Abstract
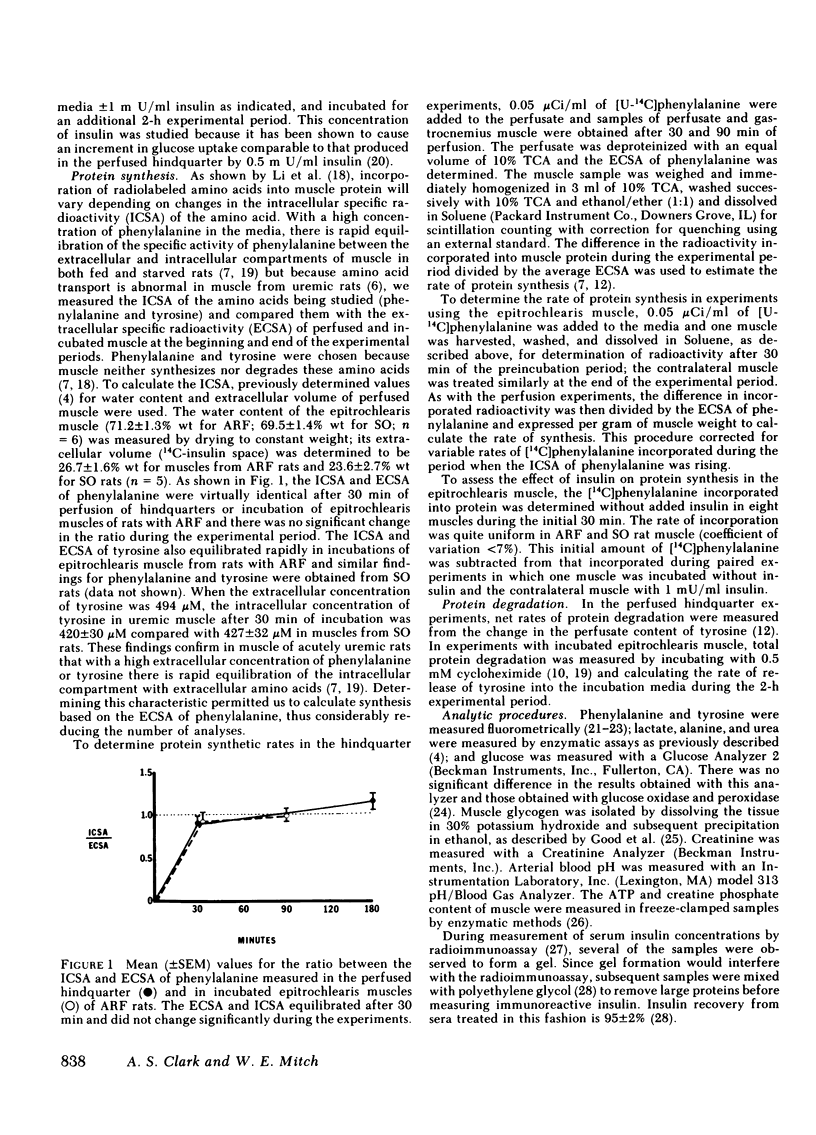
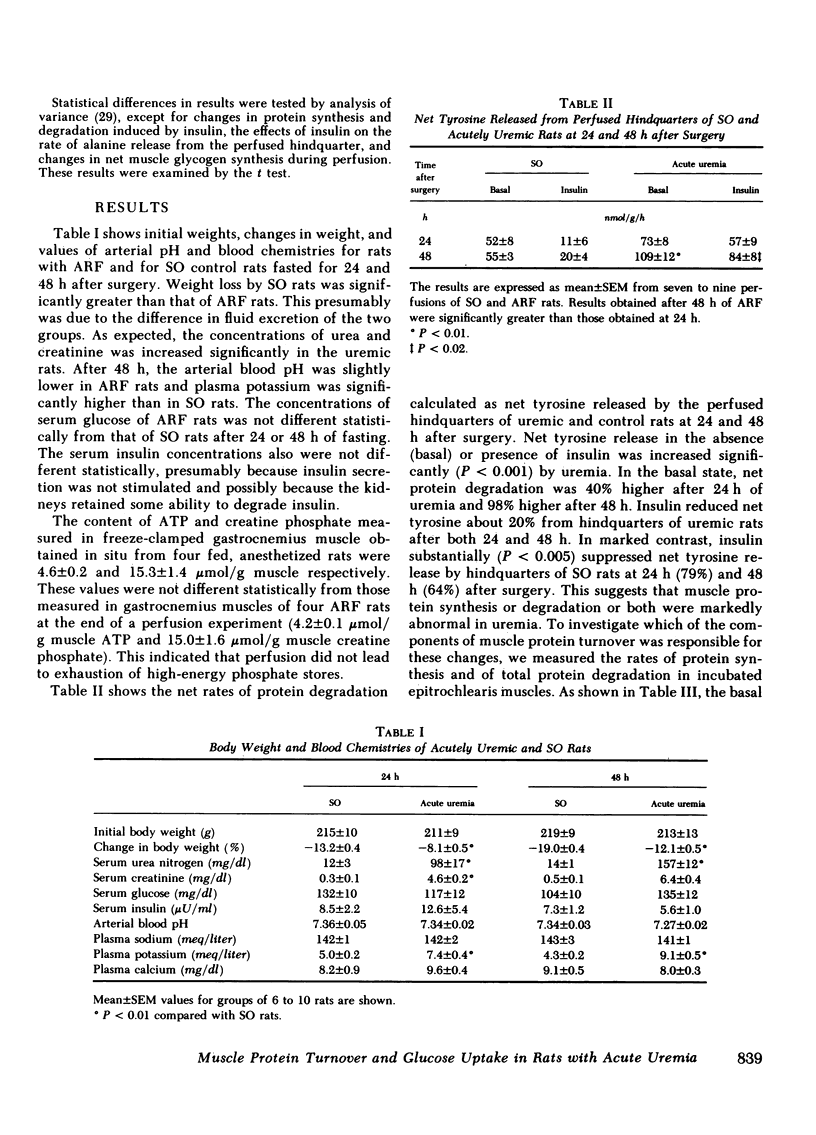
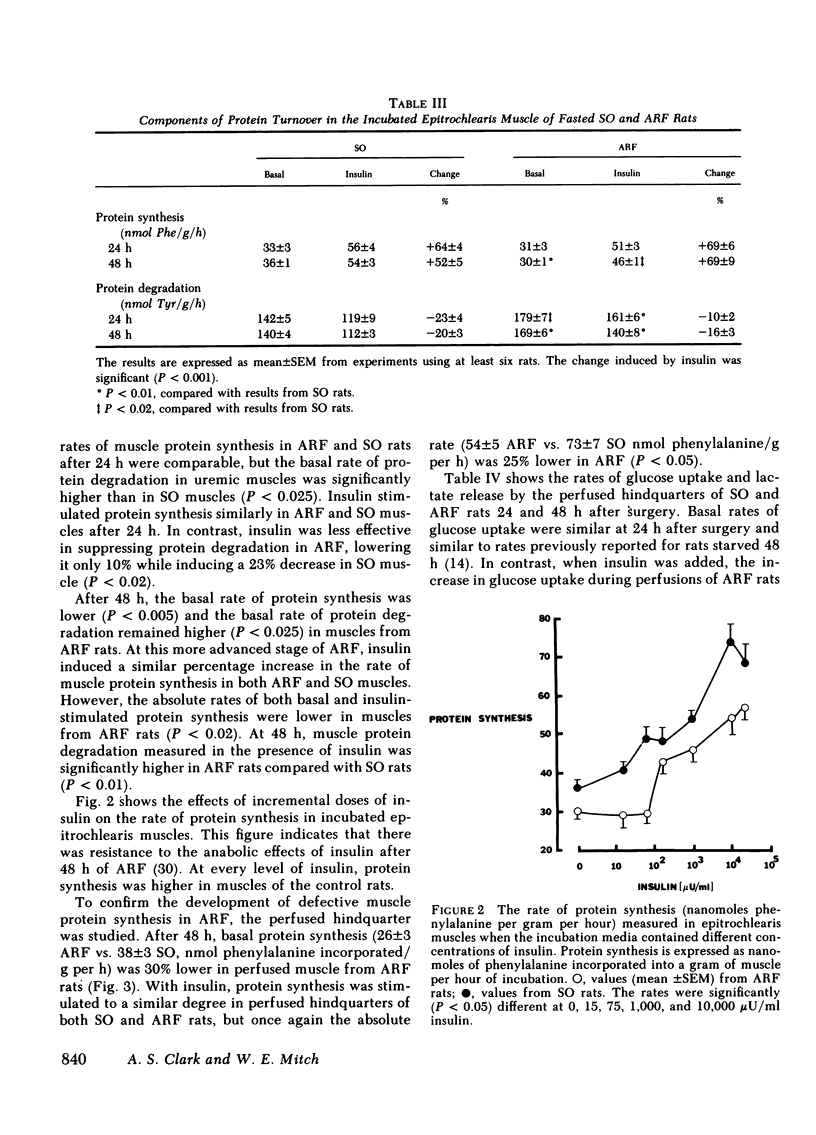
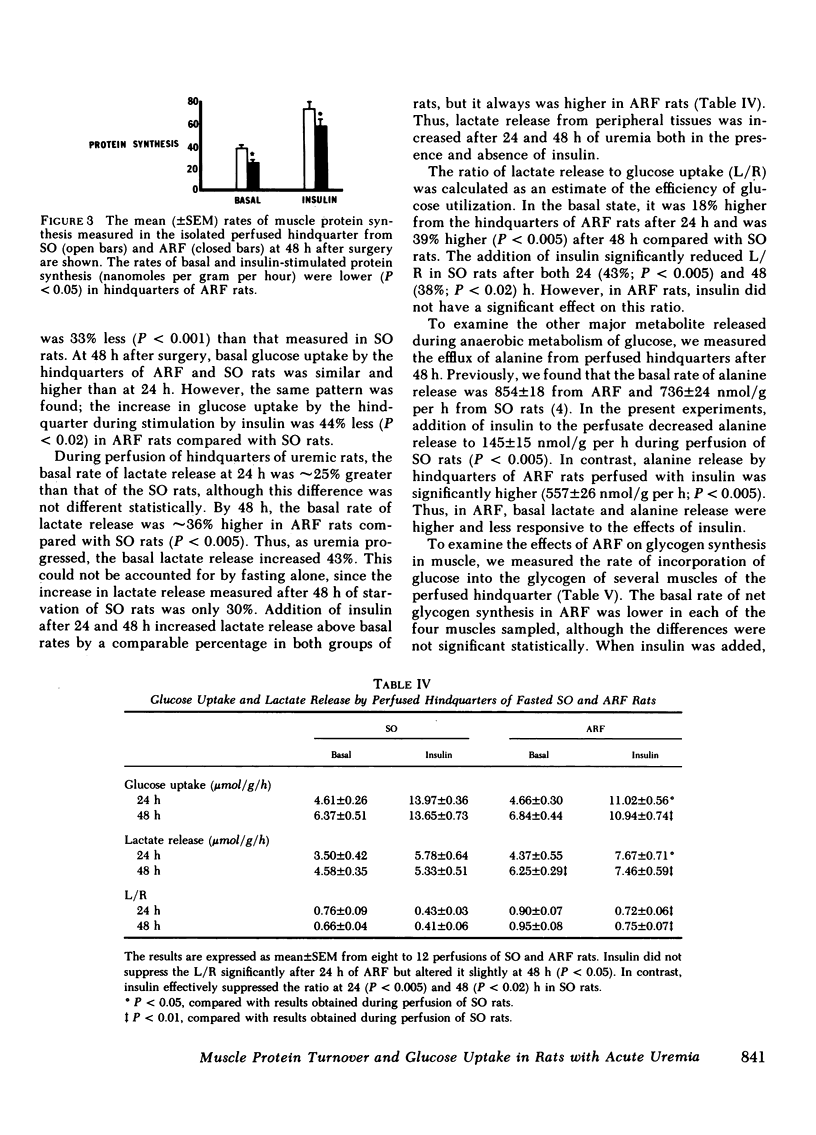
Acute renal failure (ARF) in rats is associated with increased amino acid release from peripheral tissues and insulin resistance. To study whether abnormal protein and carbohydrate metabolism are linked in ARF, the effects of insulin on net muscle protein degradation (T) and on glucose uptake were measured in the perfused hindquarters of paired ARF and sham-operated (SO) rats. The basal rate of T increased 40% after 24 and 98% after 48 h of ARF. Insulin was less effective in decreasing T in ARF (-79% SO vs. -22% ARF 24 h and -64% SO vs. -23% ARF 48 h; P less than 0.01). Protein synthesis (PS) and protein degradation (PD) were measured independently in incubated epitrochlearis muscles; the increase in T after 24 h of ARF was due specifically to increased PD, while PS was unchanged. At this stage, insulin was less effective in decreasing PD in ARF (-10% ARF vs. -23% SO; P less than 0.02), although PS responded normally. After 48 h of ARF, the further increment in T was caused by the additional appearance of depressed basal and insulin-stimulated PS. This was confirmed in the perfused hindquarter (26 +/- 3 ARF vs. 38 +/- 3 SO, basal; 54 +/- 5 ARF vs 73 +/- 7 SO, insulin-stimulated, nmol phenylalanine/g per h; P less than 0.05). Although basal glucose uptake by hindquarters of ARF and SO rats was comparable, insulin-stimulated glucose uptake was 33% less at 24 and 44% less after 48 h of ARF. After 48 h of ARF, lactate and alanine release were increased and net glycogen synthesis in muscle was depressed. These abnormalities were even more apparent in the presence of insulin. Inefficient glucose utilization, estimated as the ratio of lactate release to glucose uptake, was correlated with T (r = +0.78; P less than 0.001). In conclusion, after 24 h of ARF, both increased PD and altered glucose utilization could be detected. After 48 h of ARF, T increased further because PS was depressed. At this time, glucose utilization was clearly abnormal and the results suggest that abnormal net protein degradation in ARF may be a consequence of defective glucose utilization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Goldthorp R., Watts R. W. Fluorimetric measurement of the phenylalanine content of human granulocytes. Clin Chim Acta. 1973 Feb 12;43(3):379–387. doi: 10.1016/0009-8981(73)90477-4. [DOI] [PubMed] [Google Scholar]
- Arnold W. C., Holliday M. A. Tissue resistance to insulin stimulation of amino acid uptake in acutely uremic rats. Kidney Int. 1979 Aug;16(2):124–129. doi: 10.1038/ki.1979.113. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Goodman M. N., Kalish F. N., Ruderman N. B. Insulin binding and sensitivity in rat skeletal muscle: effect of starvation. Am J Physiol. 1981 Feb;240(2):E184–E190. doi: 10.1152/ajpendo.1981.240.2.E184. [DOI] [PubMed] [Google Scholar]
- Cernácek P., Spustová V., Dzúrik R. Inhibitor(s) of protein synthesis in uremic serum and urine: partial purification and relationship to amino acid transport. Biochem Med. 1982 Jun;27(3):305–316. doi: 10.1016/0006-2944(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Clark A. S., Mitch W. E. Comparison of protein synthesis and degradation in incubated and perfused muscle. Biochem J. 1983 Jun 15;212(3):649–653. doi: 10.1042/bj2120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich J., Schölmerich J., Hoppe-Seyler G., Maier K. P., Talke H., Schollmeyer P., Gerok W. The effect of acute uraemia on gluconeogenesis in isolated perfused rat livers. Eur J Clin Invest. 1974 Dec 5;4(6):453–458. doi: 10.1111/j.1365-2362.1974.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Garber A. J. Skeletal muscle protein and amino acid metabolism in experimental chronic uremia in the rat: accelerated alanine and glutamine formation and release. J Clin Invest. 1978 Sep;62(3):623–632. doi: 10.1172/JCI109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Goodman M. N., McElaney M. A., Ruderman N. B. Adaptation to prolonged starvation in the rat: curtailment of skeletal muscle proteolysis. Am J Physiol. 1981 Oct;241(4):E321–E327. doi: 10.1152/ajpendo.1981.241.4.E321. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hedden M. P., Buse M. G. Effects of glucose, pyruvate, lactate, and amino acids on muscle protein synthesis. Am J Physiol. 1982 Mar;242(3):E184–E192. doi: 10.1152/ajpendo.1982.242.3.E184. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- Lacy W. W. Effect of acute uremia on amino acid uptake and urea production by perfused rat liver. Am J Physiol. 1969 Jun;216(6):1300–1305. doi: 10.1152/ajplegacy.1969.216.6.1300. [DOI] [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- Mitch W. E. Amino acid release from the hindquarter and urea appearance in acute uremia. Am J Physiol. 1981 Dec;241(6):E415–E419. doi: 10.1152/ajpendo.1981.241.6.E415. [DOI] [PubMed] [Google Scholar]
- Mitch W. E., Chan W. alpha-Ketoisocaproate stimulates branched-chain amino acid transaminase in kidney and muscle. Am J Physiol. 1979 May;236(5):E514–E518. doi: 10.1152/ajpendo.1979.236.5.E514. [DOI] [PubMed] [Google Scholar]
- Mondon C. E., Dolkas C. B., Reaven G. M. The site of insulin resistance in acute uremia. Diabetes. 1978 May;27(5):571–576. doi: 10.2337/diab.27.5.571. [DOI] [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kaiser K. E., Kipnis D. M. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol. 1980 Dec;239(6):E454–E460. doi: 10.1152/ajpendo.1980.239.6.E454. [DOI] [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kipnis D. M. Epitrochlearis muscle. II. Metabolic effects of contraction and catecholamines. Am J Physiol. 1980 Dec;239(6):E461–E467. doi: 10.1152/ajpendo.1980.239.6.E461. [DOI] [PubMed] [Google Scholar]
- Pils P., Jettmar W., Adamiker D., Tragl K. H. Insulin and the in vitro protein synthesis of liver and skeletal muscle ribosomes in experimental acute uraemia. Horm Metab Res. 1981 Feb;13(2):89–91. doi: 10.1055/s-2007-1019181. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. Rates of protein synthesis in skin and bone, and their importance in the assessment of protein degradation in the perfused rat hemicorpus. Biochem J. 1981 Jan 15;194(1):373–376. doi: 10.1042/bj1940373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Goodman M. N., Conover C. A., Berger M. Substrate utilization in perfused skeletal muscle. Diabetes. 1979 Jan;28 (Suppl 1):13–17. doi: 10.2337/diab.28.1.s13. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear L. Internal redistribution of tissue protein synthesis in uremia. J Clin Invest. 1969 Jul;48(7):1252–1257. doi: 10.1172/JCI106090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Tischler M. E. Is regulation of proteolysis associated with redox-state changes in rat skeletal muscle? Biochem J. 1980 Dec 15;192(3):963–966. doi: 10.1042/bj1920963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wang M., Vyhmeister I., Kopple J. D., Swendseid M. E. Effect of protein intake on weight gain and plasma amino acid levels in uremic rats. Am J Physiol. 1976 May;230(5):1455–1459. doi: 10.1152/ajplegacy.1976.230.5.1455. [DOI] [PubMed] [Google Scholar]


