Abstract
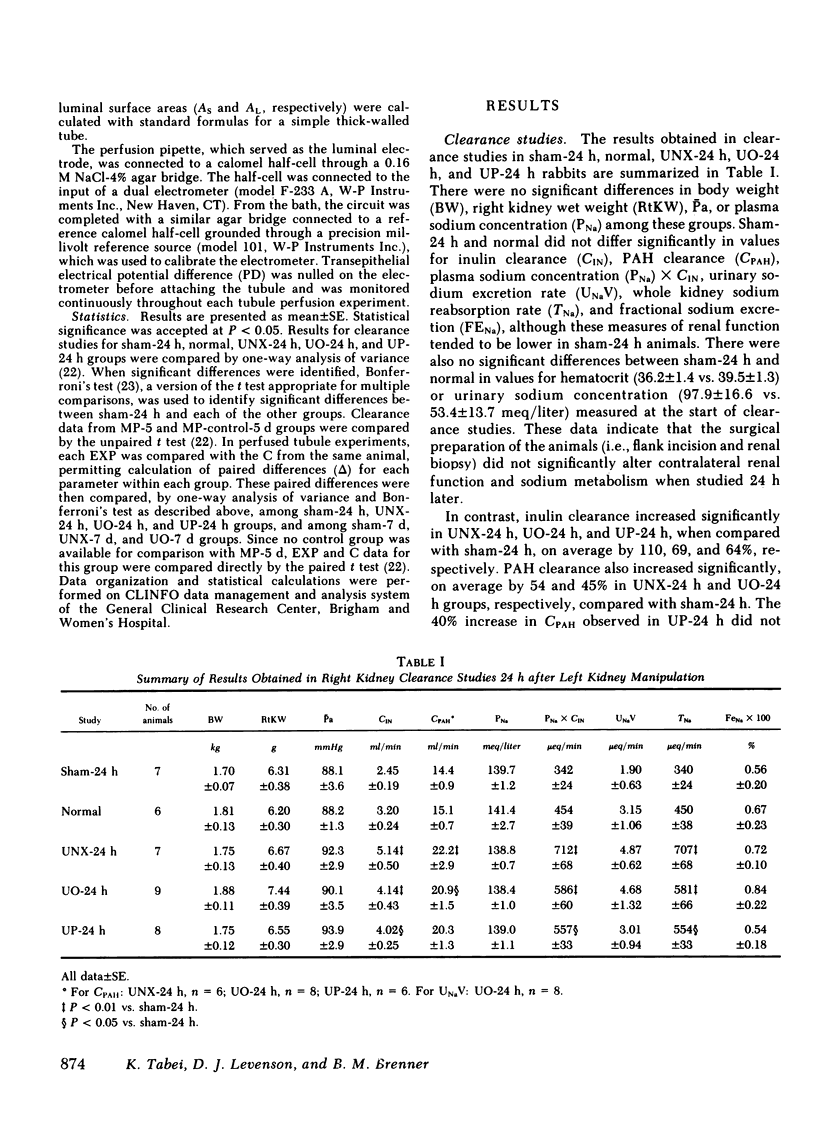
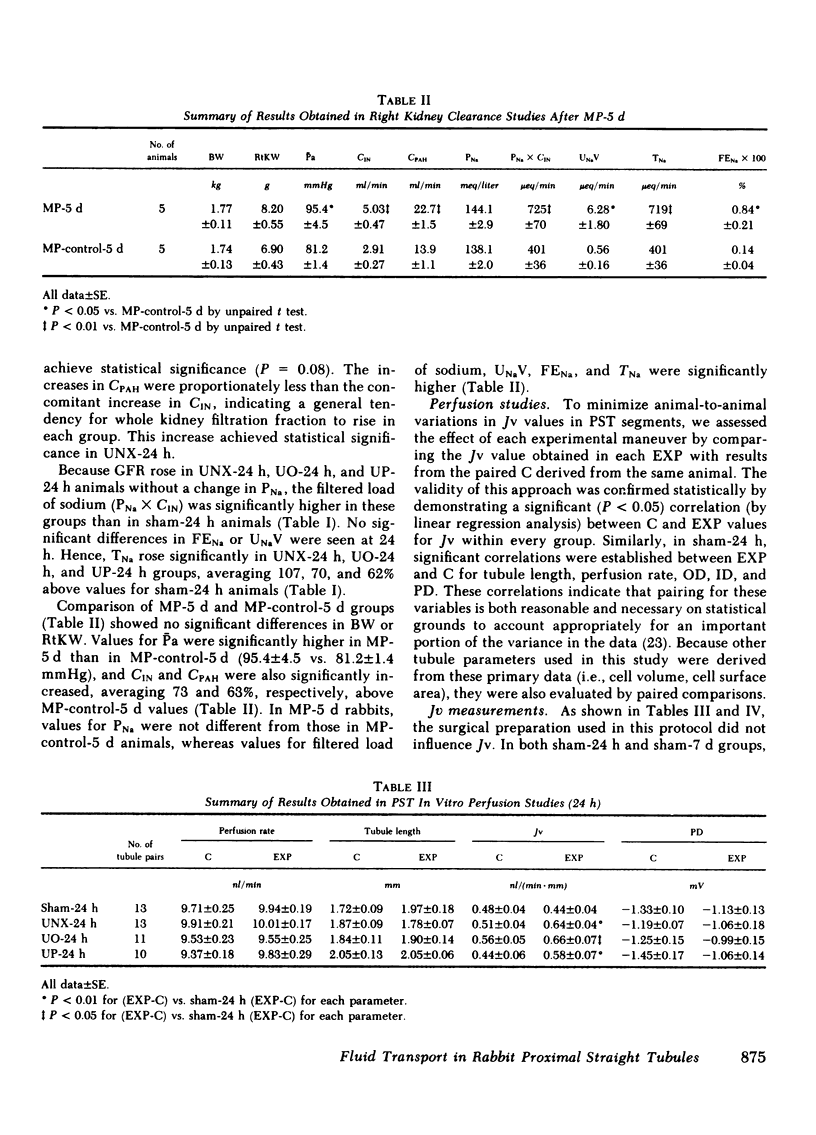
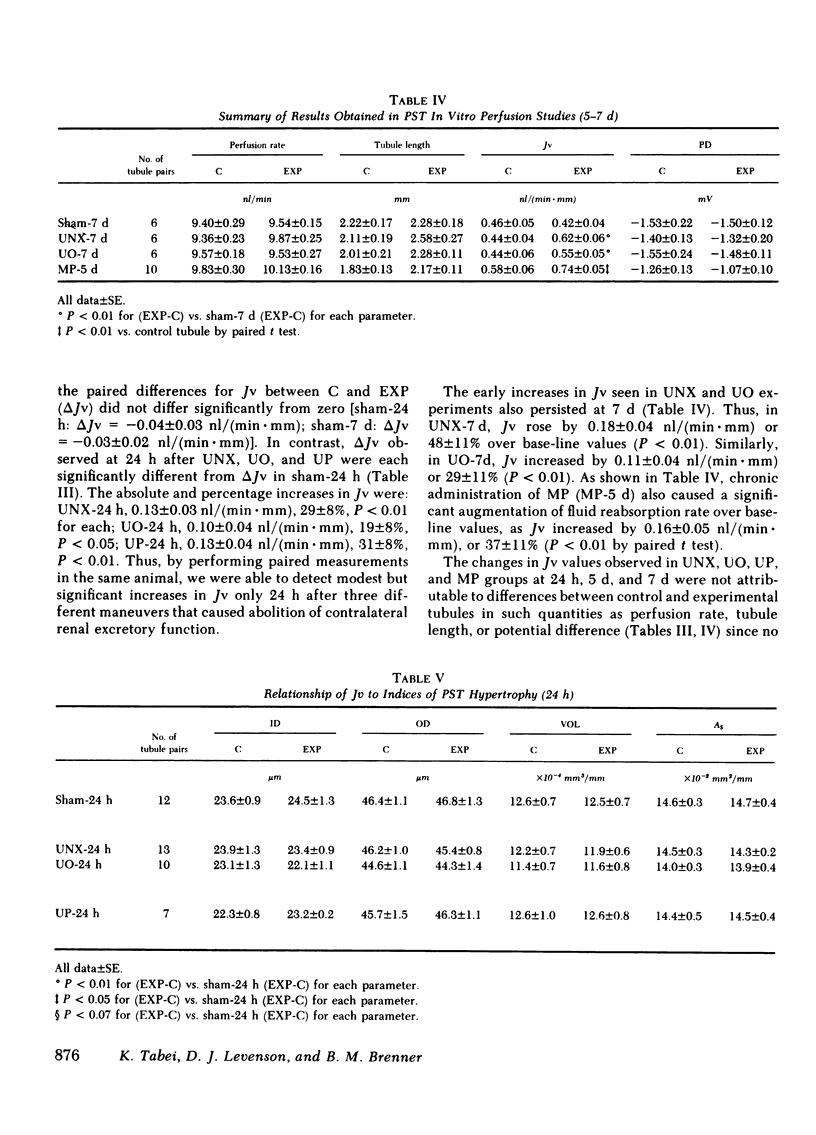
To assess the renal functional adaptation to reduced excretory capacity, we studied whole kidney and single nephron function in anesthetized volume-replete rabbits after unilateral (left kidney) nephrectomy (UNX), ureteral obstruction (UO), or ureteroperitoneostomy (UP). At 24 h, despite the absence of measurable hypertrophy of the contralateral (right) kidney, these procedures significantly increased p-aminohippurate clearance (45-54%) and inulin clearance (CIN) (64-110%) compared with sham-operated control animals. In each group, whole kidney sodium reabsorption increased in proportion to the rise in CIN. To determine whether the intrinsic transport capacity of proximal tubule segments is altered by these maneuvers, we measured fluid volume reabsorption rate (Jv) in isolated superficial proximal straight tubule (PST) segments perfused in vitro, comparing each control tubule (obtained by biopsy of the left kidney immediately before an experimental maneuver) with a corresponding tubule segment obtained 24 h or 7 d later from the contralateral kidney. Control tubule Jv in sham-24 h animals averaged 0.48 +/- 0.04 nl/(min X mm). Jv did not change significantly at 24 h or 7 d after sham maneuvers but increased significantly at 24 h after UNX [delta Jv = 0.13 +/- 0.03 nl/(min X mm)], UO [delta Jv = 0.10 +/- 0.04 nl/(min X mm)], and UP [delta Jv = 0.13 +/- 0.04 nl/(min X mm)]. Jv remained increased by similar amounts at 7 d after UNX and UO. To evaluate whether an increase in glomerular filtration rate (GFR) might be the stimulus to this augmentation in Jv values, methylprednisolone (MP) (15 mg/kg per d) was administered daily to sham-operated animals, a maneuver which induced a 73% rise in CIN by day 5. This procedure also produced a significant increase in Jv in PST at 5 d [delta Jv = 0.16 +/- 0.05 nl/(min X mm)]. The increase in Jv evident in each group at 5 or 7 d was paralleled by an equivalent change in tubule cell volume and apparent tubule luminal surface area in UNX-7d and MP-5d; no such increments in these indices, or in apparent tubule serosal surface area were evident at 24 h in any group. Thus, a 50% reduction in renal excretory function in the rabbit provokes adjustments in renal plasma flow rate and GFR in the contralateral kidney, which are evident by 24 h. The concurrent change in Jv in PST is closely related to CIN or some associated hemodynamic process, but does not appear to require an increase in tubule cell volume or apparent surface area. The ability to detect these small in vivo changes in Jv may derive from the enhanced sensitivity of paired-kidney experiments using tubule segments obtained by renal biopsy.
Full text
PDF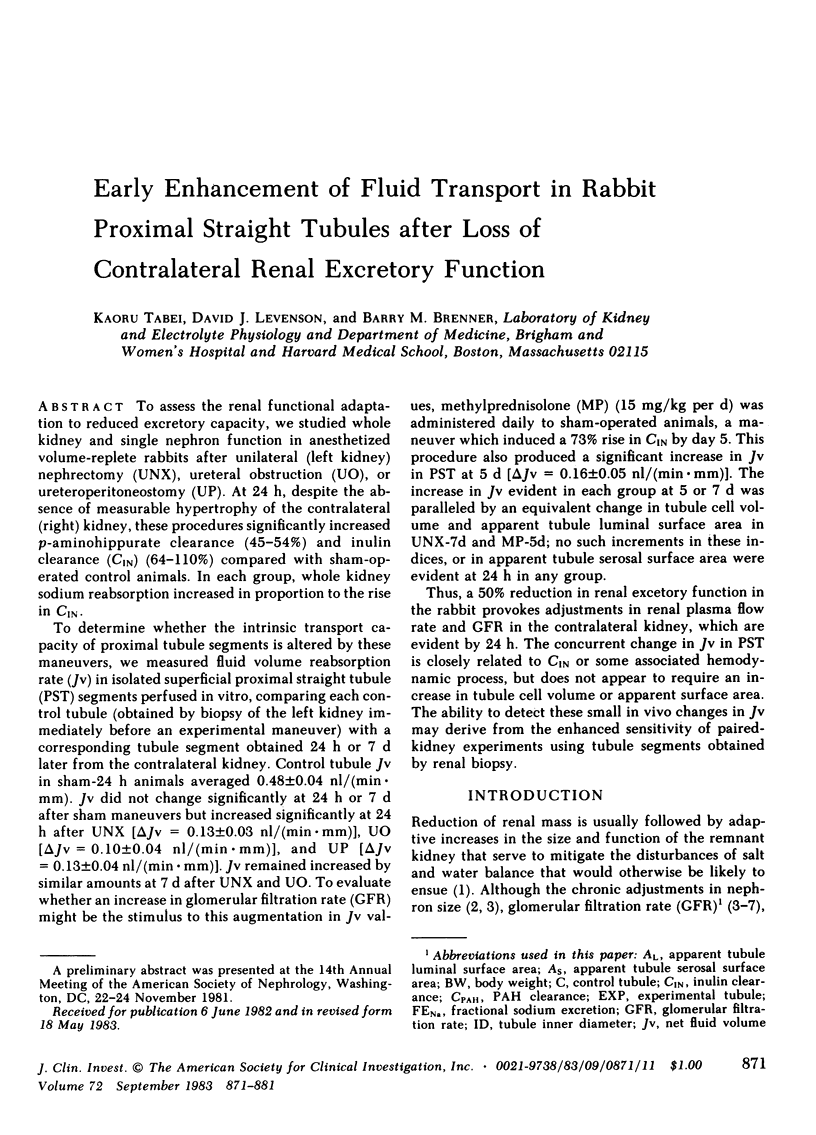






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Brenner B. M. Mechanism of the glucocorticoid-induced increase in glomerular filtration rate. Am J Physiol. 1978 Feb;234(2):F166–F170. doi: 10.1152/ajprenal.1978.234.2.F166. [DOI] [PubMed] [Google Scholar]
- Brazy P. C., McKeown J. W., Harris R. H., Dennis V. W. Comparative effects of dietary phosphate, unilateral nephrectomy, and parathyroid hormone on phosphate transport by the rabbit proximal tubule. Kidney Int. 1980 Jun;17(6):788–800. doi: 10.1038/ki.1980.91. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Control of fluid absorption in the renal proximal tubule. J Clin Invest. 1968 Sep;47(9):2016–2024. doi: 10.1172/JCI105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Maddox D. A., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am J Physiol. 1974 Sep;227(3):556–562. doi: 10.1152/ajplegacy.1974.227.3.556. [DOI] [PubMed] [Google Scholar]
- Diezi J., Michoud P., Grandchamp A., Giebisch G. Effects of nephrectomy on renal salt and water transport in the remaining kidney. Kidney Int. 1976 Dec;10(6):450–462. doi: 10.1038/ki.1976.132. [DOI] [PubMed] [Google Scholar]
- Emmanouel D. S., Lindehimer M. D., Katz A. I. Urinary concentration and dilution after unilateral nephrectomy in the rat. Clin Sci Mol Med. 1975 Dec;49(6):563–572. doi: 10.1042/cs0490563. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Trizna W., Bourgoignie J. J., Bricker N. S. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978 Jun;61(6):1508–1518. doi: 10.1172/JCI109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. Chemical aspects of compensatory renal hypertrophy. Cancer Res. 1965 Dec;25(11):1882–1887. [PubMed] [Google Scholar]
- Harris R. H., Best C. F. Circulatory retention of urinary factors as a stimulus to renal growth. Kidney Int. 1977 Nov;12(5):305–312. doi: 10.1038/ki.1977.117. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P. Functional adaptation to reduction in renal mass. Physiol Rev. 1979 Jan;59(1):137–164. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968 Apr;47(4):774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Mechanism of change in the excretion of sodium per nephron when renal mass is reduced. J Clin Invest. 1969 Jun;48(6):1002–1006. doi: 10.1172/JCI106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Burg M. B. Effect of parathyroid hormone on bicarbonate absorption by proximal tubules in vitro. Am J Physiol. 1979 Apr;236(4):F387–F391. doi: 10.1152/ajprenal.1979.236.4.F387. [DOI] [PubMed] [Google Scholar]
- Johnson H. A., Vera Roman J. M. Compensatory renal enlargement. Hypertrophy versus hyperplasia. Am J Pathol. 1966 Jul;49(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. Relation of glomerular filtration rate and sodium reabsorption to kidney size in compensatory renal hypertrophy. Yale J Biol Med. 1967 Dec;40(3):222–230. [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. M., DiMeola H. J., Siegel N. J., Lytton B., Kashgarian M., Hayslett J. P. Compensatory adaptation of structure and function following progressive renal ablation. Kidney Int. 1974 Jul;6(1):10–17. doi: 10.1038/ki.1974.72. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Burg M. B. Increased fluid absorption and cell volume in isolated rabbit proximal straight tubules after in vivo DOCA administration. Am J Physiol. 1981 Nov;241(5):F502–F508. doi: 10.1152/ajprenal.1981.241.5.F502. [DOI] [PubMed] [Google Scholar]
- Malt R. A. Compensatory growth of the kidney. N Engl J Med. 1969 Jun 26;280(26):1446–1459. doi: 10.1056/NEJM196906262802606. [DOI] [PubMed] [Google Scholar]
- Obertop H., Malt R. A. Lost mass and excretion as stimuli to parabiotic compensatory renal hypertrophy. Am J Physiol. 1977 May;232(5):F405–F408. doi: 10.1152/ajprenal.1977.232.5.F405. [DOI] [PubMed] [Google Scholar]
- Pabico R. C., McKenna B. A., Freeman R. B. Renal function before and after unilateral nephrectomy in renal donors. Kidney Int. 1975 Sep;8(3):166–175. doi: 10.1038/ki.1975.96. [DOI] [PubMed] [Google Scholar]
- Potter D. E., Leumann E. P., Sakai T., Holliday M. A. Early responses of glomerular filtration rate to unilateral nephrectomy. Kidney Int. 1974 Feb;5(2):131–136. doi: 10.1038/ki.1974.17. [DOI] [PubMed] [Google Scholar]
- Rous S. N., Wakim K. G. Kidney function before, during and after compensatory hypertrophy. J Urol. 1967 Jul;98(1):30–35. doi: 10.1016/S0022-5347(17)62817-9. [DOI] [PubMed] [Google Scholar]
- Sachtjen E., Rabinowitz L., Binkerd P. E. Renal concentrating ability in the uninephrectomized rat. Am J Physiol. 1977 Nov;233(5):F428–F437. doi: 10.1152/ajprenal.1977.233.5.F428. [DOI] [PubMed] [Google Scholar]
- Schultze R. G., Shapiro H. S., Bricker N. S. Studies on the control of sodium excretion in experimental uremia. J Clin Invest. 1969 May;48(5):869–877. doi: 10.1172/JCI106045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley D. G., Skinner J. Acute compensatory adaptation of renal function following contralateral kidney exclusion in Brattleboro rats with diabetes insipidus. J Physiol. 1978 Oct;283:425–438. doi: 10.1113/jphysiol.1978.sp012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Elkan I. O., Weerts C., Bricker N. S. Studies on the characteristics of the control system governing sodium excretion in uremic man. J Clin Invest. 1968 Mar;47(3):521–530. doi: 10.1172/JCI105748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toback F. G., Lowenstein L. M. Thymidine metabolism during normal and compensatory renal growth. Growth. 1974 Mar;38(1):35–44. [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Phospholipid metabolism in the initiation of renal compensatory growth after acute reduction of renal mass. J Clin Invest. 1974 Jul;54(1):91–97. doi: 10.1172/JCI107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizna W., Yanagawa N., Bar-Khayim Y., Houston B., Fine L. G. Functional profile of the isolated uremic nephron. Evidence of proximal tubular "memory" in experimental renal disease. J Clin Invest. 1981 Sep;68(3):760–767. doi: 10.1172/JCI110312. [DOI] [PMC free article] [PubMed] [Google Scholar]


