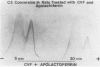
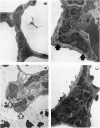
Abstract
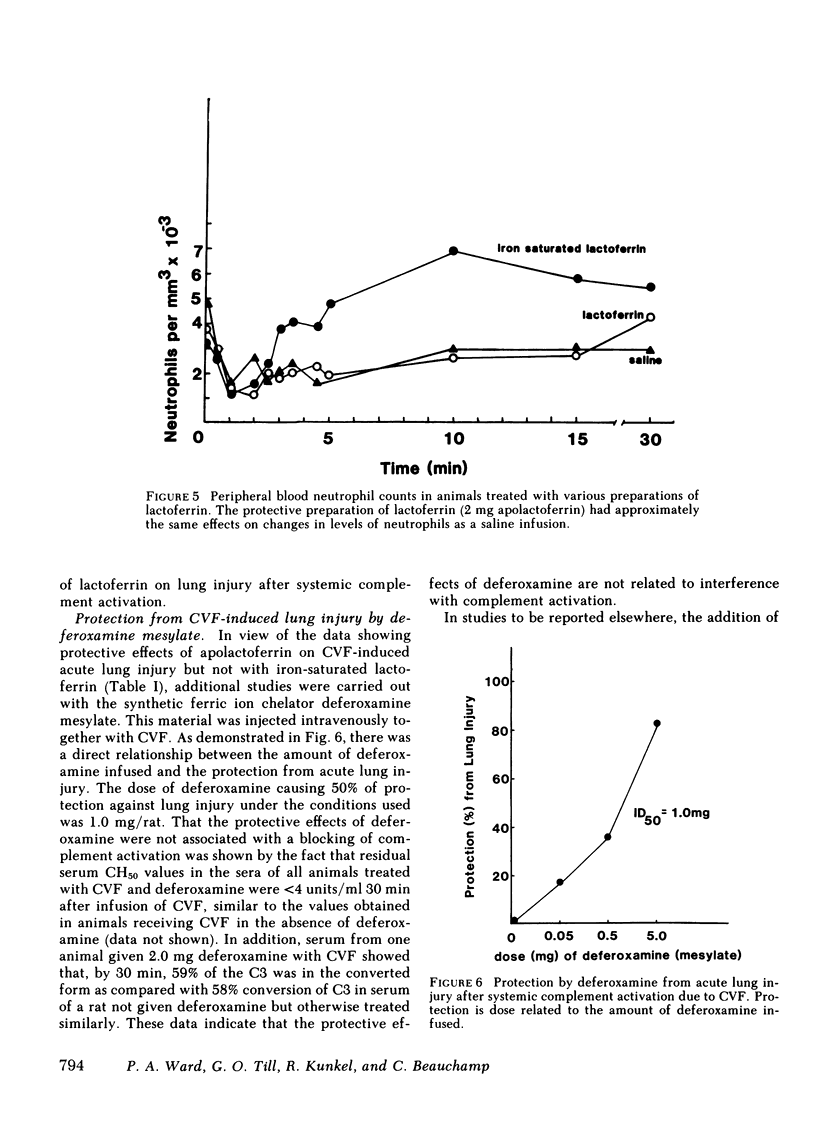
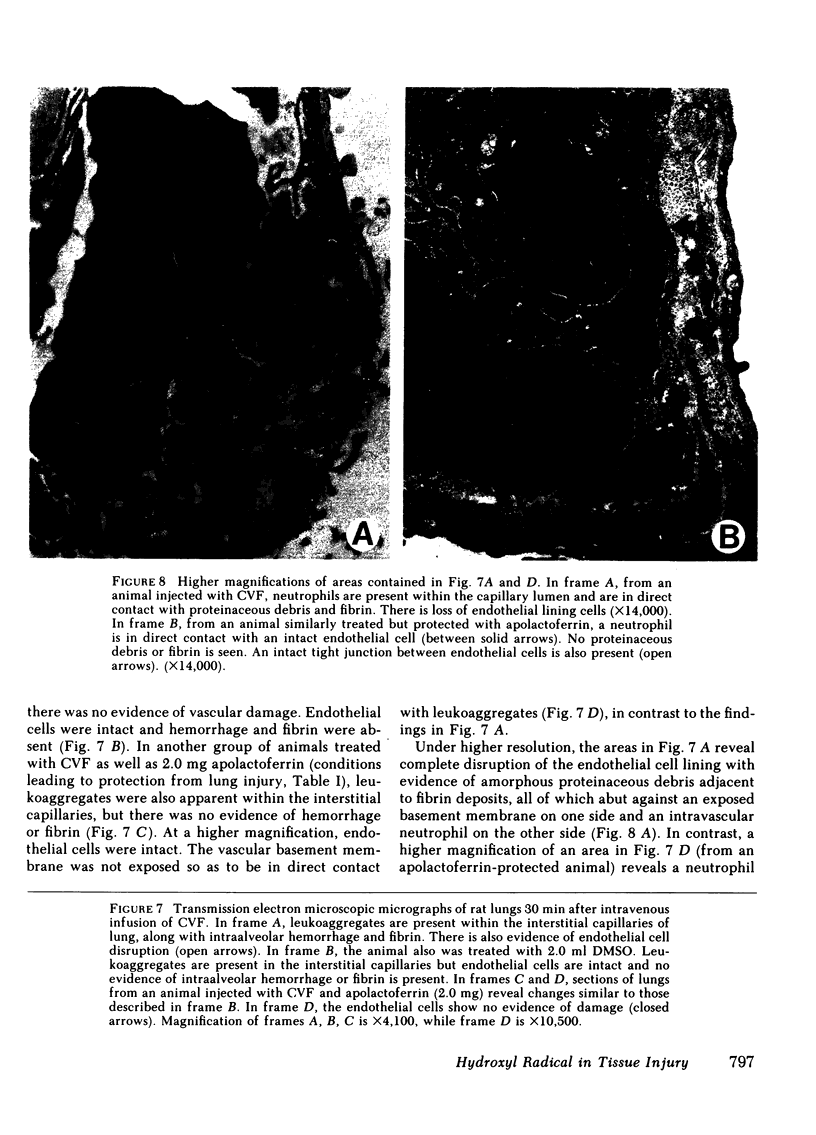
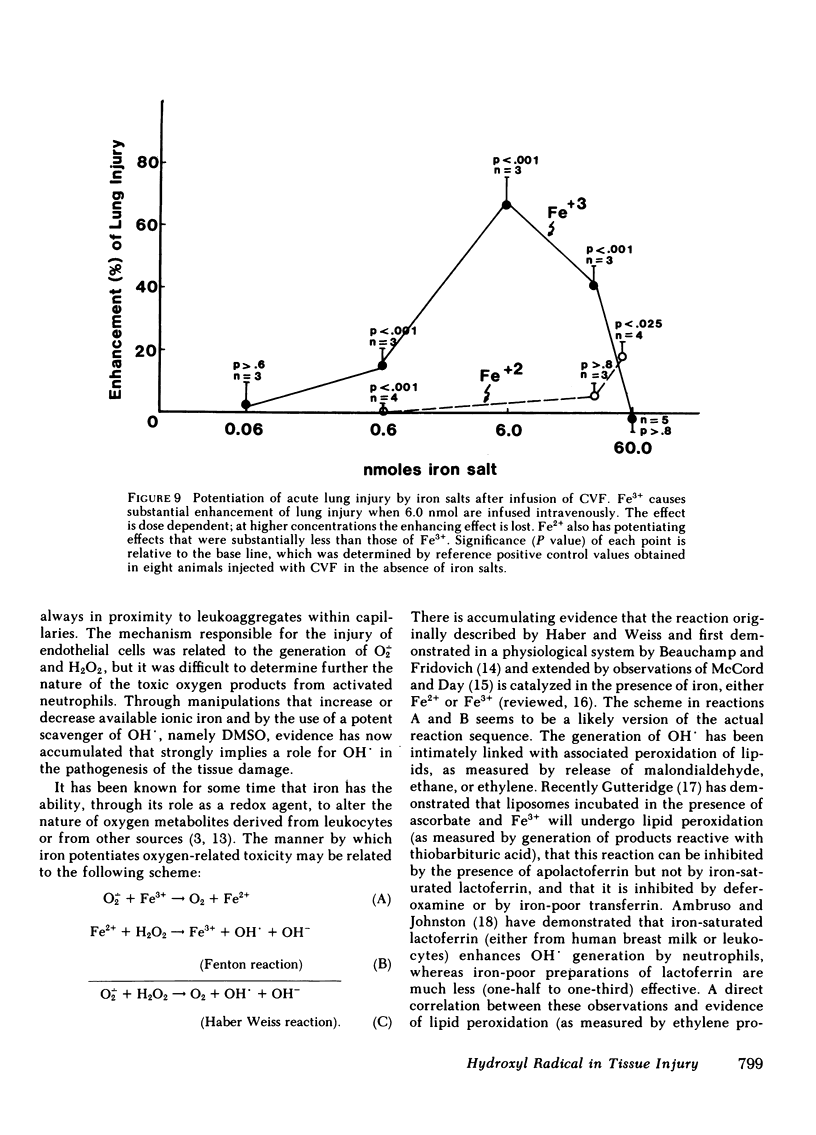
Using our recently described model of acute lung injury in rats after systemic activation of complement by cobra venom factor (CVF), we demonstrated that pretreatment of animals with human milk apolactoferrin (in its native or derivatized form), but not iron-saturated lactoferrin, provides significant protection against complement- and neutrophil-mediated lung injury. The synthetic iron chelator deferoxamine mesylate also affords protection from lung injury. The protective effects of apolactoferrin are not related to a blocking of CVF-induced complement activation. We also demonstrated that infusion of ionic iron, especially Fe3+, greatly potentiates lung vascular injury after systemic complement activation. Finally, protection from lung injury occurs in animals pretreated with the potent scavenger of hydroxyl radicals (OH.), dimethyl sulfoxide. Based on transmission electron microscopy, CVF-treated rats show leukoaggregates and endothelial cell destruction in interstitial pulmonary capillaries, along with intraalveolar hemorrhage and fibrin deposition. In animals protected with apolactoferrin, deferoxamine mesylate, or dimethyl sulfoxide, the morphological studies reveal leukoaggregates but no endothelial cell damage, hemorrhage, or fibrin deposition. These data support the concept that tissue injury that is complement and neutrophil dependent may be related to generation of OH. derived from H2O2 after leukocytic activation.
Full text
PDF

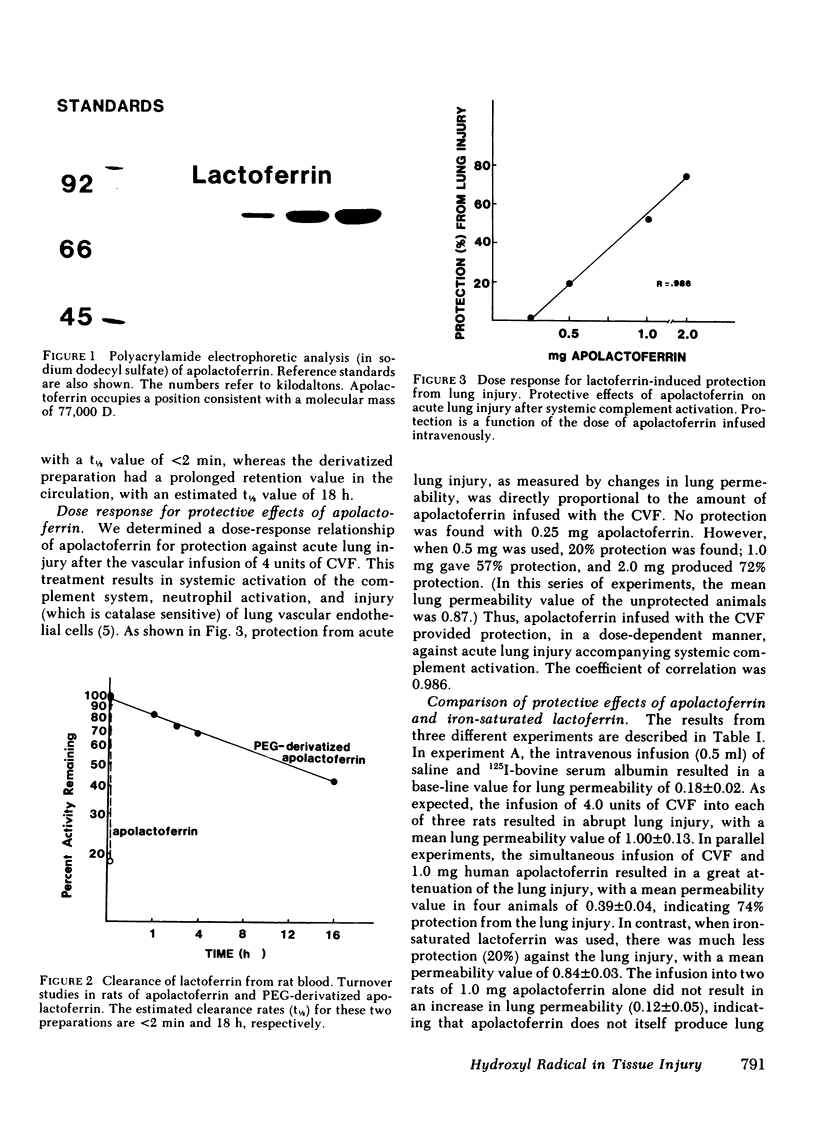
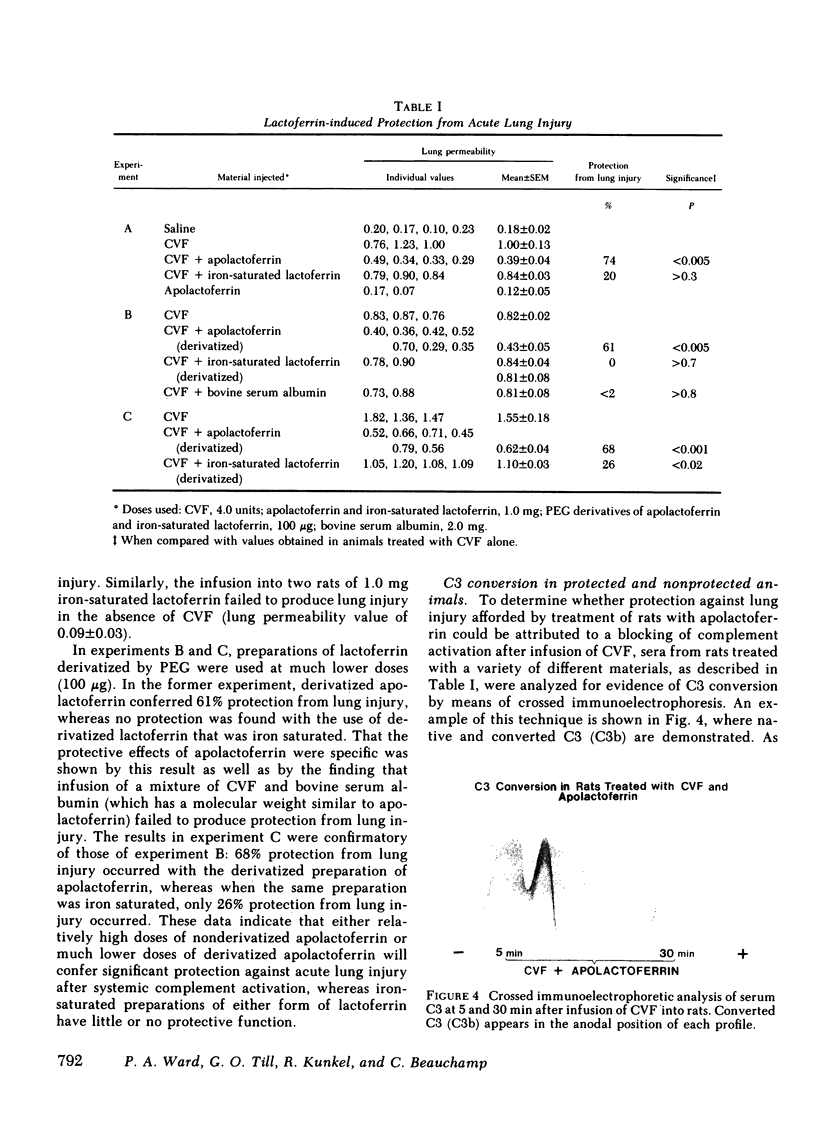






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C. O., Gonias S. L., Menapace D. P., Pizzo S. V. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and alpha 2-macroglobulin. Anal Biochem. 1983 May;131(1):25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bläckberg L., Hernell O. Isolation of lactoferrin from human whey by a single chromatographic step. FEBS Lett. 1980 Jan 14;109(2):180–183. doi: 10.1016/0014-5793(80)81081-7. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Björkstén B., Björk J., Yang H. H., Allen J. M., Baehner R. L. Neutropenia induced by systemic infusion of lactoferrin. J Lab Clin Med. 1982 Jun;99(6):866–872. [PubMed] [Google Scholar]
- Bucher J. R., Tien M., Aust S. D. The requirement for ferric in the initiation of lipid peroxidation by chelated ferrous iron. Biochem Biophys Res Commun. 1983 Mar 29;111(3):777–784. doi: 10.1016/0006-291x(83)91366-9. [DOI] [PubMed] [Google Scholar]
- Chapman W. E., Ward P. A. The complement profile in babesiosis. J Immunol. 1976 Sep;117(3):935–938. [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Croft W. A., Hoekstra W. G. Effects of ferrous chloride and iron dextran on lipid peroxidation in vivo in vitamin E and selenium adequate and deficient rats. J Nutr. 1981 Oct;111(10):1784–1796. doi: 10.1093/jn/111.10.1784. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fogel B. J., Von Doenhoff A. E., Jr, Cooper N. R., Fife E. H., Jr Complement in acute experimental malaria. I. Total hemolytic activity. Mil Med. 1966 Sep;131(9 Suppl):1173–1179. [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Kijlstra A., Jeurissen S. H. Modulation of classical C3 convertase of complement by tear lactoferrin. Immunology. 1982 Oct;47(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. The iron-H2O2-iodide cytotoxic system. J Exp Med. 1982 Oct 1;156(4):1262–1267. doi: 10.1084/jem.156.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]