Abstract
Mitochondria are the energy-producing organelles of mammalian cells with critical involvement in metabolism and signaling. Studying their regulation in pathological conditions may lead to the discovery of novel drugs to treat, for instance, cardiovascular or neurological diseases, which affect high-energy-consuming cells such as cardiomyocytes, hepatocytes, or neurons. Mitochondria possess both protein-coding and noncoding RNAs, such as microRNAs, long noncoding RNAs, circular RNAs, and piwi-interacting RNAs, encoded by the mitochondria or the nuclear genome. Mitochondrial RNAs are involved in anterograde-retrograde communication between the nucleus and mitochondria and play an important role in physiological and pathological conditions. Despite accumulating evidence on the presence and biogenesis of mitochondrial RNAs, their study continues to pose significant challenges. Currently, there are no standardized protocols and guidelines to conduct deep functional characterization and expression profiling of mitochondrial RNAs. To overcome major obstacles in this emerging field, the EU-CardioRNA and AtheroNET COST Action networks summarize currently available techniques and emphasize critical points that may constitute sources of variability and explain discrepancies between published results. Standardized methods and adherence to guidelines to quantify and study mitochondrial RNAs in normal and disease states will improve research outputs, their reproducibility, and translation potential to clinical application.
Keywords: MT: Non-coding RNAs, noncoding RNAs, mitochondria, gene expression, guidelines, microRNAs
Graphical abstract
Devaux and colleagues discuss current pitfalls and challenges in the molecular profiling and functional characterization of mitochondrial noncoding RNAs. They summarize available techniques and provide guidelines for the establishment of standardized protocols for the study of these RNAs under normal and pathological conditions.
Introduction
Mitochondria are cellular membrane-bound organelles originally derived from a proteobacterium during the evolution of the eukaryotic cell.1 They are responsible for the energy supply to the cells and are enriched in high-energy-demanding cells such as cardiomyocytes, hepatocytes, and neurons. Mitochondrial dysfunction is associated with cardiac, liver, and neurological diseases, as well as cancer, and is a potential source of novel therapeutic targets, which is currently under active investigation.
Human mitochondria possess a circular genome, which is highly condensed and contains only 13 protein-coding genes, 2 transfer RNAs (tRNAs), and 2 ribosomal RNAs (rRNAs). Similar to nuclear DNA, mitochondrial DNA (mtDNA) is uniquely regulated by epigenetic factors such as DNA methylation and noncoding RNAs (ncRNAs).2,3,4,5,6,7 While ncRNAs lack protein-coding potential, they have extensive regulatory properties and act at multiple layers of gene expression.8,9 The improvement of RNA sequencing (RNA-seq) techniques has led to an increasing number of ncRNAs, including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and piwi-interacting RNAs (piRNAs), being connected physically and/or functionally to the mitochondrial compartment (Figure 1).10,11
Figure 1.
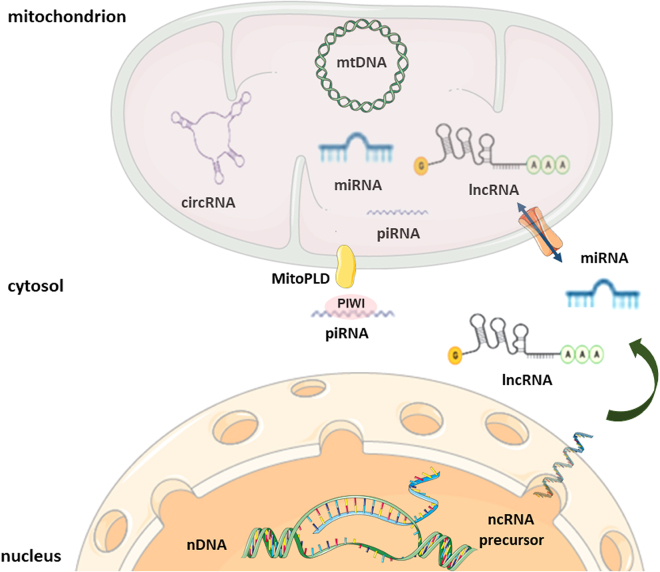
Biogenesis and targeting of mitochondria-related ncRNAs
Mitochondria-related ncRNAs are of nuclear (nDNA) or mitochondrial DNA (mtDNA) origin. Nuclear genome-encoded lncRNAs and miRNAs are being processed in the cytosol and regulate mitochondrial physiology (anterograde signaling) by targeting transcripts/proteins outside or inside the organelle. The mechanism of import of ncRNAs in the mitochondrial matrix remains elusive. piRNAs encoded by the nuclear genome are being processed by MitoPLD at the surface of mitochondria. The mitochondrial genome encodes lncRNAs, miRNAs, piRNAs, and circRNAs that regulate mitochondrial and nuclear (retrograde signaling) gene expression. Whether mitochondria are autonomous for the maturation of these ncRNAs or they depend on cytosolic enzymes is a matter of debate (diagrams were adapted with permission from Servier Medical Art library, available under Creative Commons license).
miRNAs are small ncRNAs with an average length of 22 nucleotides. They are transcribed as primary miRNAs and processed to precursor and mature miRNAs. In most cases, they mediate gene repression by interacting with the 3′ UTR of their pre-mRNA target and recruiting Argonaute (Ago) proteins to form the RNA-induced silencing complex (RISC).12,13,14 lncRNAs are noncoding transcripts that are longer than 200 nucleotides. They can be intergenic, intronic, antisense in protein-coding genes, or derived from pseudogenes. Many lncRNAs are spliced and polyadenylated. They participate in all aspects of genome organization, cell structure, and regulation of gene expression via interactions with other RNA molecules, DNA, and/or proteins.9,15,16 piRNAs are small ncRNAs of 24–31 nucleotides that interact with PIWI proteins, the germline subclass of Ago proteins. They mediate transposon silencing, preserve germline genome integrity, and participate in chromatin remodeling and mRNA degradation.17,18 circRNAs are single stranded, covalently closed RNA molecules, generated by the back-splicing of pre-mRNAs. Some circRNAs can be translated into peptides, whereas others act as transcriptional regulators, sponges for miRNAs, protein scaffolds, decoys, or recruiters.19,20 Table 1 summarizes key mitochondria-related ncRNAs and their roles in the regulation of mitochondrial genes and pathways. Further information on miRNAs targeting specific mitochondrial mRNAs can be found in Table S1 and the review paper by Jusic et al.4
Table 1.
Summary of mitochondria-related ncRNAs and their role in mitochondrial physiology
| ncRNA | Origin | Regulatory role in mitochondria | Reference |
|---|---|---|---|
| miR-30 | nDNA | inhibits mitochondrial fission by suppressing p53 expression and its downstream target dynamin-related protein 1 (DRP1) | Li et al.21 |
| miR-140 | nDNA | promotes mitochondrial fission by suppressing mitofusin 1 (Mfn1) expression | Li et al.22 |
| miR-125b | nDNA | reduces mitochondrial respiration and promotes mitochondrial elongation by silencing BCL2-interacting killer (BIK) and mitochondrial fission process protein 1 (MTP18), respectively | Duroux-Richard et al.23 |
| miR-181c | nDNA | mediates respiratory complex IV remodeling by regulating gene expression of mitochondrial cyclooxygenase 1 and 2 (mt-COX1/2) | Das et al.24 |
| miR-378 | nDNA | downregulates the F0 component of ATP6 | Jagannathan et al.5 |
| miR-34a | nDNA | inhibits mitophagy by suppressing PTEN-induced putative kinase 1 (PINK1) expression | Tai et al.25 |
| miR-27a and miR-27b | nDNA | inhibits mitophagy by silencing PINK1 | Kim et al.26 |
| miR-338 | nDNA | decreases respiration of axonal mitochondria by targeting the 3′ UTR of cytochrome c oxidase IV (COXIV) and reducing its mRNA levels | Aschrafi et al.27 |
| miR-15b | nDNA | prevents mitochondrial depolarization and mitochondrial ROS generation by silencing sirtuin 4 (SIRT4), and inducing its downstream targets cytochrome c, mitochondrial transcription factor 1 (TFAM), and nuclear respiratory factor 1 (NRF1) | Lang et al.28 |
| let-7a | nDNA | destabilizes the mRNA of mitochondrial NADH dehydrogenase subunit 4 (ND4) and mediates metabolic reprogramming | Sharma et al.29 |
| miR-1 | nDNA | promotes the translation of cytochrome c oxidase subunit 1 (COX1) and mitochondrial NADH-ubiquinone oxido-reductase chain 1 (ND1) in differentiating myoblasts | Zhang et al.30 |
| miR-21 | nDNA | promotes the translation of cytochrome b (CYTB) in cardiomyocytes | Li et al.31 |
| miR-5787 | nDNA | promotes the translation of cytochrome c oxidase subunit 3 (COX3) and mediates metabolic reprogramming | Chen et al.32 |
| miR-2392 | nDNA | represses transcription of mtDNA and downregulates mitochondrial NADH-ubiquinone oxido-reductase chains 2, 4, and 5 (ND2-5), CYTB, and COX1 | Fan et al.33 |
| lncND5, lncND6 and lncCyt b | mtDNA | stabilizes their complementary ND5, NADH dehydrogenase subunit 6 (ND6), and CYTB mRNAs, respectively, by forming RNA-RNA duplexes | Jusic et al.,4,11,34 Ren et al.,4,11,34 Rackham et al.4,11,34 |
| LIPCAR | mtDNA | regulates atrial fibrosis via TGF-β/Smad pathway | Wang et al.35 |
| Kcnq1ot1 | nDNA | reduces miR-378a levels and rescues ATP6 expression | Durr et al.36 |
| Cerox1 | nDNA | binds to miR-488-3p and promotes the expression and activity of mitochondrial respiratory complex I | Sirey et al.37 |
| circRNA SCAR | mtDNA | binds to mitochondrial ATP synthase subunit β (ATP5B) and inhibits mitochondrial ROS production | Zhao et al.38 |
| mecciND1 and mecciND5 | mtDNA | mediates mitochondrial entry of proteins | Liu et al.39 |
| mcPGK1 | mtDNA | interacts with translocase of outer mitochondrial membrane 40 (TOMM40) and promotes mitochondrial import of phosphoglycerate kinase 1 (PGK1) to mediate metabolic shift from oxidative phosphorylation to glycolysis | Chen et al.40 |
| circPUM1 | nDNA | binds to ubiquinol-cytochrome c reductase core protein 2 (UQCRC2) and modulates mitochondrial respiratory complex III assembly | Gong et al.41 |
mtDNA, mitochondrial DNA; nDNA, nuclear DNA; LIPCAR, long intergenic noncoding RNA predicting cardiac remodeling; SCAR, steatohepatitis-associated circRNA ATP5B regulator.
Among the above-described ncRNAs, mitochondria-related miRNAs are the most studied and can be separated into three classes based on their genetic origin and subcellular localization: (1) nuclear-encoded miRNAs targeting mitochondria-related transcripts in the cytoplasm, (2) nuclear-encoded miRNAs translocating to mitochondria, and (3) mtDNA-encoded miRNAs.4,42 The two latter classes are also designated as mitochondrial miRNAs (mitomiRs). For instance, class 1 members, such as miR-30, miR-140, and miR-125b, regulate mitochondrial dynamics by targeting different components of the fusion/fission machinery.21,22,23 As an example of a class 2 miRNA, miR-181c was shown to translocate into the mitochondria of mouse cardiac myocytes and to regulate gene expression of mitochondrial cyclooxygenase 1 and 2.24 Kuthethur et al. identified 13 differentially expressed mitochondrial genome-encoded miRNAs (class 3) in breast cancer cell lines in comparison to a non-malignant breast epithelial cell line, and in breast cancer compared with normal tissue specimens.43 NcRNA-805, previously known as miR-805, was shown to be induced in response to cellular stress and to play a role in the regulation of the tricarboxylic acid cycle.44 In response to type 1 diabetes-induced hyperglycemia, mitomiR-378 regulates the mitochondrial-encoded F0 component of ATP6 in cardiomyocyte HL-1 cells.5 Multiple studies suggest an association of mitomiRs with various diseases including cancer and cardiovascular diseases indicating their clinical importance as biomarkers or therapeutic targets.4,45
In addition to miRNAs, several lncRNAs have been related to mitochondria.6,46,47,48 lncRNAs potentially encoded by the mitochondrial genome have been identified in publicly available transcriptome databases or detected by northern blot and reverse-transcription quantitative PCR (RT-qPCR).34,49 Novel lncRNAs have been associated with cardiovascular and liver diseases. For instance, long intergenic noncoding RNA predicting cardiac remodeling has been proposed as a biomarker for patients with heart failure.50,51 Liu et al. reported that mtDNA-encoded circRNAs serve as molecular chaperones for the folding of proteins imported into mitochondria, and mediate mitochondria-to-nucleus communication.39 Mitochondria-localized circRNA steatohepatitis-associated circRNA ATP5B regulator binds to ATP synthase subunit β and inhibits mitochondrial reactive oxygen species (ROS) production and activation of liver fibroblasts, a critical step in the progression of non-alcoholic steatohepatitis.38,52 Latest next-generation sequencing (NGS) analyses leveraged piRNAs generated from mitochondrial tRNA genes in mouse primordial germ cells and somatic cells, as well as in human normal and cancer cell lines.53,54 It was suggested that these mtDNA-encoded piRNAs play a role in anterograde and retrograde signaling between mitochondria and the nucleus. Of note, mitochondria have been described as essential to piRNAs biogenesis, which involves the nuclease activity of MitoPLD/Zucchini, a mitochondria-anchored member of the phospholipase D superfamily.55,56,57
Despite accumulating evidence on the existence of mitochondrial ncRNAs, there are significant conceptual and technical challenges in studying their biological impact under physiological and pathological conditions. Our knowledge of the biogenesis and processing of these RNAs, and their transport in and out of mitochondria, is limited by important gaps and numerous controversies (Figure 1).10,58 To date, there is no proof of the presence of enzymatic activities involved in the processing and maturation of ncRNAs inside the mitochondrial matrix. Although components of the RISC including Ago2 have been shown to localize in mitochondria, there is no conclusive evidence of miRNA-mediated regulation of mitochondrial transcripts taking place in the mitochondria matrix or in the cytoplasm.30,59,60 Different facilitators of RNA import into the mitochondrial compartment have been suggested, including the ribonuclease polynucleotide phosphorylase and the RNA binding protein GRSF1, but how ncRNAs are being shuttled between mitochondria and the cytoplasm or the nucleus remains elusive.61,62 Last, but not least, it is well established that miRNA-mediated repression of gene expression is relatively mild.63,64 Many newly identified mitochondrial ncRNAs show low abundance, which renders their functional relevance questionable, at least in the specific biological context (cell type, growth conditions) they are being studied.65,66
Currently, there are no standardized protocols and guidelines to conduct expression profiling of mitochondrial RNAs and deep functional characterization. The main obstacles to studying mitochondrial RNAs are (1) a lack of techniques allowing isolation of uncontaminated mitochondria/mitoplasts from other membrane-bound vesicles, especially from small amounts of tissue, (2) mitochondrial ribonuclease activities degrading mitochondrial RNAs during the isolation process, (3) a lack of standardized assays for RNA-seq and RT-qPCR, and (4) a lack of reliable reference genes for relative expression quantification. These technical challenges hinder the accurate profiling of RNAs (especially ncRNAs) in the mitochondrion, which explains the ongoing debate and controversies on the existence of different types and functions of RNAs in this organelle.
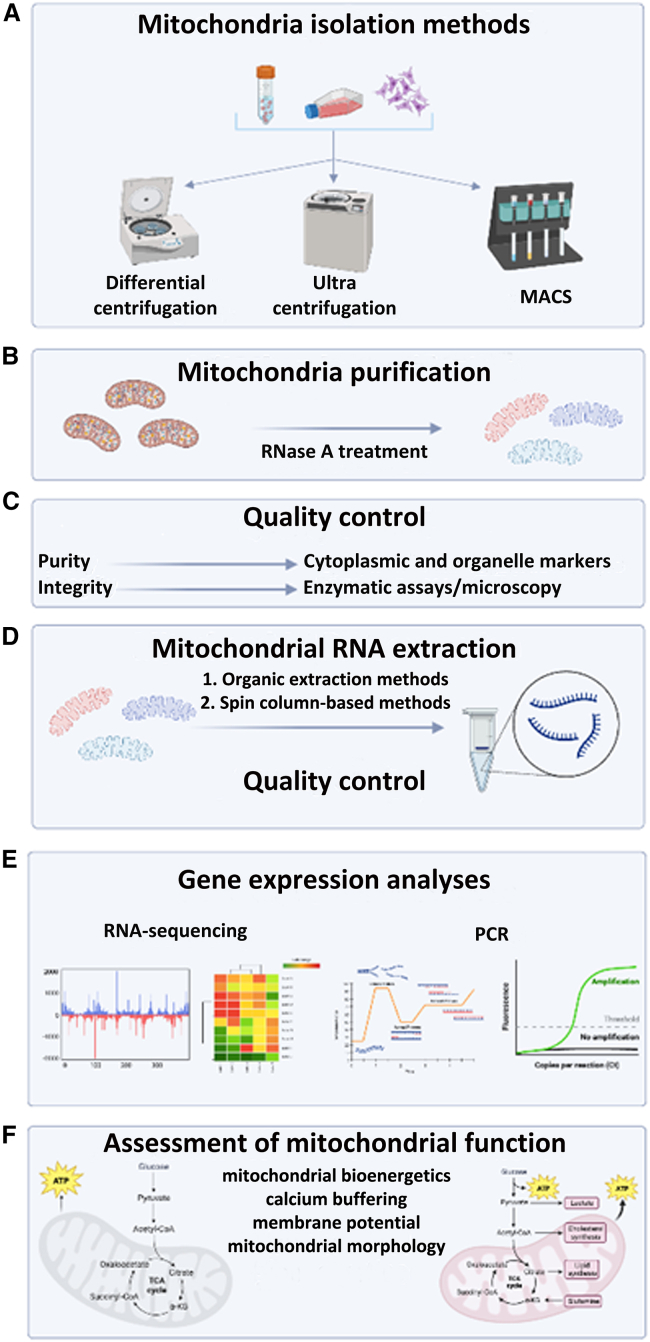
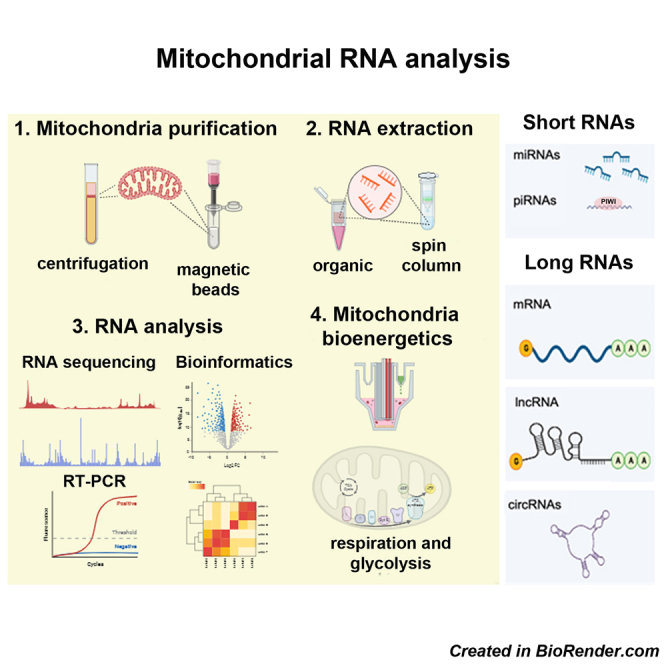
To address these challenges, experts from the EU-CardioRNA and AtheroNET COST Actions networks summarize current techniques with an emphasis on critical steps during the experimental procedure that are prone to introduce bias and/or artifacts in the study of mitochondrial ncRNAs.67 Figure 2 displays the workflow of mitochondrial RNA expression profiling, comprising five steps that constitute the first technical sections of this article, starting from (1) mitochondria isolation to (2) mitochondrial purification, (3) quality control, (4) RNA extraction and quality control, and (5) RNA expression profiling. The last section includes recommendations on the assessment of the role of RNAs in the regulation of mitochondrial function as well as recent advances in detecting and targeting these ncRNAs inside the mitochondrial compartment (see functional analyses, Figure 2F).
Figure 2.
Workflow of mitochondrial RNA expression profiling
(A) Isolation of mitochondria by differential centrifugation, ultra-centrifugation using density gradients, or antibody-mediated capture using magnetic separation (MACS). (B) Mitochondria purification after RNase A treatment to remove cytosolic RNAs from the outer mitochondrial membrane. (C) Quality control of mitochondrial preparations using enzymatic assays, microscopy, and enrichment analysis of mitochondrial markers. (D) Mitochondrial RNA extraction and quality control. (E) Gene expression analyses including RNA sequencing and RT-qPCR. (F) Assessment of mitochondrial function (created with BioRender.com).
Mitochondria isolation methods
The starting and critical point in mitochondrial RNA expression profiling and functional characterization is the isolation of highly pure mitochondria (Figure 2A). Different techniques have been developed to extract mitochondria from cells and tissues with most of them relying on differential centrifugation (DC), ultra-centrifugation with density gradients, affinity purification of the organelle by magnetic sorting, or free-flow electrophoresis (FFE).
DC
DC is a widely employed technique for the isolation and purification of mitochondria for functional analyses such as the measurement of O2 consumption, transmembrane potential, ROS formation, ATP production, and swelling.68 The DC method is based on a gentle homogenization of the sample followed by a series of centrifugations of increasing centrifugal force to pellet crude mitochondria. Several DC protocols for mitochondria isolation have been described previously.68,69,70,71,72
An important factor to consider during mitochondria isolation for RNA expression profiling studies using DC is the mechanical force applied for tissue or cell homogenization, which varies by cell and tissue type. For example, soft tissues such as the kidney, brain, and liver require gentle mechanical forces, while harder tissues such as cardiac and skeletal muscles require much stronger mechanical forces.73 Of note, all buffers used for homogenization and centrifugation should be ice-cold and have a physiologically relevant pH with an ionic and osmotic strength compatible with the cytosol. Fernández-Vizarra et al. described a detailed protocol for mitochondria isolation for biogenetic studies from brain, heart, kidney, liver, and cultured cells.73 The advantage of DC is that the technique is inexpensive and relatively quick (1–2 h) compared with other techniques. However, this technique has some limitations: (1) it requires a large amount of sample, restricting its use for tissue biopsies or primary cells and (2) mitochondria can be contaminated with other cellular components which require additional washing and centrifugation steps.
Several commercially available mitochondria isolation kits exist that are based on the DC approach. These kits require standard laboratory equipment and constitute theoretically quick and simple procedures, yielding pure and functional mitochondria from cell culture and tissue samples that can be further used in almost any downstream application. Kits are attractive when limited starting material is available and a large number of samples must be processed in a reproducible manner in terms of mitochondrial quantity and quality. Mitochondrial integrity is well preserved, but the yield may be lower compared with manual procedures. Although no special equipment is required, the relatively high cost of kits can be a limiting factor for their systematic use.70,74
Ultra-centrifugation with density gradients
Density gradient centrifugation (DGC) relies on the combination of basic mitochondrial extraction using DC with further purification employing one or more gradients.75,76 A detailed protocol on mitochondrial isolation using DGC has been described by Graham.77 In DGC, the tissue or cellular extract is layered over a sucrose or a Percoll cushion and centrifuged at a certain speed (17–31,000 × g) causing the mitochondria to be isolated from other cellular components according to their densities. This method is often used to isolate brain mitochondria with very low contamination from synaptosomes. Thus, while the purity of mitochondria isolated by DGC is higher than for DC and can meet criteria for some applications, such as mitochondrial proteomics analyses, investigation of mitochondrial morphology and mitochondrial apoptosis, a limitation of DGC is the lower yield of purified mitochondria compared with DC.78 Contamination by other subcellular particles may necessitate additional washing and centrifugation steps (see quality control of mitochondrial preparations). In addition, the DGC method is more laborious and time-consuming than DC.72
Mitochondria isolation based on magnetic-activated cell sorting
Mitochondria isolation based on magnetic-activated cell sorting (MACS) is a separation technique commonly used for isolating different types of cells or organelles based on binding to antibodies coupled to paramagnetic beads. The MACS technique ensures the isolation of mitochondria of good purity. It uses magnetic beads coated with antibodies targeting the translocase of the outer mitochondrial membrane protein 22 (TOMM22) at the surface of mitochondria.79 When optimized, this procedure can be completed in less than 30–45 min with a success rate, purity, and integrity significantly higher compared with DGC for neuronal synaptosomal mitochondria although not for total brain mitochondria.80 In addition, the MACS method allows the extraction of mitochondria from tissues and cells with low mitochondrial content.
Among the current mitochondrial extraction and purification methods, the magnetic bead method shows better performance in impurities elimination including microsomes and peroxisomes compared with the DC method.81 Reports also suggest that isolated mitochondria by MACS protocols are well suited for mitochondrial RNA expression profiling studies.59,82 MACS can be automated and standardized. However, the MACS method requires substantial amounts of antibodies, which can lead to prohibitive costs for studies including numerous samples. Also, the quantity of tissue or cells that can be used as input material is limited, thereby reducing the yield of isolated mitochondria.
FFE
FFE is a well-established method that allows the isolation of mitochondria with high resolution and purity compared with other methods.83,84 In FFE, a sample is continuously fed into a chamber filled with a flowing buffer. An electric field is applied perpendicular to the flow, causing charged particles to separate into distinct streams based on their mobility. However, commercial FFE systems typically require large sample volumes, which can be restrictive when only small sample sizes are available, such as rare cells or small tissue samples.85 In addition, extended exposure to electric fields and chemicals could potentially compromise the structural integrity of mitochondria due to their delicate nature.85
Advantages and limitations of mitochondria isolation methods are summarized in Table 2.
Table 2.
Advantages and limitations of mitochondria isolation methods
| Mitochondria isolation | ||
| Method | Advantages | Limitations |
| differential centrifugation | ✓cost-effective ✓quick |
✕ a large amount of sample required ✕ high contamination by other organelles |
| ultracentrifugation with density gradients | ✓high mitochondria purity | ✕ time-consuming ✕ laborious ✕ low yield of mitochondria |
| affinity purification by magnetic sorting | ✓high reproducibility ✓quick and effective |
✕ limited sample quantity ✕ expensive at large scale |
| free-flow electrophoresis | ✓high purity and reproducibility | ✕ prolonged exposure to electromagnetic field can damage mitochondrial integrity ✕ requires high sample input |
Mitochondria purification
Successful mitochondrial RNA expression profiling depends on the preparation of highly purified mitochondria with low amounts of contaminating cytosol and organelles, as other organelles also contain RNAs. Additional purification procedures (Figure 2B) to remove outer mitochondrial membrane-associated RNAs using digestion with RNase A, followed by incubation with sodium dodecyl sulfate (SDS) and/or proteinase K to inactivate exogenous and resident RNases are required.
Detailed protocols to eliminate cytosolic RNAs from the outer mitochondrial membrane using RNase A treatment described previously.59,86,87 For downstream mitochondrial RNA isolation, RNase A must be removed to prevent later degradation of the purified intra-mitochondrial RNA. This can be achieved by proteinase K treatment and requires subsequent washing twice in suspension buffer followed by recovery by centrifugation (e.g., 13,000 × g for 2 min at 4°C) to remove the protease. While proteinase K and SDS combined with elevated temperatures (100°C) can be used in protocols for mitochondria preparation for RNA extraction, these conditions impact RNA stability. To overcome this obstacle, Huang and Wang proposed a method using SDS lysis coupled with a milder temperature (70°C) incubation, which denatures most proteins without causing RNA instability.88 A subsequent proteinase K digestion degrades surface proteins, providing a reliable protocol to isolate mammalian mitochondrial RNA suitable for RT-qPCR and other downstream assays. For each experimental procedure, it is important to assess the potential consequences of incomplete RNase A inactivation on mitochondrial RNA expression profiling.
Quality control of mitochondrial preparations
Quality control of mitochondrial preparations constitutes an obligatory step in any pipeline investigating mitochondrial RNA expression. The quality control of mitochondrial preparations (Figure 2C) includes evaluation of purity, defined as relative enrichment in mitochondrial markers and by the absence of contaminating proteins from other organelles such as lysosomes, peroxisomes, endoplasmic reticulum, and endosomes. Possible contaminants of mitochondrial preparations by other organelles can be assessed by measuring the activity of acid phosphatase (lysosomes), glucose-6-phosphatase (microsomes), or catalase (peroxisomes).89
Mitochondrial integrity is defined by the measurement of functional characteristics and may be monitored by measuring activity of the mitochondrial matrix enzyme citrate synthase and the inner membrane protein cytochrome c oxidase.59 Integrity of mitochondrial membrane structure may also be assessed by confocal microscopy following immunostaining against mitochondrial markers, but also transmission electron microscopy or atomic force microscopy to detect ultrastructural abnormalities, such as disrupted cristae and mitochondrial swelling.90,91,92 However, many published studies investigating mitochondrial RNA expression did not include these quality control steps systematically. Since sequencing techniques are very sensitive, they can easily detect small amounts of contaminating cytosolic RNAs leading to possible data misinterpretation. Thus, obtaining pure mitochondrial preparations and an RNA pool devoid of cytosolic RNAs is a prerequisite for reliable RNA studies.
Commonly used methods for monitoring the purity of mitochondria regarding RNA and protein content are RT-PCR and western blot analysis.90 In experimental RT-PCR settings, RNA isolated from cytoplasmic and pure mitochondria fractions are analyzed for the presence of, e.g., glyceraldehyde 3-phosphate dehydrogenase (unambiguous cytoplasmic marker) and mitochondrial genome-encoded markers (e.g., 16S rRNA, MT-CYB). Similar markers can be used for western blotting.
RNA extraction from mitochondria and quality control
RNA extraction methods
Numerous protocols have been proposed to isolate RNA from mitochondria based on organic extraction, spin columns, or paramagnetic bead-based extraction methods (Figure 2D).93 Geiger and Dalgaard described the isolation of mitochondrial RNAs from rat liver tissue and HepG2 cells based on phenol-guanidinium isothiocyanate-chloroform RNA extraction.87 An important factor to consider for the selection of appropriate procedures for mitochondrial RNA isolation is the low input of mitochondria for RNA extraction. Although mitochondria are highly abundant in brain, heart, or liver tissues, pre-processing preparation steps and RNase A treatment of mitochondria before RNA extraction result in significant loss of material, which consequently may lead to low yield of mitochondrial RNA. In line with that, several phenol-free commercial kits allowing RNA extraction from low input of mitochondrial samples may be recommended (Table 3).
Table 3.
Commercially available automated and manual extraction kits for the isolation of total RNA including small RNAs from low-input cell and tissue samples
| RNA extraction kit | Manufacturer | Input | Method | RNA biotypes |
|---|---|---|---|---|
| QIAzol TRI Reagent TRIzol |
QIAGEN Sigma-Aldrich Thermo Fisher Scientific |
up to 100 mg per 1 mL reagent or 1 mL per 107 cells | manual | total RNA including miRNAs |
| RNeasy Plus Micro and Mini Kits | QIAGEN | 5 × 105 cells or 5 mg (Micro kit) 107 cells or 30 mg tissue (mini kit) |
manual and automated | total RNA including miRNAs |
| RNAqueous Total RNA and Micro Isolation Kit | Thermo Fisher Scientific | 1–75 mg of tissue or from 102–107 cells | manual | total RNA including miRNAs |
| Arcturus PicoPure RNA Isolation Kit | Thermo Fisher Scientific | laser-capture micro-dissected samples and larger samples | manual | total RNA |
| MagMax mirVana Kit | Thermo Fisher Scientific | various low-input samples | manual and automated | total RNA including miRNAs |
| Total RNA Purification Plus Kit | Norgen | various low-input samples | manual | total RNA including miRNAs |
Organic extraction methods, such as the phenol-guanidinium isothiocyanate-chloroform method, offer superior RNA yields and stable isolation suitable for downstream applications. However, these protocols are susceptible to RNA contamination with phenol and other contaminants, and they are generally time-consuming compared with spin-column-based methods. On the other hand, spin-column-based methods provide reduced contamination risk due to the enclosed system but may provide lower RNA amounts compared with organic extraction.94,95 Commercial kits designed for mitochondrial RNA isolation enable RNA extraction from low-input mitochondrial samples. However, they tend to be more expensive than manual extraction methods, and there is variability in performance among different kits.
Overall, the choice of RNA isolation method should consider factors such as the RNA species of interest (e.g., short or long RNAs), downstream applications (e.g., NGS or PCR), and considerations of time, cost, and sample input. Each method has its advantages and limitations, and the selection of the most appropriate method for specific study requirements should be carefully considered.
Quality control of mitochondrial RNA
Fast, accurate, sensitive, and specific quantification of integrity, quality, and purity of mitochondrial RNAs for downstream gene expression analyses can be achieved using Qubit Fluorometers (high-sensitivity and/or microRNA kits) and automated electrophoresis methods (Agilent Bioanalyzers or TapeStation).96,97 Due to low RNA yields from mitochondria, standard optical density methods (OD260nm) may not be applicable for accurate quality control. RNA integrity is critical for subsequent profiling, and ongoing degradation in low quality samples often leads to overestimation of RNA concentration.98
Mitochondrial RNA expression analyses
Molecular profiling methods of mitochondrial RNAs include NGS, PCR-based methods, and microarrays (Figure 2E). NGS is a time-consuming approach, requiring a great investment in subsequent bioinformatic analyses, but can generate information on novel miRNAs, and detect different isoforms (isomiRs) and post-transcriptional modifications. PCR-based analyses and microarrays on the other hand are compatible with quantification of known RNAs. A summary of the advantages and limitations of these approaches is presented in Table 4.
Table 4.
Advantages and limitations of different approaches for mitochondrial RNA expression analysis
| RNA expression analysis | ||
| Method | Advantages | Limitations |
| next-generation sequencing | ✓high throughput ✓identification of novel ncRNAs and their modifications |
✕ laborious and long bioinformatic analysis ✕ bias introduced during library preparation |
| single-molecule real-time sequencing/Nanopore sequencing | ✓generation of full-length transcripts ✓identification of novel ncRNAs and their modifications ✓amplification bias bypassed |
✕ laborious and long bioinformatic analysis ✕ high cost |
| qPCR | ✓rapid ✓cost-effective ✓absolute (standard curve required) and relative quantification |
✕ limited to known ncRNAs ✕ lack of reliable reference RNAs ✕ sensitive to RNA quality |
| droplet digital PCR | ✓no standard curve required for absolute quantification ✓discriminatory power for low-input or low-quality samples |
✕ limited to known ncRNAs ✕ low dynamic range compared with qPCR ✕ high cost |
NGS
Small RNA-seq has become a popular method for high-throughput miRNA profiling.99,100 Nevertheless, NGS data on mitochondrial ncRNAs often fail to be reproduced, and comparison of datasets obtained by different library preparation approaches requires great caution.101,102,103,104,105 Efficient representation of miRNAs in the library to be sequenced depends on the RNA isolation method (the use of spin columns capable of retaining RNA molecules that are greater than 10 nucleotides is highly recommended), rRNA depletion and enzymatic ligation of adapters to the RNA molecules.106,107,108 RNA ligases show differential preference for miRNAs based on their structure, sequence, strand orientation, and post-transcriptional modifications, leading to adapter ligation bias, and subsequent reverse transcription and amplification biases.109,110 Thus, some miRNA species or variants of a given miRNA may be favored during library preparation at the expense of others, compromising their quantitative analysis. The use of randomized adapters and/or the addition of polyethylene glycol in the ligation reaction allow overcoming the ligation bias.101,102,103 The sensitivity of commercially available library preparation kits has been greatly improved to overcome the limitations of low-input mitochondrial RNA (input <100 ng of total RNA or sub-ng amounts of miRNA). However, none of these kits is able to accurately reflect the relative amounts of all miRNAs in the original sample.103 Increasing sequencing depth improves sensitivity, but also favors the detection of transcriptional noise. Indeed, a substantial number of novel miRNAs identified by RNA-seq fail to be confirmed in subsequent studies.101,102 Experimental validation of newly identified miRNAs is required prior to any further functional study and involves the detection of both the precursor and mature forms of these RNAs (at endogenous levels or after ectopic expression) by northern blot.
Third-generation, long-read sequencing approaches, such as single-molecule, real-time sequencing (available from Pacific Biosciences), and Nanopore Sequencing (available from Oxford Nanopore Technologies), provide full-length transcripts, bypassing the amplification bias and the need of assembly.111,112,113 Their use in the study of mitochondrial ncRNAs is so far very limited due to their high cost, but they hold great promise for the identification of novel RNA molecules/isoforms and RNA modifications.49,114
PCR-based methods
RT-qPCR is considered a routine technique to measure gene expression in various sample types including mitochondria. Although RT-qPCR is widely used, several factors may lead to quantification bias including: (1) variations in protocols, reagents, sample quality, and instruments, (2) inconsistent data analysis, (3) the investigation of samples containing low amounts of the template with small expression differences of 2-fold or less, (4) sample quality heterogeneity, which affects the efficiency of RT-qPCR, and (5) differences in interpretation or data-analytical protocols within and across laboratories.115,116
These limitations also apply to RNA-seq and some of them may be overcome using droplet digital PCR (ddPCR), a method that provides ultrasensitive nucleic acid detection and absolute quantification. Although both techniques, RT-qPCR and ddPCR, utilize Taq polymerase in a standard PCR reaction and pre-validated primer or primer/probe assays, they have major differences: (1) in ddPCR, samples are portioned into thousands of individual droplets that undergo PCR amplification and (2) following PCR, each droplet is analyzed using Poisson statistic to determine the target gene concentration in the tested sample.115 ddPCR allows direct and independent quantification of target genes without standard curves and resolves quantification of low abundance targets, which are below the detection limits of other PCR platforms. Thereby, an advantage of ddPCR technology is its discriminatory power for low-target quantitation (quantification cycle [Cq] ≥ 29) including mitochondrial RNAs as well as low-input samples and/or samples containing variable amounts of chemical and protein contaminants.117 A low dynamic range as compared with other PCR methods can be a limitation of ddPCR, and its use at a routine level is restrained by the elevated costs for instrumentation and maintenance.
Gene expression analysis
The two most widely used methods to analyze RT-qPCR data are absolute quantification and relative quantification. While absolute quantification determines the input copy number, usually by relating the PCR signal to a standard curve, relative quantification relates the PCR signal of the target transcript to that of another transcript used as a control. This process is known as normalization and is a crucial step in RT-qPCR analysis.
The most common method to normalize RNA expression data is to use stably expressed reference genes as internal controls for monitoring RNA extraction, reverse transcription, and qPCR efficiency.118 It is recommended to use multiple reference genes instead of a single one to obtain more accurate results.118,119 However, to date, no universal reference genes and no common rules for PCR normalization have been defined, especially for mitochondrial RNAs, and even less for miRNAs and other ncRNAs. This gap limits the comparison of results between studies and is still challenging to address. Different normalization strategies may give different results and even lead to misinterpretation of data.120,121 Reference genes that are stable under some conditions may change significantly in other conditions or diseases. Thus, validation of the optimal reference genes in each individual experimental and clinical setting is important to limit biases.118,122 Strikingly, only a few studies justify the choice of reference gene(s) and report its/their stability in their experimental setting. Different algorithms, including Normfinder, geNorm, and BestKeeper, can also be used for the selection of optimal normalization genes for specific experimental conditions.119,123,124 In a comparative study, these algorithms converged to the same most stable and less stable reference genes, but the ranking was different depending on correction against PCR efficiency.125
As far as mitochondrial RNAs and particularly mitomiRs are concerned, the choice of optimal reference genes depends on whether the mitomiR expression is studied in mitochondrial, whole-cell, tissue extracts or plasma/serum. U6 and other small nucleolar RNAs (snRNAs) are commonly used for the normalization of miRNA expression in cells and tissues, and they have been used for the normalization of mitomiRs expression as well.5,29,33,43,126,127,128,129 The use of nucleus-enriched snRNAs as normalizers for RNA species purified from mitochondrial extracts is inappropriate, because they are substantially, yet variably, depleted in mitochondrial extracts. Using TaqMan miRNA RT-qPCR arrays and the global mean normalization method, Wang et al. observed that U6 snRNA levels were 13-fold lower in mitochondria compared with the cytosolic fraction of hippocampal tissue.130,131 Moreover, the expression of snRNAs may not be stable in all pathological conditions and their use as reference genes may introduce bias in miRNA expression analysis.121,122 Furthermore, snRNAs have different biochemical properties compared with miRNAs, which may lead to different efficiencies in RNA extraction, RT reaction, and qPCR. 5S and 12S rRNAs are also commonly used as reference genes for miRNA quantification.32,43,132 12S rRNA is a mitochondrial gene product and 5S rRNA is present in both cytosolic and mitochondrial fractions. Das et al. used 12S rRNA as a reference gene when comparing miR-181c expression in mitochondrial compared with total heart tissue fraction and 5S rRNA when comparing miR-181c expression in cytosolic and mitochondrial fractions.132 A similar normalization strategy has been used in cancer cell lines.32
Stably expressed miRNAs, robustly present in mitochondria, would be preferable reference genes for expression analysis of mitomiRs.65,122 Zheng et al. used miR-320a to normalize mitomiR expression in mitochondrial extracts as it was abundantly expressed in mitochondrial extracts and did not change during osteogenic differentiation of human mesenchymal stem cells.126 MiR-103a was used to normalize mitomiR expression in mitochondrial extracts of colorectal adenomas.133 If multiple subcellular fractions are to be compared, it may not be possible to find an appropriate reference gene due to the different RNA composition of subcellular fractions. To overcome this problem, Wang et al. used the global mean normalization method for comparing miRNA expression in multiple subcellular fractions and found that miR-19b levels did not differ between cytosolic and mitochondrial fractions. They consequently used this miRNA to normalize miR-155 and miR-223 expression in hippocampal cytosolic and mitochondrial fractions.130
The addition of synthetic spike-in miRNA standards is a good alternative allowing normalization against an external control.65,122 Spike-in miRNAs are added at determined concentrations (within the linear dynamic range of the assay) immediately after lysis of the original sample, and therefore serve as readouts of technical variation throughout the whole experimental procedure, from RNA purification to RT-qPCR or library preparation and RNA-seq. On the contrary, spike-in standards do not allow correction against biological variability between samples, and their combined use with internal reference genes is recommended when comparing independent experiments.
Quantification of miRNA molecules per mitochondria or per cell is more informative on the biological relevance of gene expression changes. This approach involves the generation of standard curves for the miRNA of interest, as well as mitochondrial and nuclear DNA that are used for normalization.65 Alternatively, the expression levels of miRNAs per cell or mitochondria can be assessed by quantitative fluorescence imaging (see functional analyses).
Bioinformatic analyses
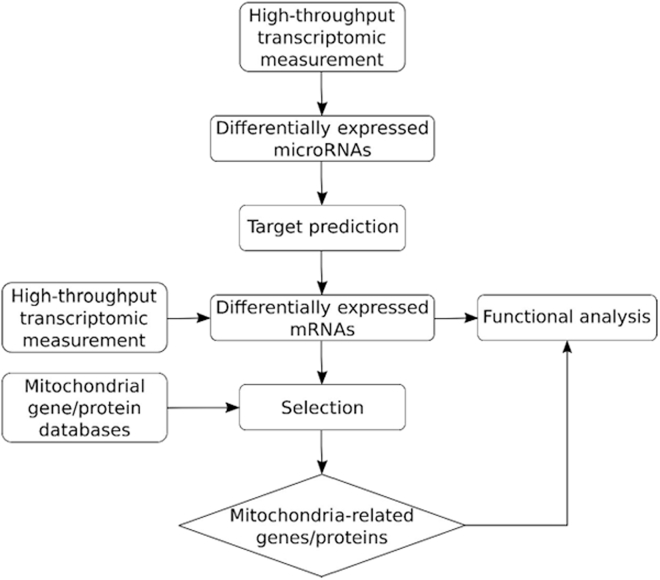
Bioinformatic methodologies could be applied to narrow down the bulk transcriptomic data to the expression profile of mitochondria-related RNAs. For this purpose, the most widespread approach is to use mitochondrial gene/protein databases that integrate various sources on mitochondrial involvement.134,135,136 Mitochondrial gene/protein databases, such as MitoCarta3.0 or MitoMiner4.0, provide information on genes encoding mitochondria-localized proteins or proteins involved in mitochondria-related processes, based on experimental data (e.g., green fluorescent protein tagging and mass spectrometry), manual curation (literature and public databases), and computational evidence (Table 5).134,135,136,137,138,139 Using such mitochondrial gene/protein databases, differentially expressed genes derived from RNA-seq can be filtered for mitochondria-related differentially expressed genes (Figure 3).140,141,142,143
Table 5.
Mitochondrial gene/protein databases for identifying mitochondria-related genes in bulk transcriptomic datasets
| Database name (latest release) | Available species | Evidence category | Reference |
|---|---|---|---|
| MitoCarta3.0 2020 | human and mouse |
|
Rath et al.139 |
| MitoMiner4.0 2018 | several species including human, mouse, and rat |
|
Smith and Robinson138 |
| Integrated Mitochondrial Protein Index (IMPI) 2021 | several species including human, mouse, and rat |
|
Smith et al.144 |
| MitoProteome 2022 | several species including human, mouse, and rat |
|
Cotter et al.145 |
Figure 3.
In silico analysis of mitochondria-related ncRNAs
Possible approaches to retrieve expression profiles of mitochondria-related RNAs from bulk transcriptomic data. Appropriate functional tests will confirm the biological relevance of the differentially expressed miRNAs and validate the link between these miRNAs and their predicted mRNA targets.
Alternatively, non-specific sources (e.g., UniProt, large-scale projects like Human Protein Atlas), pathway (e.g., Reactome, Kyoto Encyclopedia of Genes and Genomes), and functional (e.g., Gene Ontology, MSigDB) databases also provide information on subcellular localization and function of mitochondrial RNA-encoded proteins, and thus can be utilized to examine bulk transcriptomic datasets to identify dysregulated mitochondria-related genes.134,146,147,148,149,150,151,152,153 These non-specific sources are often integrated in specific mitochondrial gene/protein databases as well.138 Gene ontology and pathway enrichment analysis tools can be used to analyze the narrowed-down mitochondria-related gene sets to provide further specific functional information on the genes of interest.142,143,154,155
As ncRNAs and especially miRNAs constitute major regulators of gene expression, identification of differentially expressed mitochondria-related genes can be obtained indirectly from miRNA-seq data by predicting mitochondria-related targets for differentially expressed miRNAs.156,157,158 With genome-wide target prediction using experimentally validated miRNA-target interaction databases (e.g., miRTarBase, miRecords) and/or prediction algorithms (e.g., PicTar, miRanda, DIANA-microT, TargetScan), genes likely regulated by the miRNAs of interest can be retrieved.159,160,161,162,163,164 Sequence-based prediction tools for miRNA targets rely on complementarity of base pairing and evolutionary conservation. There is no perfect algorithm, especially for identifying targets of novel miRNAs. Combining multiple tools increases specificity of miRNA target recognition at the expense of sensitivity. Selection of the appropriate tool also depends on the mRNA region (UTR, CDS) to be scanned.165,166,167,168,169 As an alternative approach, mitochondrial genome-wide miRNA-target predictions rely on different sequence-based prediction algorithms to scan the mitochondrial genome for possible targets of the investigated miRNAs.157,170,171
These approaches can be used to define possible actions of mitochondrial RNAs. Although bioinformatic approaches that are capable of determining the localization of RNAs in mitochondria exist, the accuracy and the coverage of these approaches could be further increased.172,173
Functional analyses
Mitochondria-related ncRNAs have been linked to the regulation of different aspects of mitochondrial physiology, in particular mitochondrial bioenergetics, metabolic reprogramming, and the expression/function of specific electron transport chain (ETC) subunits (summarized in Tables 1 and S1).4,42,46,174,175 Regulation of mitochondrial metabolism by ncRNAs is mediated at multiple levels of gene expression, including transcription, RNA degradation, translation, protein import into mitochondria, and assembly of respiratory complexes.
As previously discussed, the majority of known mitochondria-related miRNAs affect the stability of their mRNA targets, leading to the downregulation of ETC subunits, reduced mitochondrial respiration and increased ROS production.5,24,27,29,36 Other miRNAs, such as miR-1, miR-21, and miR-5787, regulate mitochondrial respiratory chain complexes by promoting the translation of specific subunits.30,31,32 miR-2392 downregulates mitochondrial genome-encoded ETC subunits by repressing mtDNA transcription.33 circRNA mcPGK1 mediates a metabolic switch from oxidative phosphorylation to glycolysis by promoting the import of PGK1 into mitochondria, whereas circPUM1 regulates mitochondrial respiratory chain complex III assembly by directly binding to UQCRC2.40,41 Lastly, some examples of cross-regulation between ncRNAs have been documented. In particular, lncRNAs Kcnq1ot1 and Cerox1 inhibit miR-378a and miR-488-3p, respectively, and consequently upregulate the expression of the mRNA targets of these miRNAs.36,37
In the following paragraphs, we discuss the available systems and methodology to study the impact of ncRNAs on the function of mitochondria (Figure 2F). We emphasize critical points that could explain discrepancies among studies and should thus be considered when addressing the functional role of ncRNAs in mitochondria. We also discuss bioimaging approaches for the detection and quantification of mitochondria-localized ncRNAs and the current methodology for modulating their expression levels.
Experimental systems and spatial considerations for functional analyses of RNAs
Both in vivo (animal models) and in vitro (primary cell cultures, cell lines, differentiated cells from embryonic stem cells, or induced pluripotent stem cells, 2D/3D models) can be used for the study of RNAs in mitochondria (for studies in cardiomyocytes, neurons, and hepatocytes).176,177,178,179,180,181 Combining information from different models is essential for elucidating the biological function and deciphering the molecular mechanisms by which specific ncRNAs affect mitochondrial physiology.
Mature cells such as high-energy-demanding cardiomyocytes, neurons, and hepatocytes display spatially distinct mitochondrial subpopulations.182,183,184 In cardiac cells, mitochondria are classified as subsarcolemmal, nuclear, and/or interfibrillar. Subsarcolemmal and nuclear mitochondria lack specific organization. On the contrary, interfibrillar mitochondria are mainly tubular and are highly organized within contractile filaments. In addition to their morphological differences, these subpopulations show distinct metabolic profiles and differential responses to physiological stimuli, such as apoptotic signals.185,186,187,188 As expected, interfibrillar mitochondria display high oxidative phosphorylation activity to provide the necessary energy for contraction. These features suggest that ncRNAs may have differential effects on cardiac mitochondrial subpopulations and underline the importance of using specific protocols to isolate and analyze separately these subpopulations.188,189 Such an approach demonstrated that a pool of mitomiRs, including miR-378 that targets the ATP synthase subunit ATP6, shows differential redistribution between subsarcolemmal and interfibrillar cardiac mitochondria in response to diabetes.5,30
A novel approach, permeabilized cell mitochondrial function sequencing (PMF-seq), was recently developed to study the genetic profile of distinct mitochondrial subpopulations.190 In PMF-seq, cells are subjected to gentle permeabilization, which preserves mitochondrial physiology, selected for desired bioenergetic parameters using flow cytometry, and analyzed by NGS. This approach was successfully used to screen for genes that regulate mitochondrial respiration in a pool of CRISPR-mutagenized cells, but could also be combined with mitochondrial ncRNA profiling in the future.
Bioimaging of mitochondria and mitochondrial ncRNAs
Several imaging strategies for RNAs have been developed. One of them is single-molecule fluorescence in situ hybridization (smFISH), which gives information on RNA localization and quantification within the cell. The disadvantages of this method are linked to fluorophore limitations and rapid bleaching of the signal by lasers as well as to the necessary fixation (live imaging is not possible). When combined with immunostaining strategies, smFISH can be used to colocalize fluorescently labeled RNA into a specific cell compartment.191 This approach allows monitoring the localization of both exogenous and endogenous RNA. The smFISH technique was used to build the Multiplexed Error-Robust Fluorescence in situ Hybridization tool (Merscope). This novel platform works in high-throughput mode with high spatial resolution and sensitivity and its multiplexing is enhanced due to combinatorial labeling.192
Hybridization chain reaction has been lately connected with the photoacoustic imaging technique to detect miR-21, for instance.193 The advantage of this approach is its specificity to miR-21 (down to 1 base pair mutation) with a detection limit of 148 pM. Moreover, hybridization chain reaction combined with mass spectrometry was able to detect miR-21 at femto-level concentration (via accumulation of ultrasmall up-conversion nanoparticles).194
Another interesting platform that can be used for high-throughput imaging of mitochondrial RNAs in single living cells is Multiplexed Organelles Portrait Barcodes. In this system, core-shell mesoporous silica nanoparticles are biofunctionalized with the organelle-targeting peptide. Linking nanoparticles to dyes such as Cy3, Cy5, or AMCA at different ratios and combining them with molecular beacon detection probes allowed to create a tool that recognized mitomiR-155, -146a, -210, and -34a by confocal laser scanning microscopy. This approach was also successfully tested for miRNA alteration recognition in mitochondria and the endoplasmic reticulum related to Ca2+ homeostasis modulation. An advantage of this method is the ability to track real-time dynamic changes that can be applied in pathophysiological research.195
Assessing the impact of ncRNAs on mitochondria bioenergetics
Mitochondria bioenergetics is primarily analyzed using in vitro models, namely intact cells, permeabilized cells, whole-cell homogenates, or mitochondria-enriched fractions. The size of available biological material (tissue biopsy, isolated cells, mitochondrial fractions) is critical for the design of research protocols. The use of permeabilized cells combines several advantages: all mitochondria subpopulations are taken into account, mitochondrial networks and their interactions with other organelles are maintained, and, as opposed to the use of intact cells, the experimenter has direct control on the medium surrounding mitochondria.196 A titration assay of the permeabilization reagent (saponin) is required to avoid outer mitochondrial membrane damage (evidenced by cytochrome c release), taking into account that the assay buffer used for the subsequent analysis may have an additive effect on the degree of permeabilization.197 On the other hand, the use of mitochondrial fractions allows identifying defects on specific components of the ETC, which can be further confirmed and characterized by specialized enzymatic and assembly assays of mitochondrial respiratory complexes.198,199
Mitochondrial respiration can be analyzed by a variety of respirometry methods that measure oxygen consumption rate as a readout of oxidative phosphorylation.200,201,202,203,204 Seahorse analyzers are adapted for small samples, such as human biopsies. They allow rapid metabolic profiling of cells that goes beyond oxidative phosphorylation (glycolysis, fatty acid oxidation, glutaminolysis) and are ideal for high-throughput screens. Their use is limited by their high cost and the small number of possible injections (up to four). Clark-type electrodes, on the other hand, are inexpensive tools that allow multiple injections for a more precise characterization of the mitochondrial respiration phenotype. They are not suitable for small samples or for comparing multiple conditions simultaneously. The procedure is quite long, and the quality of the sample may decline during the analysis. Oxygraph 2k respirometry shares the same advantages with Clark-type electrode respirometry, with the addition of high sensitivity and precision, and minimal noise. The user benefits from great flexibility to adapt the protocol to specific questions and can combine respirometry with fluorometry to measure Ca2+ buffering and mitochondrial membrane potential. Recently, respirometry protocols have been optimized and adapted for the use of thawed samples to overcome the challenges of immediate sample processing and bioenergetics analysis in the clinical setting.205,206
Overall, these methods can provide insight into different aspects of how RNAs regulate mitochondrial bioenergetics. Their combined use can give complementary information. In addition to the nature of the sample used, the composition of the assay buffer can influence the analysis. Data correction is an important parameter to consider to avoid discrepancies and, when possible, normalization against mitochondrial content (mtDNA, citrate synthase activity) should be applied instead of normalization against total protein or tissue mass.206
Finally, other aspects of mitochondrial physiology that are tightly linked to mitochondrial respiration, such as mitochondrial Ca2+ import, mitochondrial ROS production, and mitochondrial membrane potential, should be analyzed to get a better insight into the regulation by RNAs of the observed phenotypes.207,208 For example, overexpression of miR-181c led to the reduction of its target, mt-COX1, as well as other mitochondrial respiratory complex IV subunits, and to complex IV remodeling. Surprisingly, the effect on mitochondrial respiration was the opposite of what would have been expected, as it was enhanced, due to a parallel increase in mitochondrial Ca2+ import.24
Characterization of mitochondrial bioenergetics in vivo is possible using non-invasive, magnetic resonance spectroscopy (MRS)-based methods.209 31P-MRS allows measuring the phosphocreatine versus ATP (PCr/ATP) ratio, which is a readout of the energetic state of the heart. When combined with 13C-MRS it detects oxidation products and allows the evaluation of mitochondria coupling of fatty acid oxidation with ATP synthesis. These methods are far from being widely applied as they are time-consuming and lack resolution and mechanistic insight.
Mitochondria-targeted RNA therapies and delivery strategies
As mentioned previously, mitochondrial ncRNAs play crucial roles in controlling various disease processes by modulating glycolysis, mitochondrial respiration, and the expression of genes involved in mitochondrial metabolism and homeostasis. Standardizing methods for targeting/modulating mitochondrial ncRNAs has great potential in enhancing our understanding of the underlying mechanisms linking fluctuations in their expression levels to disrupted mitochondrial function and specific disease phenotypes, as well as in promoting the development of mitochondria-targeted RNA therapies.
RNA-based therapies have shown remarkable efficacy in treating certain well-defined genetic conditions, and include RNA interference, aptamers, antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), CRISPR-based gene editing, and mRNA therapeutics.210,211 The number of FDA-approved RNA drugs has seen a rapid increase over the past decade (Table S2); also, hundreds of novel investigational drugs are in the preclinical stage or already progressing in the advanced phase of clinical trials. Although RNA-based therapies for mitochondrial diseases have yet to receive approval, targeting mitochondrial RNAs holds promise for therapeutic interventions, by leveraging (1) ASOs and siRNAs to decrease mutant mitochondrial protein levels, (2) mRNA-based drugs to substitute defective mitochondrial RNAs and proteins, and (3) anti-replicative RNAs to mitigate mutant mtDNA levels in cells.212 Delivery of siRNAs, mitomiR mimics, or ASOs to mitochondria can modulate mitochondrial gene expression for specific therapeutic purposes. For instance, nanocarriers have been utilized to transport let-7b to mitochondria, reducing the expression of COX1 and COX2 in non-small cell lung cancer cells.213 ASOs have also been transported to the mitochondrial matrix using MITO-porter, effectively decreasing COX2 mRNA expression.214
In addition, gene editing approaches offer the potential for treating mitochondrial diseases. Various tools have been developed for modifying mtDNA, including bacterial toxin derivatives such as DddA-derived cytosine base editor, which can introduce single-base changes.215 Protein-only nucleases, such as mitoTALEN (transcription activator-like effector nuclease), equipped with mitochondrial targeting sequences, can be imported into the mitochondrial matrix for specific mtDNA editing.216 In addition, CRISPR-Cas9 systems have been adapted for targeting mtDNA (mitoCRISPR).217 While there is growing evidence of evolving mitochondrial therapeutic delivery systems, RNA import into mitochondria remains largely understudied. Further research is necessary to elucidate the fundamental principles underlying RNA import into mitochondria.
Conclusion
Mitochondria are organelles of utmost importance in all cells, particularly in high-energy-demanding cells. The cellular transcriptome is much more complex than previously anticipated, with many RNAs having regulatory properties. ncRNAs unable to encode proteins regulate gene expression at multiple stages and thereby participate in virtually all pathophysiological processes. A detailed knowledge of the role of RNAs may lead to the discovery of novel therapeutic targets. Circulating RNAs also have shown some biomarker potential in many diseases. Mitochondria possess a pool of protein-coding and ncRNAs with poorly understood functions. This partial and sometimes debated knowledge arising from inconsistent studies may come from the use of diverse protocols for mitochondria purification, RNA extraction and quantification, and functional assessments. By standardizing these methods, we can certainly reduce findings inconsistency, improve the state-of-the-art of the role of mitochondrial RNAs in disease development and progression, and generate reliable and robust research findings that can be translated to clinical application for the benefit of patients. Fulfilment of technical and experimental guidelines has the potential to move personalized medicine a step forward. After the use of RNAs for vaccine and diagnostic tests during the COVID-19 pandemic, RNAs may well become the next generation of drugs and biomarkers.
Acknowledgments
This article is based upon work from COST Action EU-CardioRNA, CA17129, and COST Action AtheroNET, CA21153, supported by COST (European Cooperation in Science and Technology). A.J. is funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Actions individual fellowship (grant agreement 893435). L.T.D. is funded by the Innovation Fund Denmark (1044-00139B) and the Novo Nordisk Foundation (NNF22OC0078203). Y.D. is funded by the EU Horizon 2020 project COVIRNA (grant agreement 101016072), the National Research Fund (grant nos. C14/BM/8225223, C17/BM/11613033, and COVID-19/2020-1/14719577/miRCOVID), the Ministry of Higher Education and Research of Luxembourg, and the Heart Foundation-Daniel Wagner of Luxembourg. DdG-C has received financial support from the Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), co-funded by the European Union. CIBERES (CB07/06/2008) is an initiative of the Instituto de Salud Carlos III. P.L. is funded by the Aarne Koskelo Foundation, The Finnish Foundation for Cardiovascular Research, The Finnish Society of Clinical Chemistry, and the Finnish Foundation for Laboratory Medicine. K.F. is funded by the National Centre for Research and Development, Poland, grant no. DWM/WPC2/285/2020 and NCN Miniatura 5 2021/05/X/NZ3/01013. P.F. and B.Á. were funded by project no. RRF-2.3.1-21-2022-00003 that has been implemented with the support provided by the European Union. The 2020-1.1.5-GYORSÍTÓSÁV-2021-00011 project was funded by the Ministry for Innovation and Technology with support from the National Research Development and Innovation Fund under the 2020-1.1.5-GYORSÍTÓSÁV call programme. This study was funded by the by grant 2020-1.1.6-JÖVŐ-2021-00013 (“Befektetés a jövőbe” NKFIH). This project has received funding from the HUN-REN Hungarian Research Network.
Author contributions
A.J. and Y.D. conceptualized the outline of the manuscript. A.J., Z.E., and Y.D. critically revised the main manuscript draft. All authors participated in writing this article and approved it for publication.
Declaration of interests
A.J. is employed by HAYA Therapeutics SA, Switzerland. Y.D. holds patents related to diagnostic and therapeutic applications of RNAs and is member of the Scientific Advisory Board of Firalis SA. P.F. is the founder and CEO of Pharmahungary Group, a group of research and development (R&D) companies (www.pharmahungary.com). B.Á. is employed by Pharmahungary Group.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2024.102262.
Supplemental information
References
- 1.Lane N., Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 2.Stoccoro A., Coppedè F. Mitochondrial DNA Methylation and Human Diseases. IJMS. 2021;22:4594. doi: 10.3390/ijms22094594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordoni L., Petracci I., Mlodzik-Czyzewska M., Malinowska A.M., Szwengiel A., Sadowski M., Gabbianelli R., Chmurzynska A. Mitochondrial DNA and Epigenetics: Investigating Interactions with the One-Carbon Metabolism in Obesity. Oxid. Med. Cell. Longev. 2022;2022:9171684–9171712. doi: 10.1155/2022/9171684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jusic A., Devaux Y., EU-CardioRNA COST Action CA17129 Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res. Cardiol. 2020;115:23. doi: 10.1007/s00395-020-0783-5. [DOI] [PubMed] [Google Scholar]
- 5.Jagannathan R., Thapa D., Nichols C.E., Shepherd D.L., Stricker J.C., Croston T.L., Baseler W.A., Lewis S.E., Martinez I., Hollander J.M. Translational Regulation of the Mitochondrial Genome Following Redistribution of Mitochondrial MicroRNA in the Diabetic Heart. Circ. Cardiovasc. Genet. 2015;8:785–802. doi: 10.1161/CIRCGENETICS.115.001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W., Lu Y., Zhang H., Zhang J., Fang X., Wang J., Li M. Mitochondrial Non-Coding RNAs Are Potential Mediators of Mitochondrial Homeostasis. Biomolecules. 2022;12:1863. doi: 10.3390/biom12121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang L., Ju H.Q., Yang Z., Ge Q., Zhang Z., Liu F., Yang L., Gong H., Shi C., Qu L., et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 2021;3:90–106. doi: 10.1038/s42255-020-00325-z. [DOI] [PubMed] [Google Scholar]
- 8.Gomes C.P.D.C., Schroen B., Kuster G.M., Robinson E.L., Ford K., Squire I.B., Heymans S., Martelli F., Emanueli C., Devaux Y., et al. Regulatory RNAs in Heart Failure. Circulation. 2020;141:313–328. doi: 10.1161/CIRCULATIONAHA.119.042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statello L., Guo C.-J., Chen L.-L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vendramin R., Marine J.C., Leucci E. Non-coding RNA s: the dark side of nuclear–mitochondrial communication. EMBO J. 2017;36:1123–1133. doi: 10.15252/embj.201695546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren B., Guan M.-X., Zhou T., Cai X., Shan G. Emerging functions of mitochondria-encoded noncoding RNAs. Trends Genet. 2023;39:125–139. doi: 10.1016/j.tig.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Shang R., Lee S., Senavirathne G., Lai E.C. microRNAs in action: biogenesis, function and regulation. Nat. Rev. Genet. 2023;24:816–833. doi: 10.1038/s41576-023-00611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 14.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019;20:5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 15.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.-L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023;24:430–447. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchese F.P., Raimondi I., Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luteijn M.J., Ketting R.F. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat. Rev. Genet. 2013;14:523–534. doi: 10.1038/nrg3495. [DOI] [PubMed] [Google Scholar]
- 18.Ozata D.M., Gainetdinov I., Zoch A., O’Carroll D., Zamore P.D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 2019;20:89–108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- 19.Dragomir M., Calin G.A. Circular RNAs in Cancer – Lessons Learned From microRNAs. Front. Oncol. 2018;8:179. doi: 10.3389/fonc.2018.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene J., Baird A.-M., Brady L., Lim M., Gray S.G., McDermott R., Finn S.P. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Front. Mol. Biosci. 2017;4:38. doi: 10.3389/fmolb.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Donath S., Li Y., Qin D., Prabhakar B.S., Li P. miR-30 Regulates Mitochondrial Fission through Targeting p53 and the Dynamin-Related Protein-1 Pathway. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Li Y., Jiao J., Wang J., Li Y., Qin D., Li P. Mitofusin 1 Is Negatively Regulated by MicroRNA 140 in Cardiomyocyte Apoptosis. Mol. Cell Biol. 2014;34:1788–1799. doi: 10.1128/MCB.00774-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duroux-Richard I., Roubert C., Ammari M., Présumey J., Grün J.R., Häupl T., Grützkau A., Lecellier C.-H., Boitez V., Codogno P., et al. miR-125b controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Blood. 2016;128:3125–3136. doi: 10.1182/blood-2016-02-697003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S., Bedja D., Campbell N., Dunkerly B., Chenna V., Maitra A., Steenbergen C. miR-181c Regulates the Mitochondrial Genome, Bioenergetics, and Propensity for Heart Failure In Vivo. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai Y., Pu M., Yuan L., Guo H., Qiao J., Lu H., Wang G., Chen J., Qi X., Tao Z., Ren J. miR-34a-5p regulates PINK1-mediated mitophagy via multiple modes. Life Sci. 2021;276 doi: 10.1016/j.lfs.2021.119415. [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Fiesel F.C., Belmonte K.C., Hudec R., Wang W.-X., Kim C., Nelson P.T., Springer W., Kim J. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1) Mol. Neurodegener. 2016;11:55. doi: 10.1186/s13024-016-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aschrafi A., Schwechter A.D., Mameza M.G., Natera-Naranjo O., Gioio A.E., Kaplan B.B. MicroRNA-338 Regulates Local Cytochrome c Oxidase IV mRNA Levels and Oxidative Phosphorylation in the Axons of Sympathetic Neurons. J. Neurosci. 2008;28:12581–12590. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang A., Grether-Beck S., Singh M., Kuck F., Jakob S., Kefalas A., Altinoluk-Hambüchen S., Graffmann N., Schneider M., Lindecke A., et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging. 2016;8:484–505. doi: 10.18632/aging.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P., Sharma V., Ahluwalia T.S., Dogra N., Kumar S., Singh S. Let-7a induces metabolic reprogramming in breast cancer cells via targeting mitochondrial encoded ND4. Cancer Cell Int. 2021;21:629. doi: 10.1186/s12935-021-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Zuo X., Yang B., Li Z., Xue Y., Zhou Y., Huang J., Zhao X., Zhou J., Yan Y., et al. MicroRNA Directly Enhances Mitochondrial Translation during Muscle Differentiation. Cell. 2014;158:607–619. doi: 10.1016/j.cell.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Zhang X., Wang F., Zhou L., Yin Z., Fan J., Nie X., Wang P., Fu X.-D., Chen C., Wang D.W. MicroRNA-21 Lowers Blood Pressure in Spontaneous Hypertensive Rats by Upregulating Mitochondrial Translation. Circulation. 2016;134:734–751. doi: 10.1161/CIRCULATIONAHA.116.023926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W., Wang P., Lu Y., Jin T., Lei X., Liu M., Zhuang P., Liao J., Lin Z., Li B., et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics. 2019;9:5739–5754. doi: 10.7150/thno.37556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan S., Tian T., Chen W., Lv X., Lei X., Zhang H., Sun S., Cai L., Pan G., He L., et al. Mitochondrial miRNA Determines Chemoresistance by Reprogramming Metabolism and Regulating Mitochondrial Transcription. Cancer Res. 2019;79:1069–1084. doi: 10.1158/0008-5472.CAN-18-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rackham O., Shearwood A.-M.J., Mercer T.R., Davies S.M.K., Mattick J.S., Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA. 2011;17:2085–2093. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Song T., Zhao Y., Zhao J., Wang X., Fu X. Long non-coding RNA LICPAR regulates atrial fibrosis via TGF-β/Smad pathway in atrial fibrillation. Tissue Cell. 2020;67 doi: 10.1016/j.tice.2020.101440. [DOI] [PubMed] [Google Scholar]
- 36.Durr A.J., Hathaway Q.A., Kunovac A., Taylor A.D., Pinti M.V., Rizwan S., Shepherd D.L., Cook C.C., Fink G.K., Hollander J.M. Manipulation of the miR-378a/mt-ATP6 regulatory axis rescues ATP synthase in the diabetic heart and offers a novel role for lncRNA Kcnq1ot1. Am. J. Physiol. Cell Physiol. 2022;322:C482–C495. doi: 10.1152/ajpcell.00446.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sirey T.M., Roberts K., Haerty W., Bedoya-Reina O., Rogatti-Granados S., Tan J.Y., Li N., Heather L.C., Carter R.N., Cooper S., et al. The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. Elife. 2019;8 doi: 10.7554/eLife.45051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q., Liu J., Deng H., Ma R., Liao J.-Y., Liang H., Hu J., Li J., Guo Z., Cai J., et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell. 2020;183:76–93.e22. doi: 10.1016/j.cell.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Wang X., Li J., Hu S., Deng Y., Yin H., Bao X., Zhang Q.C., Wang G., Wang B., et al. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci. China Life Sci. 2020;63:1429–1449. doi: 10.1007/s11427-020-1631-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., He Q., Lu T., Wu J., Shi G., He L., Zong H., Liu B., Zhu P. mcPGK1-dependent mitochondrial import of PGK1 promotes metabolic reprogramming and self-renewal of liver TICs. Nat. Commun. 2023;14:1121. doi: 10.1038/s41467-023-36651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong W., Xu J., Wang Y., Min Q., Chen X., Zhang W., Chen J., Zhan Q. Nuclear genome-derived circular RNA circPUM1 localizes in mitochondria and regulates oxidative phosphorylation in esophageal squamous cell carcinoma. Sig Transduct Target Ther. 2022;7:40. doi: 10.1038/s41392-021-00865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gusic M., Prokisch H. ncRNAs: New Players in Mitochondrial Health and Disease? Front. Genet. 2020;11:95. doi: 10.3389/fgene.2020.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuthethur R., Shukla V., Mallya S., Adiga D., Kabekkodu S.P., Ramachandra L., Saxena P.U.P., Satyamoorthy K., Chakrabarty S. Expression analysis and function of mitochondrial genome-encoded microRNAs. J. Cell Sci. 2022;135 doi: 10.1242/jcs.258937. [DOI] [PubMed] [Google Scholar]
- 44.Blumental-Perry A., Jobava R., Bederman I., Degar A.J., Kenche H., Guan B.J., Pandit K., Perry N.A., Molyneaux N.D., Wu J., et al. Retrograde signaling by a mtDNA-encoded non-coding RNA preserves mitochondrial bioenergetics. Commun. Biol. 2020;3:626. doi: 10.1038/s42003-020-01322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purohit P.K., Saini N. Mitochondrial microRNA (MitomiRs) in cancer and complex mitochondrial diseases: current status and future perspectives. Cell. Mol. Life Sci. 2021;78:1405–1421. doi: 10.1007/s00018-020-03670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X., Shan G. Mitochondria Encoded Non-coding RNAs in Cell Physiology. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.713729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran K.-V., Brown E.L., DeSouza T., Jespersen N.Z., Nandrup-Bus C., Yang Q., Yang Z., Desai A., Min S.Y., Rojas-Rodriguez R., et al. Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473. Nat. Metab. 2020;2:397–412. doi: 10.1038/s42255-020-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar J., Mohammad G., Alka K., Kowluru R.A. Mitochondrial Genome–Encoded Long Noncoding RNA and Mitochondrial Stability in Diabetic Retinopathy. Diabetes. 2023;72:520–531. doi: 10.2337/db22-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao S., Tian X., Chang H., Sun Y., Wu Z., Cheng Z., Dong P., Zhao Q., Ruan J., Bu W. Two novel lncRNAs discovered in human mitochondrial DNA using PacBio full-length transcriptome data. Mitochondrion. 2018;38:41–47. doi: 10.1016/j.mito.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Meessen J.M.T.A., Bär C., Di Dona F.M., Staszewsky L.I., Di Giulio P., Di Tano G., Costa A., Leonardy J., Novelli D., Nicolis E.B., et al. LIPCAR Is Increased in Chronic Symptomatic HF Patients. A Sub-Study of the GISSI-HF Trial. Clin. Chem. 2021;67:1721–1731. doi: 10.1093/clinchem/hvab197. [DOI] [PubMed] [Google Scholar]
- 51.Kumarswamy R., Bauters C., Volkmann I., Maury F., Fetisch J., Holzmann A., Lemesle G., De Groote P., Pinet F., Thum T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients With Heart Failure. Circ. Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 52.Yan L., Chen Y.G. One Ring to Rule Them All: Mitochondrial Circular RNAs Control Mitochondrial Function. Cell. 2020;183:11–13. doi: 10.1016/j.cell.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Barreñada O., Larriba E., Fernández-Pérez D., Brieño-Enríquez M.Á., Del Mazo Martínez J. Unraveling mitochondrial piRNAs in mouse embryonic gonadal cells. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-14414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon C., Tak H., Rho M., Chang H.R., Kim Y.H., Kim K.T., Balch C., Lee E.K., Nam S. Detection of PIWI and piRNAs in the mitochondria of mammalian cancer cells. Biochem. Biophys. Res. Commun. 2014;446:218–223. doi: 10.1016/j.bbrc.2014.02.112. [DOI] [PubMed] [Google Scholar]
- 55.Ipsaro J.J., Haase A.D., Knott S.R., Joshua-Tor L., Hannon G.J. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–283. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimasu H., Ishizu H., Saito K., Fukuhara S., Kamatani M.K., Bonnefond L., Matsumoto N., Nishizawa T., Nakanaga K., Aoki J., et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–287. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe T., Chuma S., Yamamoto Y., Kuramochi-Miyagawa S., Totoki Y., Toyoda A., Hoki Y., Fujiyama A., Shibata T., Sado T., et al. MITOPLD Is a Mitochondrial Protein Essential for Nuage Formation and piRNA Biogenesis in the Mouse Germline. Dev. Cell. 2011;20:364–375. doi: 10.1016/j.devcel.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gammage P.A., Moraes C.T., Minczuk M. Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. 2018;34:101–110. doi: 10.1016/j.tig.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bandiera S., Rüberg S., Girard M., Cagnard N., Hanein S., Chrétien D., Munnich A., Lyonnet S., Henrion-Caude A. Nuclear Outsourcing of RNA Interference Components to Human Mitochondria. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beitzinger M., Peters L., Zhu J.Y., Kremmer E., Meister G. Identification of Human microRNA Targets From Isolated Argonaute Protein Complexes. RNA Biol. 2007;4:76–84. doi: 10.4161/rna.4.2.4640. [DOI] [PubMed] [Google Scholar]
- 61.Wang G., Chen H.-W., Oktay Y., Zhang J., Allen E.L., Smith G.M., Fan K.C., Hong J.S., French S.W., McCaffery J.M., et al. PNPASE Regulates RNA Import into Mitochondria. Cell. 2010;142:456–467. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noh J.H., Kim K.M., Abdelmohsen K., Yoon J.-H., Panda A.C., Munk R., Kim J., Curtis J., Moad C.A., Wohler C.M., et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016;30:1224–1239. doi: 10.1101/gad.276022.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 64.Baek D., Villén J., Shin C., Camargo F.D., Gygi S.P., Bartel D.P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chugh P., Dittmer D.P. Potential pitfalls in microRNA profiling. WIREs RNA. 2012;3:601–616. doi: 10.1002/wrna.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palazzo A.F., Lee E.S. Non-coding RNA: what is functional and what is junk? Front. Genet. 2015;6 doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson E.L., Emanueli C., Martelli F., Devaux Y. Leveraging non-coding RNAs to fight cardiovascular disease: the EU-CardioRNA network. Eur. Heart J. 2021;42:4881–4883. doi: 10.1093/eurheartj/ehab326. [DOI] [PubMed] [Google Scholar]
- 68.Yin Y., Shen H. Common methods in mitochondrial research (Review) Int. J. Mol. Med. 2022;50:126. doi: 10.3892/ijmm.2022.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Timmons M.D., Bradley M.A., Lovell M.A., Lynn B.C. Procedure for the isolation of mitochondria, cytosolic and nuclear material from a single piece of neurological tissue for high-throughput mass spectral analysis. J. Neurosci. Methods. 2011;197:279–282. doi: 10.1016/j.jneumeth.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 70.Azimzadeh P., Asadzadeh Aghdaei H., Tarban P., Akhondi M.M., Shirazi A., Khorram Khorshid H.R. Comparison of three methods for mitochondria isolation from the human liver cell line (HepG2) Gastroenterol. Hepatol. Bed Bench. 2016;9:105–113. [PMC free article] [PubMed] [Google Scholar]
- 71.Caldeira D.D.A.F., de Oliveira D.F., Cavalcanti-de-Albuquerque J.P., Nascimento J.H.M., Zin W.A., Maciel L. Isolation of Mitochondria From Fresh Mice Lung Tissue. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.748261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frezza C., Cipolat S., Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 73.Fernández-Vizarra E., Ferrín G., Pérez-Martos A., Fernández-Silva P., Zeviani M., Enríquez J.A. Isolation of mitochondria for biogenetical studies: An update. Mitochondrion. 2010;10:253–262. doi: 10.1016/j.mito.2009.12.148. [DOI] [PubMed] [Google Scholar]
- 74.Hartwig S., Feckler C., Lehr S., Wallbrecht K., Wolgast H., Müller-Wieland D., Kotzka J. A critical comparison between two classical and a kit-based method for mitochondria isolation. Proteomics. 2009;9:3209–3214. doi: 10.1002/pmic.200800344. [DOI] [PubMed] [Google Scholar]
- 75.Sims N.R., Anderson M.F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. Protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 76.Liao P.-C., Bergamini C., Fato R., Pon L.A., Pallotti F. Methods in Cell Biology. Elsevier; 2020. Isolation of mitochondria from cells and tissues; pp. 3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graham J.M. Purification of a Crude Mitochondrial Fraction by Density-Gradient Centrifugation. CP Cell Biology. 2001;4 doi: 10.1002/0471143030.cb0304s04. Unit 3.4. [DOI] [PubMed] [Google Scholar]
- 78.Forner F., Arriaga E.A., Mann M. Mild Protease Treatment as a Small-Scale Biochemical Method for Mitochondria Purification and Proteomic Mapping of Cytoplasm-Exposed Mitochondrial Proteins. J. Proteome Res. 2006;5:3277–3287. doi: 10.1021/pr060361z. [DOI] [PubMed] [Google Scholar]
- 79.Franko A., Baris O.R., Bergschneider E., Von Toerne C., Hauck S.M., Aichler M., Walch A.K., Wurst W., Wiesner R.J., Johnston I.C.D., de Angelis M.H. Efficient Isolation of Pure and Functional Mitochondria from Mouse Tissues Using Automated Tissue Disruption and Enrichment with Anti-TOM22 Magnetic Beads. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hubbard W.B., Harwood C.L., Prajapati P., Springer J.E., Saatman K.E., Sullivan P.G. Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci. Rep. 2019;9:9656. doi: 10.1038/s41598-019-45568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hornig-Do H.-T., Günther G., Bust M., Lehnartz P., Bosio A., Wiesner R.J. Isolation of functional pure mitochondria by superparamagnetic microbeads. Anal. Biochem. 2009;389:1–5. doi: 10.1016/j.ab.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 82.Barrey E., Saint-Auret G., Bonnamy B., Damas D., Boyer O., Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kostal V., Fonslow B.R., Arriaga E.A., Bowser M.T. Fast determination of mitochondria electrophoretic mobility using micro free-flow electrophoresis. Anal. Chem. 2009;81:9267–9273. doi: 10.1021/ac901508x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zischka H., Lichtmannegger J., Jägemann N., Jennen L., Hamöller D., Huber E., Walch A., Summer K.H., Göttlicher M. In: 2D PAGE: Sample Preparation and Fractionation Methods in Molecular Biology™. Posch A., editor. Humana Press; 2008. Isolation of Highly Pure Rat Liver Mitochondria with the Aid of Zone-Electrophoresis in a Free Flow Device (ZE-FFE) pp. 333–348. [DOI] [PubMed] [Google Scholar]
- 85.He Y.-C., Kong F.-Z., Fan L.-Y., Wu J.Y., Liu X.-P., Li J., Sun Y., Zhang Q., Yang Y., Wu X.-J., et al. Preparation of intact mitochondria using free-flow isoelectric focusing with post-pH gradient sample injection for morphological, functional and proteomics studies. Anal. Chim. Acta. 2017;982:200–208. doi: 10.1016/j.aca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Kren B.T., Wong P.Y.-P., Sarver A., Zhang X., Zeng Y., Steer C.J. MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Biol. 2009;6:65–72. doi: 10.4161/rna.6.1.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geiger J., Dalgaard L.T. In: Mitochondrial Bioenergetics Methods in Molecular Biology. Palmeira C.M., Moreno A.J., editors. Springer; 2018. Isolation and Analysis of Mitochondrial Small RNAs from Rat Liver Tissue and HepG2 Cells; pp. 337–350. [DOI] [PubMed] [Google Scholar]
- 88.Huang J., Wang G. Improved Mammalian Mitochondrial RNA Isolation. Bio. Protoc. 2019;9 doi: 10.21769/BioProtoc.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartwig S., Kotzka J., Lehr S. In: Mitochondrial Medicine Methods in Molecular Biology. Weissig V., Edeas M., editors. Springer; New York: 2015. Isolation and Quality Control of Functional Mitochondria; pp. 9–23. [DOI] [PubMed] [Google Scholar]
- 90.Chinopoulos C., Zhang S.F., Thomas B., Ten V., Starkov A.A. In: Neurodegeneration Methods in Molecular Biology. Manfredi G., Kawamata H., editors. Humana Press; 2011. Isolation and Functional Assessment of Mitochondria from Small Amounts of Mouse Brain Tissue; pp. 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farah N.K., Liu X., Wu C.H., Wu G.Y. An Improved Method for Preparation of Uniform and Functional Mitochondria from Fresh Liver. J. Clin. Transl. Hepatol. 2019;7:46–50. doi: 10.14218/JCTH.2018.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee G.-J., Park H.-K. In: Mitochondrial Medicine Methods in Molecular Biology. Weissig V., Edeas M., editors. Springer; New York: 2015. Atomic Force Microscopy-Based Shape Analysis of Heart Mitochondria; pp. 397–406. [DOI] [PubMed] [Google Scholar]
- 93.Nakhle J., Özkan T., Lněničková K., Briolotti P., Vignais M.-L. Methods for simultaneous and quantitative isolation of mitochondrial DNA, nuclear DNA and RNA from mammalian cells. Biotechniques. 2020;69:436–442. doi: 10.2144/btn-2020-0114. [DOI] [PubMed] [Google Scholar]
- 94.Scholes A.N., Lewis J.A. Comparison of RNA isolation methods on RNA-Seq: implications for differential expression and meta-analyses. BMC Genom. 2020;21:249. doi: 10.1186/s12864-020-6673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sellin Jeffries M.K., Kiss A.J., Smith A.W., Oris J.T. A comparison of commercially-available automated and manual extraction kits for the isolation of total RNA from small tissue samples. BMC Biotechnol. 2014;14:94. doi: 10.1186/s12896-014-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright K., De Silva K., Purdie A.C., Plain K.M. Comparison of methods for miRNA isolation and quantification from ovine plasma. Sci. Rep. 2020;10:825. doi: 10.1038/s41598-020-57659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Elias A., Alloza L., Puigdecanet E., Nonell L., Tajes M., Curado J., Enjuanes C., Díaz O., Bruguera J., Martí-Almor J., et al. Defining quantification methods and optimizing protocols for microarray hybridization of circulating microRNAs. Sci. Rep. 2017;7:7725. doi: 10.1038/s41598-017-08134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Becker C., Hammerle-Fickinger A., Riedmaier I., Pfaffl M.W. mRNA and microRNA quality control for RT-qPCR analysis. Methods. 2010;50:237–243. doi: 10.1016/j.ymeth.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 99.Williams Z., Ben-Dov I.Z., Elias R., Mihailovic A., Brown M., Rosenwaks Z., Tuschl T. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc. Natl. Acad. Sci. USA. 2013;110:4255–4260. doi: 10.1073/pnas.1214046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hagemann-Jensen M., Abdullayev I., Sandberg R., Faridani O.R. Small-seq for single-cell small-RNA sequencing. Nat. Protoc. 2018;13:2407–2424. doi: 10.1038/s41596-018-0049-y. [DOI] [PubMed] [Google Scholar]
- 101.Chiang H.R., Schoenfeld L.W., Ruby J.G., Auyeung V.C., Spies N., Baek D., Johnston W.K., Russ C., Luo S., Babiarz J.E., et al. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev. 2010;24:992–1009. doi: 10.1101/gad.1884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alles J., Fehlmann T., Fischer U., Backes C., Galata V., Minet M., Hart M., Abu-Halima M., Grässer F.A., Lenhof H.-P., et al. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019;47:3353–3364. doi: 10.1093/nar/gkz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heinicke F., Zhong X., Zucknick M., Breidenbach J., Sundaram A.Y.M., T. Flåm S., Leithaug M., Dalland M., Farmer A., Henderson J.M., et al. Systematic assessment of commercially available low-input miRNA library preparation kits. RNA Biol. 2020;17:75–86. doi: 10.1080/15476286.2019.1667741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giraldez M.D., Spengler R.M., Etheridge A., Godoy P.M., Barczak A.J., Srinivasan S., De Hoff P.L., Tanriverdi K., Courtright A., Lu S., et al. Comprehensive multi-center assessment of small RNA-seq methods for quantitative miRNA profiling. Nat. Biotechnol. 2018;36:746–757. doi: 10.1038/nbt.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dard-Dascot C., Naquin D., d’Aubenton-Carafa Y., Alix K., Thermes C., Van Dijk E. Systematic comparison of small RNA library preparation protocols for next-generation sequencing. BMC Genom. 2018;19:118. doi: 10.1186/s12864-018-4491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun Y.-M., Chen Y.-Q. Principles and innovative technologies for decrypting noncoding RNAs: from discovery and functional prediction to clinical application. J. Hematol. Oncol. 2020;13:109. doi: 10.1186/s13045-020-00945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown R.A.M., Epis M.R., Horsham J.L., Kabir T.D., Richardson K.L., Leedman P.J. Total RNA extraction from tissues for microRNA and target gene expression analysis: not all kits are created equal. BMC Biotechnol. 2018;18:16. doi: 10.1186/s12896-018-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baran-Gale J., Kurtz C.L., Erdos M.R., Sison C., Young A., Fannin E.E., Chines P.S., Sethupathy P. Addressing Bias in Small RNA Library Preparation for Sequencing: A New Protocol Recovers MicroRNAs that Evade Capture by Current Methods. Front. Genet. 2015;6 doi: 10.3389/fgene.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jayaprakash A.D., Jabado O., Brown B.D., Sachidanandam R. Identification and remediation of biases in the activity of RNA ligases in small-RNA deep sequencing. Nucleic Acids Res. 2011;39:e141. doi: 10.1093/nar/gkr693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hafner M., Renwick N., Brown M., Mihailović A., Holoch D., Lin C., Pena J.T.G., Nusbaum J.D., Morozov P., Ludwig J., et al. RNA-ligase-dependent biases in miRNA representation in deep-sequenced small RNA cDNA libraries. RNA. 2011;17:1697–1712. doi: 10.1261/rna.2799511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ardui S., Ameur A., Vermeesch J.R., Hestand M.S. Single molecule real-time (SMRT) sequencing comes of age: applications and utilities for medical diagnostics. Nucleic Acids Res. 2018;46:2159–2168. doi: 10.1093/nar/gky066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eid J., Fehr A., Gray J., Luong K., Lyle J., Otto G., Peluso P., Rank D., Baybayan P., Bettman B., et al. Real-Time DNA Sequencing from Single Polymerase Molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 113.Clarke J., Wu H.-C., Jayasinghe L., Patel A., Reid S., Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009;4:265–270. doi: 10.1038/nnano.2009.12. [DOI] [PubMed] [Google Scholar]
- 114.Liu J., Jiang H., Zan J., Bao Y., Dong J., Xiong L., Nie L. Single-molecule long-read transcriptome profiling of Platysternon megacephalum mitochondrial genome with gene rearrangement and control region duplication. RNA Biol. 2018;15:1244–1249. doi: 10.1080/15476286.2018.1521212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor S.C., Laperriere G., Germain H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Sci. Rep. 2017;7:2409. doi: 10.1038/s41598-017-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lakkisto P., Dalgaard L.T., Belmonte T., Pinto-Sietsma S.-J., Devaux Y., De Gonzalo-Calvo D., EU-CardioRNA COST Action CA17129 Development of circulating microRNA-based biomarkers for medical decision-making: a friendly reminder of what should NOT be done. Crit. Rev. Clin. Lab Sci. 2023;60:141–152. doi: 10.1080/10408363.2022.2128030. [DOI] [PubMed] [Google Scholar]
- 117.Gohel D., Sripada L., Prajapati P., Currim F., Roy M., Singh K., Shinde A., Mane M., Kotadia D., Tassone F., et al. Expression of expanded FMR1-CGG repeats alters mitochondrial miRNAs and modulates mitochondrial functions and cell death in cellular model of FXTAS. Free Radic. Biol. Med. 2021;165:100–110. doi: 10.1016/j.freeradbiomed.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 118.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 119.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faraldi M., Gomarasca M., Sansoni V., Perego S., Banfi G., Lombardi G. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 2019;9:1584. doi: 10.1038/s41598-019-38505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gee H.E., Buffa F.M., Camps C., Ramachandran A., Leek R., Taylor M., Patil M., Sheldon H., Betts G., Homer J., et al. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br. J. Cancer. 2011;104:1168–1177. doi: 10.1038/sj.bjc.6606076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarzenbach H., Da Silva A.M., Calin G., Pantel K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015;61:1333–1342. doi: 10.1373/clinchem.2015.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 124.Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 125.De Spiegelaere W., Dern-Wieloch J., Weigel R., Schumacher V., Schorle H., Nettersheim D., Bergmann M., Brehm R., Kliesch S., Vandekerckhove L., Fink C. Reference Gene Validation for RT-qPCR, a Note on Different Available Software Packages. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng H., Liu J., Yu J., McAlinden A. Expression profiling of mitochondria-associated microRNAs during osteogenic differentiation of human MSCs. Bone. 2021;151 doi: 10.1016/j.bone.2021.116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giuliani A., Cirilli I., Prattichizzo F., Mensà E., Fulgenzi G., Sabbatinelli J., Graciotti L., Olivieri F., Procopio A.D., Tiano L., Rippo M.R. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging. 2018;10:2855–2873. doi: 10.18632/aging.101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bian Z., Li L.-M., Tang R., Hou D.-X., Chen X., Zhang C.-Y., Zen K. Identification of mouse liver mitochondria-associated miRNAs and their potential biological functions. Cell Res. 2010;20:1076–1078. doi: 10.1038/cr.2010.119. [DOI] [PubMed] [Google Scholar]
- 129.Bai M., Chen H., Ding D., Song R., Lin J., Zhang Y., Guo Y., Chen S., Ding G., Zhang Y., et al. MicroRNA-214 promotes chronic kidney disease by disrupting mitochondrial oxidative phosphorylation. Kidney Int. 2019;95:1389–1404. doi: 10.1016/j.kint.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 130.Wang W.-X., Prajapati P., Nelson P.T., Springer J.E. The Mitochondria-Associated ER Membranes Are Novel Subcellular Locations Enriched for Inflammatory-Responsive MicroRNAs. Mol. Neurobiol. 2020;57:2996–3013. doi: 10.1007/s12035-020-01937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.D’haene B., Mestdagh P., Hellemans J., Vandesompele J. In: Next-Generation MicroRNA Expression Profiling Technology Methods in Molecular Biology. Fan J.-B., editor. Humana Press; 2012. miRNA Expression Profiling: From Reference Genes to Global Mean Normalization; pp. 261–272. [DOI] [PubMed] [Google Scholar]
- 132.Das S., Ferlito M., Kent O.A., Fox-Talbot K., Wang R., Liu D., Raghavachari N., Yang Y., Wheelan S.J., Murphy E., Steenbergen C. Nuclear miRNA Regulates the Mitochondrial Genome in the Heart. Circ. Res. 2012;110:1596–1603. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wallace L., Aikhionbare K., Banerjee S., Peagler K., Pitts M., Yao X., Aikhionbare F. Differential Expression Profiles of Mitogenome Associated MicroRNAs Among Colorectal Adenomatous Polyps. Cancer Res. J. 2021;9:23–33. [PMC free article] [PubMed] [Google Scholar]
- 134.Gómez-Serrano M., Camafeita E., Loureiro M., Peral B. Mitoproteomics: Tackling Mitochondrial Dysfunction in Human Disease. Oxid. Med. Cell. Longev. 2018;2018:1–26. doi: 10.1155/2018/1435934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cappa R., De Campos C., Maxwell A.P., McKnight A.J. “Mitochondrial Toolbox” – A Review of Online Resources to Explore Mitochondrial Genomics. Front. Genet. 2020;11:439. doi: 10.3389/fgene.2020.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Labory J., Fierville M., Ait-El-Mkadem S., Bannwarth S., Paquis-Flucklinger V., Bottini S. Multi-Omics Approaches to Improve Mitochondrial Disease Diagnosis: Challenges, Advances, and Perspectives. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.590842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith A.C., Robinson A.J. MitoMiner, an Integrated Database for the Storage and Analysis of Mitochondrial Proteomics Data. Mol. Cell. Proteomics. 2009;8:1324–1337. doi: 10.1074/mcp.M800373-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith A.C., Robinson A.J. MitoMiner v4.0: an updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 2019;47:D1225–D1228. doi: 10.1093/nar/gky1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rath S., Sharma R., Gupta R., Ast T., Chan C., Durham T.J., Goodman R.P., Grabarek Z., Haas M.E., Hung W.H.W., et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49:D1541–D1547. doi: 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wingo T.S., Liu Y., Gerasimov E.S., Vattathil S.M., Wynne M.E., Liu J., Lori A., Faundez V., Bennett D.A., Seyfried N.T., et al. Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nat. Commun. 2022;13:4314. doi: 10.1038/s41467-022-31873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li N., Li Y., Hu J., Wu Y., Yang J., Fan H., Li L., Luo D., Ye Y., Gao Y., et al. A Link Between Mitochondrial Dysfunction and the Immune Microenvironment of Salivary Glands in Primary Sjogren’s Syndrome. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.845209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Farahani R.A., Zhu X.-Y., Tang H., Jordan K.L., Lerman L.O., Eirin A. Renal ischemia alters expression of mitochondria-related genes and impairs mitochondrial structure and function in swine scattered tubular-like cells. Am. J. Physiol. Renal Physiol. 2020;319:F19–F28. doi: 10.1152/ajprenal.00120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang T., Wang K., Li Y., Ye Y., Chen Y., Wang J., Yao C. Construction of a Novel Ferroptosis-Related Gene Signature of Atherosclerosis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.800833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith A.G., Smith A.C., Palmer O.C., Smith C., Szukszto M., Minczuk M., Robinson A.J. A Curated Collection of Human Mitochondrial Proteins— the Integrated Mitochondrial Protein Index (IMPI) SSRN J. 2022 doi: 10.2139/ssrn.4042282. [DOI] [Google Scholar]
- 145.Cotter D., Guda P., Fahy E., Subramaniam S. MitoProteome: mitochondrial protein sequence database and annotation system. Nucleic Acids Res. 2004;32:463D–D467. doi: 10.1093/nar/gkh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu H., Yu M., Li Z., Zhang E., Ma H. Identification and analysis of mitochondria-related key genes of heart failure. J. Transl. Med. 2022;20:410. doi: 10.1186/s12967-022-03605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 149.Gillespie M., Jassal B., Stephan R., Milacic M., Rothfels K., Senff-Ribeiro A., Griss J., Sevilla C., Matthews L., Gong C., et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50:D687–D692. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kanehisa M., Furumichi M., Sato Y., Kawashima M., Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–D592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gene Ontology Consortium The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–D334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aksu-Menges E., Akkaya-Ulum Y.Z., Dayangac-Erden D., Balci-Peynircioglu B., Yuzbasioglu A., Topaloglu H., Talim B., Balci-Hayta B. The Common miRNA Signatures Associated with Mitochondrial Dysfunction in Different Muscular Dystrophies. Am. J. Pathol. 2020;190:2136–2145. doi: 10.1016/j.ajpath.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 155.Gao H., Xing F. A novel signature model based on mitochondrial-related genes for predicting survival of colon adenocarcinoma. BMC Med. Inform. Decis. Mak. 2022;22:277. doi: 10.1186/s12911-022-02020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meng Y., Eirin A., Zhu X.Y., Tang H., Chanana P., Lerman A., Van Wijnen A.J., Lerman L.O. Obesity-induced mitochondrial dysfunction in porcine adipose tissue-derived mesenchymal stem cells. J. Cell. Physiol. 2018;233:5926–5936. doi: 10.1002/jcp.26402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Khorsandi S.E., Salehi S., Cortes M., Vilca-Melendez H., Menon K., Srinivasan P., Prachalias A., Jassem W., Heaton N. An in silico argument for mitochondrial microRNA as a determinant of primary non function in liver transplantation. Sci. Rep. 2018;8:3105. doi: 10.1038/s41598-018-21091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schreckenberg R., Klein J., Kutsche H.S., Schulz R., Gömöri K., Bencsik P., Benczik B., Ágg B., Sághy É., Ferdinandy P., Schlüter K.D. Ischaemic post-conditioning in rats: Responder and non-responder differ in transcriptome of mitochondrial proteins. J. Cell Mol. Med. 2020;24:5528–5541. doi: 10.1111/jcmm.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lewis B.P., Shih I.h., Jones-Rhoades M.W., Bartel D.P., Burge C.B. Prediction of Mammalian MicroRNA Targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 160.Huang H.-Y., Lin Y.-C.-D., Cui S., Huang Y., Tang Y., Xu J., Bao J., Li Y., Wen J., Zuo H., et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2022;50:D222–D230. doi: 10.1093/nar/gkab1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiao F., Zuo Z., Cai G., Kang S., Gao X., Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krek A., Grün D., Poy M.N., Wolf R., Rosenberg L., Epstein E.J., MacMenamin P., Da Piedade I., Gunsalus K.C., Stoffel M., Rajewsky N. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 163.Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kiriakidou M., Nelson P.T., Kouranov A., Fitziev P., Bouyioukos C., Mourelatos Z., Hatzigeorgiou A. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004;18:1165–1178. doi: 10.1101/gad.1184704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Quillet A., Anouar Y., Lecroq T., Dubessy C. Prediction methods for microRNA targets in bilaterian animals: Toward a better understanding by biologists. Comput. Struct. Biotechnol. J. 2021;19:5811–5825. doi: 10.1016/j.csbj.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gumienny R., Zavolan M. Accurate transcriptome-wide prediction of microRNA targets and small interfering RNA off-targets with MIRZA-G. Nucleic Acids Res. 2015;43:1380–1391. doi: 10.1093/nar/gkv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fridrich A., Hazan Y., Moran Y. Too Many False Targets for MicroRNAs: Challenges and Pitfalls in Prediction of miRNA Targets and Their Gene Ontology in Model and Non-model Organisms. Bioessays. 2019;41 doi: 10.1002/bies.201800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sethupathy P., Megraw M., Hatzigeorgiou A.G. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat. Methods. 2006;3:881–886. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- 169.Fan X., Kurgan L. Comprehensive overview and assessment of computational prediction of microRNA targets in animals. Brief. Bioinform. 2015;16:780–794. doi: 10.1093/bib/bbu044. [DOI] [PubMed] [Google Scholar]
- 170.Shinde S., Bhadra U. A Complex Genome-MicroRNA Interplay in Human Mitochondria. BioMed Res. Int. 2015;2015:206382–206413. doi: 10.1155/2015/206382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dasgupta N., Peng Y., Tan Z., Ciraolo G., Wang D., Li R. miRNAs in mtDNA-less cell mitochondria. Cell Death Discov. 2015;1 doi: 10.1038/cddiscovery.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cui T., Dou Y., Tan P., Ni Z., Liu T., Wang D., Huang Y., Cai K., Zhao X., Xu D., et al. RNALocate v2.0: an updated resource for RNA subcellular localization with increased coverage and annotation. Nucleic Acids Res. 2022;50:D333–D339. doi: 10.1093/nar/gkab825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garg A., Singhal N., Kumar R., Kumar M. mRNALoc: a novel machine-learning based in-silico tool to predict mRNA subcellular localization. Nucleic Acids Res. 2020;48:W239–W243. doi: 10.1093/nar/gkaa385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Srinivasan H., Das S. Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can. J. Physiol. Pharmacol. 2015;93:855–861. doi: 10.1139/cjpp-2014-0500. [DOI] [PubMed] [Google Scholar]
- 175.Kobayashi A., Takeiwa T., Ikeda K., Inoue S. Roles of Noncoding RNAs in Regulation of Mitochondrial Electron Transport Chain and Oxidative Phosphorylation. IJMS. 2023;24:9414. doi: 10.3390/ijms24119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savoji H., Mohammadi M.H., Rafatian N., Toroghi M.K., Wang E.Y., Zhao Y., Korolj A., Ahadian S., Radisic M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials. 2019;198:3–26. doi: 10.1016/j.biomaterials.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oh J.G., Ishikawa K. In: Experimental Models of Cardiovascular Diseases Methods in Molecular Biology. Ishikawa K., editor. Springer; 2018. Experimental Models of Cardiovascular Diseases: Overview; pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 178.Pereira I., Lopez-Martinez M.J., Samitier J. Advances in current in vitro models on neurodegenerative diseases. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1260397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Slanzi A., Iannoto G., Rossi B., Zenaro E., Constantin G. In vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020;8:328. doi: 10.3389/fcell.2020.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Polidoro M.A., Ferrari E., Marzorati S., Lleo A., Rasponi M. Experimental liver models: From cell culture techniques to microfluidic organs-on-chip. Liver Int. 2021;41:1744–1761. doi: 10.1111/liv.14942. [DOI] [PubMed] [Google Scholar]
- 181.Blaszkiewicz J., Duncan S.A. Use of stem cell-derived hepatocytes to model liver disease. J. Hepatol. 2024;80:826–828. doi: 10.1016/j.jhep.2023.11.029. [DOI] [PubMed] [Google Scholar]
- 182.Hom J., Sheu S.-S. Morphological dynamics of mitochondria — A special emphasis on cardiac muscle cells. J. Mol. Cell. Cardiol. 2009;46:811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pekkurnaz G., Wang X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022;4:802–812. doi: 10.1038/s42255-022-00594-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kang S.W.S., Cunningham R.P., Miller C.B., Brown L.A., Cultraro C.M., Harned A., Narayan K., Hernandez J., Jenkins L.M., Lobanov A., et al. A spatial map of hepatic mitochondria uncovers functional heterogeneity shaped by nutrient-sensing signaling. Nat. Commun. 2024;15:1799. doi: 10.1038/s41467-024-45751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Riva A., Tandler B., Loffredo F., Vazquez E., Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H868–H872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- 186.Williamson C.L., Dabkowski E.R., Baseler W.A., Croston T.L., Alway S.E., Hollander J.M. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H633–H642. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suh J.H., Heath S.-H., Hagen T.M. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic. Biol. Med. 2003;35:1064–1072. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Palmer J.W., Tandler B., Hoppel C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 189.Dabkowski E.R., Williamson C.L., Hollander J.M. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic. Biol. Med. 2008;45:855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 190.To T.-L., McCoy J.G., Ostriker N.K., Sandler L.S., Mannella C.A., Mootha V.K. PMF-seq: a highly scalable screening strategy for linking genetics to mitochondrial bioenergetics. Nat. Metab. 2024;6:687–696. doi: 10.1038/s42255-024-00994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Paramasivam P., Stöter M., Corradi E., Dalla Costa I., Höijer A., Bartesaghi S., Sabirsh A., Lindfors L., Yanez Arteta M., Nordberg P., et al. Quantitative intracellular retention of delivered RNAs through optimized cell fixation and immunostaining. RNA. 2022;28:433–446. doi: 10.1261/rna.078895.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xia C., Fan J., Emanuel G., Hao J., Zhuang X. Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc. Natl. Acad. Sci. USA. 2019;116:19490–19499. doi: 10.1073/pnas.1912459116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Borum R.M., Moore C., Chan S.K., Steinmetz N.F., Jokerst J.V. A Photoacoustic Contrast Agent for miR-21 via NIR Fluorescent Hybridization Chain Reaction. Bioconjug. Chem. 2022;33:1080–1092. doi: 10.1021/acs.bioconjchem.1c00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu X., Zhang S.-Q., Cheng Z.-H., Wei X., Yang T., Yu Y.-L., Chen M.-L., Wang J.-H. Highly Sensitive Detection of MicroRNA-21 with ICPMS via Hybridization Accumulation of Upconversion Nanoparticles. Anal. Chem. 2018;90:12116–12122. doi: 10.1021/acs.analchem.8b03038. [DOI] [PubMed] [Google Scholar]
- 195.Wei W., Lu H., Dai W., Zheng X., Dong H. Multiplexed Organelles Portrait Barcodes for Subcellular MicroRNA Array Detection in Living Cells. ACS Nano. 2022;16:20329–20339. doi: 10.1021/acsnano.2c06252. [DOI] [PubMed] [Google Scholar]
- 196.Kuznetsov A.V., Javadov S., Margreiter R., Hagenbuchner J., Ausserlechner M.J. Analysis of Mitochondrial Function, Structure, and Intracellular Organization In Situ in Cardiomyocytes and Skeletal Muscles. IJMS. 2022;23:2252. doi: 10.3390/ijms23042252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hwang H.V., Sandeep N., Nair R.V., Hu D.Q., Zhao M., Lan I.S., Fajardo G., Matkovich S.J., Bernstein D., Reddy S. Transcriptomic and Functional Analyses of Mitochondrial Dysfunction in Pressure Overload-Induced Right Ventricular Failure. JAHA. 2021;10 doi: 10.1161/JAHA.120.017835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Timón-Gómez A., Pérez-Pérez R., Nyvltova E., Ugalde C., Fontanesi F., Barrientos A. Protocol for the Analysis of Yeast and Human Mitochondrial Respiratory Chain Complexes and Supercomplexes by Blue Native Electrophoresis. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2020.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spinazzi M., Casarin A., Pertegato V., Salviati L., Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 200.Cantó C., Garcia-Roves P.M. High-Resolution Respirometry for Mitochondrial Characterization of Ex Vivo Mouse Tissues. CP Mouse Biology. 2015;5:135–153. doi: 10.1002/9780470942390.mo140061. [DOI] [PubMed] [Google Scholar]
- 201.Lanza I.R., Nair K.S. Mitochondrial metabolic function assessed in vivo and in vitro. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:511–517. doi: 10.1097/MCO.0b013e32833cc93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Divakaruni A.S., Rogers G.W., Murphy A.N. Measuring Mitochondrial Function in Permeabilized Cells Using the Seahorse XF Analyzer or a Clark-Type Oxygen Electrode. CP Toxicology. 2014;60:25.2.1–25.2.16. doi: 10.1002/0471140856.tx2502s60. [DOI] [PubMed] [Google Scholar]
- 203.Zhang J., Nuebel E., Wisidagama D.R.R., Setoguchi K., Hong J.S., Van Horn C.M., Imam S.S., Vergnes L., Malone C.S., Koehler C.M., Teitell M.A. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 2012;7:1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schmidt C.A., Fisher-Wellman K.H., Neufer P.D. From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Acin-Perez R., Benador I.Y., Petcherski A., Veliova M., Benavides G.A., Lagarrigue S., Caudal A., Vergnes L., Murphy A.N., Karamanlidis G., et al. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 2020;39 doi: 10.15252/embj.2019104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Osto C., Benador I.Y., Ngo J., Liesa M., Stiles L., Acin-Perez R., Shirihai O.S. Measuring Mitochondrial Respiration in Previously Frozen Biological Samples. CP Cell Biology. 2020;89:e116. doi: 10.1002/cpcb.116. [DOI] [PubMed] [Google Scholar]
- 207.Dedkova E.N., Blatter L.A. Measuring mitochondrial function in intact cardiac myocytes. J. Mol. Cell. Cardiol. 2012;52:48–61. doi: 10.1016/j.yjmcc.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Connolly N.M.C., Theurey P., Adam-Vizi V., Bazan N.G., Bernardi P., Bolaños J.P., Culmsee C., Dawson V.L., Deshmukh M., Duchen M.R., et al. Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. 2018;25:542–572. doi: 10.1038/s41418-017-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Abdurrachim D., Prompers J.J. Evaluation of cardiac energetics by non-invasive 31P magnetic resonance spectroscopy. Biochim. Biophys. Acta, Mol. Basis Dis. 2018;1864:1939–1948. doi: 10.1016/j.bbadis.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 210.Sparmann A., Vogel J. RNA -based medicine: from molecular mechanisms to therapy. EMBO J. 2023;42 doi: 10.15252/embj.2023114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chernega T., Choi J., Salmena L., Andreazza A.C. Mitochondrion-targeted RNA therapies as a potential treatment strategy for mitochondrial diseases. Mol. Ther. Nucleic Acids. 2022;30:359–377. doi: 10.1016/j.omtn.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maghsoudnia N., Baradaran Eftekhari R., Naderi Sohi A., Norouzi P., Akbari H., Ghahremani M.H., Soleimani M., Amini M., Samadi H., Dorkoosh F.A. Mitochondrial delivery of microRNA mimic let-7b to NSCLC cells by PAMAM-based nanoparticles. J. Drug Target. 2020;28:818–830. doi: 10.1080/1061186X.2020.1774594. [DOI] [PubMed] [Google Scholar]
- 214.Yamada Y., Akita H., Kamiya H., Kogure K., Yamamoto T., Shinohara Y., Yamashita K., Kobayashi H., Kikuchi H., Harashima H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim. Biophys. Acta. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 215.Guo J., Chen X., Liu Z., Sun H., Zhou Y., Dai Y., Ma Y., He L., Qian X., Wang J., et al. DdCBE mediates efficient and inheritable modifications in mouse mitochondrial genome. Mol. Ther. Nucleic Acids. 2022;27:73–80. doi: 10.1016/j.omtn.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bacman S.R., Kauppila J.H.K., Pereira C.V., Nissanka N., Miranda M., Pinto M., Williams S.L., Larsson N.-G., Stewart J.B., Moraes C.T. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 2018;24:1696–1700. doi: 10.1038/s41591-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schmiderer L., Yudovich D., Oburoglu L., Hjort M., Larsson J. Site-specific CRISPR-based mitochondrial DNA manipulation is limited by gRNA import. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-21794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.