Abstract
We examined the associations between lung function and incident dementia and cognitive decline in 12,688 participants in the ARIC Study who provided lung function measurements in 1990–1992. Cognitive tests were administered up to 7 times, and dementia was ascertained through 2019. We used shared parameter models to jointly fit proportional hazard models and linear mixed-effect models to estimate lung-function–associated dementia rate and cognitive change, respectively. Higher forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were associated with reduced dementia (n = 2,452 persons developed dementia); hazard ratios per 1-L increase in FEV1 and FVC were 0.79 (95% confidence interval (CI): 0.71, 0.89) and 0.81 (95% CI: 0.74, 0.89), respectively. Each 1-L increase in FEV1 and FVC was associated with a 0.08–standard deviation (SD) (95% CI: 0.05, 0.12) and a 0.05-SD (95% CI: 0.02, 0.07) attenuation of 30-year cognitive decline, respectively. A 1% increase in FEV1/FVC ratio was associated with 0.008-SD (95% CI: 0.004, 0.012) less cognitive decline. We observed statistical interaction between FEV1 and FVC, suggesting that cognitive declines depended on values of specific FEV1 and FVC (as compared with FEV1, FVC, or FEV1/FVC ratio models that suggested linear incremental associations). Our findings may have important implications for reducing the burden of cognitive decline that is attributable to environmental exposures and associated lung function impairment.
Keywords: cognitive decline, dementia, lung function, shared parameter models
Abbreviations
- APOE*ε4
apolipoprotein E gene epsilon 4 allele
- ARIC
Atherosclerosis Risk in Communities
- BP
blood pressure
- CI
confidence interval
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HR
hazard ratio
- LLN
lower limit of normal
- SD
standard deviation
- SPM
shared parameter model
Later-life cognitive impairment is associated with a substantial public health and financial burden, which is likely to increase with the aging of the population (1, 2). With no foreseeable cure or disease-modifying interventions for severe forms of cognitive impairment, including dementia, research on modifiable risk factors for cognitive decline may have important public health implications.
Prior studies have linked changes in lung function measures, as well as clinical respiratory diseases, with adverse cognitive outcomes, including dementia (3–6). Systemic inflammatory processes underlying reduced lung function and chronic hypoxia-mediated neuropathological alterations have been suggested as potential mechanisms for the association between lung function and cognitive impairment (7–12). Although a handful of studies have shown somewhat consistent links between suboptimal lung function and dementia (4), findings have been inconclusive for cognitive decline (13–18). Further, the majority of studies evaluating cognitive change had short follow-up times, which are likely inadequate to capture cognitive decline. Those studies also did not account for cohort attrition that may have biased findings, especially for studies that recruited older participants (13). Given that reduced lung function in adulthood may be partially preventable, elucidating the association between lung function and cognition and underlying mechanisms may have important implications for dementia prevention.
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing prospective cohort study that, at inception, included middle-aged community-dwelling adults. Previously, 2 prospective investigations on associations of lung function with dementia and cognitive decline were conducted using ARIC data (16, 19). While both studies found some evidence for associations between poor lung function and risk of dementia (median follow-up, 23 years) (19) or related hospitalizations (median follow-up, 14 years) (16), there was no evidence of association with 6- to 16-year cognitive decline (16). Since then, the ARIC Study has performed more cognitive examinations and accumulated additional dementia cases. With extended follow-up data from over 30 years, we conducted an updated analysis to examine lung function status, including clinical conditions, in relation to incident dementia and cognitive decline in the ARIC Study (i.e., potential etiological associations between lung function and these outcomes).
METHODS
Study population
The ARIC Study recruited adults aged 45–64 years from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and suburbs of Minneapolis, Minnesota. The study details have been published elsewhere (20, 21). Briefly, 15,792 participants were enrolled in 1987–1989 (visit 1). Participants were reexamined in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), 2011–2013 (visit 5), 2016–2017 (visit 6), and 2018–2019 (visit 7). Additionally, participants were followed up through annual or semiannual (since 2012) telephone interviews and hospitalization and mortality surveillance. The study was approved by institutional review boards at participating study centers, and all participants provided informed consent.
For the current analysis, we considered visit 2 (when 3 brief cognitive assessments were first introduced) the index visit. Of the 14,348 visit 2 participants, 12,688 participants were included in this analysis after exclusion of those with missing data on lung function (n = 417), those with race/ethnicity other than Black or White (n = 42), Black participants from the Minnesota and Maryland centers (n = 49), those with missing visit 2 cognition data or prevalent dementia (n = 154), and those with missing data on other covariates (n = 998). Race/ethnicity-based exclusions were made because of small numbers. Of the 12,688 participants (with complete visit 2 cognition data) included in our analysis, we had cognitive data available from 1,857 at visit 3 (cognitive examinations were conducted only in a subset of visit 3 participants); 9,993 at visit 4; 5,401 at visit 5; 3,285 at visit 6; and 2,765 at visit 7 (see Web Table 1, available at https://doi.org/10.1093/aje/kwad140).
Lung function measures
Lung function was measured at visit 2 using the Collins Survey II water-seal spirometer (Collins Medical, Inc., Braintree, Massachusetts) coupled with the Pulmo-Screen II system (PDS Healthcare Products, Inc., Louisville, Colorado) by trained and certified technicians; details on measurement procedures, quality control, and acceptability and reproducibility criteria are provided elsewhere (22). Briefly, up to 8 forced expirations were performed to achieve 2 reproducible spirograms (volumes within ±5%) out of 3 acceptable maneuvers. The best spirogram, selected by computer software and later confirmed by a technician, was used to obtain lung function measures. The current analysis used 3 measures of lung function—forced vital capacity (FVC; the total volume of air that can be forcefully exhaled after deep inspiration), forced expiratory volume in 1 second (FEV1; the volume of air exhaled in the first second of the FVC maneuver), and the FEV1/FVC ratio (expressed as a percentage).
We also created 4 clinical categories based on predicted FVC, FEV1/FVC ratio, and clinical symptoms reported at visit 2, as previously described (19). Specifically, the age-, sex-, and height-specific lower limit of normal (LLN) values were estimated using the Global Lung Function Initiative’s equation for “other race” (23). While the use of race-specific thresholds is a standard practice, this may not be appropriate, since it is based on the unfounded assumption that racial differences in lung function are biological. The Global Lung Function Initiative “other” equation averages estimates from multiple races and has been used previously to estimate race-neutral thresholds (24, 25). Obstructive lung disease (or chronic obstructive pulmonary disease) was defined as FEV1/FVC < LLN, and restrictive lung disease was defined as FEV1/FVC ≥ LLN and FVC < LLN. We classified the participants who did not belong to either the restrictive or obstructive category (“normal”) as “normal without respiratory symptoms” and “normal with respiratory symptoms” based on respiratory symptoms (cough, wheeze, and phlegm) self-reported at visit 2 (19).
Neurocognitive assessment and dementia ascertainment
Three cognitive tests, including the Delayed Word Recall Test (a measure of verbal learning and memory), the Digit Symbol Substitution Test (a measure of psychomotor speed and sustained attention), and the Word Fluency Test (a measure of verbal functioning), were administered at ARIC visits 2–7 by trained examiners (more detail is provided in Web Appendix 1) (26). All participants were eligible for cognitive examinations at all visits, except for visit 3 (when only those from North Carolina and Mississippi underwent cognitive examinations as part of a brain imaging substudy). The raw scores for each cognitive test at each visit were standardized as z scores, using the visit 2 mean value and standard deviation (SD). The 3 test-specific z scores were then averaged and standardized to calculate composite cognition z scores for each visit in SD units. Dementia was ascertained from several sources, including in-person neuropsychological assessment, informant interviews, and expert adjudication, as well as telephone cognitive screenings, and surveillance of hospitalizations and deaths (more detail is provided in Web Appendix 1) (27).
Covariates
Information on sex, site-race (Maryland White, Minnesota White, Mississippi Black, North Carolina Black, and North Carolina White participants), height, education (less than high school, high school or equivalent, or more than high school), leisure-time physical activity (score on the sport index of the Baecke Physical Activity Questionnaire (28)), and apolipoprotein E gene epsilon 4 allele (APOE*ε4) status (any ε4 allele vs. no ε4 allele) was obtained from the visit 1 examination. Information on other covariates was obtained from the visit 2 examination: age, cigarette smoking status (current, former, or never smoker), pack-years of smoking, secondhand smoking, waist:hip ratio, diabetes (defined as fasting glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, self-reported physician-diagnosed diabetes, or use of antidiabetic medication), blood pressure (BP) status (hypertension (defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medication), prehypertension (defined as systolic BP ≥120 mm Hg and <140 mm Hg), or normal BP), total cholesterol level, prevalent stroke, and coronary heart disease.
Statistical analysis
We used a shared parameter model (SPM) approach (29, 30) for the related outcomes of dementia and cognitive decline. SPMs can be used to simultaneously model time-to-event outcomes (such as dementia and/or death events) and longitudinal repeated-measures outcomes (such as cognitive scores). The sharing of information between these 2 models (often through a set of latent characteristics) allows the longitudinal outcome to inform the event outcome process and the event outcome to inform the longitudinal trajectory. SPMs have been shown to be preferable to the related separate analyses, since they provide less biased and more efficient estimates (30). Another benefit of SPMs is their ability to more appropriately account for missing data (29). Specifically, in ARIC, participants with poorer lung function and lower cognitive test scores at visit 2 were more likely to have missing cognitive data over time (Web Table 1, Web Table 2), mainly due to loss to follow-up associated with dementia, death, or dropout. In such a scenario, where attrition is likely to be related to the outcome of interest, longitudinal data analysis approaches such as generalized estimating equations and standard mixed models (which assume missingness completely at random and missingness at random, respectively) may lead to biased results. Under the extended missing-at-random assumption (which assumes missingness depends on observed data and a function of missing values), SPM allows more tractable handling of missingness by incorporating additional external information related to the outcome and missingness/dropout. Details on these models have been provided elsewhere (29), and they are briefly described in Web Appendix 2. Interpretations of SPM estimates obtained from the joint time-to-event submodels and longitudinal mixed submodels remain similar to those of estimates obtained from the separate models (30).
Our primary SPM was constructed using 1) a linear mixed submodel to estimate the association of lung function with repeated measurements of cognition over 30 years, 2) a parametric Weibull survival submodel to estimate hazard ratios (HRs) for the association of lung function with incident dementia, and 3) a secondary parametric Weibull survival submodel to account for death. For our longitudinal submodel, we used a linear mixed model with both random intercepts and slopes (a full model description is given in Web Appendix 3). Our longitudinal time terms incorporated linear splines with a knot at the sixth year from the index visit (following the ARIC Study’s analytical guidance to account for observed temporal nonlinearities in the cognitive trajectories and to account for a long gap until visit 5 (visit 4 occurred roughly 6 years after visit 2)). We used terms for interaction between lung function measures and these time components to estimate associations of lung function with cognitive declines. We present the average difference in composite cognition z score over a 30-year period. All 3 of the submodels adjusted for age, sex, site-race, height, smoking status, waist:hip ratio, education, physical activity, diabetes, hypertension, total cholesterol, heart disease, stroke, and APOE*ε4 status. Linear mixed models in our primary analyses also incorporated interactions of time with baseline age (median split at 54 years), smoking status, diabetes, education, waist:hip ratio, total cholesterol, and APOE*ε4 status. However, we also present the results from the linear mixed models that included only the terms for interaction between lung function measures and time (i.e., without additional terms for the interaction between time and covariates). We used Akaike’s information criterion, the Bayesian information criterion, and likelihood ratio test statistics to assist with model selection.
We fitted SPM models separately for FEV1, FVC, FEV1/FVC ratio, and categorical clinical outcomes (i.e., modeled each measure separately). To additionally illustrate the combined information available in the FEV1 and FVC measurements, we performed analyses with FEV1 and FVC in the same model and estimated differences in predicted change in 30-year cognitive performance associated with varying changes to FEV1, FVC, or both; in other words, we examined the combined associations of FEV1 and FVC (i.e., associations of FVC at specific values of FEV1 and vice versa) with cognitive decline. The best-fitting SPM model for this approach included dementia and death event submodels utilizing FEV1 as an exposure and a longitudinal submodel utilizing FEV1 and FVC, as well as 2-way interactions of FEV1 and FVC with the linear time spline terms (full model descriptions are given in Web Appendix 4).
Further, to facilitate the interpretation of our results, we compared the magnitude of associations between lung function measures and cognitive outcomes with that of associations between baseline age and cognitive outcomes. The age estimates were obtained from separate survival and linear mixed models adjusting for sex, site-race, education, and smoking (inclusion of all covariates considered for lung function models may lead to overadjustment for age estimates). The linear mixed model for age association included terms for the interaction between baseline age and time components.
We also conducted several sensitivity analyses. We examined APOE*ε4 carrier status and race/ethnicity as effect modifiers. Because smoking is a strong predictor of poor lung function and death and potentially contributes to dementia, we restricted the analysis to never smokers, additionally adjusting for secondhand exposure to tobacco smoke. Adjusting for pack-years of smoking in addition to smoking status yielded results similar to those of the primary analysis. We also examined associations with incident dementia and cognitive decline using standard, separate survival and linear mixed models. Lastly, while for longitudinal models our primary outcome of interest was composite cognition z score, we also present results for cognitive-domain–specific analyses. We used STATA, version 16.0 (StataCorp LLC, College Station, Texas) for data analyses; we used the GSEM command in STATA for SPM models. The software code with which to run SPMs (similar to our analysis) in STATA, SAS, and R is available elsewhere (29).
RESULTS
The mean age of the participants at visit 2 was 57 (SD, 5.7) years; 45% were male, 24% were Black, and 79% had at least a high school or equivalent education (Table 1). Over the 30 years of follow-up (median, 23.8 years), 19% (n = 2,452) of participants developed dementia; older, female, and Black participants and those with less education were more likely to have poor lung function, as well as to develop dementia (Table 1, Web Table 3). The Spearman correlation between FEV1 and FVC was 0.93. All lung function measures except FEV1/FVC ratio were associated with baseline cognition (Web Table 4).
Table 1.
Visit 2 Characteristics of Participants in the Atherosclerosis Risk in Communities Study (n = 12,688), by Dementia Status, 1990–2019a
| Dementia Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (n = 12,688) | No Dementia (n = 10,236) | Dementia (n = 2,452) | ||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| FEV1, L | 2.71 (0.75) | 2.73 (0.76) | 2.61 (0.70) | ||||||
| FVC, L | 3.65 (0.97) | 3.69 (0.98) | 3.50 (0.92) | ||||||
| FEV1/FVC ratio, % | 74.44 (8.11) | 74.30 (8.24) | 75.01 (7.54) | ||||||
| Lung function category | |||||||||
| Normal (no respiratory symptoms) | 6,228 | 49.1 | 4,988 | 48.7 | 1,240 | 50.6 | |||
| Normal with respiratory symptoms | 3,449 | 27.2 | 2,741 | 26.8 | 708 | 28.9 | |||
| Restrictive lung disease | 667 | 5.3 | 537 | 5.3 | 130 | 5.3 | |||
| COPD | 2,344 | 18.5 | 1,970 | 19.2 | 374 | 15.3 | |||
| Age, years | 57.0 (5.71) | 56.4 (5.63) | 59.5 (5.31) | ||||||
| Male sex | 5,662 | 44.6 | 4,667 | 45.6 | 995 | 40.6 | |||
| Cigarette smoking status | |||||||||
| Never smoker | 5,077 | 40.0 | 3,964 | 38.7 | 1,113 | 45.4 | |||
| Former smoker | 4,788 | 37.7 | 3,907 | 38.2 | 881 | 35.9 | |||
| Current smoker | 2,823 | 22.2 | 2,365 | 23.1 | 458 | 18.7 | |||
| Black race/ethnicity | 2,997 | 23.6 | 2,300 | 22.5 | 697 | 28.4 | |||
| ARIC study center | |||||||||
| Forsyth, North Carolina | 3,240 | 25.5 | 2,708 | 26.5 | 532 | 21.7 | |||
| Jackson, Mississippi | 2,658 | 20.9 | 2,015 | 19.7 | 643 | 26.2 | |||
| Minneapolis, Minnesota | 3,498 | 27.6 | 2,915 | 28.5 | 583 | 23.8 | |||
| Washington County, Maryland | 3,292 | 25.9 | 2,598 | 25.4 | 694 | 28.3 | |||
| Education | |||||||||
| Less than high school | 2,649 | 20.9 | 1,948 | 19.0 | 701 | 28.6 | |||
| High school | 5,305 | 41.8 | 4,314 | 42.1 | 991 | 40.4 | |||
| More than high school | 4,734 | 37.3 | 3,974 | 38.8 | 760 | 31.0 | |||
| Body mass indexb | 27.9 (5.36) | 27.9 (5.33) | 28.2 (5.46) | ||||||
| Waist:hip ratio | 0.93 (0.08) | 0.92 (0.08) | 0.93 (0.08) | ||||||
| Height, cm | 168.5 (9.32) | 168.8 (9.31) | 167.3 (9.25) | ||||||
| Physical activity indexc | 2.39 (0.57) | 2.39 (0.57) | 2.37 (0.58) | ||||||
| Coronary heart disease | 699 | 5.5 | 595 | 5.8 | 104 | 4.2 | |||
| Stroke | 197 | 1.6 | 156 | 1.5 | 41 | 1.7 | |||
| Diabetes | 1,847 | 14.6 | 1,436 | 14.0 | 411 | 16.8 | |||
| Blood pressure | |||||||||
| Normal | 5,685 | 44.8 | 4,760 | 46.5 | 925 | 37.7 | |||
| Prehypertension | 2,560 | 20.2 | 2,015 | 19.7 | 545 | 22.2 | |||
| Hypertension | 4,443 | 35.0 | 3,461 | 33.8 | 982 | 40.0 | |||
| Total cholesterol level, mmol/L | 5.43 | 1.0 | 5.41 | 1.0 | 5.53 | 1.0 | |||
| APOE*ε4 | 3,915 | 30.9 | 2,861 | 28.0 | 1,054 | 43.0 | |||
Abbreviations: APOE*ε4, apolipoprotein E gene epsilon 4 allele; ARIC, Atherosclerosis Risk in Communities; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
a Percentages in subgroups may not sum to 100% because of rounding.
b Weight (kg)/height (m)2.
b Score on the sport index of the Baecke Physical Activity Questionnaire (range, 1–4) (28).
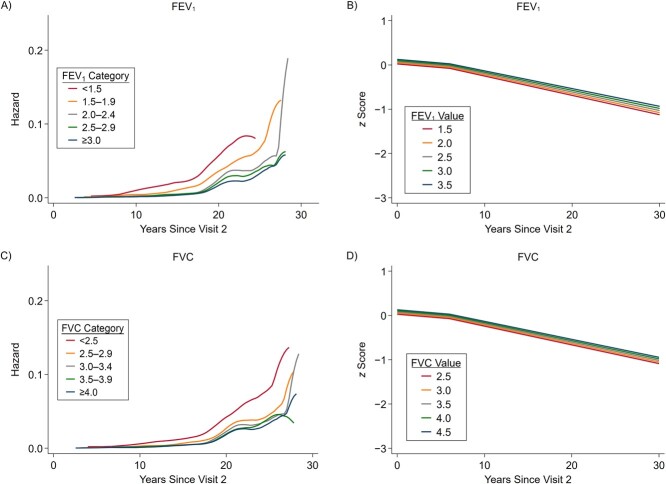
After adjustment for potential confounders, higher FEV1 and FVC were associated with 21% and 19% lower rates of dementia per 1-L increase in FEV1 and FVC, respectively (HR = 0.794 (95% confidence interval (CI): 0.710, 0.888) and HR = 0.812 (95% CI: 0.739, 0.892), respectively) (Table 2, Figures 1A and 1B). Web Table 5 provides the HR estimates associated with a 1-SD increase in FEV1 and FVC. Continuous FEV1/FVC ratio was not associated with dementia. We observed a 30% higher dementia rate associated with restrictive lung disease (HR = 1.305, 95% CI: 1.023, 1.663) and a 23% higher dementia rate associated with chronic obstructive pulmonary disease (HR = 1.228, 95% CI: 1.056, 1.428). In comparison, a 1-year increase in baseline age was associated with a 15% increase in the dementia rate (HR = 1.150, 95% CI: 1.140, 1.160).
Table 2.
Associations of Lung Function Measures at Visit 2 With Dementia Events and Cognitive Decline Over 30 Years (n = 12,688), Atherosclerosis Risk in Communities Study, 1990–2019a
| Lung Function Measure | Dementia | Cognitive Decline Attenuation Over 30 Years b | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | β | 95% CI | P Value | |
| FEV1, per 1-L increase | 0.794 | 0.710, 0.888 | <0.001 | 0.083 | 0.049, 0.116 | <0.001 |
| FVC, per 1-L increase | 0.812 | 0.739, 0.892 | <0.001 | 0.046 | 0.021, 0.072 | <0.001 |
| FEV1/FVC ratio, per 1% increase | 0.994 | 0.986, 1.001 | 0.104 | 0.008 | 0.004, 0.012 | <0.001 |
| FEV1/FVC ratio category | ||||||
| Normal without respiratory symptoms | 1.000 | Referent | 0.000 | Referent | ||
| Normal with respiratory symptoms | 1.111 | 0.987, 1.251 | 0.082 | −0.003 | −0.059, 0.053 | 0.919 |
| Restrictive lung disease | 1.305 | 1.023, 1.663 | 0.032 | 0.070 | −0.050, 0.191 | 0.250 |
| Chronic obstructive pulmonary disease | 1.228 | 1.056, 1.428 | 0.008 | −0.039 | −0.108, 0.030 | 0.265 |
Abbreviations: APOE*ε4, apolipoprotein E gene epsilon 4 allele; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HR, hazard ratio.
a All estimates were obtained from shared parameter models. All of the models adjusted for continuous age, sex, site-race, education, smoking, height, waist:hip ratio, physical activity, total cholesterol, hypertension status, diabetes, heart disease, stroke, and APOE*ε4 status. The linear mixed model additionally included terms for the interaction of time with age (split at the median age of 54 years), smoking, diabetes, education, waist:hip ratio, total cholesterol, and APOE*ε4 status.
b Positive estimates (>0) indicate attenuation of cognitive decline, whereas negative estimates indicate faster cognitive decline.
Figure 1.
Smoothed hazard estimates for the associations between dementia and forced expiratory volume in 1 second (FEV1) (A) and forced vital capacity (FVC) (C) and predicted composite z scores for FEV1 (B) and FVC (D), Atherosclerosis Risk in Communities Study, 1990–2019. FEV1 and FVC values are expressed in liters. Panels A and C show smooth hazard estimates for dementia for discrete categories of FEV1 and FVC, respectively (e.g., persons with FEV1 < 1.5 L and FVC < 2.5 L have the highest incidence of dementia). Panels B and D show predicted cognitive declines for continuous FEV1 and FVC values, respectively. The longitudinal model incorporated linear splines with a knot at the sixth year from visit 2, as reflected by different slopes of predicted cognitive declines. The full-scale versions of panels B and D with data points are presented as Web Figures 1 and 2.
Better lung function measures were also associated with attenuation in 30-year cognitive decline; each 1-L increase in FEV1 and FVC was respectively associated with 0.083 SD (95% CI: 0.049, 0.116) and 0.046 SD (95% CI: 0.021, 0.072) attenuation of the 30-year decline in composite cognition z scores (Table 2). Further, each 1% increase in FEV1/FVC ratio was associated with 0.008 SD (95% CI: 0.004, 0.012) less decline in 30-year composite z scores. In comparison, a 1-year increase in baseline age was associated with −0.050 SD (95% CI: −0.054, −0.046) faster decline in composite z scores. Overall decline was steeper in later life than in midlife (Figures 1C and 1D). The results did not support associations between clinical categories and cognitive decline. These associations were similar when we did not include terms for the interaction of time with baseline covariates (Web Table 6).
When assessing the combined associations of FVC and FEV1 within the same model, we found that the greatest attenuation in cognitive decline was observed where the difference between FVC and FEV1 was small, which is in line with the FEV1/FVC ratio findings (Table 3, Figures 2 and 3). For example, Figure 2 shows that cognitive decline in later life was greatest and steepest among persons with the highest difference between FVC and FEV1. The model combining FEV1 and FVC demonstrated that cognitive decline depended on joint levels of specific FEV1 and FVC (i.e., suggested that these lung function measures work synergistically with each other in a nonlinear fashion; P for interaction between FEV1 and FVC ≤ 0.001), adding on to the FEV1/FVC ratio finding that simply depicted a linear relationship with cognitive decline (Table 3, Figure 2). In Figure 3, we provide results for selected data points as examples tied to the values presented in Table 3; for example, compared with persons with an FVC of 2.5 L and an FEV1 of 1.5 L, cognitive decline was 0.207 SD (95% CI: 0.128, 0.287) less for those with an FVC of 2.5 L and an FEV1 of 2.5 L. The attenuation diminished to 0.104 SD (95% CI: 0.064, 0.143) for persons with the same FVC level and FEV1 of 2.0 L. However, those with a higher FVC of 3.5 L and an FEV1 of 3.5 L had the greatest attenuation (0.362 SD, 95% CI: 0.254, 0.470).
Table 3.
Predicted Attenuationa,b of 30-Year Cognitive Decline Across Varying Levels of FEV1 and FVC Obtained From Shared Parameter Models, Atherosclerosis Risk in Communities Study, 1990–2019
| FEV 1 , L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FVC, L | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | |||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| 2.5 | 0.000 | Referent | 0.104 | 0.064, 0.143 | 0.207 | 0.128, 0.287 | NAc | NA | NA | NA |
| 3.0 | −0.006 | −0.037, 0.025 | 0.093 | 0.072, 0.114 | 0.191 | 0.136, 0.246 | 0.290 | 0.196, 0.383 | NA | NA |
| 3.5 | −0.011 | −0.074, 0.051 | 0.082 | 0.047, 0.116 | 0.175 | 0.135, 0.215 | 0.269 | 0.198, 0.340 | 0.362 | 0.254, 0.470 |
| 4.0 | −0.017 | −0.110, 0.076 | 0.071 | 0.009, 0.133 | 0.159 | 0.115, 0.204 | 0.248 | 0.191, 0.304 | 0.336 | 0.249, 0.422 |
| 4.5 | NA | NA | 0.060 | −0.031, 0.151 | 0.143 | 0.079, 0.208 | 0.226 | 0.170, 0.282 | 0.310 | 0.237, 0.382 |
Abbreviations: APOE*ε4, apolipoprotein E gene epsilon 4 allele; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NA, not available.
a All of the models adjusted for continuous age, sex, site-race, education, smoking, height, waist:hip ratio, physical activity, total cholesterol, hypertension status, diabetes, heart disease, stroke, and APOE*ε4 status. The linear mixed model additionally included terms for the interaction of time with age (split at the median age of 54 years), smoking, diabetes, education, waist:hip ratio, total cholesterol, and APOE*ε4 status. In the shared parameter model, the dementia and death submodels included only FEV1 as the lung function measure in the model. The longitudinal submodel included both FEV1 and FVC; specifically, the model was:
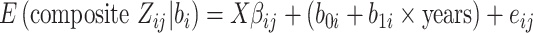
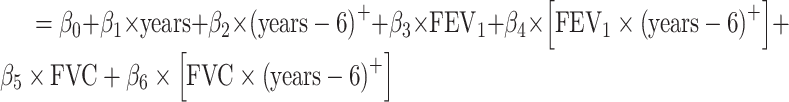
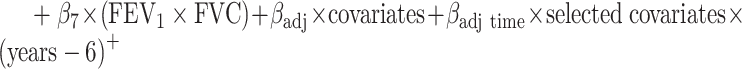
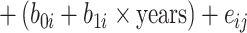 . (See Web Appendix 4 for details and notation.)
. (See Web Appendix 4 for details and notation.)
b Positive estimates (>0) indicate attenuation of cognitive decline, whereas negative estimates indicate faster cognitive decline.
c No data/implausible data.
Figure 2.
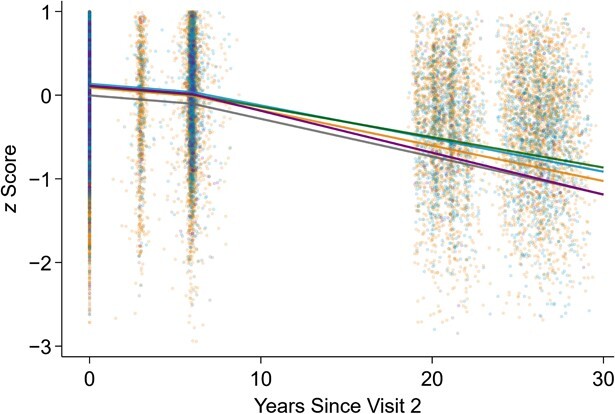
Predicted cognitive declines over time for varying levels of continuous forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), Atherosclerosis Risk in Communities (ARIC) Study, 1990–2019. Data were obtained from a linear mixed model with prediction computed at years 0, 6, 10, 15, 20, 25, and 30 (the model incorporated linear splines with a knot at the sixth year from ARIC visit 2). Plausible values of FEV1 and FVC were chosen. The gray, orange, blue, green, and purple lines represent the cognition trajectories for the groups “both low” (FEV1 = 1.5 L, FVC = 2.5 L), “both medium” (FEV1 = 2.5 L, FVC = 3.5 L), “both high” (FEV1 = 3.5 L, FVC = 4.5 L), “equal” (FEV1 = 3.5 L, FVC = 3.5 L), and “FVC − FEV1 > 2” (FEV1 = 1.5 L, FVC = 4.0 L), respectively. The 30-year decline was steepest among persons with the highest difference between FVC and FEV1.
Figure 3.
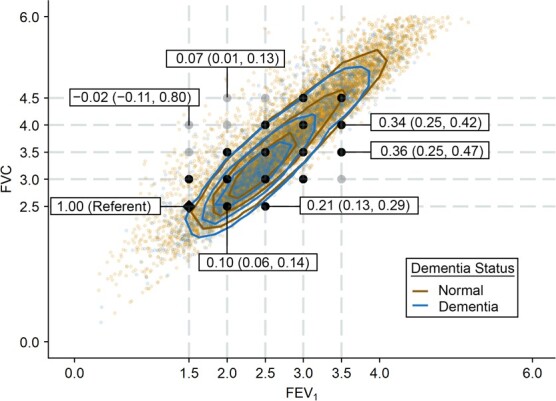
Joint distribution of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) with contour plots by dementia status and data points showing predicted attenuation of 30-year cognitive decline, Atherosclerosis Risk in Communities Study, 1990–2019. The contours represent percentiles of the bivariate probability distribution of FEV1 and FVC. We present the 25th (innermost lines), 50th, and 75th (outermost lines) percentile contour lines separately for persons who developed dementia and those who remained dementia-free. Participants who developed dementia were more likely to have lower FEV1 and FVC values (contours pulled toward the bottom) than those who did not develop dementia. Text boxes show the magnitude of predicted attenuation/acceleration of 30-year cognitive decline (and 95% confidence intervals) obtained from the model examining the combined association of FEV1 and FVC (see Table 3 for details; black and gray points indicate the data points presented in Table 3, and the black diamond shows the referent). Compared with the referent group (FVC = 2.5 L, FEV1 = 1.5 L), the predicted attenuation in cognitive decline was 0.207 standard deviation (SD) (95% CI: 0.128, 0.287) for persons with an FVC of 2.5 L and an FEV1 of 2.5 L (numbers shown on the figure are rounded). The attenuation diminished to 0.104 SD (95% CI: 0.064, 0.143) for those with the same FVC level and an FEV1 of 2.0 L. No attenuation was observed in those with a higher FVC of 4.0 L and a lower FEV1 of 1.5 L (−0.017 SD, 95% CI: −0.110, 0.076).
In the analyses restricted to never smokers (n = 5,077), the findings were similar (Web Table 7). The associations did not differ by APOE*ε4 allele status or race/ethnicity for either dementia or cognitive decline (data not shown).
The results from survival models (Cox proportional hazards or Weibull models) and linear mixed models that were fitted separately without SPM supported the primary results (Web Table 8); however, parameter estimates from SPM models were stronger. Lastly, in domain-specific analyses, we found that higher FEV1 was associated with attenuation of cognitive decline in all 3 domains (i.e., memory, language, and processing speed and attention), while FVC was associated only with language and FEV1/FVC ratio was associated only with memory and processing speed and attention (Web Table 9).
DISCUSSION
In this study of community-dwelling adults, we found that better lung function in midlife was associated with very modest reductions in the rate of dementia and cognitive decline. For instance, 1-L increases in FEV1 and FVC were associated with reduced rates of dementia (about 21% and 19%, respectively) and a slowing of cognitive decline over 30 years that was equivalent to being 1–2 years younger. The combined analysis of FEV1 and FVC underscored that cognitive declines depended on joint levels of specific FEV1 and FVC. Further, the associations persisted in the analyses restricted to participants who had never smoked, suggesting the findings were not entirely confounded by smoking.
Our finding of an association between better lung function and reduced dementia rate is consistent with the findings of earlier ARIC investigations (16, 19) and other studies (3, 4). In the most recent ARIC investigation that examined visit 1 lung function and dementia (1,407 cases identified through 2013), Lutsey et al. (19) reported that 1-SD decreases in FEV1 and FVC percent predicted were associated with a modestly elevated dementia rate, and the associations were stronger when analyses were restricted to adjudicated dementia cases. The study also found higher odds of dementia for restrictive lung disease and chronic obstructive pulmonary disease pattern when analyses were restricted to adjudicated dementia. Other prior prospective studies have also found somewhat consistent associations between poor lung function and dementia, although only a handful used objective lung function measures (4). Further, in most larger studies, dementia cases were ascertained solely from death certificates and/or electronic health records (3, 31, 32), and thus outcome misclassification may have biased the results. Our current findings based on dementia cases identified mostly from comprehensive neuropsychological evaluations from long-term follow-up provide more robust evidence for associations of lung function with dementia.
Unlike a prior ARIC investigation that did not find associations of lung function with cognitive decline over 16 years of follow-up from late midlife (16), the current analysis using extended follow-up data found associations with multiple domains, including memory, language, and processing speed/attention. The current findings are also somewhat in line with a coordinated analysis that used data from 8 longitudinal studies (overall n = 20,586, with follow-up periods ranging from 5 to 15 years) to study the associations between lung function measures and cognitive decline (33). The study found some evidence for a link between simultaneous longitudinal changes in both lung function and cognition in multiple cognitive domains across the studies in study-specific analyses, as well as in their meta-analysis. Other prior studies on lung function and cognitive decline have shown mixed associations overall (13), as well as across neuropsychological domains (6, 14, 17); further, their limitations included shorter follow-up periods with limited cognitive assessments, inadequate control of confounding, smaller sample sizes, and attrition bias (13, 33).
While we found that FEV1, FVC, and FEV1/FVC ratio were all associated with attenuation in cognitive decline (consistent with a few prior studies) (15, 33), our findings from the combined inclusion of FEV1 and FVC underscored that FEV1 may be a stronger predictor. Further, the findings demonstrated that cognitive decline depended on joint levels of specific FEV1 and FVC, with these working together synergistically in a nonlinear fashion, imparting additional information than that provided by the FEV1/FVC ratio model (which simply suggests linear relationships with cognitive decline). While the analysis using clinical lung disease categories did not find any association with cognitive decline, the analysis combining FEV1 and FVC suggested that cognitive declines were steepest among persons with the highest differences between FVC and FEV1, providing more insights on the association.
Prior studies, including ARIC, have also linked poor lung function with a range of dementia-associated neuropathological changes, including reduced brain volume, white matter integrity, microbleeds, and infarcts, providing some mechanistic support for the lung function–cognition association (34–38). Mechanistically, stroke and inflammation could be common pathological processes linking lung function to cognitive outcomes (8, 10, 39, 40). Our findings persisted after adjustment for available inflammatory marker serum fibrinogen level (data not shown) and stroke at baseline. Likewise, hypoxia has been shown to induce pathophysiological changes, including amyloid β accumulation, τ phosphorylation, calcium ion homeostasis dysregulation, neuroinflammation, and modulation of neurotransmission (7, 9).
Our study had several limitations. First, we employed one-time point lung function to examine associations with dementia rate and cognitive decline. Lung function may change with age and in response to external environmental exposures. Evaluation of longitudinal changes in lung function and cognitive decline may provide additional insights into this relationship. Second, cohort attrition associated with death and poor health is inevitable in studies with long-term follow-up such as ARIC. Although we cannot completely rule out bias due to selective attrition, we observed consistent associations across multiple models, including a joint modeling SPM approach which has been shown to mitigate bias compared with standard longitudinal modeling approaches (29). Third, we used prebronchodilator lung function measures to categorize lung diseases, whereas postbronchodilator measures are used in the clinical setting. Nonetheless, our study had several strengths, including a large sample size, 30-year follow-up, and the availability of comprehensive information on cardiovascular risk factors and cognition. Additionally, the majority of prior studies focused on populations with European ancestry (5, 6, 18, 32); here, we demonstrated lung-function–associated cognitive decline and dementia in a cohort of White and Black participants.
In conclusion, our findings support the hypothesis that better lung function at midlife is associated with more favorable cognitive outcomes in later life. While use of FEV1 alone was moderately associated with both dementia and cognitive decline, associations were stronger for participants with highly disparate levels of FEV1 versus FVC when the 2 measures were used in combination. While lung-function–associated cognitive change may appear modest on average, at the population level our findings may have important implications for reducing the burden of cognitive impairment that is attributable to environmental exposures (given their ubiquity—for example, smoking, air pollution) and associated lung function impairment. Further, future studies should explore the implications of potential interventions designed to improve lung function, such as physical and respiratory exercises, in dementia prevention.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson, Mississippi, United States (Srishti Shrestha, Xiaoqian Zhu, Kevin J. Sullivan, B. Gwen Windham, Michael E. Griswold, Thomas H. Mosley, Jr.); Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina, United States (Stephanie J. London); and Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States (Pamela L. Lutsey).
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Collection of neurocognitive data is supported by grants U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the National Institutes of Health (NHLBI, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders), and previous brain magnetic resonance imaging examinations were funded by grant R01-HL70825 from the NHLBI. P.L.L. was also supported by NHLBI grant K24-HL159246. S.J.L. was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (grant ZO1 ES043012).
The ARIC Study data used for this analysis are available to qualified investigators upon request. Details on data availability and study protocols can be accessed at the ARIC website (https://sites.cscc.unc.edu/aric/).
We thank the staff and participants of the ARIC Study for their important contributions.
This work was presented at the 2021 Gerontological Society of America Annual Scientific Meeting, Phoenix, Arizona, November 10–13, 2021.
Conflict of interest: none declared.
REFERENCES
- 1. Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaugler J, James B, Johnson T, et al. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. [Google Scholar]
- 3. Gilsanz P, Mayeda ER, Flatt J, et al. Early midlife pulmonary function and dementia risk. Alzheimer Dis Assoc Disord. 2018;32(4):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russ TC, Kivimaki M, Batty GD. Respiratory disease and lower pulmonary function as risk factors for dementia: a systematic review with meta-analysis. Chest. 2020;157(6):1538–1558. [DOI] [PubMed] [Google Scholar]
- 5. Guo X, Waern M, Sjogren K, et al. Midlife respiratory function and incidence of Alzheimer’s disease: a 29-year longitudinal study in women. Neurobiol Aging. 2007;28(3):343–350. [DOI] [PubMed] [Google Scholar]
- 6. Qiao H, Chen M, Li S, et al. Poor lung function accelerates cognitive decline in middle-aged and older adults: evidence from the English Longitudinal Study of Ageing. Arch Gerontol Geriatr. 2020;90:104129. [DOI] [PubMed] [Google Scholar]
- 7. Daulatzai MA. Death by a thousand cuts in Alzheimer’s disease: hypoxia—the prodrome. Neurotox Res. 2013;24(2):216–243. [DOI] [PubMed] [Google Scholar]
- 8. Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lall R, Mohammed R, Ojha U. What are the links between hypoxia and Alzheimer’s disease? Neuropsychiatr Dis Treat. 2019;15:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker KA, Ficek BN, Westbrook R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Nerosci. 2019;10(8):3340–3342. [DOI] [PubMed] [Google Scholar]
- 11. Lahousse L, Tiemeier H, Ikram MA, et al. Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med. 2015;109(11):1371–1380. [DOI] [PubMed] [Google Scholar]
- 12. Austin V, Crack PJ, Bozinovski S, et al. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond). 2016;130(13):1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duggan EC, Graham RB, Piccinin AM, et al. Systematic review of pulmonary function and cognition in aging. J Gerontol B Psychol Sci Soc Sci. 2020;75(5):937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weuve J, Glymour MM, Hu H, et al. Forced expiratory volume in 1 second and cognitive aging in men. J Am Geriatr Soc. 2011;59(7):1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giltay EJ, Nissinen A, Giampaoli S, et al. Apolipoprotein E genotype modifies the association between midlife lung function and cognitive function in old age. Dement Geriatr Cogn Disord. 2009;28(5):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pathan SS, Gottesman RF, Mosley TH, et al. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011;18(6):888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richards M, Strachan D, Hardy R, et al. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005;67(4):602–608. [DOI] [PubMed] [Google Scholar]
- 18. Vidal JS, Aspelund T, Jonsdottir MK, et al. Pulmonary function impairment may be an early risk factor for late-life cognitive impairment. J Am Geriatr Soc. 2013;61(1):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lutsey PL, Chen N, Mirabelli MC, et al. Impaired lung function, lung disease, and risk of incident dementia. Am J Respir Crit Care Med. 2019;199(11):1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 21. Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk in Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The ARIC Investigators . Atherosclerosis Risk in Communities Study Manual 4: Pulmonary Function. Chapel Hill, NC: National Heart, Lung, and Blood Institute Collaborative Studies Coordinating Center; 1987. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Pulmonary_Function_Assessment.1_4.pdf. Accessed February 20, 2021. [Google Scholar]
- 23. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baugh AD, Shiboski S, Hansel NN, et al. Reconsidering the utility of race-specific lung function prediction equations. Am J Respir Crit Care Med. 2022;205(7):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elmaleh-Sachs A, Balte P, Oelsner EC, et al. Race/ethnicity, spirometry reference equations, and prediction of incident clinical events: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Am J Respir Crit Care Med. 2022;205(6):700–710. [DOI] [PubMed] [Google Scholar]
- 26. Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology. 1998;44(2):95–105. [DOI] [PubMed] [Google Scholar]
- 27. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baecke JE, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. [DOI] [PubMed] [Google Scholar]
- 29. Griswold ME, Talluri R, Zhu X, et al. Reflection on modern methods: shared-parameter models for longitudinal studies with missing data. Int J Epidemiol. 2021;50(4):1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asar O, Ritchie J, Kalra PA, et al. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. Int J Epidemiol. 2015;44(1):334–344. [DOI] [PubMed] [Google Scholar]
- 31. Alonso A, Jacobs DR Jr, Menotti A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J Neurol Sci. 2009;280(1-2):79–83. [DOI] [PubMed] [Google Scholar]
- 32. Russ TC, Starr JM, Stamatakis E, et al. Pulmonary function as a risk factor for dementia death: an individual participant meta-analysis of six UK general population cohort studies. J Epidemiol Community Health. 2015;69(6):550–556. [DOI] [PubMed] [Google Scholar]
- 33. Duggan EC, Piccinin AM, Clouston S, et al. A multi-study coordinated meta-analysis of pulmonary function and cognition in aging. J Gerontol A Biol Sci Med Sci. 2019;74(11):1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dodd JW, Chung AW, van den Broek MD, et al. Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med. 2012;186(3):240–245. [DOI] [PubMed] [Google Scholar]
- 35. Lahousse L, Vernooij MW, Darweesh SK, et al. Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam Study. Am J Respir Crit Care Med. 2013;188(7):783–788. [DOI] [PubMed] [Google Scholar]
- 36. Liao D, Higgins M, Bryan NR, et al. Lower pulmonary function and cerebral subclinical abnormalities detected by MRI: the Atherosclerosis Risk in Communities Study. Chest. 1999;116(1):150–156. [DOI] [PubMed] [Google Scholar]
- 37. Guo X, Pantoni L, Simoni M, et al. Midlife respiratory function related to white matter lesions and lacunar infarcts in late life: the prospective population study of women in Gothenburg, Sweden. Stroke. 2006;37(7):1658–1662. [DOI] [PubMed] [Google Scholar]
- 38. Murray AD, Staff RT, Shenkin SD, et al. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237(1):251–257. [DOI] [PubMed] [Google Scholar]
- 39. Portegies ML, Lahousse L, Joos GF, et al. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med. 2016;193(3):251–258. [DOI] [PubMed] [Google Scholar]
- 40. Silvestre OM, Nadruz W Jr, Querejeta Roca G, et al. Declining lung function and cardiovascular risk: the ARIC Study. J Am Coll Cardiol. 2018;72(10):1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.