threatening metabolic acidosis in exclusively breast-fed infants. Nutrition. 2016;32:213–216. doi: 10.1016/j.nut.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Nannapaneni S, Ramar K, Surani S. Effect of obstructive sleep apnea on type 2 diabetes mellitus: A comprehensive literature review. World J Diabetes. 2013;4:238–244. doi: 10.4239/wjd.v4.i6.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altaf QA, Ali A, Piya MK, Raymond NT, Tahrani AA. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. J Diabetes Complications. 2016;30:1315–1320. doi: 10.1016/j.jdiacomp.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Zhang P, Zhao F, Han X, Ji L. Association of Diabetic Microvascular Complications and Parameters of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Diabetes Technol Ther. 2016;18:415–420. doi: 10.1089/dia.2015.0433. [DOI] [PubMed] [Google Scholar]
- 41.Leong WB, Jadhakhan F, Taheri S, Thomas GN, Adab P. The Association between Obstructive Sleep Apnea on Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Sleep. 2016;39:301–308. doi: 10.5665/sleep.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Altaf QA, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013;36:3718–3725. doi: 10.2337/dc13-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furukawa S, Saito I, Yamamoto S, Miyake T, Ueda T, Niiya T, Torisu M, Kumagi T, Sakai T, Minami H, Miyaoka H, Sakurai S, Matsuura B, Onji M, Tanigawa T. Nocturnal intermittent hypoxia as an associated risk factor for microalbuminuria in Japanese patients with type 2 diabetes mellitus. Eur J Endocrinol. 2013;169:239–246. doi: 10.1530/EJE-13-0086. [DOI] [PubMed] [Google Scholar]
- 44.Shiba T, Maeno T, Saishin Y, Hori Y, Takahashi M. Nocturnal intermittent serious hypoxia and reoxygenation in proliferative diabetic retinopathy cases. Am J Ophthalmol. 2010;149:959–963. doi: 10.1016/j.ajo.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Abbas A, Embarak S, Al-nashar H, Farag S. The relationship between obstructive sleep apnea and diabetic retinopathy in type 2 diabetes patients, Zagazig University Hospitals, Egypt. Egypt J Chest Dis Tuberc. 2019;68:290. [Google Scholar]
- 46.Chew M, Tan NYQ, Lamoureux E, Cheng CY, Wong TY, Sabanayagam C. The associations of objectively measured sleep duration and sleep disturbances with diabetic retinopathy. Diabetes Res Clin Pract. 2020;159:107967. doi: 10.1016/j.diabres.2019.107967. [DOI] [PubMed] [Google Scholar]
- 47.Chang AC, Fox TP, Wang S, Wu AY. RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA AND THE PRESENCE AND SEVERITY OF DIABETIC RETINOPATHY. Retina. 2018;38:2197–2206. doi: 10.1097/IAE.0000000000001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altaf QA, Dodson P, Ali A, Raymond NT, Wharton H, Fellows H, Hampshire-Bancroft R, Shah M, Shepherd E, Miah J, Barnett AH, Tahrani AA. Obstructive Sleep Apnea and Retinopathy in Patients with Type 2 Diabetes. A Longitudinal Study. Am J Respir Crit Care Med. 2017;196:892–900. doi: 10.1164/rccm.201701-0175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaba Q, Tai F, Al-Awadi A, Somani S. Examining the Relationship Between Diabetic Macular Edema, and Obstructive Sleep Apnea. Clin Ophthalmol. 2022;16:1215–1223. doi: 10.2147/OPTH.S354087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shechter A, Foster GD, Lang W, Reboussin DM, St-Onge MP, Zammit G, Newman AB, Millman RP, Wadden TA, Jakicic JM, Strotmeyer ES, Wing RR, Pi-Sunyer FX, Kuna ST Sleep Ahead Research Group of the Look Ahead Research Group. Effects of a lifestyle intervention on REM sleep-related OSA severity in obese individuals with type 2 diabetes. J Sleep Res. 2017;26:747–755. doi: 10.1111/jsr.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgoulis M, Yiannakouris N, Kechribari I, Lamprou K, Perraki E, Vagiakis E, Kontogianni MD. Cardiometabolic Benefits of a Weight-Loss Mediterranean Diet/Lifestyle Intervention in Patients with Obstructive Sleep Apnea: The "MIMOSA" Randomized Clinical Trial. Nutrients. 2020;12 doi: 10.3390/nu12061570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carneiro-Barrera A, Amaro-Gahete FJ, Guillén-Riquelme A, Jurado-Fasoli L, Sáez-Roca G, Martín-Carrasco C, Buela-Casal G, Ruiz JR. Effect of an Interdisciplinary Weight Loss and Lifestyle Intervention on Obstructive Sleep Apnea Severity: The INTERAPNEA Randomized Clinical Trial. JAMA Netw Open. 2022;5:e228212. doi: 10.1001/jamanetworkopen.2022.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurnool S, McCowen KC, Bernstein NA, Malhotra A. Sleep Apnea, Obesity, and Diabetes - an Intertwined Trio. Curr Diab Rep. 2023;23:165–171. doi: 10.1007/s11892-023-01510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond) 2016;40:1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra A, Bednarik J, Chakladar S, Dunn JP, Weaver T, Grunstein R, Fietze I, Redline S, Azarbarzin A, Sands SA, Schwab RJ, Bunck MC. Tirzepatide for the treatment of obstructive sleep apnea: Rationale, design, and sample baseline characteristics of the SURMOUNT -OSA phase 3 trial. Contemp Clin Trials. 2024;141:107516. doi: 10.1016/j.cct.2024.107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprung VS, Kemp GJ, Wilding JP, Adams V, Murphy K, Burgess M, Emegbo S, Thomas M, Needham AJ, Weimken A, Schwab RJ, Manuel A, Craig SE, Cuthbertson DJ. Randomised, cOntrolled Multicentre trial of 26 weeks subcutaneous liraglutide (a glucagon-like peptide-1 receptor Agonist), with or without contiNuous positive airway pressure (CPAP), in patients with type 2 diabetes mellitus (T2DM) and obstructive sleep apnoEa (OSA) (ROMANCE): study protocol assessing the effects of weight loss on the apnea-hypnoea index (AHI) BMJ Open. 2020;10:e038856. doi: 10.1136/bmjopen-2020-038856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Oweidat K, Toubasi AA, Tawileh RBA, Tawileh HBA, Hasuneh MM. Bariatric surgery and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2023;27:2283–2294. doi: 10.1007/s11325-023-02840-1. [DOI] [PubMed] [Google Scholar]
- 59.Ding L, Fan Y, Li H, Zhang Y, Qi D, Tang S, Cui J, He Q, Zhuo C, Liu M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes Rev. 2020;21:e13030. doi: 10.1111/obr.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med. 2019;15:301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, Shekelle P Clinical Guidelines Committee of the American College of Physicians. Management of obstructive sleep apnea in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471–483. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 62.Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, Malhotra A. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herth J, Sievi NA, Schmidt F, Kohler M. Effects of continuous positive airway pressure therapy on glucose metabolism in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. Eur Respir Rev. 2023;32 doi: 10.1183/16000617.0083-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aurora RN, Rooney MR, Wang D, Selvin E, Punjabi NM. Effects of Positive Airway Pressure Therapy on Glycemic Variability in Patients With Type 2 Diabetes and OSA: A Randomized Controlled Trial. Chest. 2023;164:1057–1067. doi: 10.1016/j.chest.2023.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zamarrón E, Jaureguizar A, García-Sánchez A, Díaz-Cambriles T, Alonso-Fernández A, Lores V, Mediano O, Troncoso-Acevedo F, Cabello-Pelegrín S, Morales-Ruíz E, Ramírez-Prieto MT, Valiente-Díaz MI, Gómez-García T, Casitas R, Martínez-Cerón E, Galera R, Cubillos-Zapata C, García-Río F. Continuous Positive Airway Pressure Effect on Albuminuria Progression in Patients with Obstructive Sleep Apnea and Diabetic Kidney Disease: A Randomized Clinical Trial. Am J Respir Crit Care Med. 2023;207:757–767. doi: 10.1164/rccm.202206-1091OC. [DOI] [PubMed] [Google Scholar]
- 66.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med Rev. 2017;35:113–123. doi: 10.1016/j.smrv.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenda ED, Henrina J, Samosir J, Amalia R, Yulianti M, Pitoyo CW, Setiati S. Machine learning-based COVID-19 acute respiratory distress syndrome phenotyping and clinical outcomes: A systematic review. Heliyon. 2023;9:e17276. doi: 10.1016/j.heliyon.2023.e17276. [DOI] [PMC free article] [PubMed] [Google Scholar]