Abstract
Objective
To (1) estimate the lifetime prevalence of suspected and diagnosed traumatic brain injury (TBI) based on parent report overall and select sociodemographic characteristics; and (2) describe differences in prevalence of health conditions and health-related risk factors by whether a child had a lifetime history of diagnosed TBI.
Study design
We analyzed data from the 2020 National Survey of Children’s Health, a cross-sectional address-based survey of US households. A categorical variable was created on the basis of parent responses to 3 questions inquiring about their suspicion of their child having a brain injury, if they sought medical care, and if the health care provider provided a diagnosis. Parents also were asked to report on their child’s additional health conditions, functional indicators, school and social factors, and health care access and service use.
Results
The prevalence of lifetime diagnosed TBI was 4.2% (95% CI 3.8-4.5). Children with a parent-reported lifetime history of diagnosed TBI were more likely to have a variety of health conditions, special health care needs, disabilities, activity limitations, missed days of school, and unmet care coordination needs, compared with those without a history. However, they were more likely to have a usual source of sick care and to receive more health-related services.
Conclusions
For school-aged children, a history of TBI is associated with parent-reported health needs and conditions, as well as missed days from school. It is particularly important for parents to seek care when they suspect their child has experienced a TBI to receive a diagnosis and monitor the impacts of the TBI.
Keywords: children, traumatic brain injury
Traumatic brain injury (TBI) among children is a significant health problem, as indicated by the high rate of emergency department visits, hospitalizations, and deaths,1, 2, 3 as well as increased risk for long-term negative effects, such as changes in cognition and behavior, that can notably affect a child’s learning, school, and recreational participation.4, 5, 6, 7 Current methods of surveillance that rely on administrative health care datasets may underestimate the true prevalence of TBI among children. These data sources identify those seen for care in hospital settings but do not capture those who seek care elsewhere or do not seek care at all.1, 2, 3 Previous national parent and self-report surveys show lifetime TBI prevalence may be as high as 7.0% among children aged 0-17 years and 18.4% among adolescents aged 13 years and older.8, 9, 10 However, estimates of TBI vary regarding who responds to the survey (eg, parent proxy vs child self-report), question wording (eg, use of terms such as head injury vs concussion vs TBI), and mode of survey administration (eg, in-person interview, telephone survey, web-based survey).11,12
A previous study examining data from the National Survey of Children’s Health (NSCH) provided a lifetime estimate of parent-reported diagnosed TBI among children aged 0-17 years of 2.5%, defined by one question that asked parents, “Has a doctor or other health care professional ever told you that (your child) had a brain injury or concussion?”8 Several health care conditions were associated with a parent-reported history of diagnosed TBI, corroborating research showing that children who have sustained a TBI are at risk for developing a range of health conditions that can affect their participation in school and community activities.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26
Recent estimates of both diagnosed and suspected TBI prevalence in addition to associations of TBI with critical health conditions and health-related risk factors are needed to inform timely prevention and treatment efforts. Although there are estimates of TBI-related visits to the emergency department and hospitalizations among children, as well as parent-reported history of diagnosed TBIs, there is a lack of recent data on suspected but undiagnosed TBI.27,28 To address these gaps, this study examined a nationally representative sample of US school-aged children to (1) estimate the prevalence of parent-reported lifetime suspected TBI and diagnosed TBI, overall and by selected sociodemographic characteristics, and (2) describe associations between lifetime history of diagnosed TBIs, critical health conditions, and risk factors, including neurobehavioral conditions, chronic health conditions indicating persistent effects continue over time, functional indicators, school and social factors, and health care service access and use.
Methods
Data Source
Data from the 2020 NSCH were analyzed.29 The survey samples a random selection of household addresses across the 50 states and the District of Columbia and solicits participation by mailing instructions to access an online survey; additionally, some addresses receive a paper version of the questionnaire.29,30 In 2020, the overall response rate was 42.4%; among households reporting the presence of 1 or more child, a parent or other primary caregiver (hereafter referred to as “parent”) answered detailed questions about 1 randomly selected child, leading to an interview completion rate of 81.2%. The analytic sample included school-aged children aged 6-17 years with valid responses to our primary outcome of interest, lifetime suspected and diagnosed TBI (n = 30 481). The NSCH Research Ethics Review Board and NORC/University of Chicago Institutional Review Board approved all study procedures and modifications. Participants provided verbal informed consent.
Measures
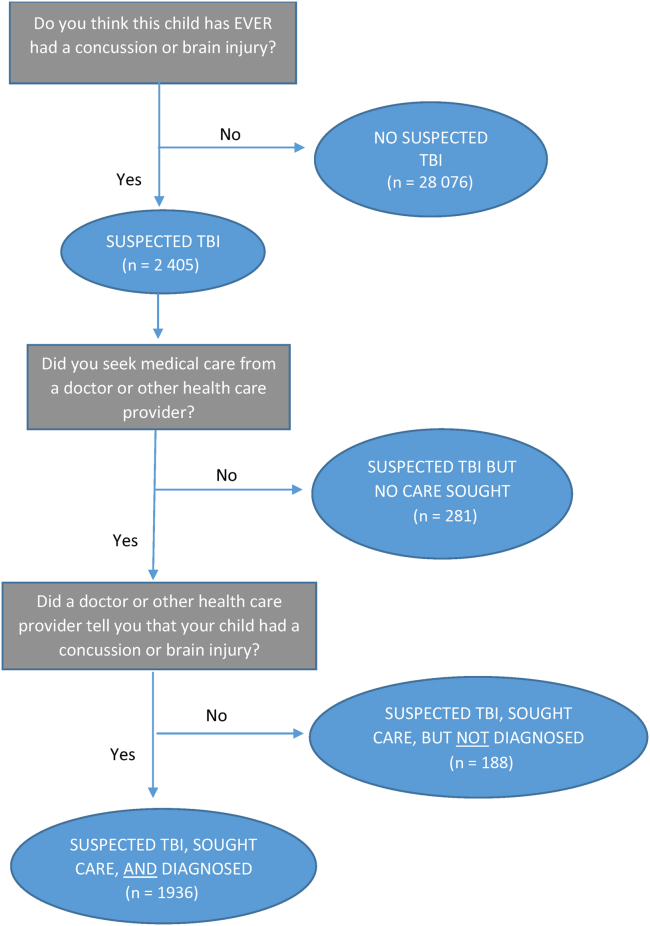
We created a categorical TBI variable on the basis of parent responses to a series of 3 questions (Figure). First, respondents were asked about the randomly selected child, “Do you think this child has ever had a concussion or brain injury?” If a parent responded “Yes,” they were then asked, “Did you seek medical care from a doctor or other health care provider?” If a parent responded “Yes” to seeking care, they were asked, “Did a doctor or other health care provider tell you that your child had a concussion or brain injury?” On the basis of these responses, we categorized children into 4 groups: “No Suspected TBI,” “Suspected TBI but No Care Sought,” “Suspected TBI, Sought Care, but Not Diagnosed,” and “Suspected TBI, Sought Care, and Diagnosed.”
Figure.
Flow chart depicting parent-reported responses to 3 TBI-related questions among US children aged 6-17 years (n = 30 481)—2020 NSCH.
Parents reported child and family-related sociodemographic characteristics including age, sex, race and ethnicity, health insurance status, family income and structure, and highest achieved level of parent education. Family income to poverty ratio was calculated as the proportion of reported total family income to the family poverty threshold, which is derived from the Census Bureau’s poverty thresholds. Missing or invalid responses on poverty ratio were replaced with single imputed values.29
Parents reported whether their child had ever received a diagnosis from a health care provider for several neurobehavioral conditions and other chronic health conditions. Parents also were asked 5 questions to determine whether the child had special health care needs.31 Similarly, respondents were asked whether their child had any of 5 disability types (ie, hearing, vision, cognition, mobility, and/or self-care); activity limitations; school and social factors (ie, school engagement, grade repetition, missed school days, difficulty making friends); and health care access and service use (ie, medical home, usual source of care, unmet need for care coordination and mental health care, preventive health visits, mental health treatment among those who need it, specialist treatment among those who need it, special education or early intervention plans).
Statistical Analysis
SAS-callable SUDAAN, Version 9.4 (RTI International), was used to complete analyses for the complex survey design. The analyses focused on children within the categories of “No Suspected TBI” and “Suspected TBI, Sought Care, and Diagnosed” as a result of the limited sample size in the other 2 groups (“Suspected TBI but No Care Sought” and “Suspected TBI, Care Sought, but Not Diagnosed”). We calculated weighted prevalence estimates of TBI by sociodemographic characteristics. We also calculated weighted prevalence estimates of neurobehavioral and other chronic health conditions, functional indicators, school and social factors, and health care access and service use, and these were stratified by the 2 primary TBI categories of interest. Associations were examined between diagnosed TBI and health conditions and health-related characteristics using weighted adjusted prevalence ratios (aPRs) comparing children with “Suspected TBI, Sought Care, and Diagnosed” with the reference group of children with “No Suspected TBI.” All prevalence ratios were adjusted for age, sex, and race and ethnicity consistent with previous analyses using NSCH data.9 In addition, we adjusted prevalence ratios for outcomes related to health conditions and health care access/use by health insurance type. All estimates are presented with a measure of statistical reliability (95% CI) and all sample sizes are greater than 30. Estimates with relative SEs greater than 30% were flagged for potential instability and estimates with relative SEs greater than 50% were suppressed.
Results
Prevalence of TBI among Children Aged 6-17 Years
Overall, among the 6- to 17-year-old age group, 5.4% (95% CI 4.9-5.8) of parents reported their child had a suspected TBI within his or her lifetime. Within the group of children with a suspected TBI, 88.6% (95% CI 85.4-91.1) sought care from a doctor or other health care provider; 87.6% (95% CI 83.2-91.0) among those who sought care received a TBI diagnosis. Altogether, 4.2% (95% CI 3.8-4.5) of children aged 6-17 years were diagnosed with a TBI in their lifetime (Table I).
Table I.
Prevalence of TBI categories and sociodemographic characteristics of US children aged 6-17 years (n = 30 481)—2020 NSCH
| Characteristics | Unweighted, No. | Weighted prevalence, % | 95% CI |
|---|---|---|---|
| TBI | |||
| No suspected TBI | 28 076 | 94.7 | 94.2-95.1 |
| Suspected TBI, sought care, and diagnosed with TBI | 1936 | 4.2 | 3.8-4.5 |
| Suspected TBI, sought care, but not diagnosed | 188 | 0.6 | 0.4-0.8 |
| Suspected TBI but no care sought | 281 | 0.6 | 0.5-0.8 |
| Child age groups, y | |||
| 6-11 | 13 097 | 49.1 | 47.8-50.3 |
| 12-14 | 7926 | 26.1 | 25.0-27.2 |
| 15-17 | 9613 | 24.9 | 23.8-26.0 |
| Child sex | |||
| Male | 15 853 | 51.1 | 49.8-52.3 |
| Female | 14 783 | 48.9 | 47.7-50.2 |
| Child race/ethnicity | |||
| Hispanic | 4117 | 26.3 | 24.9-27.7 |
| White, non-Hispanic | 20 237 | 49.6 | 48.3-50.8 |
| African American/Black, non-Hispanic | 2201 | 13.7 | 12.8-14.6 |
| Asian, non-Hispanic | 1709 | 4.7 | 4.2-5.1 |
| American Indian/Alaska Native, non-Hispanic | 201 | 0.4 | 0.3-0.5 |
| Native Hawaiian/Other Pacific Islander, non-Hispanic | 89 | 0.3 | 0.2-0.4 |
| Two or more races, non-Hispanic | 2082 | 5.2 | 4.8-5.7 |
| Child current health insurance status | |||
| Only private insurance | 21 064 | 58.1 | 56.8-59.5 |
| Only public insurance | 6289 | 29.1 | 27.8-30.4 |
| Other (public and private) | 1224 | 5.0 | 4.4-5.7 |
| Uninsured | 1580 | 7.7 | 6.9-8.6 |
| FPR, % above or below∗ | |||
| <100% FPR (lower income) | 3834 | 17.7 | 16.6-18.8 |
| 200%-299% FPR | 5207 | 22.5 | 21.4-23.7 |
| 300%-399% FPR | 9295 | 29.4 | 28.3-30.6 |
| ≥400% FPR (greater income) | 12 300 | 30.4 | 29.4-31.4 |
| Family structure | |||
| Two parents | 21 739 | 69.7 | 68.4-70.9 |
| Single mother | 5176 | 19.4 | 18.4-20.5 |
| Single father | 1675 | 5.7 | 5.1-6.3 |
| Other (nonparent caregivers) | 1210 | 5.2 | 4.7-5.9 |
| Highest parent education | |||
| Less than high school | 887 | 10.4 | 9.3-11.7 |
| High school diploma | 4273 | 20.4 | 19.4-21.6 |
| More than high school | 25 476 | 69.2 | 67.8-70.5 |
FPR, family poverty ratio.
FPR was based on self-reported income and number of people in the household, categorized as 0%-99% FPL, 100%-199% FPL, 200%-399% FPL, and ≥400% FPL. Missing or invalid responses on income or number of people in the household were replaced with single imputed values.30
The prevalence of a lifetime TBI diagnosis differed by child and family sociodemographic characteristics (Table II). The prevalence of TBI was greatest among older adolescents aged 15-17 years (7.8%; 95% CI 7.0-8.7) and lowest among children aged 6-11 years (2.2%; 95% CI 1.8-2.8). In addition, the prevalence of TBI diagnosis was slightly greater among male than female children (4.8%; 95% CI 4.3-5.5 vs 3.6%; 95% CI 3.1-4.0). The prevalence of a lifetime TBI diagnosis was greatest among non-Hispanic White children (5.8%; 95% CI 5.3-6.3) and lowest among non-Hispanic Asian children (1.2%, 95% CI 0.7-2.0); non-Hispanic American Indian/Alaska Native children also had a high prevalence, but the estimate was unstable. Greater proportions of children with private insurance (5.1%; 95% CI 4.6-5.6 among those with private only and 6.4%; 95% CI 3.7-11.0 among those with private and public) had a diagnosed TBI compared with children with public-only (2.8%; 95% CI 2.3-3.5) and no insurance (2.1%; 95% CI 1.3-3.4). Children’s lifetime prevalence of diagnosed TBI increased with each consecutive increase in level of household income, ranging from 2.9% (95% CI 2.1-3.9) at the lowest level (<100% family poverty ratio) to 5.7% (95% CI 5.1-6.3) at the highest level (≥400%).
Table II.
Prevalence of US children aged 6-17 years with a suspected TBI who sought care and received a diagnosis, by sociodemographic characteristics—2020 NSCH
| Characteristics | n | Weighted prevalence, % | 95% CI |
|---|---|---|---|
| Child age groups, y | |||
| 6-11 | 364 | 2.2 | 1.8-2.8 |
| 12-14 | 514 | 4.5 | 3.8-5.3 |
| 15-17 | 1058 | 7.8 | 7.0-8.7 |
| Child sex | |||
| Male | 1123 | 4.8 | 4.3-5.5 |
| Female | 813 | 3.6 | 3.1-4.0 |
| Child race/ethnicity | |||
| Hispanic | 186 | 2.5 | 1.8-3.5 |
| White, non-Hispanic | 1492 | 5.8 | 5.3-6.3 |
| African American/Black, non-Hispanic | 61 | 2.3 | 1.6-3.5 |
| Asian, non-Hispanic | 37 | 1.2 | 0.7-2.0 |
| American Indian/Alaska Native, non-Hispanic | 19 | 12.8∗ | 6.4-24.0 |
| Native Hawaiian/Other Pacific Islander, non-Hispanic | 5 | -† | - |
| Two or more races, non-Hispanic | 136 | 5.1 | 3.3-7.9 |
| Child current health insurance status | |||
| Only private insurance | 1445 | 5.1 | 4.6-5.6 |
| Only public insurance | 314 | 2.8 | 2.3-3.5 |
| Other (public and private) | 86 | 6.4 | 3.7-11.0 |
| Uninsured | 70 | 2.1 | 1.3-3.4 |
| FPR, % above or below‡ | |||
| <100% FPR (lower income) | 169 | 2.9 | 2.1-3.9 |
| 200%-299% FPR | 266 | 3.0 | 2.3-4.0 |
| 300%-399% FPR | 573 | 4.3 | 3.7-5.1 |
| ≥400% FPR (greater income) | 928 | 5.7 | 5.1-6.3 |
| Family structure | |||
| Two parents | 1431 | 4.5 | 4.1-4.9 |
| Single mother | 343 | 4.3 | 3.3-5.6 |
| Single father | 69 | 3.1 | 1.9-5.1 |
| Other (nonparent caregivers) | 57 | 2.3 | 1.4-3.6 |
| Highest parent education | |||
| Less than high school | 18 | 1.3∗ | 0.6-2.6 |
| High school diploma | 191 | 3.0 | 2.3-3.8 |
| More than high school | 1,727 | 5.0 | 4.6-5.5 |
Relative SE (RSE) is between 30% and 50%; therefore, estimate should be interpreted with caution.
Estimate suppressed; RSE is >50%.
FPR was based on self-reported income and number of people in the household, categorized as 0%-99% FPL, 100%-199% FPL, 200%-399% FPL, and ≥400% FPL. Missing or invalid responses on income or number of people in the household were replaced with imputed values.30
Prevalence of Neurobehavioral Conditions, Other Chronic Health Conditions, and Special Health Care Needs by TBI Status
Parent-reported neurobehavioral conditions of children with diagnosed TBI included anxiety problems (24.2%; 95% CI 20.9-27.8), attention-deficit/hyperactivity disorder (17.0%; 95% CI 14.3-20.0), learning disability (16.6%; 95% CI 12.9-21.1), behavioral/conduct problems (16.1%; 95% CI 12.5-20.6), depression (14.7%; 95% CI 12.1-17.9), developmental delay (12.9%; 95% CI 9.3-17.5), and speech/language disorder (11.1%; 95% CI 7.7-15.8) (Table III). Relative to children with no suspected TBI, children with parent-reported diagnosed TBI were more likely to have lifetime diagnoses of co-occurring mental health conditions including depression (aPR 1.9; 95% CI 1.5-2.3), anxiety (aPR 1.6; 95% CI 1.3-1.8), and behavioral/conduct problems (aPR 1.5; 95% CI 1.2-1.9). Children with diagnosed TBI were also more likely to have ever been diagnosed with a developmental delay (aPR 1.7; 95% CI 1.2-2.2) and learning disability (aPR 1.6; 95% CI 1.2-2.1) compared with those without suspected TBI.
Table III.
Observed prevalence and aPRs of neurobehavioral conditions, other chronic health conditions, and special health care needs by TBI category among US children aged 6-17 years—2020 NSCH
| Prevalences | No suspected TBI (ref) |
Suspected TBI, sought care, and diagnosed |
|||||
|---|---|---|---|---|---|---|---|
| No. | Weighted size | % (95% CI) | No. | Weighted size | % (95% CI) | aPR∗ (95% CI) | |
| Neurobehavioral conditions† | |||||||
| Attention deficit hyperactivity disorder | 3700 | 5 442 405 | 11.8 (11.0-12.5) | 351 | 345 015 | 17.0 (14.3-20.0) | 1.2 (1.0-1.4) |
| Behavioral/conduct problems | 2856 | 4 838 629 | 10.4 (9.6-11.3) | 297 | 328 445 | 16.1 (12.5-20.6) | 1.5 (1.2-1.9) |
| Depression | 1898 | 2 576 385 | 5.6 (5.0-6.2) | 319 | 299 768 | 14.7 (12.1-17.9) | 1.9 (1.5-2.3) |
| Anxiety problems | 4112 | 5 508 909 | 11.9 (11.1-12.7) | 523 | 491 821 | 24.2 (20.9-27.8) | 1.6 (1.3-1.8) |
| Autism spectrum disorder | 991 | 1 408 748 | 3.1 (2.7-3.5) | 72 | 73 401 | 3.6 (2.5-5.2) | 1.0 (0.6-1.4) |
| Tourette syndrome | 90 | 123 671 | 0.3 (0.2-0.4) | 10 | 10 092 | 0.5 (0.2-1.2)‡ | 1.6 (0.7-3.7)‡ |
| Learning disability | 2514 | 4 194 120 | 9.1 (8.3-9.8) | 293 | 336 875 | 16.6 (12.9-21.1) | 1.6 (1.2-2.1) |
| Developmental delay | 2060 | 3 453 966 | 7.4 (6.8-8.1) | 204 | 261 370 | 12.9 (9.3-17.5) | 1.7 (1.2-2.2) |
| Intellectual disability | 356 | 703 784 | 1.5 (1.2-1.9) | 38 | 76 279 | -§ | -§ |
| Speech/language disorder | 2256 | 3 915 001 | 8.4 (7.7-.2) | 199 | 226 947 | 11.1 (7.7-15.8) | 1.4 (1.0-1.9) |
| Other chronic health¶ conditions | |||||||
| Epilepsy | 296 | 464 091 | 1.0 (0.8-1.2) | 60 | 99 406 | 4.9 (2.9-8.2) | 4.2 (2.3-8.0)‡ |
| Frequent/severe headaches | 1302 | 2 092 503 | 4.5 (4.0-5.1) | 265 | 305 015 | 15.0 (12.3-18.1) | 2.8 (2.2-3.5) |
| Chronic pain (past 12 mo) | 1932 | 3 209 133 | 6.9 (6.3-7.6) | 345 | 409 142 | 20.1 (16.7-24.1) | 2.6 (2.1-3.2) |
| Meets criteria for special health care needs∗∗ | 7659 | 10 725 637 | 23.0 (22.1-24.1) | 778 | 786 067 | 38.5 (34.2-43.1) | 1.5 (1.3-1.7) |
| Meets criteria for special health care needs∗∗ | 7659 | 10 725 637 | 23.0 (22.1-24.1) | 778 | 786 067 | 38.5 (34.2-43.1) | 1.5 (1.3-1.7) |
Prevalence ratios for neurobehavioral conditions and other chronic health conditions were adjusted for age, sex, race and ethnicity, and health insurance type. The prevalence ratios for meets criteria for special health care needs were adjusted for age, sex, and race and ethnicity.
Neurobehavioral conditions were identified by respondents of NSCH when asked “Has a doctor or other health care provider ever told you that this child has… attention-deficit/hyperactivity disorder, depression, anxiety problems, autism spectrum disorder, or Tourette syndrome?” The survey also asked parents, “Has a doctor, other health care provider, or educator ever told you that this child has… behavioral or conduct problems, learning disability, developmental delay, intellectual disability (formerly known as mental retardation), or speech or other language disorder?”
RSE is between 30% and 50%; therefore, estimate should be interpreted with caution.
Estimate suppressed; RSE is >50%.
Chronic health conditions were identified by respondents of NSCH when asked “Has a doctor or other health care provider ever told you that this child has…epilepsy or seizure disorder, or frequent or severe headaches, including migraine?” Respondents were also asked “During the past 12 months has this child had frequent or chronic difficulty with any of the following…Repeated or chronic physical pain, including headaches or other back or body pain?”
NSCH uses the children with special health care needs (CSHCN) screener to identify children with special health care needs.32 The CSHCN screener is a 5-item, parent-reported tool categorized as: (1) need or use of prescription medications, (2) need or use of services, (3) need or use of specialized therapies, (4) functional difficulties, and (5) emotional, developmental, or behavioral problems for which treatment or counseling is needed. It identifies children across the range and diversity of childhood chronic conditions and special needs, allowing a more comprehensive and robust assessment of children's needs and health care system performance than is attainable by focusing on a single diagnosis or type of special need.
Parent-reported other chronic health conditions of children with diagnosed TBI included chronic pain (20.1%; 95% CI 16.7-24.1) and frequent/severe headaches (15.0%; 95% CI 12.3-18.1). Children with diagnosed TBI were more likely than children with no suspected TBI to have epilepsy (aPR 4.2; 95% CI 2.3-8.0), frequent/severe headaches (aPR 2.8; 95% CI 2.2-3.5), and chronic pain (aPR 2.6; 95% CI 2.1-3.2). More than one-third (38.5%; 95% CI 34.2-43.1) of children with diagnosed TBI met the criteria for special health care needs compared with approximately one-quarter of children with no suspected TBI (23.0%; 95% CI 22.1-24.1; aPR: 1.5; 95% CI 1.3-1.7).
Prevalence of Functional Indicators, School and Social Factors, and Health Care Access and Service Use by TBI Status
Children with diagnosed TBI were more likely to have parent-reported cognitive disability (17.4% vs 9.7%; aPR 1.7; 95% CI 1.3-2.2) and difficulty with self-care (5.1% vs 1.5%; aPR 3.8; 95% CI 1.7-8.9) compared with children with no suspected TBI (Table IV). In addition, parents of children with diagnosed TBI were more than 3 times as likely to report their child’s daily activities were “usually or always affected” by health conditions than children with no suspected TBI (10.1% vs 2.9%; aPR 3.2; 95% CI 2.0-5.3). Children with diagnosed TBI were 3 times as likely to miss 11 or more days of school than children without a suspected TBI (10.1% vs 3.1%; aPR 3.0; 95% CI 2.3-4.0).
Table IV.
Observed prevalence and aPRs of functional indicators, school and social factors, and health care access and service use by TBI category among US children aged 6-17 years—2020 NSCH
| Prevalences | No suspected TBI (ref) |
Suspected TBI, sought care, and diagnosed |
|||||
|---|---|---|---|---|---|---|---|
| No. | Weighted size | % (95% CI) | No. | Weighted size | % (95% CI) | aPR∗ (95%CI) | |
| Functional indicators | |||||||
| Functional disability type† | |||||||
| Deafness or problems with hearing | 351 | 533 080 | 1.2 (1.0-1.4) | 35 | 24 317 | 1.2 (0.7-1.9) | 1.0 (0.6-1.7) |
| Blindness or problems with seeing | 428 | 1 006 585 | 2.2 (1.7-2.7) | 51 | 52 394 | 2.6 (1.7-4.0) | 1.4 (0.8-2.2) |
| Cognitive | 2846 | 4 480 242 | 9.7 (9.0-10.5) | 328 | 353 815 | 17.4 (13.7-21.9) | 1.7 (1.3-2.2) |
| Mobility | 184 | 275 863 | 0.6 (0.5-0.8) | 46 | 92 399 | 4.6 (1.9-10.4)‡ | -§ |
| Self-care | 380 | 669 034 | 1.5 (1.2-1.8) | 54 | 102 719 | 5.1 (2.3-10.7)‡ | 3.8 (1.7-8.9)‡ |
| Activity limitation—past 12 mo¶ | |||||||
| No health conditions | 18 861 | 32 219 526 | 69.7 (68.5-70.9) | 928 | 1 006 163 | 49.4 (44.9-53.9) | 0.8 (0.7-0.8) |
| Daily activities never affected | 4718 | 7 524 252 | 16.3 (15.4-17.2) | 438 | 442 928 | 21.8 (18.4-25.5)‡ | 1.3 (1.1-1.5) |
| Sometimes affected | 3526 | 5 143 091 | 11.1 (10.3-12.0) | 421 | 381 655 | 18.7 (16.1-21.7) | 1.5 (1.3-1.8) |
| Usually or always affected | 888 | 1 344 140 | 2.9 (2.6-3.3) | 146 | 205 650 | 10.1 (6.7-14.9) | 3.2 (2.0-5.3) |
| School and social factors | |||||||
| School engagement∗∗ | |||||||
| Always | 11 551 | 19 861 836 | 43.7 (42.4-45.1) | 676 | 753 606 | 37.6 (33.3-42.1) | 0.9 (0.8-1.0) |
| Usually | 10 979 | 17 022 079 | 37.6 (36.3-38.9) | 769 | 770 329 | 38.5 (34.3-42.8) | 1.0 (0.9-1.1) |
| Sometimes/never | 5018 | 8 482 587 | 18.7 (17.6-19.8) | 466 | 479 406 | 24.0 (19.9-28.5) | 1.2 (1.0-1.4) |
| Repeated grade (ever) | 1346 | 2 958 546 | 6.5 (5.8-7.2) | 102 | 116 944 | 5.8 (3.9-8.4) | 0.8 (0.5-1.2) |
| Missed school days (past 12 mo) | |||||||
| No days missed | 9722 | 18 492 425 | 40.6 (39.3-42.0) | 404 | 451 899 | 22.4 (18.9-26.5) | 0.6 (0.5-0.7) |
| 1-3 d | 11 617 | 17 650 438 | 38.8 (37.5-40.1) | 759 | 759 109 | 37.7 (33.6-42.0) | 1.0 (0.9-1.1) |
| 4-6 d | 3817 | 5 855 668 | 12.9 (12.1-13.7) | 358 | 432 583 | 21.5 (17.3-26.4) | 1.7 (1.4-2.1) |
| 7-10 d | 1478 | 2 086 031 | 4.6 (4.1-5.1) | 191 | 167 954 | 8.3 (6.5-10.7) | 1.7 (1.3-2.3) |
| 11+ d | 910 | 1 416 646 | 3.1 (2.7-3.6) | 201 | 202 551 | 10.1 (8.0-12.6) | 3.0 (2.3-4.0) |
| Difficulty making and keeping friends | |||||||
| No difficulties | 20 928 | 35 554 956 | 77.8 (76.7-78.9) | 1359 | 1 501 904 | 74.1 (70.6-77.4) | 1.0 (0.9-1.0) |
| A little difficulty | 5408 | 8 139 178 | 17.8 (16.8-18.9) | 428 | 409 788 | 20.2 (17.3-23.5) | 1.0 (0.9-1.2) |
| A lot of difficulty | 1308 | 1 981 882 | 4.3 (3.8-4.9) | 131 | 114 079 | 5.6 (4.2-7.5) | 1.1 (0.8-1.5) |
| Health care access and service use | |||||||
| Access to care | |||||||
| Receipt of care in a medical home†† | 14 475 | 20 703 602 | 44.6 (43.3-45.8) | 1052 | 1 096 861 | 53.8 (49.2-58.3) | 1.1 (1.0-1.2) |
| Usual source of sick care in a doctor’s office-clinic-or health center | 22 225 | 33 917 396 | 73.7 (72.4-75.0) | 1708 | 1 781 245 | 87.8 (84.6-90.5) | 1.1 (1.1-1.2) |
| Unmet need for care coordination‡‡ | 4046 | 6 762 134 | 14.7 (13.7-15.7) | 448 | 455 829 | 22.4 (18.6-26.9) | 1.6 (1.3-1.9) |
| Unmet need for mental health care service use | 358 | 610 384 | 1.3 (1.0-1.8) | 45 | 52 765 | 2.6 (1.7-4.0) | 2.0 (1.1-3.4) |
| Service use | |||||||
| Preventive health visit (past 12 mo) | 21 457 | 34 045 852 | 73.6 (72.3-74.8) | 1680 | 1 751 878 | 86.1 (81.2-89.9) | 1.2 (1.1-1.2) |
| Mental health treatment, among those who needed it (past 12 mo)§§ | 4013 | 5 636 861 | 12.2 (11.3-13.1) | 461 | 400 312 | 19.7 (17.0-22.7) | 1.3 (1.1-1.6) |
| Specialist treatment, among those who needed it (past 12 mo)¶¶ | 4220 | 5 729 462 | 12.4 (11.7-13.2) | 584 | 630 722 | 31.1 (26.8-35.8) | 2.1 (1.8-2.5) |
| Special education services (current)∗∗∗ | 3232 | 5 258 715 | 11.4 (10.6-12.2) | 303 | 348 635 | 17.1 (13.4-21.6) | 1.4 (1.1-1.8) |
Prevalence ratios for functional indicators and school and social factors were adjusted for age, sex, and race and ethnicity. The prevalence ratios for meets criteria for health care access and service use were adjusted for age, sex, race and ethnicity, and health insurance type.
The NSCH asked parents to report if their child had “any of the following…Deafness or problems hearing; Blindness or problems with seeing-even when wearing glasses; Serious difficulty concentrating, remembering, or making decisions because of a physical, mental, or emotional condition (cognitive); Serious difficulty walking or climbing stairs (mobility); or Difficulty dressing or bathing (self-care).” Disability types are not mutually exclusive, ie, children may have more than 1 disability type.
RSE is between 30% and 50%; therefore, estimate should be interpreted with caution.
Estimate suppressed; RSE is >50%.
Respondents were asked, “During the last 12 months, how often have this child’s health conditions or problems affected their ability to do things other children their age do?” Response options included, “This child does not have any health conditions,” “Never,” “Sometimes,” “Usually,” or “Always.”
School engagement was defined as a composite measure based on responses to 2 questions: “How often does this child care about doing well in school?” and “How often does this child do all required homework?”.
Receipt of care in a medical home was defined as a composite measure composed of 5 subcomponents (usual source of sick care, personal doctor or nurse, referral access, receipt of care coordination, and receipt of family-centered care).
Unmet care coordination measure was defined as a composite measure based on responses to questions about communication between doctors when needed, communication between doctors and schools when needed, and getting needed help coordinating care. Children who did not see more than 1 health care provider were coded as not needing care coordination.
Respondents were asked, “During the last 12 months-has this child received any treatment or counseling from a mental health professional?” NSCH defined mental health professionals as psychiatrists, psychologists, psychiatric nurses, and clinical social workers.
Respondents were asked, “During the past 12 months, did this child see a specialist other than a mental health professional?” NSCH defined specialists as doctors like surgeons, heart doctors, allergy doctors, and others who specialize in 1 area of health care.
Respondents were asked, “Has this child EVER had a special education or early intervention plan? Children receiving these services often have an Individualized Family Service Plan (IFSP) or Individualized Education Plan (IEP).” If respondents answered yes, they were then asked, “Is this child CURRENTLY receiving services under one of these plans?”.
Parents of children with a TBI were more likely to report their child had a usual source of sick care in a doctor’s office, clinic, or health center compared with those without a suspected TBI (87.8% vs 73.7%; aPR 1.1; 95% CI 1.1-1.2). However, parents of children with a diagnosed TBI were more likely to report that their child had an unmet need for care coordination (aPR 1.6; 95% CI 1.3-1.9) compared with children without a suspected TBI. With respect to health care service use, 86.1% (95% CI 81.2-89.9) of children with a diagnosed TBI had at least 1 preventive check-up with a health care professional in the last 12 months compared with 73.6% (95% CI 72.3-74.8) of children with no suspected TBI. Parents of children with a diagnosed TBI were more likely to report their child received mental health treatment (19.7%; 95% CI 17.0-22.7) compared with children with no suspected TBI (12.2% 95% CI 11.3-13.1). Children with a diagnosed TBI were more than twice as likely to have parent-reported receipt of specialist treatment than children without a suspected TBI (aPR 2.1; 95% CI 1.8-2.5). Finally, a greater proportion of children with a diagnosed TBI (17.1%, 95% CI 13.4-21.6) received special education services than those with no suspected TBI (11.4%, 95% CI 10.6-12.2).
Discussion
This study examined a nationally representative dataset to better understand parent-reported lifetime prevalence of suspected and diagnosed TBI in school-aged children, as well as associations between diagnosed TBI and a range of neurobehavioral and chronic health conditions, functional indicators, school and social factors, health care access, and service use. The prevalence of lifetime suspected TBI in this population was 5.4%, and the prevalence of diagnosed TBI was 4.2%. Most parents reported seeking care for suspected TBIs and parents who sought care were also likely to receive a diagnosis of TBI from a health care provider, yet there were some notable disparities in diagnosis and care-seeking behaviors.
Our findings showed relatively low prevalence of diagnosed TBI among Hispanic, non-Hispanic Black, and non-Hispanic Asian children compared with non-Hispanic White children and among those publicly insured or uninsured relative to those privately insured. This pattern of findings may reflect potential inequities contributing to underidentification of TBI among these populations. Previous research has built a critical foundation demonstrating racial and ethnic disparities related to experiencing a TBI, including delays in acute care and diagnosis, poorer post-TBI recovery, and increased likelihood of long-term negative outcomes.32 Children who are members of racial and ethnic minority groups may be less likely to receive a TBI diagnosis after an injury as the result of mistrust of health care practitioners or institutions, social stigma and discrimination, or lack of access to appropriate health care, which may impede TBI care-seeking behaviors.32,33 This is particularly concerning because children from racial and ethnic minority groups are more likely to experience more severe TBIs and TBI outcomes compared with non-Hispanic White children.32, 33, 34
Children with a parent-reported diagnosed TBI had greater prevalence of neurobehavioral conditions (behavioral/conduct problems, depression, anxiety), which is consistent with other studies, even those studies that examined mild TBI.14,35, 36, 37 In addition, children with diagnosed TBIs more often had other parent-reported comorbid health conditions (epilepsy, chronic pain, headaches), disabilities (learning or cognitive, developmental delay, and functional limitations), impacts to daily activities, missed days of school, and special health care needs compared with children with no suspected TBI. Those with diagnosed TBIs were more likely to report health service use such as preventive check-ups, mental health treatment, and specialist treatment yet greater rates of unmet needs for care coordination, which supports previous work that showed potential gaps in services for children with TBI.14,15 Recent study findings showed younger students, females, and students not injured in school sports are at risk for delayed identification and management of concussions, a form of mild TBI.37 Although most parents of children with diagnosed TBIs reported their child had an annual preventive check-up and a usual source of sick care, children may experience symptoms like headaches and chronic pain that are not reported to health care providers or not adequately treated, which can affect their participation in school and social activities. These symptoms have been reported even among children with mild TBI.6,7,38 Research on symptoms beyond the initial injury and unmet needs for care suggests experiencing a TBI of any severity can lead to chronic health conditions in children.4,7 Having a medical home, or a place for routine medical care that also provides family-centered care may be an important factor in reducing negative long-term impacts following a TBI.5,39
The current study findings indicate that children who experience a TBI may benefit from follow-up services for in both health care and school settings. Although children with a TBI diagnosis were more likely to receive health care and special education services, they were also more likely to have unmet needs for care coordination, suggesting even their elevated access to services is insufficient to meet their needs. Support and follow-up in school settings are helpful because this is where children spend most of their time. Although many children with mild TBI may recover within a few months, some experience ongoing academic issues and may benefit from continued monitoring and accomodations.7 Children who experience a TBI may benefit from improved processes to ensure successful transitions between grades and schools.5
Limitations
The NSCH relies on parent report of health care provider–diagnosed brain injury or concussion and other health conditions and does not examine medical records or include those with suspected and undiagnosed TBI, which may be affected by insurance status, parent education, and income. In addition, parent response to surveys is more likely for White, well-educated parents. While it’s true that White and college-educated households are more likely to respond to the NSCH, when survey sampling weights are incorporated to account for non-response, the non-response bias is reduced and these small biases in sampling frame translate to even smaller biases in key survey estimates. The Census Bureau has conducted extensive analyses and concluded that there is no strong or consistent evidence of nonresponse bias in the NSCH (https://www2.census.gov/programs-surveys/nsch/technical-documentation/nonresponse/2020-NSCH-Nonresponse-Bias-Analysis.pdf). Families with children who have experienced TBI and who have cognitive impairment or learning disabilities associated with TBI may have a different response rate to surveys and perhaps be more motivated to answer surveys regarding children’s health, a point that could affect the prevalence estimates. We have also learned that children in families seeking asylum in the US may have experienced injuries that were not seen in health care.40
Health care provider diagnosis was inferred by parents’ response to survey questions; however, these estimates may have been affected by difficulties in recall and by challenges related to communicating the diagnosis between the health care provider and the parent. Second, the survey did not ask about TBI severity, so estimating the proportion of children who experienced mild compared with severe TBI is not possible. Third, because of the cross-sectional nature of the survey, we could not ascribe a temporal or causal relationship between TBIs and other comorbid conditions, functional limitations, or school and social impacts. Fourth, factors such as parent mental and ability to recall events, injury status (mild-moderate-severe), and child premorbid characteristics were not examined in this study. These factors may contribute to what is learned about children in this age group. Finally, because of small cell sizes, we were unable to further explore the sample of children whose parents suspected a TBI but did not seek health care for the injury.
Conclusions
In this study, an estimated 4.2% of US children aged 6-17 years were ever diagnosed with a TBI. Children with a diagnosed TBI were more likely to have various health conditions, disabilities, functional limitations, as well as school and social impacts, relative to those with no TBI history. The findings about TBI effects and potential for health conditions and functional limitations support the importance of health care providers discussing the effects of TBI with parents, at the time of the injury, as well as facilitating ongoing monitoring of the child’s health and behavior. Health care providers can include anticipatory guidance to encourage parents to seek medical care for suspected TBIs, support parents by providing education about the effects of TBI and the importance of informing their child’s school of a confirmed TBI and recommend potential symptom-based accommodations to aid in a child’s return to school. Future directions to better educate parents about the effects of the injury and need to seek care. Additional research can explore how to better reach all parents for survey completion to ensure an accurate prevalence estimate. Both the Maternal and Child Health Bureau (MCHB) and Census Bureau support our methodology to incorporate survey weights adjusting for non-response which helps produce accurate prevalence estimates despite the differential response rates.
CRediT authorship contribution statement
Juliet Haarbauer-Krupa: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Conceptualization. Allison P. Wray: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization. Lydie A. Lebrun-Harris: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Robyn A. Cree: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Lindsay S. Womack: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization.
Declaration of Competing Interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Health and Human Services, the Centers for Disease Control and Prevention, or the Health Resources and Services Administration. The authors have no conflicts of interest relevant to this article.
Acknowledgments
We appreciate Dr Jill Daugherty (Division of Injury Prevention, National Center for Injury Prevention and Control) and Dr Rebecca H. Bitsko (Division of Human Development and Disability, National Center on Birth Defects and Developmental Disabilities) for their valuable contributions during the planning and development of the manuscript.
References
- 1.Centers for Disease Control and Prevention . National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Atlanta (GA): 2019. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2014. [Google Scholar]
- 2.Centers for Disease Control and Prevention Surveillance report of traumatic brain injury-related hospitalizations and deaths by age group, sex, and mechanism of injury—United States, 2016 and 2017. 2021. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-surveillance-report-2016-2017-508.pdf
- 3.Centers for Disease Control and Prevention Surveillance report of traumatic brain injury-related deaths by age group, sex, and mechanism of injury—United States, 2018 and 2019. 2022. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-surveillance-report-2018-2019-508.pdf
- 4.Babikian T., Merkley T., Savage R.C., Giza C.C., Levin H. Chronic aspects of pediatric traumatic brain injury: review of the literature. J Neurotrauma. 2015;32:1849–1860. doi: 10.1089/neu.2015.3971. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Atlanta (GA): 2018. Report to Congress: the management of traumatic brain injury in children. [Google Scholar]
- 6.Haarbauer-Krupa J., Pugh M.J., Prager E.M., Harmon N., Wolfe J., Yaffe K. Epidemiology of chronic effects of traumatic brain injury. J Neurotrauma. 2021;38:3235–3247. doi: 10.1089/neu.2021.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurowski B., Haarbauer-Krupa J., Giza C.C. When traumatic brain injuries in children become chronic health conditions. J Head Trauma Rehabil. 2023;38:348–350. doi: 10.1097/HTR.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haarbauer-Krupa J., Lee A.H., Bitsko R.H., Zhang X., Kresnow-Sedacca M.-J. Prevalence of parent-reported traumatic brain injury in children and associated health conditions. JAMA Pediatr. 2018;172:1078–1086. doi: 10.1001/jamapediatrics.2018.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black L.I., Zammitti E.P., Hoffman H.J., Li C.M. Parental report of significant head injuries in children aged 3-17 Years: United States, 2016. NCHS Data Brief. 2018:1–8. [PubMed] [Google Scholar]
- 10.Veliz P., McCabe S.E., Eckner J.T., Schulenberg J.E. Prevalence of concussion among US adolescents and correlated factors. JAMA. 2017;318:1180–1182. doi: 10.1001/jama.2017.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veliz P. Variation in national survey estimates and youth traumatic brain injury—where does the truth lie? JAMA Pediatr. 2019;173:399. doi: 10.1001/jamapediatrics.2019.0001. [DOI] [PubMed] [Google Scholar]
- 12.Haarbauer-Krupa J., Lebrun-Harris L.A., Black L.I., Veliz P., Daugherty J., Desrocher R., et al. Comparing prevalence estimates of concussion/head injury in US children and adolescents in national surveys. Ann Epidemiol. 2021;54:11–20. doi: 10.1016/j.annepidem.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esterov D., Witkowski J., McCall D.M., Wi C.-I., Weaver A.L., Brown A.W. Risk factors for development of long-term mood and anxiety disorder after pediatric traumatic brain injury: a population-based, birth cohort analysis. Brain Inj. 2022;36:722–732. doi: 10.1080/02699052.2022.2077987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledoux A.-A., Webster R.J., Clarke A.E., Fell D.B., Knight B.D., Gardner W., et al. Risk of mental health problems in children and youths following concussion. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Max J.E., Troyer E.A., Arif H., Vaida F., Wilde E.A., Bigler E.D., et al. Traumatic brain injury in children and adolescents: psychiatric disorders 24 years later. J Neuropsychiatry Clin Neurosci. 2022;34:60–67. doi: 10.1176/appi.neuropsych.20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson V., Beauchamp M.H., Yeates K.O., Crossley L., Ryan N., Hearps S.J.C., et al. Social competence at two years after childhood traumatic brain injury. J Neurotrauma. 2017;34:2261–2271. doi: 10.1089/neu.2016.4692. [DOI] [PubMed] [Google Scholar]
- 17.Allonsius F., de Kloet A., Bedell G., van Markus-Doornbosch F., Rosema S., Meesters J., et al. Participation restrictions among children and young adults with acquired brain injury in a pediatric outpatient rehabilitation cohort: the patients’ and parents’ perspective. Int J Environ Res Publ Health. 2021;18:1625. doi: 10.3390/ijerph18041625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Câmara-Costa H., Francillette L., Opatowski M., Toure H., Brugel D., Laurent-Vannier A., et al. Participation seven years after severe childhood traumatic brain injury. Disabil Rehabil. 2020;42:2402–2411. doi: 10.1080/09638288.2019.1594398. [DOI] [PubMed] [Google Scholar]
- 19.Renaud M.I., van de Port I.G., Catsman-Berrevoets C.E., Jellema K., Lambregts S.A., van Heugten C.M. Activities and participation in the first 6 months after mild traumatic brain injury in children and adolescents. J Head Trauma Rehabil. 2020;35:E501–E512. doi: 10.1097/HTR.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 20.Ryan N.P., Anderson V.A., Bigler E.D., Dennis M., Taylor H.G., Rubin K.H., et al. Delineating the nature and correlates of social dysfunction after childhood traumatic brain injury using common data elements: evidence from an international multi-cohort study. J Neurotrauma. 2021;38:252–260. doi: 10.1089/neu.2020.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumane S., Câmara-Costa H., Francillette L., AraujoHa M., Toura A., Brugel D., et al. Functional outcome after severe childhood traum atic brain injury: results of the TGE prospective longitudinal study. Ann Phys Rehabil Med. 2021;64 doi: 10.1016/j.rehab.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Prigatano G.P., Gupta S. Friends after traumatic brain injury in children. J Head Trauma Rehabil. 2006;21:505–513. doi: 10.1097/00001199-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Haarbauer-Krupa J., Pugh M.J., Prager E.M., Harmon N., Wolfe J., Yaffe K.C. Epidemiology of chronic effects of traumatic brain injury. J Neurotrauma. 2021;38:3235–3247. doi: 10.1089/neu.2021.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuentes M.M., Wang J., Haarbauer-Krupa J., Yeates K.O., Durbin D., Zonfrillo M.R., et al. Unmet rehabilitation needs after hospitalization for traumatic brain injury. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slomine B.S., McCarthy M.L., Ding R., Mackenzie E.J., Jaffe K.M., Aitken M.E., et al. Health care utilization and needs after pediatric traumatic brain injury. Pediatrics. 2006;117:e663–e674. doi: 10.1542/peds.2005-1892. [DOI] [PubMed] [Google Scholar]
- 26.Lundine J.P., Todis B., Gau J.M., Mccart M., Wade S.L., Yeates K.O., et al. Return to school following TBI: educational services received 1 year after injury. J Head Trauma Rehabil. 2021;36:E89–E96. doi: 10.1097/HTR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 27.Womack L.S., Breiding M.J., Daugherty J. Concussion evaluation patterns among US adults. J Head Trauma Rehabil. 2022;37:303–310. doi: 10.1097/HTR.0000000000000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setnick L., Bazarianm J.J. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj. 2007;21:1–9. doi: 10.1080/02699050601111419. [DOI] [PubMed] [Google Scholar]
- 29.US Census Bureau 2020 National survey of children’s health: data users frequently asked questions (FAQs) https://www2.census.gov/programs-surveys/nsch/technical-documentation/methodology/2020-NSCH-FAQs.pdf
- 30.US Census Bureau 2020 National survey of children’s health: methodology report. https://www2.census.gov/programs-surveys/nsch/technical-documentation/methodology/2020-NSCH-Methodology-Report.pdf
- 31.Bethell C.D., Read D., Stein R.E.K., Blumberg S.J., Wells N., Newacheck P. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Saadi A., Bannon S., Watson E., Vranceanu A.M. Racial and ethnic disparities associated with traumatic brain injury across the continuum of care: a narrative review and directions for future research. J Racial Ethn Health Disparities. 2022;9:786–799. doi: 10.1007/s40615-021-01017-4. [DOI] [PubMed] [Google Scholar]
- 33.Son C., Tarasiewicz I. Short-term outcomes of ethnic and racial minority pediatric patients following traumatic brain injury in the state of Texas. Cureus. 2021;13 doi: 10.7759/cureus.16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piatt J. Racial disparities in mortality after severe traumatic brain injury in childhood: mediators identified by Oaxaca-Blinder decomposition of trauma registry data. Inj Epidemiol. 2021;8:1. doi: 10.1186/s40621-020-00295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry R., Haarbauer-Krupa J.K., Breiding M.J., Thigpen S., Rasberry C.N., Lee S.M. Concussion and academic impairment among US high school students. Am J Prev Med. 2019;57:733–740. doi: 10.1016/j.amepre.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ledoux A., Webster J., Clarke A.E., Fell D.B., Knight B.D., Gardner W., et al. Risk of mental health problems in children and youths following concussion. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babcock L., Byczkowski T., Wade S.L., Ho M., Mookerjee S., Bazarian J.J. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013;167:156–161. doi: 10.1001/jamapediatrics.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price Snedaker K.P., Lundine J.P., Ciccia A.H., Haider M.N., O’Brien K.H. Gaps in concussion management across school-aged children. Brain Inj. 2022;36:714–721. doi: 10.1080/02699052.2022.2034954. [DOI] [PubMed] [Google Scholar]
- 39.Strickland B., McPherson M., Weissman G., van Dyck P., Huang Z.J., Newacheck P. Access to the medical home: results of the national survey of children with special health care needs. Pediatrics. 2004;113:1485–1492. [PubMed] [Google Scholar]
- 40.McMurry H.A., Tsang D.C., Lin N., Symes S.N., Dong C., Teshamae S.M. Head injury and neuropsychiatric sequelae in asylum seekers. Neurology. 2020;95:e2605–e2609. doi: 10.1212/WNL.0000000000010929. [DOI] [PubMed] [Google Scholar]