Abstract
Although interleukin-4 (IL-4) has been implicated in respiratory syncytial virus (RSV)-enhanced disease, the mechanism by which it modulates immune responses to primary RSV infection remains unclear. We have developed a system to investigate the effect of IL-4 on RSV epitope-specific cytotoxic T-lymphocyte (CTL) effector function in vivo, using an H-2Kd-restricted RSV M2 epitope. BALB/c mice were infected with recombinant vaccinia virus (rVV) constructed to express RSV M2 protein (vvM2) alone or coexpress M2 and IL-4 (vvM2/IL-4). Splenocytes were assessed for M2-specific CTL activity in a direct 51Cr release assay and intracellular gamma interferon (IFN-γ) production by fluorescence-activated cell sorting analysis. Mice infected with vvM2/IL-4 had less M2-specific primary CTL activity than those infected with vvM2. M2-specific CTL frequency, as measured by M2 peptide-induced intracellular IFN-γ production, was diminished in the vvM2/IL-4 group, partially accounting for the reduction of CTL activity. Mice immunized with either construct were challenged intravenously with RSV 4 weeks postimmunization, and direct CTL were measured. These results demonstrate that local expression of IL-4, at the time of antigen presentation, diminishes the cytolytic activity of primary and memory CD8+ RSV-specific CTL responses in vivo.
Cytokine production patterns of T-cell subsets can dramatically affect the pathogenesis of infectious diseases. CD4+ (Th) T-helper cells and CD8+ (Tc) cells can be classified as members of two distinct subsets based on their production of cytokines: type 1 CD4+ Th lymphocytes characteristically secrete gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor beta, while type 2 CD4+ Th lymphocytes are distinguished by the synthesis of IL-4, IL5, IL-6, IL-10, and IL-13 (11, 22). Similarly, CD8+ Tc1 cells secrete IL-2 and IFN-γ, and Tc2 cells secrete IL-4, IL-5, IL-6, and IL-10 (6, 31). Both patterns of cytokine secretion are self-propagating and can inhibit activation and proliferation of the complementary subset. In many animal models of infectious diseases, a predominance of type 2 cytokines has been correlated with disease progression, while a type 1 polarized immune response results in disease resolution (7, 10, 14, 28, 29, 33). Although IL-4 is known to inhibit CD4+ Th1 differentiation, its role in CD8+ T-cell effector function is not fully defined.
During cytotoxic T-lymphocyte (CTL) development naive precursors (CTLp) are activated by the recognition and binding of the antigen-specific T-cell receptor to processed peptides presented on major histocompatibility complex (MHC) class I. A second signal is mediated by CD28 on the T cell and B7.1/B7.2 (CD80/CD86) on the antigen-presenting cell (1, 16, 30). In the absence of CD28 costimulation, T cells become unresponsive to cytokines (9, 24). Once activated, CTLp expand in response to IL-2, generally supplied by nearby CD4+ Th cells (13, 17). Although the importance of IL-2 in T-cell induction is well documented, other cytokines, such as IL-12, IFN-γ, IL-6, IL-7, and IL-15, have also been shown to promote CTL induction (18, 19, 21, 40).
In the murine model of respiratory syncytial virus (RSV), aberrant high levels of IL-4 production are associated with delayed viral clearance and enhanced disease. In humans, RSV-specific immunoglobulin E (IgE) antibody detected in children with severe illness indirectly suggests that IL-4 may play a role in determining disease severity (39). Previously we have shown that systemic production of IL-4 by IL-4-overexpressing mice caused a delay in viral clearance and diminished RSV-specific CTL activity compared to wild-type controls (7). We have also shown that immunization with formalin-inactivated RSV vaccine promotes increased expression of IL-4 by lung lymphocytes upon subsequent challenge with RSV and that treatment with anti-IL-4 diminishes illness and increases CTL activity after challenge (34). Thus, there is evidence suggesting that in both humans and mice, IL-4 may be associated with an increase in RSV disease severity. To better understand the role of IL-4 in RSV-specific immune responses, we designed a system to investigate the effect of local IL-4 production at the time of antigen presentation on primary RSV-specific CD8+ CTL development in vivo. BALB/c (H-2d) mice infected with RSV recognize the viral matrix protein M2 as a major target antigen for induction of CD8+ CTL (5). It has been shown that the M2 protein of RSV contains an immunodominant H-2Kd-restricted CTL epitope consisting of amino acid residues 82 to 90 (SYIGSINNI) shared by RSV subgroups A and B (15). Recombinant vaccinia viruses (rVV) expressing RSV antigen M2 (vvM2) or coexpressing M2 and IL-4 (vvM2/IL-4) were constructed to ensure that IL-4 was present at the site of antigen presentation. Since in vitro restimulation of primed CTL relies on addition of exogenous stimuli that can stimulate nonspecific proliferation of lymphocytes, we assayed splenocytes directly (without in vitro manipulation). Splenocytes were directly assayed for cytolytic activity, using M2 peptide-sensitized P815 target cells. Levels of CTL activity were significantly lower in vvM2-infected mice than in mice infected with vvM2. Virus replication curves in the spleen were similar in the two groups of mice, suggesting that decreased antigen load was not responsible for diminished CTL activity. Fewer M2 peptide-specific IFN-γ-producing cells were detected in vvM2/IL-4-infected mice than in vvM2-infected mice, indicating that reduced expansion of CTL effectors may partially explain the diminished cytolytic activity. Mice challenged with RSV 4 weeks after immunization with vvM2/IL-4 experienced decreased magnitude of secondary RSV-specific CTL activity compared to vvM2 immunized mice, suggesting that expansion of the CTL memory population was also diminished. These data indicate that increased levels of IL-4 cytokine at the time of infection or immunization can significantly diminish subsequent CD8+ CTL activity.
MATERIALS AND METHODS
Mice.
Eight- to ten-week-old pathogen-free BALB/c mice, purchased from Harlan Laboratories (Indianapolis, Ind.), were housed and cared for in accordance with Guide for the Care and Use of Laboratory Animals (4a) as previously described (8).
Cell lines.
P815 (H-2d), a mastocytoma cell line derived from a DBA/2 mouse, and EL-4 (H-2b), a mouse lymphoma cell line, were maintained in Eagle’s minimal essential medium containing 10% fetal bovine serum (10% EMEM). 143B, a human osteosarcoma cell line, was maintained in 10% EMEM and used for preparation of both vaccinia virus stocks and virus titration. HEp-2 cells were maintained in 10% EMEM.
Viruses.
rVV (WR strain) containing the RSV M2 protein interrupting the thymidine kinase (TK) gene (vvM2) was a gift from Peter L. Collins, National Institutes of Health, Bethesda, Md. rVV containing β-galactosidase (rVVLac) was a gift from Bernard Moss (National Institutes of Health). Plasmid pFBX-mIL-4 expressing murine IL-4 was provided by Ian A. Ramshaw, Australia National University. The vvIL-4 plasmid was constructed with the cytokine gene and the herpes simplex virus TK gene flanked by vaccinia virus HindIII-F sequences, allowing selection of TK+ recombinant viruses. rVV constructs expressing RSV M2 and murine IL-4 were constructed according to the published protocol (3, 4, 27), with modification. Briefly, 143B cells were infected with vvM2 (multiplicity of infection of 0.1) and cotransfected with pFBX-mIL-4 precipitated by incubation in 60 μg of DNA, 0.6 ml of 10× Hanks’ balanced salt solution (Atlantic Biologics, Norcross, Ga.), 0.3 ml of 2.5 M CaCl2, and 5 ml of sterile distilled H2O for 45 min at room temperature. After 2.5 h, the monolayers were washed and selection medium (10% EMEM containing 3 μM methotrexate, 15 μM thymidine, 50 μM adenosine, 50 μM guanosine, and 10 μM glycine) was added. Virus was selected by serial passage and plaque purification prior to production of working stocks. Each stock was titered by plaque assay and evaluated for mycoplasma contamination by PCR (American Type Culture Collection) before use. IL-4 secretion was confirmed by enzyme-linked immunosorbent assay (ELISA) on infected cell culture supernatants by using commercial kits purchased from Endogen, Inc. (Cambridge, Mass.) (data not shown).
Antibodies.
11B.11, a monoclonal antibody against murine IL-4, was kindly provided by the Biological Response Modifiers Program, National Cancer Institute (Frederick, Md.). Hybridoma HB151, a monoclonal antibody against human HLA-Dr5 (American Type Culture Collection), was used as an irrelevant antibody control. Monoclonal antibodies were administered intraperitoneally at 200 μg/dose on 5 successive days starting 2 days before rVV infection.
Synthetic peptides.
Peptides synthesized by Bio-synthesis, Inc. (Lewisville, Tex.), included H-2Kd-restricted RSV M2 82-90 (SYIGSINNI), derived from M2 protein of RSV strain A2, and FLU 147-155 (TYQRTRALV), derived from influenza virus A/Puerto Rico/8/34 nucleoprotein (NP) (15).
Experimental design.
For primary CTL assays, mice were injected in the tail vein with 5 × 106 PFU of rVV. Splenocytes were isolated to measure CTL activity by a direct 51Cr release assay and for intracellular IFN-γ production by fluorescence-activated cell sorting (FACS) analysis at the indicated times postinfection. For challenge experiments, the same mice were injected 4 weeks later with 107 PFU of live RSV in the tail vein. Isolated lymphocytes were assayed for CTL activity and intracellular IFN-γ production on day 5 after challenge.
Plaque assays.
Animals were sacrificed, and lung and spleen tissues were removed and quick-frozen in 10% EMEM. Thawed tissues were kept chilled while individually ground. Dilutions of clarified supernatant were inoculated on 80% confluent 143B cell monolayers and overlaid with 0.75% methylcellulose in 10% EMEM. After incubation for 2 days at 37°C, the monolayers were fixed and stained with 0.1% crystal violet in 20% methanol, and PFU were counted and expressed as log10 PFU per gram of tissue.
CTL assay.
Lymphocytes were isolated by centrifugation (1,000 × g) on a cushion of Ficoll-Hypaque (specific gravity of 1.09) at room temperature, washed twice, and resuspended in RPMI containing 10% fetal bovine serum. H-2d P815 target or H-2b EL-4 target cells were incubated with 50 μl of relevant peptides (0.1 mg/ml) and 51Cr (100 μCi/107 cells) for 60 min at 37°C, washed three times in 10% EMEM, and distributed in V-bottom 96-well plates (Costar, Cambridge, Mass.) at 2 × 104 cells/100 μl per well. Splenic effector cells (2 × 106/100 μl) were added at an effector/target (E:T) ratio of 100:1 and serially diluted to 3:1 in triplicate. The plate was centrifuged at 150 × g for 30 s before incubation at 37°C for 4 h. The cells were gently pelleted, and 100 μl of the supernatant was counted in a gamma counter (Packard, Meriden, Conn.). Spontaneous and total release was measured by treating the targets cells with 10% RPMI or with 5% Triton X-100 detergent, respectively. Specific release of 51Cr from target cells is defined as 100 × [(sample cpm − background cpm)/(total cpm − background cpm)]. One lytic unit (LU) was defined as the number of lymphocytes needed to achieve 50% specific lysis.
Intracellular staining and flow cytometry.
A total of 2 × 106 spleen cells in complete medium were cultured in 6-ml Falcon round-bottom tubes (Becton Dickinson Labware, Lincoln Park, N.J.) and incubated with either FLU 147-155 (TYQRTRALV) or RSV M2 peptide at a concentration of 0.5 μg/ml. Cells were incubated in the presence of peptide for 2 h before supplementation with 0.66 μl of monensin (Golgistop; Pharmingen, San Diego, Calif.)/2 × 106 cells for an additional 8 h. Cells were washed once in staining buffer (phosphate-buffered saline, 0.1% sodium azide, 2% fetal calf serum) and surface stained with Cy-Chrome-conjugated monoclonal rat anti-mouse CD8α (clone 53-6.7) antibody and fluorescein isothiocyanate-conjugated monoclonal rat anti-mouse CD4 (clone GK1.5). Cells were washed two times in staining buffer and stained intracellularly by using a staining kit as instructed by the manufacturer (Pharmingen). For intracellular IFN-γ staining phycoerythrin-conjugated monoclonal rat anti-mouse IFN-γ antibody (clone XMG1.2) and an isotype control antibody (rat IgG1) (Pharmingen) were used. Positive control cells for intracellular cytokine staining were stained with a kit provided by Pharmingen according to the manufacturer’s instructions. Three-color analysis was performed on a FACSCaliber (Becton Dickinson, San Jose, Calif.) argon ion laser at 15 mW and 488 nm; 40,000 events were collected at an average of 1,000 events/s. Data were analyzed by using CellQuest version 3.1 (Becton Dickinson).
Western blotting.
A total of 4 × 106 HEp-2 cells were infected at a multiplicity of infection of 5. Virus was allowed to adsorb for 1 h at room temperature, and then the culture was incubated at 37°C for 24 or 48 h. Cells were lysed in a buffer (150 mM NaCl, 10 mM HEPES, 0.6% NP-40, 1 mM EDTA) containing protease inhibitors (5 μg each of leupeptin and aprotinin per ml and 0.5 mM phenylmethylsulfonyl fluoride [Sigma, St. Louis, Mo.]). Protein concentrations were determined by the Bio-Rad (Hercules, Calif.) protein assay according to the manufacturer’s instructions. Twenty micrograms of protein was loaded on a sodium dodecyl sulfate–12% polyacrylamide gel. M2 was detected with a primary rabbit RSV polyclonal antibody (a gift from Jim Crowe, Vanderbilt University, Nashville, Tenn.) and a secondary goat anti-rabbit IgG coupled to alkaline phosphatase (Sigma). Untreated HEp-2 cells and vvIL-4-infected cells were controls for nonspecific binding. Bands were visualized by using the substrate FAST Red TR/Naphthol AS-MX (Sigma) according to the manufacturer’s instructions.
Statistics.
The two-tailed Student t test was used for comparison of means, using Corel QuattroPro version 6.0 for Windows. Scheffe and Fischer’s protected least significant difference analysis of variance was used to evaluate primary M2-specific T-cell receptor-bearing CD8+ cell frequency detected by FACS analysis. The Mann-Whitney analysis of the data in Fig. 5 was performed with InStat 2.01 (GraphPad Software). Values of P < 0.05 were considered statistically significant.
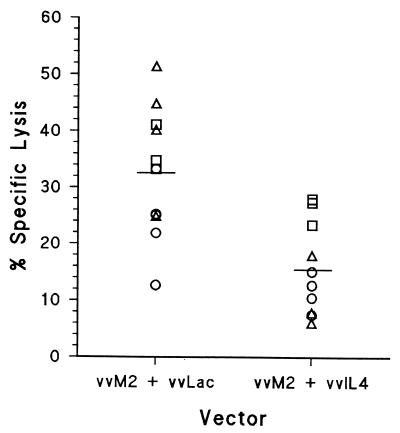
FIG. 5.
M2-specific CTL activity of mice coinjected with vvM2 and vvIL-4. CTL activity was measured in splenocytes on day 6 postinfection. Mice were coinjected with vvM2 plus vvIL-4 or vvM2 plus vvLac. Scatter plot data are values for individual mice at E:T = 100:1, and the horizontal bar indicates the arithmetic mean of the group. The data are derived from three independent experiments, each designated by a unique symbol. P = 0.001 by Mann-Whitney test.
RESULTS
Kinetics of rVV replication and cytokine production in lungs and spleens.
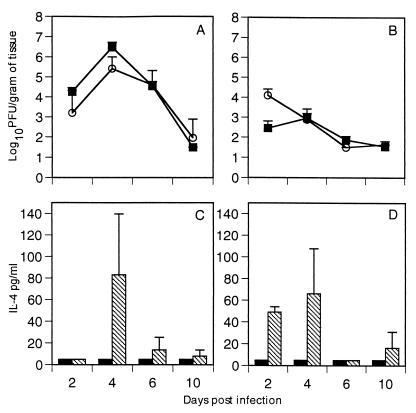
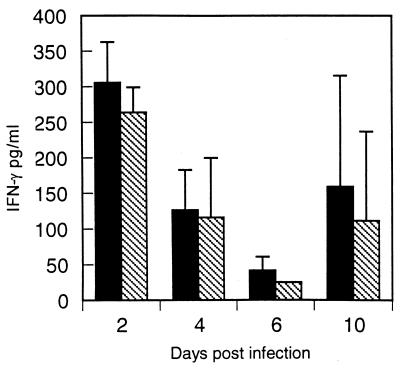
Mice were injected with either vvM2 or vvM2/IL-4 and sacrificed on days 2, 4, 6, and 10 postinfection. Lung and spleen supernatant were assayed for viral replication and cytokine production (Fig. 1). The two vaccinia virus constructs replicated in the lungs and spleens with similar kinetics, and virus was cleared by day 10 in both groups (Fig. 1A and B). Virus-derived IL-4 secretion could be detected in the spleen 2 days after infection, and peak IL-4 levels were attained on day 4 in both lungs and spleens of mice infected with vvM2/IL-4 (Fig. 1C and D). No IL-4 was detected in mice infected with vvM2. Analysis of IFN-γ levels in mice injected with either vvM2 or vvM2/IL-4 showed no significant difference at any time point (Fig. 2). Similarly, there was no significant difference in IL-2 production between groups (data not shown).
FIG. 1.
Kinetics of viral replication and cytokine production in the lungs and spleens. Mice were sacrificed on days 2, 4, 6, and 10 postinfection. vvM2 (■) and vvM2/IL-4 (○) viral replication was measured in the lung (A) and spleen (B) by standard plaque assay. IL-4 protein in the lung (C) and spleen (D) was measured by ELISA. Solid bars, vvM2; hatched bars, vvM2/IL-4. The limit of detection for virus replication is 1.8 log10 PFU/g of tissue. Data are representative of three independent experiments with four mice per group (n = 4).
FIG. 2.
IFN-γ levels in mice infected with vvM2 (solid bars) or vvM2/IL-4 (hatched bars) on days 2, 4, 6, and 10 postinfection. IFN-γ was measured from spleen supernatant by ELISA. Data represent two independent experiments.
IL-4 down-regulates virus-specific CTL.
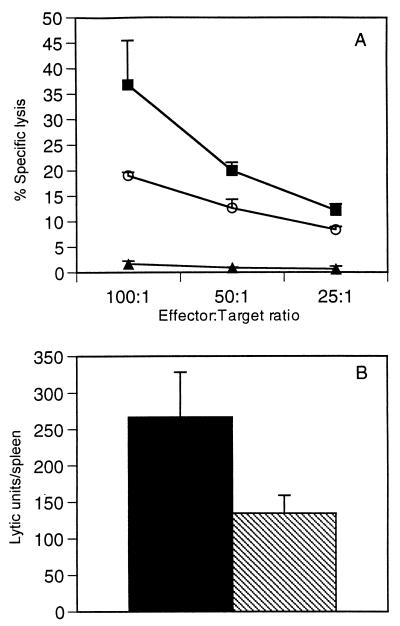
To determine if IL-4 diminished M2-specific CTL activity, we measured CTL cell lysis by using a direct CTL assay. On day 6, splenic effector cell cytotoxicity was measured by incubation with specific peptide-sensitized P815 target cells in a direct 51Cr release assay (Fig. 3A). M2-specific CTL activity in mice infected with vvM2 averaged 37% ± 8.6% (E:T = 100:1); in contrast, in mice receiving vvM2/IL-4 CTL lysis was diminished to 19% ± 0.7% (E:T = 100:1; P <0.05). No specific lysis was observable in mice receiving vvIL-4 (Fig. 3A), influenza virus hemagglutinin peptide-loaded P815 cells, or MHC-unmatched EL-4 (H-2b) target cells (data not shown). To adjust for lymphocyte numbers in the spleen, LU were calculated on a per-spleen basis (Fig. 3B) where 1 LU is equal to the number of lymphocytes needed to achieve 50% lysis. The number of LU in the vvM2/IL-4 group (134 ± 24) was significantly lower than that in the vvM2 group (267 ± 61; P <0.05).
FIG. 3.
M2-specific CTL activity. (A) CTL activity in splenocytes was measured by determination of specific lysis on day 6 postinfection in mice injected with 5 × 106 PFU of vvIL-4 (▴), vvM2 (■), or vvM2/IL-4 (○) (P <0.05 between vvM2 and vvM2/IL-4 for E:T ratios of 100:1 and 50:1). (B) LU measured on a per-spleen basis. Solid bars, vvM2; hatched bars, vvM2/IL-4 (P <0.05). Results are representative of six independent experiments.
Anti-IL-4 restores M2-specific CTL activity.
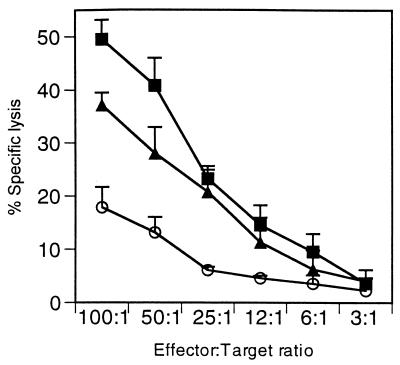
To determine if the observed decrease in M2-specific CTL response involved secreted IL-4, we administered anti-IL-4 intraperitoneally for 5 days starting on day −2 and ending on day +2 (Fig. 4). Peak M2-specific lysis at an E:T ratio of 100:1 was 49.5% ± 3.65%, whereas mice receiving vvM2/IL-4 experienced marked attenuation of M2-specific CTL activity (17.9% ± 2.3%). Anti-IL-4 treatment of these mice restored CTL activity from 17.9% to 37.2% ± 3.8% (E:T = 100:1; P <0.05). HB151 control antibody did not increase CTL activity (data not shown).
FIG. 4.
Anti-IL-4 treatment restores CTL activity. Direct CTL activity was measured in splenocytes on day 6 postinfection. Mice were injected with vvM2 (■), vvM2/IL-4 (○), or vvM2/IL-4 plus anti-IL-4 (▴). Results are representative of three independent experiments.
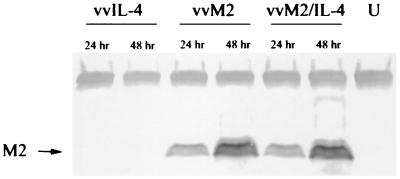
To address the possibility that decreased expression of M2 antigen in the vvM2/IL-4 construct was responsible for the diminished CTL activity, mice were injected with vvM2 plus vvLac or vvM2 plus vvIL-4. Specific lysis by effectors from individual mice are shown in Fig. 5 as a scatter plot of the results from the E:T ratio of 100:1. Effectors from mice injected with vvM2 plus vvLac induced greater specific lysis than those from mice injected with vvM2 plus vvIL-4 (P = 0.001). Western blot analysis of M2 protein expression in infected HEp-2 cells demonstrated equal expression of the M2 protein by both vvM2 and vvM2/IL-4 constructs (Fig. 6).
FIG. 6.
Western blot analysis of M2 protein expression in infected HEp-2 cells at 24 and 48 h postinfection. RSV polyclonal antibody was used to detect M2 expression in vvIL-4-, vvM2-, and vvM2/IL-4-infected cells; 20 μg of protein was loaded into each lane. Levels of M2 expression were similar for the two vectors.
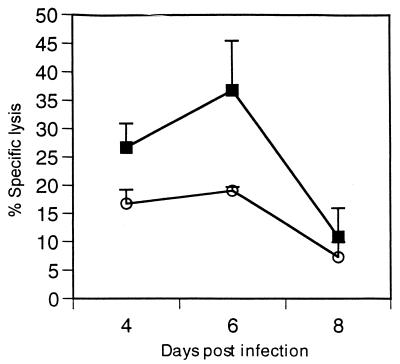
To assess whether IL-4 may delay CTL activity rather than diminish it, we measured CTL activity on days 4, 6, and 8 postinfection (Fig. 7). Kinetics for CTL activity in the vvM2/IL-4 group was similar to that of the vvM2 group, with peak CTL lysis on day 6 postinfection. These data demonstrate that anti-IL-4 cytokine is able to dampen the effects of IL-4 in the microenvironment and that the diminished CTL activity is not due to a reduced M2 antigen load delivered by the vvM2/IL-4 construct or a delay in peak CTL activity.
FIG. 7.
Kinetics of splenic CTL activity. Mice were injected with vvM2 (■) or vvM2/IL-4 (○) and sacrificed on days 4, 6, and 10 postinfection. Data represent two independent experiments (P <0.05 between groups for days 4 and 6).
Effects of IL-4 on M2-specific CD8+ T-cell frequency in the spleen.
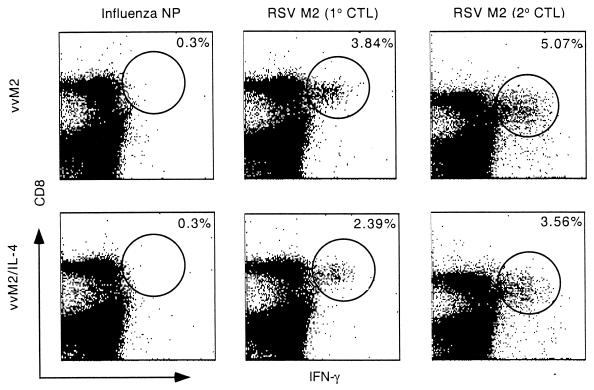
Diminished proliferation and clonal expression of M2-specific CD8+ T cells is one potential mechanism for the reduced CTL activity. Therefore, we compared the frequencies of M2-specific CD8+ T cells in spleens from mice infected with vvM2 and vvM2/IL-4. M2 peptide-stimulated splenocytes were analyzed by three-color flow cytometry for surface expression of CD4 and CD8α and intracellular IFN-γ. Combining the results of five independent experiments with four to five mice per group, we calculated that the percentages of M2 epitope-specific CD8+ T cells averaged 3.84% ± 1.1% in vvM2-infected mice, compared to 2.39% ± 0.9% (P < 0.05) in the vvM2/IL-4 group (Fig. 8). Levels of nonspecific IFN-γ production by CD8+ T cells in the vvM2- and vvM2/IL-4-infected mice, using an influenza virus NP peptide, were 0.30% ± 0.07% and 0.30% ± 0.04%, respectively (Fig. 8). Neither peptide induced IFN-γ production in CD4+ T cells (data not shown). Thus, IL-4 exerted a moderate suppression of the expansion of CD8+ M2-specific T cells, as determined by intracellular IFN-γ secretion. This diminution may partially account for the observed reduction in cytolytic activity.
FIG. 8.
Intracellular cytokine staining of M2 peptide-stimulated spleen cells. Mice were primarily infected (1° CTL) or immunized (2° CTL) with vvM2 or vvM2/IL-4; 2 × 106 spleen cells were stimulated with FLU 147-155 (TYQRTRALV) or RSV M2 82-90 (SYIGSINNI) peptide for 8 h in the presence of monensin and analyzed for IFN-γ production by flow cytometry. Data for primary CTL are representative of averages compiled from five independent experiments with n = 4 or 5 per group; data for secondary CTL are representative of two independent experiments, n = 4 (P > 0.05 between groups for both primary and secondary CTL responses).
IL-4 diminishes memory CTL activity.
We next wanted to determine if IL-4 given at the time of antigen presentation would affect the development of CTL memory. Mice immunized with vvM2 or vvM2/IL-4 were intravenously injected with RSV 4 weeks later. Determination of splenic CTL activity on day 5 after challenge showed that the vvM2/IL-4-primed mice had diminished secondary CTL responses of 31% ± 7.0% in the vvM2 group and 17.5% ± 6.4% in the vvM2/IL-4 group (E:T = 100:1; P <0.05) (Fig. 9). Consistent with this observation, IFN-γ production of M2-specific CD8+ T cells was less in the vvM2/IL-4 group (3.56% ± 1.0%) than in the vvM2 group (5.07% ± 0.9%) (Fig. 8).
FIG. 9.
Memory CD8+ T-cell activity. Mice were injected with medium (▴) or with 5 × 106 PFU of vvM2 (■) or vvM2/IL-4 (○). Four weeks later, all mice were challenged with 107 PFU of live RSV intravenously and CTL activity in splenocytes was measured on day 5 after challenge (P < 0.05 between vvM2 and vvM2/IL-4 for E:T = 100:1 and 50:1).
DISCUSSION
In this report, we show that IL-4 production during CTL development diminishes the expansion and activity of both primary and memory M2-specific CTL in vivo. We developed a system to evaluate the local cytokine effects on CTL induction by constructing rVV expressing the RSV M2 antigen (vvM2) or coexpressing M2 and IL-4 (vvM2/IL-4). Mice injected with vvM2/IL-4 experienced significant attenuation of M2-specific CTL activity whether IL-4 was expressed together (vvM2/IL-4) or separately (vvM2 plus vvIL-4). Since IL-4 is a known T- and B-cell proliferation factor (12, 23, 42), total numbers of lymphocytes were accounted for by calculating LU per spleen. Lytic activity in the vvM2/IL-4 group was twofold less than in the vvM2 group. Our data indicate that the mechanism of diminished CTL activity was not due to antigen availability, delay in CTL activity, reduction of IL-2 or IFN-γ, or a dilutional effect of excess nonspecific cells.
Recent studies using rVV, IL-4 knockout mice, or immune-complexed IL-4 have suggested that IL-4 inhibits CTL activity and delays viral clearance (7, 20, 32, 36). Many of these studies have relied on in vitro restimulation assays as a surrogate for in vivo CTL activity. Interestingly, in vitro studies of effector CTL development using purified splenocyte, thymocyte, or lymph node cells in allogeneic mixed lymphocyte culture have shown that recombinant IL-4 together with recombinant IL-2 increases CTL activity and that both IL-2 and IL-4 can function as growth factors for CD8+ T cells and CTL generation (26, 35, 37, 41). Thus, the effects of IL-4 on CTL generation in vitro may not apply to in vivo phenomena. We have shown that IL-4 present at the time of initial antigen presentation led to diminished memory CTL activity after challenge with RSV 4 weeks postimmunization by a direct CTL assay. However, a previous study in which vaccinia virus coexpressing RSV-F and IL-4 were used to study the effects of IL-4 on memory CTL response showed that there was no difference in CTL activity regardless of IL-4 expression (2). The major difference between that study and our work is that in the other study splenocytes were restimulated in vitro for 5 days (2), whereas our assays were performed by directly adding splenocytes to target cells without in vitro manipulation. Our data indicate that IL-4 diminishes peptide-specific CD8+ CTL activity in both the primary and the memory responses.
New methods to quantitate and characterize cytokine production by epitope-specific effector T cells have proven to be highly efficient and precise (25, 38). By staining for intracellular IFN-γ and surface expression of CD4 and CD8α, we showed that M2-specific CD8+ T-cell frequencies were lower in the vvM2/IL-4-infected mice than in mice receiving vvM2. Although IL-4 did not diminish overall IFN-γ production (Fig. 2), the number of IFN-γ-producing M2-specific of CD8+ T cells was diminished (Fig. 8). M2 peptide-induced IL-2 or IL-4 was not detected by intracellular staining (data not shown). Thus, IL-4 diminution of M2-specific CTL activity may be partially explained by the reduction of M2-specific CD8+ T cells in vivo.
In this study there was no evidence for a delay in vaccinia virus clearance in the lung or spleen, possibly due to the nature of immune response to vaccinia virus compared to RSV. Vaccinia virus can induce a broad set of immune responses, including activation of NK cells, cytolytic CD4+ T cells, and antiviral cytokines, that may contribute to the elimination of vaccinia virus from the spleen. These results are consistent with a study showing that BALB/cAnN mice deficient in IL-4 display an increase in CTL activity compared to wild-type controls, without a delay in viral clearance in either group (36). CTL development includes a complex series of processes including activation, proliferation, and differentiation into effector or memory CTL. Our findings suggest that the expansion of RSV M2-specific CD8+ T cells activated to produce IFN-γ is diminished by IL-4, suggesting that a step in clonal expansion has been impaired. Although this may not be the only effect that IL-4 has on the process of CD8+ CTL induction, it is a partial explanation for how IL-4 diminishes CTL activity in vivo. Induction of an antiviral T-cell response is a goal of many vaccine development efforts. Understanding the mechanism by which IL-4 exerts its effect on T-cell development will contribute to our understanding of T-cell-mediated immune response and improve vaccine approaches to viral diseases.
ACKNOWLEDGMENTS
We thank the Biological Response Modifiers Program (National Cancer Institute, Frederick, Md.) for supplying the anti-IL-4 (11B.11) monoclonal antibody. We also thank Rauf Kuli-Zade and Frances Robinson for technical assistance, David McFarland for flow cytometry expertise, and Mark Boothby for reviewing the manuscript.
This work was supported by grant RO1-AI-33933.
REFERENCES
- 1.Azuma M, Cayabyab M, Buck D, Phillips J H, Lanier L L. CD28 interaction with B7 co-stimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bembridge G P, Lopez J A, Cook R, Melero J A, Taylor G. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of the RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J Virol. 1998;72:4080–4087. doi: 10.1128/jvi.72.5.4080-4087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle D B, Couper B E H, Both G W. Multiple-cloning-site plasmids for the rapid construction of recombinant poxviruses. Gene. 1985;35:169–177. doi: 10.1016/0378-1119(85)90169-6. [DOI] [PubMed] [Google Scholar]
- 4.Boyle D B, Couper B E H. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988;65:123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- 4a.Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington, D.C: Institute of Laboratory Animal Resources, National Research Council; 1996. [Google Scholar]
- 5.Connors M, Kulkarni A B, Collins P L, Firestone C Y, Holmes K L, Morse III H C, Murphy B R. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol. 1992;66:1277–1281. doi: 10.1128/jvi.66.2.1277-1281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erard F, Wil M T, Garcia-Sanz J A, Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make Th2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 7.Fischer J E, Johnson J E, Kuli-Zade R K, Johnson T R, Aung S, Parker R A, Graham B S. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol. 1997;71:8672–8677. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham B S, Perkins M D, Wright P F, Karzon D T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 9.Guerder S, Meyerhoff J, Flavell R A. The role of the T cell costimulator B7.1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994;1:155–166. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 10.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-γ or interleukin-4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Hu-Li J, Chen H, Ben-Sasson S Z, Paul W E. IL-4 and IL-13 production in differentiated T helper type 2 cells is not IL-4 dependent. J Immunol. 1997;159:3731–3738. [PubMed] [Google Scholar]
- 12.Hu-Li J, Shevach E M, Mizuguchi J, Ohara J, Mosmann T, Paul W E. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 1987;165:157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 14.James S L, Sher A. Cell-mediated immune response to schistosomiasis. Curr Top Microbiol Immunol. 1990;155:21–30. doi: 10.1007/978-3-642-74983-4_2. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni A B, Morse III H C, Bennink J R, Yewdell J W, Murphy B R. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenschow D J, Walnus J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 17.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–267. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 18.Mizel S B. The interleukins. FASEB J. 1989;3:2379–2388. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- 19.Modlin R L, Lancki D W, Herold K C, Fitch F W. An antigen receptor-driven, interleukin 2-independent pathway for proliferation of murine cytolytic T lymphocyte clones. J Exp Med. 1986;163:1566–1582. doi: 10.1084/jem.163.6.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran T M, Isobe H, Fernandez-Sesma A, Schulman J L. Interleukin-4 causes delayed viral clearance in influenza virus-infected mice. J Virol. 1996;70:5230–5235. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T R, Coffman R L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T R, Bond M W, Coffman R L, Ohara J, Paul W E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci USA. 1986;83:5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller D L, Jenkins M K. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995;7:375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer J D, McKenzie D T, Swain S L, Dutton R W. B cell stimulatory factor 1 (interleukin 4) is sufficient for the proliferation and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Exp Med. 1987;166:1464–1470. doi: 10.1084/jem.166.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramshaw I A, Ruby J, Ramsay A, Ada G, Karupiah G. Expression of cytokines by recombinant vaccinia viruses: a model for studying cytokines in virus infections in vivo. Immunol Rev. 1992;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 29.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudd C E. Upstream-downstream: CD28 co-signaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 31.Sad S, Marcotte R, Mosmann T R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ cells secreting Th1 and Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 32.Sharma D P, Ramsay A J, Maguire D J, Rolph M S, Ramshaw I A. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y W, Graham B S. T cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J Clin Investig. 1997;99:2183–2191. doi: 10.1172/JCI119391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y W, Graham B S. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Investig. 1994;95:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trenn G, Takayama H, Hu-Li J, Paul W E, Sitkovsky M V. B cell stimulatory factor (IL-4) enhances the development of cytotoxic T cells from Lyt-2+ resting murine T lymphocytes. J Immunol. 1988;140:1101–1106. [PubMed] [Google Scholar]
- 36.Villacres M C, Bergmann C C. Enhanced cytotoxic T cell activity in IL-4-deficient mice. J Immunol. 1999;162:2663–2670. [PubMed] [Google Scholar]
- 37.Wagner H, Hardt C, Rouse B T, Rollinghoff M, Scheurich P, Pfizenmaier K. Dissection of the proliferative and differentiative signals controlling murine cytotoxic T lymphocyte responses. J Exp Med. 1982;155:1876–1881. doi: 10.1084/jem.155.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry. J Clin Investig. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welliver R C, Wong D T, Sun M, Middleton E, Jr, Vaughan R S, Ogra P L. The development of respiratory syncytial virus specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 40.Widmer M B, Bach F H. Antigen-driven helper cell-independent cloned cytolytic T lymphocytes. Nature. 1981;295:750–752. doi: 10.1038/294750a0. [DOI] [PubMed] [Google Scholar]
- 41.Widmer M B, Grabstein K H. Regulation of cytolytic T lymphocyte generation by B-cell stimulatory factor. Nature. 1987;326:795–798. doi: 10.1038/326795a0. [DOI] [PubMed] [Google Scholar]
- 42.Yokota T, Otsuka T, Mosmann T, Banchereau J, DeFrance T, Blanchard D, De Vries J E, Lee F, Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci USA. 1986;83:5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]