Abstract
Hedgehog (Hh) signaling pathway dysregulation is involved in the pathogenesis of metabolic dysfunction-associated steatohepatitis, and the sonic Hh (SHh) protein, a pivotal molecule in the Hh pathway, is expressed in ballooned hepatocytes. The present study aimed to investigate the clinicopathological significance of SHh expression in steatohepatitic hepatocellular carcinoma (SH-HCC). Reverse transcription-quantitative polymerase chain reaction and immunohistochemistry were performed to examine SHh gene and SHh protein expression in SH-HCC. Additionally, patients with conventional HCC (C-HCC) were included in the control group. Comparisons of patient and tumor characteristics were also performed. The prevalence of SH-HCC was 3% in the whole cohort, and it was significantly associated with a high prevalence of diabetes mellitus. SHh mRNA was detected in all patients with SH-HCC, but not in 23% of patients with C-HCC. Notably, SHh mRNA expression was not significantly different between patients with SH-HCC and those with C-HCC; however, high SHh protein expression was significantly more frequent in SH-HCC patients than in those with C-HCC. Although the prognosis was not significantly different between the SH-HCC and C-HCC groups, high SHh protein expression was an independent poor prognostic factor for HCC. In conclusion, SHh could potentially serve as a therapeutic target for patients with HCC.
Keywords: hedgehog signaling pathway, sonic hedgehog, hepatocellular carcinoma, steatohepatitic hepatocellular carcinoma, steatohepatitis, primary liver cancer
Introduction
Steatohepatitic hepatocellular carcinoma (SH-HCC) is a histological subtype of HCC which was first described by Salomao et al (1,2) and that accounts for 11–19% HCC cases (3–5). The association between metabolic disorders, such as diabetes mellitus (DM) and hyperlipidemia has been repeatedly reported in SH-HCC (1–5). Histologically, it is characterized by steatosis, peritumoral fibrosis, inflammatory infiltrates, balloon-like swelling of tumor cells, and Mallory-Denk body-like cytoplasmic inclusions. The name ‘steatohepatitic’ HCC comes from the histopathologic similarity to steatohepatitis, although the molecular mechanism causing these morphological changes in tumor cells is still unknown.
The Hedgehog (Hh) signaling pathway is involved in normal embryonic development and wound healing, and its dysregulation plays an important role in tumor progression (6,7). The aberrant activation of Hh signaling pathway and overexpression of related molecules such as sonic hedgehog (SHh), patched, smoothened, or glioma-associated oncogene (GLI) are associated with poor prognosis in various cancers such as triple-negative breast cancer, ovarian cancer, small cell lung carcinoma, colon cancer, and liver cancer, including HCC (8–15). Several Hh signaling inhibitors have been used to treat advanced basal cell carcinoma in clinical practice (16). The Hh pathway is also activated during non-neoplastic liver injury (17). Rangwala et al (18) reported that the SHh protein is overexpressed in ballooned hepatocytes, which is the histological hallmark of steatohepatitis. Studies have shown that immunohistochemistry of the SHh protein in patients with non-alcoholic fatty liver disease facilitates fibrosis stage prediction and ballooned hepatocyte detection (19,20).
We hypothesized that SHh molecule expression in SH-HCC is substantially higher than that in conventional HCC (C-HCC). We investigated SHh mRNA and protein expression in SH-HCC and compared them with those in C-HCC, which is not classified as a special HCC subtype. We also examined the clinical significance of SHh overexpression in patients with HCC.
Materials and methods
Subjects
We reviewed 1,360 patients with HCC cases resected at Kurume University Hospital between April 2003 and March 2020. SH-HCC diagnosis was confirmed if the HCC fulfilled all four of the following criteria: Intratumoral steatosis (>5% tumor cells), peritumoral fibrosis, intratumoral inflammatory infiltrates, and tumor cell ballooning, and the tumors with these findings are predominant (≥50%). The histological evaluation was performed using routine H&E staining, as well as Azan or Masson's trichrome staining to assess fibrosis. Archived tissue samples were used for gene expression analysis and immunohistochemistry.
Gene expression analysis by reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Frozen materials from 22 SH-HCC and 24 C-HCC (control) tissue samples were subjected to RT-qPCR to quantify the mRNA expression levels. Total RNA was isolated using the RNeasy Protect Mini Kit (Qiagen, Hilden, Germany) and quantitatively analyzed using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). Total RNA was reverse transcribed using the Reverse Transcription System (Promega, Madison, WI, USA). RT-qPCR was performed in an ABI7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using TaqMan PCR assay probe/primers for the housekeeping gene β-actin (Hs99999903_m1) and SHh (HS00179843_m1). Each PCR was performed in duplicate. The average cycle quantification (Cq) values per duplicate were calculated for SHh and the housekeeping gene, yielding ∆Cq. To determine the SHh gene expression levels relative to the housekeeping gene, we calculated the 2−∆Cq values (21).
Immunohistochemistry
Specimens (4 µm) were cut from formalin-fixed, paraffn-embedded blocks. Immunostaining for SHh (clone EP1190Y, ab53281, dilution 1:4,000; Abcam, Cambridge, MA, USA) was performed using the Ventana Benchmark (Ventana, Tucson, AZ, USA). Whole tissue sections from 40 patients with SH-HCC and tissue microarrays (containing 2×3 and 1×3 mm2 tumor and non-tumor tissue cores, respectively, per case) from 137 patients with C-HCC were used for immunohistochemistry. The positive cell percentage in the tumor was graded as diffuse (>50%), patchy (10–50%), focal (1–10%), or none (0%). The staining intensity was classified as strong, moderate, weak, or no. Staining scores were defined as 3 (diffuse strong), 2 (diffuse moderate or patchy strong), 1 (patchy moderate or focal with any intensity or weakness with any percentage of tumor cells), and 0 (none). A representative image of each score is shown in Fig. 1. Staining scores of 2 and 3 were classified as high expression, whereas scores of 0 and 1 were classified as low expression.
Figure 1.
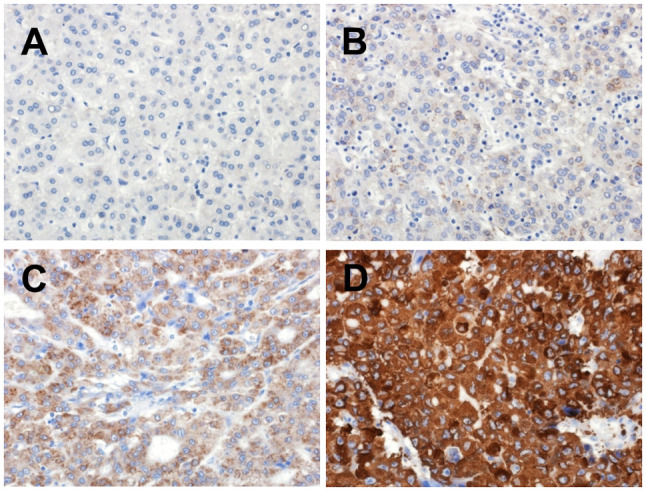
Tumor cell scoring for sonic hedgehog immunostaining. (A) Score 0: No expression. (B) Score 1: Focal and weak expression. (C) Score 2: Diffuse and moderate expression. (D) Score 3: Diffuse and strong expression.
Survival analysis
The survival analysis included patients with solitary HCC who underwent curative resection and had no previous treatment. Overall survival was defined as the time interval between the date of surgery and death.
Statistics
Data analyses were performed using the JMP pro 15.1 software (SAS Institute, Cary, NC, USA). Categorical variables were tested using the χ2 or Fisher's exact tests. Quantitative variables were tested using the Mann-Whitney U test. The Kaplan-Meier method and log-rank test were used for the survival analysis. Univariate analysis was conducted to identify the prognostic factors for overall survival, and multivariate analysis of the significant factors found in the univariate analysis was performed using Cox regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological analysis
In this cohort, we identified 40 patients with SH-HCC, with 3% incidence. The clinicopathological findings are outlined in Table I. Age, sex, viral status, serum α-fetoprotein (AFP) and des-γ-carboxy prothrombin levels, presence or absence of microvascular invasion, and intrahepatic metastasis were not significantly different between C-HCC and SH-HCC. The DM frequency was high in patients with SH-HCC.
Table I.
Clinicopathological characteristics.
| Characteristic | C-HCC (n=1,320) | SH-HCC (n=40) | P-value |
|---|---|---|---|
| Mean± SD age, years | 68.9±9.6 | 70.8±8.1 | 0.2576 |
| Male/female, n (%) | 974 (74%)/346 (26%) | 31 (78%)/9 (22%) | 0.5984 |
| HBsAg -/+, n (%) | 1,095 (83%)/225 (17%) | 34 (85%)/6 (15%) | 0.7343 |
| HCV Ab -/+, n (%) | 577 (44%)/743 (56%) | 16 (40%)/24 (60%) | 0.6409 |
| HBsAg + or HCVAb +/others, n (%) | 950 (72%)/370 (28%) | 28 (70%)/12 (30%) | 0.7848 |
| DM -/+, n (%) | 862 (65%)/458 (35%) | 16 (40%)/24 (60%) | 0.001 |
| Median AFP, ng/ml | 11.3 | 11.1 | 0.9962 |
| Median DCP, mAU/ml | 63 | 53 | 0.2522 |
| Mean ± SD maximum tumor diameter, mm | 32.8±24.3 | 24.9±9.8 | 0.1469 |
| Microvascular invasion -/+, n (%) | 652 (49%)/668 (51%) | 16 (40%)/24 (60%) | 0.2417 |
| Intrahepatic metastasis -/+, n (%) | 1140 (86%)/180 (14%) | 33 (83%)/7 (17%) | 0.4845 |
C-HCC, conventional hepatocellular carcinoma; SH-HCC, steatohepatitic hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C virus antibody; DM, diabetes mellitus; AFP, α-fetoprotein; DCP, des-γ-carboxy prothrombin.
SHh gene and protein expression
SHh mRNA expression was detected in 20 patients with SH-HCC but not in five patients with C-HCC. SHh gene expression levels were not significantly different between SH-HCC and C-HCC (Fig. 2). The scores for SHh immunostaining are presented in Table II, with 63 (46%) and two (5%) scoring 0, 30 (22%) and eight (20%) scoring 1, 21 (15%) and 12 (30%) scoring 2, and 23 (17%) and 18 (45%) scoring 3 in C-HCC and SH-HCC, respectively. Thus, the SHh staining scores were significantly different between SH-HCC and C-HCC (P=0.000003). Representative histological images of the tumor and non-tumor regions of SH-HCC, along with the immunohistochemical staining images of SHh, are presented in Fig. S1.
Figure 2.
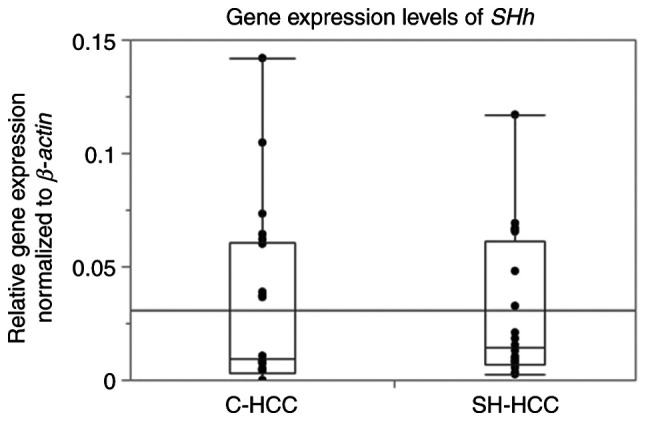
Comparison of SHh gene expression levels between C-HCC and SH-HCC. SHh, sonic hedgehog; C-HCC, conventional hepatocellular carcinoma; SH-HCC, steatohepatitic hepatocellular carcinoma.
Table II.
SHh immunoexpression in tumor cells.
| Group | - | + | ++ | +++ |
|---|---|---|---|---|
| Conventional HCC | 63 | 30 | 21 | 23 |
| Steatohepatitic HCC | 2 | 8 | 12 | 18 |
SHh, sonic hedgehog; HCC, hepatocellular carcinoma.
Prognosis
The overall survival was not significantly different between SH-HCC and C-HCC (P=0.8146; Fig. 3). Among all HCCs, including C-HCC and SH-HCC, the presence of vascular invasion, intrahepatic metastasis, high serum AFP (>200 ng/ml), and high SHh expression in immunohistochemistry were poor prognostic factors in univariate analysis. In multivariate analysis, only high SHh expression was identified as an independent poor prognostic factor (Tables III and IV). Kaplan-Meier curves comparing the high- and low-SHh expression groups are shown in Fig. 4 (P=0.0022).
Figure 3.
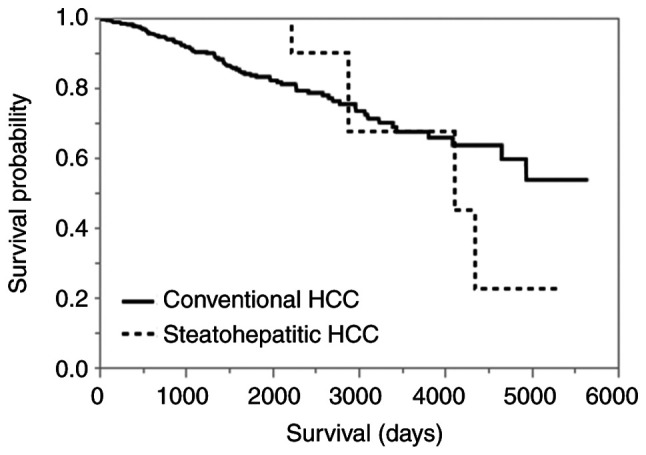
Kaplan-Meier curves for overall survival in conventional and steatohepatitic HCC (P=0.8146). HCC, hepatocellular carcinoma.
Table III.
Prognostic factors in univariate analysis for overall survival.
| Factor | P-value | HR (95% CI) |
|---|---|---|
| Steatohepatitic HCC | 0.8114 | 0.8867628 (0.2704958–2.1310151) |
| Microvascular invasion | 0.0111 | 1.7962606 (1.1475853–2.8828483) |
| Intrahepatic metastasis | 0.0028 | 2.5861964 (1.4155532–4.4294630) |
| Preoperative serum AFP >200 ng/ml | 0.0220 | 1.7185646 (1.0569048–2.7110127) |
| SHh high expression | 0.0027 | 3.2124137 (1.4932731–7.3222227) |
HCC, hepatocellular carcinoma; AFP, α-fetoprotein; SHh, sonic hedgehog.
Table IV.
Prognostic factors in the multivariate analysis of overall survival.
| Factor | P-value | HR (95% CI) |
|---|---|---|
| Microvascular invasion | 0.3217 | 1.5765628 (0.6545625–4.0347770) |
| Intrahepatic metastasis | 0.9352 | 0.9537622 (0.2631614–2.7704849) |
| Preoperative serum AFP >200 ng/ml | 0.3124 | 1.6533953 (0.5939074–3.9806890) |
| SHh high expression | 0.0069 | 3.0577992 (1.3542579–7.3759824) |
AFP, α-fetoprotein; SHh, sonic hedgehog.
Figure 4.
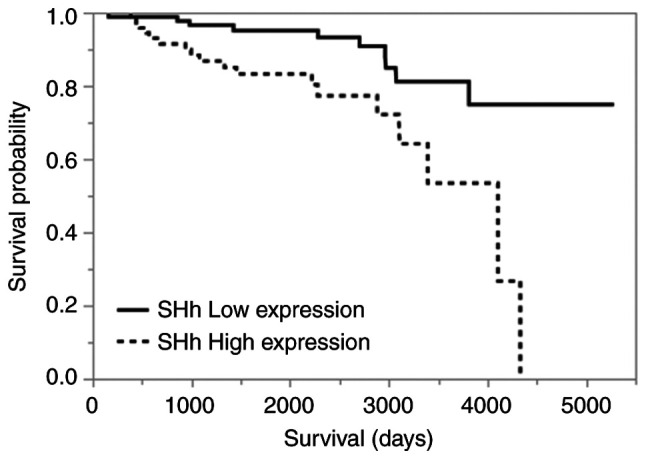
Kaplan-Meier curves for overall survival. The high SHh expression group shows worse prognosis than the low SHh expression group (P=0.0022). SHh, sonic hedgehog.
Discussion
We demonstrated a high SHh protein expression in SH-HCC and its prognostic significance in HCCs. In steatohepatitis, ballooned hepatocytes express the SHh protein, whereas non-ballooned hepatocytes do not. Therefore, SHh immunohistochemistry facilitates pathological diagnosis of steatohepatitis (18,20). Several cases in our study showed diffuse SHh expression in tumors. We confirmed consistent SHh protein expression in ballooned tumor cells and various degrees of expression in non-ballooned tumor cells, suggesting that SHh is involved in the ballooning of HCC tumor cells and ballooned hepatocytes in steatohepatitis, and can be expressed on HCC tumor cells without ballooning because of its association with cell proliferation signaling. Therefore, SHh immunohistochemistry is not always useful in discriminating SH-HCC cases from C-HCC cases. Despite many studies on Hh signaling activation in HCCs, limited reports have focused on a specific SH-HCC subgroup; thus, our study is significant in this regard (13–15). Recently, Van Treeck et al reported that the Hh pathway was upregulated in SH-HCC by comparing paired non-neoplastic liver tissues using pathway analysis (22). We confirmed SHh protein overexpression in SH-HCC than that in C-HCC. However, High SHh gene expression in SH-HCC was not significant in this study, and the correlation between gene and protein expression levels in the paired samples was unclear owing to the limited frozen tissue samples for RT-qPCR, which did not adequately represent SHh protein expression on glass slides when behaviors were heterogeneous.
High SHh protein expression in tumor cells is a significant independent poor prognostic factor. Previous studies have also reported that Hh signaling activation is significantly associated with poor prognosis in various cancers (8–12). The expression of other Hh pathway-related molecules, such as GLI-1 and GLI-2, correlate with poor prognosis in patients with HCC (13,14). Hh signaling pathway activation is associated with tumor invasiveness and progression as well as epithelial-mesenchymal transition (7). Recent reports have also suggested that Hh signaling pathway activation induces tumor immunosuppression and resists immune checkpoint inhibitors (23,24). In contrast, the presence of tumor-infiltrating lymphocytes, a definitive feature of SH-HCC, predicts the benefits of immune checkpoint inhibitors. Although we found no patients treated with immune checkpoint inhibitors after initial resection in this study, we believe that studying how these two conflicting events affect the therapeutic effect of immune checkpoint inhibitors will aid in the development of personalized treatment.
The histological presentation of steatohepatitis-like features in HCCs is not associated with patient prognosis. This outcome is consistent with that of previous studies (2,4). Our diagnostic criteria for SH-HCC were derived from the original criteria developed by Salomao et al (1) with certain modifications. Salomao et al (1) proposed five histological characteristics for SH-HCC diagnosis: intratumoral steatosis, peritumoral fibrosis, intratumoral inflammatory infiltrates, tumor cell ballooning, and Mallory-Denk body-like intracytoplasmic material in the tumor cells. We excluded Mallory-Denk body-like intracytoplasmic material from the diagnostic criteria. In cases where we identified this particular feature, the other four criteria were also present. Additionally, Mallory-Denk bodies are frequently observed in steatohepatitis but are not a mandatory criterion for steatohepatitis diagnosis (25,26). Hence, we do not consider the Mallory-Denk body-like intracytoplasmic material to be a crucial factor. Salomao et al (1,2) reported that 27% of SH-HCC cases had minimal or no fibrosis in their initial report, whereas 90% had fibrosis in subsequent reports. In this study, we found that all HCCs with the three fundamental features required for SH-HCC, showed intratumoral fibrosis, even at a very low proportion. The presence of these three fundamental findings is necessary and sufficient for SH-HCC diagnosis, although peritumoral fibrosis is a highly distinctive and definitive feature of SH-HCC compared with that of C-HCC, which is medullary and lacks fibrosis.
SH-HCC prevalence in our cohort was 3%, which is lower than the 13–19% observed in previous studies involving various etiologies (2–5) due to several potential reasons, including disparities in the cut-off used to quantify the steatohepatitic area within the tumor. Some researchers have utilized 5% cut-off, whereas we used 50% cut-off. Moreover, the viral status and frequency of metabolic diseases may have been different in each cohort. Furthermore, steatohepatitis finding interpretation by pathologists may vary. Inter-observer variability is a persistent issue in steatohepatitis diagnosis. Specifically, the assessment of ‘ballooning’ largely depends on individual subjectivity. Similar challenges may arise in the assessment of the presence of ‘tumor cell ballooning,’ leading to differences in SH-HCC prevalence. Although histological HCC subtyping does not currently affect treatment strategies, it could pose a challenge for realizing individualized therapies in the future.
Among the patient characteristics, DM frequency was the most significantly different between the C-HCC and SH-HCC groups. Individuals with SH-HCC have a higher prevalence of DM than those with C-HCC, although viral status was not significantly different. This association has been corroborated by multiple studies, highlighting the potential relationship between SH-HCC and underlying metabolic disorders that cause steatohepatitis (1–5). Nonetheless, viral etiologies without metabolic syndrome can also cause SH-HCC. Concurrent fatty liver disease and steatohepatitis can easily be overlooked in patients with chronic viral hepatitis (20). Thus, a thorough evaluation of the non-neoplastic background of the liver is increasingly important in clinical practice.
In conclusion, this study demonstrated significant upregulation of SHh protein expression in SH-HCC than that in C-HCC, which is an independent predictor of an unfavorable prognosis in HCC. Our analysis confirmed no correlation between steatohepatitis histology and patient outcome, and a strong association between steatohepatitis histology and DM incidence, which is consistent with the results of prior research. Thus, SHh could potentially serve as a promising therapeutic target for patients with HCC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
HK and RK conducted a reassessment of past pathological samples, evaluated new SHh immunostaining slides, and confirmed the authenticity of all the raw data. HK, YN, JA, ON and HY designed the research study. HK, SO, MiO and MaO collected the data. HK, RK, SO, MiO, MaO, SM, YM, YK, YY and MN analyzed the data. HK wrote the paper. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Kurume University School of Medicine (approval no. 459; Kurume, Japan) on December 24, 2020. Informed consent was not required for this retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Salomao M, Yu WM, Brown RS, Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630–1636. doi: 10.1097/PAS.0b013e3181f31caa. [DOI] [PubMed] [Google Scholar]
- 2.Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737–746. doi: 10.1016/j.humpath.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Jain D, Nayak NC, Kumaran V, Saigal S. Steatohepatitic hepatocellular carcinoma, a morphologic indicator of associated metabolic risk factors: A study from India. Arch Pathol Lab Med. 2013;137:961–966. doi: 10.5858/arpa.2012-0048-OA. [DOI] [PubMed] [Google Scholar]
- 4.Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: A clinicopathological study of Japanese patients. Histopathology. 2014;64:951–962. doi: 10.1111/his.12343. [DOI] [PubMed] [Google Scholar]
- 5.Yamaoka K, Saitoh S, Kinowaki K, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki F, et al. Clinicopathological assessment of steatohepatitic hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2022;46:101799. doi: 10.1016/j.clinre.2021.101799. [DOI] [PubMed] [Google Scholar]
- 6.Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLSE. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16:11. doi: 10.1186/s12964-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8–20. doi: 10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noman AS, Uddin M, Rahman MZ, Nayeem MJ, Alam SS, Khatun Z, Wahiduzzaman M, Sultana A, Rahman ML, Ali MY, et al. Overexpression of sonic hedgehog in the triple negative breast cancer: Clinicopathological characteristics of high burden breast cancer patients from Bangladesh. Sci Rep. 2016;6:18830. doi: 10.1038/srep18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao X, Siu MK, Au CW, Wong ES, Chan HY, Ip PP, Ngan HY, Cheung AN. Aberrant activation of hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim S, Lim SM, Kim MJ, Park SY, Kim JH. Sonic hedgehog pathway as the prognostic marker in patients with extensive stage small cell lung cancer. Yonsei Med J. 2019;60:898–904. doi: 10.3349/ymj.2019.60.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, Li X, Liu T, Leng A, Zhang G. Prognostic value of hedgehog signaling pathway in patients with colon cancer. Med Oncol. 2012;29:1010–1016. doi: 10.1007/s12032-011-9899-7. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Tan YX, Jiang BG, Pan YF, Li SX, Yang GZ, Wang M, Wang Q, Zhang J, Zhou WP, et al. The prognostic significance and therapeutic potential of hedgehog signaling in intrahepatic cholangiocellular carcinoma. Clin Cancer Res. 2013;19:2014–2024. doi: 10.1158/1078-0432.CCR-12-0349. [DOI] [PubMed] [Google Scholar]
- 13.Che L, Yuan YH, Jia J, Ren J. Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chin J Cancer Res. 2012;24:323–331. doi: 10.1007/s11670-012-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Cao L, Li Y, Lu H, Yang X, Xue P. Expression of glioma-associated oncogene 2 (Gli 2) is correlated with poor prognosis in patients with hepatocellular carcinoma undergoing hepatectomy. World J Surg Oncol. 2013;11:25. doi: 10.1186/1477-7819-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeng KS, Jeng CJ, Jeng WJ, Sheen IS, Li SY, Leu CM, Tsay YG, Chang CF. Sonic Hedgehog signaling pathway as a potential target to inhibit the progression of hepatocellular carcinoma. Oncol Lett. 2019;18:4377–4384. doi: 10.3892/ol.2019.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen A, Xie P, Litvinov IV, Lefrançois P. Efficacy and safety of sonic hedgehog inhibitors in basal cell carcinomas: An updated systematic review and meta-analysis (2009–2022) Am J Clin Dermatol. 2023;24:359–374. doi: 10.1007/s40257-023-00763-x. [DOI] [PubMed] [Google Scholar]
- 17.Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol. 2018;68:550–562. doi: 10.1016/j.jhep.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estep M, Mehta R, Bratthauer G, Alaparthi L, Monge F, Ali S, Abdelatif D, Younoszai Z, Stepanova M, Goodman ZD, Younossi ZM. Hepatic sonic hedgehog protein expression measured by computer assisted morphometry significantly correlates with features of non-alcoholic steatohepatitis. BMC Gastroenterol. 2019;19:27. doi: 10.1186/s12876-019-0951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusano H, Kondo R, Ogasawara S, Omuraya M, Okudaira M, Mizuochi S, Mihara Y, Kinjo Y, Yano Y, Nakayama M, et al. Utility of sonic hedgehog and keratin 8/18 immunohistochemistry for detecting ballooned hepatocytes. Histopathology. 2022;80:974–981. doi: 10.1111/his.14631. [DOI] [PubMed] [Google Scholar]
- 21.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 22.Van Treeck BJ, Mounajjed T, Moreira RK, Orujov M, Allende DS, Bellizzi AM, Lagana SM, Davila JI, Jessen E, Graham RP. Transcriptomic and proteomic analysis of steatohepatitic hepatocellular carcinoma reveals novel distinct biologic features. Am J Clin Pathol. 2021;155:87–96. doi: 10.1093/ajcp/aqaa114. [DOI] [PubMed] [Google Scholar]
- 23.Petty AJ, Dai R, Lapalombella R, Baiocchi RA, Benson DM, Li Z, Huang X, Yang Y. Hedgehog-induced PD-L1 on tumor-associated macrophages is critical for suppression of tumor-infiltrating CD8+ T cell function. JCI Insight. 2021;6:e146707. doi: 10.1172/jci.insight.146707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J, Ding Y, Chen Y, Lu J, Chen Y, Wu G, Xu N, Wang H, Teng L. Pan-cancer analyses reveal that increased Hedgehog activity correlates with tumor immunosuppression and resistance to immune checkpoint inhibitors. Cancer Med. 2022;11:847–863. doi: 10.1002/cam4.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 26.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.


