Abstract
High Mobility Group Box 1 (HMGB1) is a redox-sensitive molecule that plays dual roles in tissue healing and inflammation. We previously demonstrated that HMGB1 is stable when anchored by a well-characterized imidazolium-based ionic liquid (IonL), which serves as a delivery vehicle for exogenous HMGB1 to the site of injury and prevents denaturation from surface adherence. However, HMGB1 exists in different isoforms [fully reduced HMGB1 (FR), a recombinant version of FR resistant to oxidation (3S), disulfide HMGB1 (DS), and inactive sulfonyl HMGB1(SO)] that have distinct biological functions in health and disease. Thus, the goal of this study was to evaluate the effects of different recombinant HMGB1 isoforms on the host response using a rat subcutaneous implantation model. A total of 12 male Lewis rats (12–15 weeks) were implanted with titanium discs containing different treatments (n = 3/time point; Ti, Ti-IonL, Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S) and assessed at 2 and 14 days. Histological (H&E and Goldner trichrome staining), immunohistochemistry, and molecular analyses (qPCR) of surrounding implant tissues were employed for analysis of inflammatory cells, HMGB1 receptors, and healing markers. Ti-IonL-DS samples resulted in the thickest capsule formation, increased pro-inflammatory, and decreased anti-inflammatory cells, while Ti-IonL-3S samples demonstrated suitable tissue healing similar to uncoated Ti discs, as well as an upregulation of anti-inflammatory cells at 14 days compared to all other treatments. Thus, results from this study demonstrated that Ti-IonL-3S are safe alternatives for Ti biomaterials. Future studies are necessary to investigate the healing potential of Ti-IonL-3S in osseointegration scenarios.
Keywords: immunomodulation, subcutaneous, implant coating, inflammation
Graphical Abstract

1. INTRODUCTION
Titanium (Ti) orthopedic implantation is considered a relatively safe procedure to restore the function of damaged bones. However, the success of implants can be negatively affected by factors such as unregulated inflammatory response post-implantation, corrosion of the implant surface, infection in the surgical site, or even technical error during surgery that can lead to a lack of osseointegration and implant loosening.1–4 Patients have experienced failure rates between 4.9 and 14.4% after an average of 6.5 years post-implantation due to varying underlying pathologies.5 There is also an increased risk of implant failure when patients have compromised wound healing capabilities from disorders such as diabetes mellitus, which can negatively impact bone metabolism, growth factor expression, cartilage formation, and neurovascularization, often resulting in implant failure.6 Impaired bone healing can consequently lead to post-surgical complications in 25% of diabetic patients that undergo arthrodesis and osteotomy surgeries.7 Therefore, there is a significant need for multifunctional approaches targeting Ti surfaces that can address underlying mechanisms leading to implant failure at the cellular and molecular levels.
Various Ti surface modification approaches have been previously explored in an attempt to modulate osseointegration. For example, classic osteogenic factors (BMP-2 and BMP-7) have been incorporated into different carrier molecules and applied to titanium.8,9 However, the desired bioactivity of therapeutic proteins can be affected by protein adsorption onto implant surfaces, which can affect the protein interaction with anticipated host receptors.10 Without altering the bulk properties of Ti, a class of molten salts known as ionic liquids (IonL) have been increasingly investigated as multifunctional coatings for biomedical applications due to the tunability of the cation and anion structures that ultimately affect its physical, chemical, and biological properties. Gindri et al. proposed dicationic imidazolium-based IonLs bound to amino acid anions, which resulted in biocompatible Ti coatings.11 Further investigations of IonLs containing phenylalanine and methionine anions showed favorable properties as a Ti surface coating including anticorrosive, lubricative, antimicrobial, and biocompatible properties.11–14 It has been demonstrated that these specific formulations of IonLs do not interfere with normal Ti–tissue interactions and demonstrated a release profile in vivo within 14 days post-implantation in a subcutaneous animal model.14 Additionally, IonL cations and anions were shown to strongly bind to oppositely charged residues of the High Mobility Group Box 1 (HMGB1) protein in silico and in vitro.15 The purpose of this study is to further evaluate the host biological response of HMGB1 as immunomodulatory coatings that can be beneficial for constructive healing responses.
HMGB1 has been demonstrated as a key component in Ti osseointegration and bone healing post-trauma.16,17 However, HMGB1 is redox-sensitive and thus can be present in three different isoforms: “fully reduced HMGB1” (FR), “disulfide HMGB1” (DS), and inactive “sulfonyl HMGB1 (SO)”, which have distinct biological functions in health and disease.17,18 The anti-inflammatory FR is passively released by necrotic cells as a damage-associated molecular pattern (DAMP) yet can be oxidized by reactive oxygen species (ROS) in a pro-inflammatory environment and be converted into the disulfide isoform or inactivated into a sulfonyl isoform. Immunocompromised states such as cancer, diabetes mellitus, cardiovascular diseases, atherosclerosis, aging, etc., are all linked to increased ROS.19 The conversion of FR to DS results in the activation of pro-inflammatory pathways by binding to the receptor for advanced glycation end products (RAGE) and different types of Toll-like receptors (TLR), such as TLR4, which can negatively impact healing in chronic inflammatory environments.18,20 Recently, however, a recombinant fully reduced and non-oxidizable isoform of HMGB1 became commercially available (HMGBiotech, Milano, Italy) in which the three cysteines were replaced by serines (3-Serine HMGB1 (3S)), theoretically preventing oxidation from ROS.21
Although these IonL and HMGB1 isoform interactions have been previously characterized,15 the previous results were limited by the scope of in vitro and in silico models. There has been no investigation on how the presence of IonL-HMGB on Ti affects inflammation or early healing outcomes in vivo and the biological effects of using different HMGB1 isoforms in the new coating. Thus, the purpose of this study was to investigate different isoforms of HMGB1 as potential candidates for Ti immunomodulation using IonL as an anchoring thin film. To confirm the biocompatibility of the treatment, coated/uncoated Ti discs were implanted subcutaneously into the connective tissue of rats. This sterile approach is utilized to strictly assess the host response to each pre-immobilized HMGB1 isoform. Results from this study will provide insight into the most suitable isoform to be used on Ti surfaces, which will be evaluated in a more clinically relevant orthopedic osseointegration model. Considering that FR and 3S isoforms are involved in pro-healing activities by binding to CXCR4 receptors, while the DS isoform activates RAGE, thus increasing inflammatory response, it was hypothesized that inflammatory differences would be seen in the early healing response. It was also hypothesized that Ti-IonL-3S would maintain a biologically active conformation of HMGB1 and remain short-term in physiologically relevant conditions, with the potential to improve success rates. Following surgical implantation, the effect of Ti-IonL-HMGB1 on early healing was assessed using histological and molecular characterization to evaluate the impact of these treatments on healing.
2. METHODS
2.1. IonL Preparation.
1,10-Bis(3-methylimidazolium-1-yl)-decane diphenylalanine (IonL) was synthesized following the Fukumoto et al.22 and Shirota et al.23 methods and was characterized using established protocols.11,14,24,25 IonL was characterized using 1H nuclear magnetic resonance (NMR) spectroscopy (Bruker Avance III-HD 600 NMR, Bruker, Billerica, MA, USA), and the data found were in accordance with the literature.11,24,25
2.2. Sample Preparation.
Grade 5 titanium alloy (Ti6Al4V) discs (5 mm ⌀ × 2 mm, McMaster Carr, Elmhurst, IL, USA) were roughly polished with 240 grit silica paper and used as subcutaneous implants in this study. All discs were then cleaned by ultrasonication while sequentially immersed in acetone, DI water, and ethanol solutions for 15 min each. After sonication, discs were dried in an oven at 65 °C overnight and sterilized in an autoclave. Experimental samples receiving the IonL were dip coated in 50 mM ethanolic solution of IonL for 10 min. Samples were then removed from the solution at a constant rate of 60 μm/s with the assistance of a motorized stage (TA Instruments, New Castle, DE, USA) to create a uniform thin layer of IonL and then dried in an oven at 65 °C for 48 h to achieve a final dose of 0.1 μmol IonL on each disc after drying, based on the previous literature.14 Characterizations of IonL on titanium have been performed in previous studies.11,13,25–29 Samples receiving HMGB1 onto IonL were subsequently drop-coated with 2.5 μg of different isoforms of HMGB1 [disulfide (DS), fully reduced (FR), and non-oxidizable HMGB1 (3S); Tecan, AG, Switzerland] diluted in 2.5 μL of deionized water and dried for 1 h at room temperature. HMGB1 administration amount onto each disc was chosen based on previous murine studies.17 Uncoated discs were referred to as “Ti”, discs containing only IonL were referred to as “Ti-IonL”, and discs with HMGB1 and IonL were labeled as “Ti-IonL-DS”, “Ti-IonL-FR”, and “Ti-IonL-3S” for this study. For sterilization, Ti-IonL discs were placed under UV light for 1 h to maintain sample sterility before implantation and/or HMGB1 drop coating to avoid protein denaturation.
2.3. Animals.
The experimental groups consisted of 12 male Lewis Rats (Charles River Laboratories, Wilmington, MA, USA) that were 12–15 weeks old ranging from 250 to 350 g in weight. The rats were housed at the University of Texas at Dallas (Richardson, TX) vivarium throughout the study. Sterile water and dry food pellets were available to rats ad libitum. Experimental groups were separated by each treatment (Ti, Ti-Ion, Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S), as well as experimental time points (2 and 14 days after implantation: n = 6 per group).
2.4. Surgical Procedure.
Animals were weighed before each surgery to monitor body weight. The Lewis rats were anesthetized by inhalation of 4% isoflurane followed by injection of ketamine/xylazine (50 mg/kg: 20 mg/kg IP). After anesthesia, rats were placed in a ventral decubitus position. A 4 × 5 cm area of the rat dorsal side was shaved and cleaned using povidone-iodine prior to implantation. Three 2 cm incisions, avoiding fascia or underlying muscle, were made down the sagittal plane of the rat’s subcutaneous tissue approximately within 8 cm from the base of the head and tail on either side. Dissecting scissors were then inserted under the skin on the left and right sides of each incision, and connective tissues were resented away from the fascia, creating pouches of approximately 4 × 2 cm on either side of the incisions to place the implants. Each rat had a non-surgery control and received a total of five implants with the respective treatment (Ti, Ti-IonL, Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S) arbitrarily placed on either the left or the right side of the incision. The remaining sections of resected tissue without implants placed underneath the skin on the same rat were considered surgery shams, and non-resected subcutaneous tissue adjacent to the implant site was considered control tissue for baseline analysis. After implantation, the surgeon applied light pressure to the areas of resected tissue to help encourage wound closure. The incision was closed using resorbable sutures (5–0 Coated Vicryl Undyed 1X27″ RB-1). Following surgery, animals were given a subcutaneous injection of 20 mg/kg of lidocaine with 1:100,000 epinephrine (Quala Dental Products, Nashville, TN, USA) for analgesia. Animals were sacrificed at the end of the experimental periods (2 and 14 days) with previously mentioned anesthesia followed by an overdose of pentobarbital sodium (Euthanasia III Med-Pharmex Inc., Pomona, CA, USA). Each tissue section containing a disc as well as sham and control tissue was removed from the animal after sacrifice and placed in either 10% neutral buffered formalin (NBF) for histological analysis or snap frozen in dry ice for molecular analysis. All procedures were carried out with supervision and approval from the Institutional Animal Care and Use Committee (IACUC #19–03).
2.5. Histological Sample Processing.
Tissue samples collected after sacrifice were immediately placed in 10% NBF for 24 h for fixation. After fixation, they were continuously rinsed in running tap water for 24 h to prevent overfixation and subsequently placed in 70% ethanol until processing. Samples were cut down to 10mm2 sections of tissue surrounding the capsule area or selected region of interest of each and then processed for 12 h using a tissue processor (Leica ASP300 S). During processing, tissues underwent a sequence of dehydration steps in ethanol (70, 90, 100%) followed by a clearing step in xylene prior to paraffin wax infiltration. While the samples were immersed in xylene, an incision was made into the capsule containing each disc, which was carefully removed to avoid tissue damage, leaving a small pocket. Tissue samples were dissected down the middle of the pocket and mounted in paraffin. Twenty 5 μm histological sections (technical replicates) from the central region of the titanium implantation site were made per biological replicate to allow technical replicates of the capsule for standard H&E stain for histomorphometry, selecting the best three technical replicates for counting. The remaining sections in each sample were used for immunohistochemistry. Histopathological/histomorphometric analysis of H&E and immunohistochemistry followed previously established procedures.30
2.6. Quantitative Analysis of Capsule Thickness.
Tissues were stained using Goldner’s trichrome to visualize and analyze the fibrous thickness surrounding the implant using Cellsens software (Olympus, Shinjuku City, Tokyo, Japan).31 The mean capsule thickness was determined based on a previously established method using orthogonal intercepts of fibrous tissues.31,32 Each tissue section contained three technical replicates (sections) and was evaluated using the mean of six non-overlapping histological fields per section of each biological replicate. Data from mean fibrous capsule thicknesses were analyzed for statistical significance, and the results were presented as mean ± standard deviation (SDs).
2.7. Histopathological and Histomorphometric Analysis.
Macroscopic histopathological analysis was performed to identify and quantify the blood vessels, inflammatory cells, and maturation of connective tissue (fibers and fibroblasts) to determine possible differences between treatments. For H&E sections of each sample, technical replicates comprised six 173.4 μm × 130.1 μm histological fields. Regions of interest were selected in the capsule areas adjacent to the Ti disc space. Images were observed and captured using a 40× air objective (Olympus VS120, Shinjuku, Tokyo, Japan). A grid image was superimposed on each field, with nine horizontal and 12 vertical lines, which created 108 points in a quadrangular area. Quantification of histopathological analysis was conducted using ImageJ software (Version 1.51, National Institutes of Health, Bethesda, MD, USA).
2.8. Immunohistochemistry.
Immunohistochemistry was used to identify and quantify subpopulations of pro-inflammatory (M1) (CD86) and anti-inflammatory (M2) (CD163) macrophages, relevant DAMP receptor HMGB1, and chemokine receptor (CXCR4) in surrounding implant tissues. Sections were first deparaffinized and submerged for antigen retrieval in citrate buffer at pH 6.0 sustained at 95 °C for 30 min. After washing steps with 1× PBS and deionized (DI) water, tissue samples were blocked with 1% bovine serum albumin (1% BSA, in 1× phosphate-buffered saline, Sigma-Aldrich, St. Lous, MO, USA) and incubated with the selected primary antibody. Primary antibodies were obtained from Abcam and diluted in the following concentrations: Cd86 (anti-Cd86, 1:100, rabbit polyclonal (PIPA 588284) Invitrogen, Carlsbad, CA, USA), Cd163 (anti-CD163, 1:500 Rabbit polyclonal (ab182422), Abcam, Cambridge, UK), HMGB1 (anti-HMGB1, 1:400, rabbit monoclonal (ab79823), Abcam, Cambridge, UK), and Cxcr4 (anti-Cxcr4, 1:250, mouse monoclonal (sc53534), Santa Cruz Biotechnology, Dallas, TX, USA). Samples were incubated overnight in a humidified chamber with each primary antibody at 4 °C. Primary antibodies from Abcam were subsequently incubated with rabbit-specific HRP/DAB (ABC) and Micropolymer Detection IHC Kit (Abcam, Cambridge, UK). A minimum of three technical replicates from each sample were stained with each marker. Additionally, a negative control was incubated with 1% bovine serum albumin in 1× PBS (Sigma-Aldrich, St. Louis, MO, USA) instead of a primary antibody to confirm specific binding of the secondary antibody. Slides were then washed with 1× PBS and incubated for 10 min with a hydrogen peroxide solution. Slides were then washed 3 times in 1× PBS and incubated with the Micropolymer Abcam IHC kit. Finally, slides were incubated for 1 min with 3,3′- diaminobenzidine (DAB) chromogen, counterstained in Mayer’s hematoxylin for 2 min, and mounted with Permount (Fisher Scientific, Hampton, NH, USA) and a coverslip. Positively (+) stained cells for each marker were counted using the same technique employed with H&E-stained sections in ImageJ software. Positively stained cells were quantified, and the total number of points was obtained to calculate the area density (%) for each marker.
2.9. Molecular Analysis.
Snap-frozen samples from surgery were used for gene expression analysis. RNA of all samples was isolated using the RNeasy FFPE Mini-kit (Qiagen, Hilden Germany) following the manufacturer’s instructions, and the RNA concentration was verified using a spectrophotometer (NanoDrop 200, Fisher Scientific, Hampton, NH, USA). After isolation, cDNA synthesis was performed using qScript cDNA Supermix (QuantaBio, Beverly, MA, USA), and cDNA reaction products were purified with the Qiaquick Purification Kit (Qiagen, Hilden Germany). qRT-PCR was performed with cDNA and TaqMan single tube assays (Applied Biosciences, Foster City, CA, USA) to quantify genes involved in M1 (Cd80, Nos2, Tnf) or M2 polarization (Cd163, Cxcl12, Il10), as well as receptors for HMGB1 isoforms (Ager, Cxcr4) using 15 ng/μL of cDNA per reaction. Each sample reaction was performed in duplicate and contained a gDNA and reverse transcription control to confirm that there was only template-specific amplification. Data analysis was performed using ΔΔCt to compare each marker of interest with two housekeeping genes (B2M, Hprt), determining fold changes in treatment groups relative to a non-surgery control.
2.10. Statistical Analysis.
Statistical analysis of capsule thickness, histomorphometry in H&E, and IHC was performed using a two-way analysis of variance (ANOVA) with a post hoc Tukey test to evaluate the significance considering time and treatments (Ti, Ti-IonL, Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S) as factors. Analysis was run in GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA) using a significance level (α) of 0.05. Statistical significance of comparisons between different data sets was determined using the p value.
3. RESULTS
3.1. Clinical and Capsule Thickness Evaluation.
Rats exhibited no signs of hyperalgesia, with normal grooming, eating, and nesting behavior after the procedure. No infection, excessive swelling, or redness was noted at incisions or implantation sites 24 h post-surgery. Upon sacrifice and sample collection, macroscopic evaluation evidenced a thin membrane encapsulating all samples at 2 and 14 days, which were measured as previously described. Microscopic evaluation of tissues revealed residual blood clots for all samples at 2 days, with evidence of blood vessel formation at 14 days, as shown in Figure 1A. Quantitative analysis of capsules demonstrated that Ti-IonL-DS coated discs formed significantly thicker capsules (151.7 ± 34.1 μm) compared to all other treatment groups (Ti: 99.1 ± 12.9 μm, Ti-IonL: 70.8 ± 5.6 μm, Ti-IonL-FR: 76.3 ± 3.4 μm, and Ti-IonL-3S: 65.7 ± 4.4 μm) at 14 days post-implantation (Figure 1B).
Figure 1.

Capsule thickness of subcutaneous tissue representing a healing panel of uncoated (Ti) and coated [ionic liquid (Ti-IonL), disulfide HMGB1(Ti-IonL-DS), fully reduced HMGB1 (Ti-IonL-FR), and non-oxidizable HMGB1 (Ti-IonL-3S)] at 2 and 14 days post-implant placement. Scale bar = 50 μm and 200 μm, staining: GT (A). Quantitative results of capsule thickness over time are presented as mean ± SD for each parameter at implantation sites during 2 and 14 days (B). The asterisk symbol indicates statistical significance between implant treatments within the same time point (n = 3; p < 0.05).
3.2. Histological Analysis.
Microscopic evaluation of tissues stained with H&E revealed residual blood clots that created an initial provisional matrix formed by a fibrin network for all samples at 2 days (Figure 2A). The area density % of blood vessel formation was significantly higher for Ti-IonL-3S treated groups (10.8 ± 2.0) compared to the relatively consistent formation seen in other treatments (3.8–7.2 average area density %) at 14 days (Figure 2B). Tissues treated with Ti-IonL-DS discs presented significantly increased inflammatory cells (shown by positive purple staining in Figure 2A) at the 2 day time point (42.0 ± 14.6 area density %) compared to other treatment groups (14.4–21.8 average area density %) (Figure 2B). It was also noted that there were increased inflammatory cells in tissues treated with Ti-IonL-DS discs, which were seen at 14 days (p > 0.05). Connective tissue (fibers and fibroblasts) was consistent among all treatment groups, with newly formed connective tissue surrounding the Ti space after 14 days (Figure 2 A,B).
Figure 2.
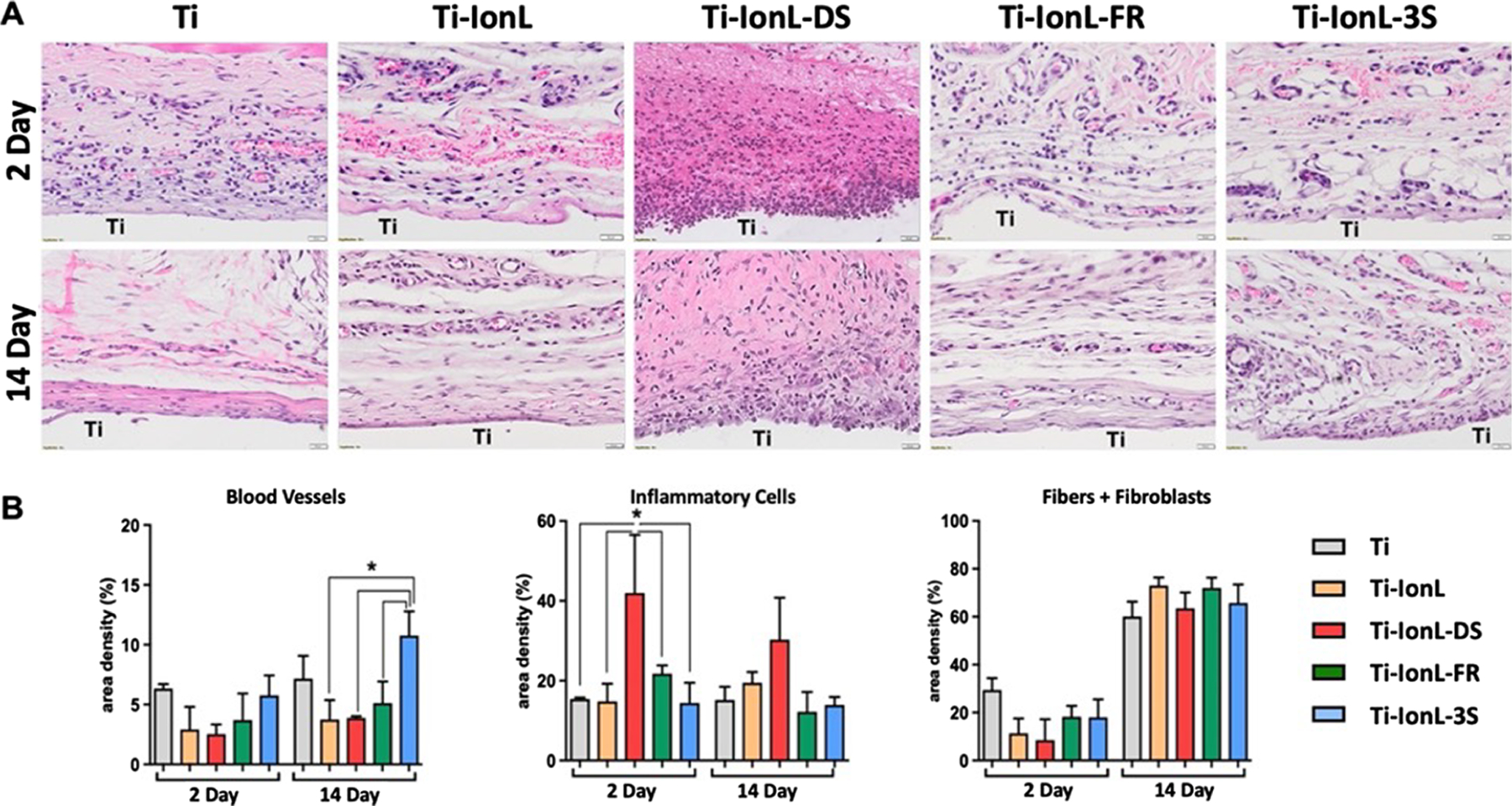
Histological evaluation of subcutaneous implantation sites representing a healing panel of uncoated (Ti) and coated [ionic liquid (Ti-IonL), disulfide HMGB1(Ti-IonL-DS), fully reduced HMGB1 (Ti-IonL-FR), and non-oxidizable HMGB1 (Ti-IonL-3S)] at 2 and 14 days post-implant placement. Scale bar = 20 μm, staining: H&E, original magnification 40× (A). Histomorphometry connective tissue parameters (blood vessels, inflammatory cells, and fibers + fibroblasts) over time (B). Quantitative results are presented as mean ± SD for each parameter at implantation sites during 2 and 14 days. The asterisk symbol indicates statistical significance between implant treatments within the same time point (n = 3; p < 0.05).
3.3. Immunohistochemistry Analysis.
Immunohistochemistry was used to quantify the presence of macrophages (CD86 was used as an M1 marker and CD163 as an M2 marker). The cell counts for CD86+ and CD163+ cells were similar at 2 days (mean average 8.6–10.1 area density %) for tissues surrounding both Ti and Ti-IonL discs. However, all tissues surrounding HMGB1 samples had increased quantities of the pro-inflammatory CD86+ cells at 2 days with a decreasing trend from Ti-IonL-DS (39.9 ± 14.3%) to Ti-IonL-FR (34 ± 5.1%) and then to Ti-IonL-3S (25.4 ± 8.0%) (Figure 3A,B). All quantities of CD86+ cells subsided by 14 days, yet Ti-IonL-DS treated tissues were the highest (24.2 ± 10.0%) compared to all other treatment groups (mean average 9.9–15.7 area density %). Considering anti-inflammatory CD163+, the quantities seen were significantly lower at 2 days for the Ti-IonL-DS group (7.3 ± 1.5%) compared to all other coated groups (mean average 19.7–25.4 area density %; p ≤ 0.05). All samples demonstrated similar behavior of CD163+ macrophages by 14 days. Nevertheless, Ti-IonL-DS (7.3 ± 1.5%) treated samples resulted in notably lower quantities of CD163+ cells, while Ti-IonL-FR (14.9 ± 3.7%) and Ti-IonL-3S (12.4 ± 1.7%) samples resulted in higher amounts compared to Ti (7.8 ± 3.2%) and Ti-IonL (8.4 ± 2.7%) samples at 14 days. Overall, Ti-IonL-DS groups resulted most populous in CD86+ pro-inflammatory macrophages, and the least in CD163+ anti-inflammatory macrophages over time, compared to other treatment groups (Figure 3A,B).
Figure 3.

Immunohistochemistry (IHC) of macrophage surface markers (CD86 and CD163) of subcutaneous tissue surrounding Ti and coated [ionic liquid (Ti-IonL), disulfide HMGB1(Ti-IonL-DS), fully reduced HMGB1 (Ti-IonL-FR), and non-oxidizable HMGB1 (Ti-IonL-3S)] discs at 2 days post-implant placement (A). Dark cells: positive labeling. Scale bar: 20 μm, original magnification 40×, counterstaining Mayer’s hematoxylin, chromogen DAB. Quantification of markers identified by immunohistochemical analysis is shown as means ± SD for area density (%) at the implant site (* = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001) between treatments (n = 3) (B). Quantitative results are presented as mean ± SD for each parameter at implant sites after 2 and 14 days.
Immunohistochemistry was also performed to quantify the presence of HMGB1 and the HMGB1 receptor CXCR4 at 2 and 14 days. At 2 days, HMGB1 present within tissues was particularly higher in Ti-IonL-HMGB1 samples [Ti-IonL-FR (4.2 ± 0.9%), Ti-IonL-DS (3.9 ± 1.5%), and Ti-IonL-3S (3.9 ± 1.4%)], compared to Ti (1.6 ± 1.1%) or Ti-IonL (2.4 ± 0.9%) treated samples (Figure 4A,B). However, at 14 days, Ti-IonL-DS samples remained consistently higher than all other treatment groups (3.3 ± 0.9%) and was significantly higher than the least populous Ti-IonL-3S samples (0.3 ± 1.7%). A similar trend was seen between HMGB1+ and CD86+ cells at 14 days; Ti-IonL-DS had the highest area density %, followed by similar counts between Ti-IonL and Ti-IonL-FR, followed by Ti and Ti-IonL-3S treated tissues (Figure 4A,B). Conversely, CXCR4 cells surrounding Ti-IonL-DS samples were quantified as the smallest area density % at 2 days in comparison to all other treatment groups. Ti-IonL-DS samples (3.3 ± 2.2%) were significantly lower than Ti-IonL-FR (17.0 ± 4.2%; p ≤ 0.0001) and Ti-IonL-3S samples at 2 days (12.1 ± 1.1%; p ≤ 0.01). Ti-IonL-FR samples were also significantly higher than Ti (9.6 ± 1.3%; p ≤ 0.05) and Ti-IonL (8.4 ± 4.6%; p ≤ 0.01) samples. At 14 days, the CXCR4 density of all samples was reduced to quantities similar to non-coated Ti samples (0.3 ± 0.4%; p > 0.05) (Figure 4A,B).
Figure 4.

Immunohistochemistry (IHC) of HMGB1 and CXCR4 in subcutaneous tissue surrounding Ti and coated [ionic liquid (Ti-IonL), disulfide HMGB1(Ti-IonL-DS), fully reduced HMGB1 (Ti-IonL-FR), and non-oxidizable HMGB1 (Ti-IonL-3S)] discs at 2 days post-implant placement (A). Dark cells: positive labeling. Scale bar: 20 μm, original magnification 40×, counterstaining Mayer’s hematoxylin, chromogen DAB. Quantification of markers identified by immunohistochemical analysis is shown as means ±SD for area density (%) at the implant site (* = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001) between implant treatments (n = 3) (B). Quantitative results are presented as mean ± SD for each parameter at implantation sites during 2 and 14 days.
3.4. qPCR.
qPCR was performed to analyze eight markers representative of the upregulation of M1 (Cd80, Nos2, and Tnf) and M2 (Cd163, Cxcl12, and Il10) macrophages and DS (Ager) and FR (Cxcr4) receptors compared to non-surgery controls (Figure 5). At 2 days, uncoated samples displayed an upregulation of M2 markers (Cd163 and Cxcl12) and a downregulation of pro-inflammatory markers (Nos2 and Tnf) and HMGB1 receptor markers (Ager and Cxcr4) compared to non-surgery controls. From 2 to 14 days, gene expression remained constant for Cd80, Nos2, Tnf, Ager, and Cxcr4 markers and was downregulated for Cd163, Cxcl12, and Il10 for Ti samples. Ti-IonL samples had an upregulation of all M1 macrophage markers (Cd80, Nos2, and Tnf), Il10, and HMGB1 receptor markers (Ager and Cxcr4) and a downregulation of M2 markers (Cd163 and Cxcl12) at 2 days that subsided at 14 days compared to non-coated Ti samples. Considering the response of Ti-IonL-HMGB1 samples (Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S) to Ti samples, the fold change in expression was similar for Cd80, Nos2, and Il10. The response of Ti-IonL-HMGB1 samples was upregulated for Tnf (for only Ti-IonL-DS and Ti-IonL-FR), Ager (only Ti-IonL-3S), and Cxcr4 (only Ti-IonL-DS) while significantly downregulated for Cd163 (Ti-IonL-DS, Ti-IonL-FR; p ≤ 0.01, and Ti-IonL-3S; p ≤ 0.0001), Cxcl12 (Ti-IonL-DS; p ≤ 0.01, and Ti-IonL-FR; p ≤ 0.001), and Cxcr4 (Ti-IonL-FR and Ti-IonL-3S; p ≤ 0.05) (Figure 5). At 14 days, all treated samples demonstrated a downregulation of gene expression compared to Ti samples except for Ti-IonL-3S samples that had significantly higher expression of anti-inflammatory markers Cd163 (p ≤ 0.01) and Cxcl12 (p ≤ 0.0001) compared to Ti samples (Figure 5).
Figure 5.

Fold change of inflammatory and wound healing gene expression in Ti and coated [ionic liquid (Ti-IonL), disulfide HMGB1(Ti-IonL-DS), fully reduced HMGB1 (Ti-IonL-FR), and non-oxidizable HMGB1 (Ti-IonL-3S)] discs at 2 and 14 days post-implant placement, relative to a non-surgery control. Quantification of expression is shown as means ±SD for area density (%) at the implant site (* = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001) between treatments (n = 3). Quantitative results are presented as mean ± SD for each parameter at implantation sites during 2 and 14 days.
4. DISCUSSION
Considering the increasing number of aging patients that have underlying conditions and will require implants, it is imperative that the inflammatory microenvironment be geared toward a favorable healing response for successful integration of implant systems;33 this is crucial to achieving both mechanical stability and fracture healing.34 Osseointegration involves initial protein adsorption on the implant surface, which triggers migration, proliferation, and differentiation of key cells related to bone healing (i.e., pre-osteoblasts and M2 macrophages).16,35,36 Current Ti modifications have utilized changes in topography, wettability, and coating approaches (ceramic, synthetic, or peptide coatings) to improve tissue response in the initial steps of inflammation and cell differentiation, ultimately improving tissue healing and osseointegration outcomes.37,38 However, complications from tissues in response to various approaches have motivated the drive to improve the predictability of implanted biomaterials. Peptide coatings have been used to functionalize surfaces and harness the immune system.39 Thus, a well-characterized, multifunctional IonL11,13,14,25–29,40 was used as a method to immobilize key immunomodulatory proteins, such as HMGB1, which was previously studied in vitro and in silico by our group.15,41 However, the behavior of this coating after in vivo implantation is still not well known.
Subcutaneous implantation models are relatively simple surgeries used to test the host response to different biomaterials, coatings, biocompatibility, or drug screening.42 In this study, we used a previously established subcutaneous model of Ti disc implantation in Lewis rats14 to characterize and evaluate the healing response of IonLs as a protein anchoring/delivery coating of different HMGB1 isoforms. The preceding model demonstrated that the selected IonL was biocompatible by not interfering with the foreign body response in comparison to an uncoated Ti control. This model also verified that the IonL was eluting into surrounding tissues and/or resorbed by macrophages by the 14 day time point.14 Although the area density % values differ between this study and previous results,14 we observed similar characteristics and trends [in terms of capsule thickness (Figure 1), histomorphometry (Figure 2), and immunohistochemical staining (Figures 3 and 4)] between the control Ti and the Ti-IonL discs. Considering that the main goal of this study was to evaluate the HMGB1 isoforms on the IonL thin film, both Ti-IonL and uncoated Ti discs were used as control groups.
Upon implantation, the innate immune system is activated, which is characterized by an influx of blood clots and inflammatory cells (primarily neutrophils and monocytes) to the site of injury (Figures 1 and 2). Inflammatory cells then subside, and macrophages (differentiated from monocytes) become predominant, ingest the foreign material, and recruit other cell types (such as fibroblasts) to aid in wound healing.43 In our study, we observed that during later stages of the immune response, new blood vessels and densely packed connective tissue (comprising fibers and fibroblasts) are formed, creating a fibrous capsule surrounding the implanted biomaterial in both control groups (Ti and Ti-IonL) (Figures 1 and 2).
Previous in vitro and in silico research from our lab validated that the IonL used in this study could pre-immobilize HMGB1 in a biologically active conformation.15 Considering the different HMGB1 coating formulations, Ti-IonL-DS samples demonstrated the thickest fibrous encapsulation after 14 days compared to all other treatment groups (Figure 1). Correspondingly, histological results showed an increased area density % of inflammatory cells for Ti-IonL-DS samples at both 2 and 14 days and demonstrated that these tissues were potentially exposed to a particular irritant (Figure 2) compared to all other treatment groups. These results are reasonable findings because DS promotes the pro-inflammatory cytokine and chemokine expression in macrophages due to binding with TLR4 or the receptor for advanced glycation end products.21,44 Additionally, DS has no action on the CXCL12/CXCR4 pathway (Figure 5) but triggers pro-inflammatory effects by binding the receptor for advanced glycation end products (RAGE) and different types of Toll-like receptors (TLR), which can mitigate any beneficial effects, thus failing to recruit CXCR4 to the site of healing.18,20 Upon cellular trauma, the pro-inflammatory environment also creates oxidative stress that activates RAGE and induces NADPH oxidase, thus increasing ROS at the site of injury.45,46 HMGB1 that is released by necrotic cells is secreted in an FR isoform and becomes exposed to increased ROS that oxidize the redox-sensitive protein into a dimerized DS isoform, which further induces the pro-inflammatory response,47 as demonstrated through Tnf expression at 14 days (Figure 5). However, secreted FR is involved in the recruitment mechanisms of macrophages and mesenchymal stem cells to the site of injury in the acute inflammatory response, thus contributing to tissue repair and healing.16,17,48 Macrophages are phagocytic cells that recognize components from the adherent protein layer on the titanium surface.49 In optimal conditions, activated mesenchymal stem cells interact with macrophages to maintain homeostasis of the inflammatory microenvironment, which consists of a controlled balance of pro-inflammatory (M1) and anti-inflammatory (M2) macrophage phenotypes. In this study, there was an increase in positive staining of M1 marker CD86 in all HMGB1 samples (Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S), indicating the impact of exogenous HMGB1 in the recruitment of pro-inflammatory cells to the injury site (Figure 3). This recruitment could have likely been stimulated by the induction of tumor necrosis factor (TNF) production (a cytokine involved in inflammation, cell proliferation, and healing),50 which was upregulated for Ti-IonL, Ti-IonL-DS, and Ti-IonL-FR coated samples compared to uncoated samples (Figure 5). However, considering that the pro-inflammatory CD86+ cells resolved for all samples except for Ti-IonL-DS at 14 days suggests that Ti-IonL-DS samples experienced chronic inflammation in response to the coating. This finding is in accordance with disproportionate inflammation in chronic wounds corresponding to an excess of immune cell infiltration, which prevents resolution.51
Quantities of HMGB1-positive cells resulted in similar trends for CD86+ cells at both 2 and 14 days (Figure 4). Increased HMGB1+ staining for HMGB1-coated samples (Ti-IonL-DS, Ti-IonL-FR, and Ti-IonL-3S) at 2 days could have been attributed to the exogenously administered HMGB1 being absorbed into the tissues. However, the high positive staining of HMGB1 in Ti-IonL-DS coated samples at 14 days could have resulted from the inhibition of alarmins (endogenous molecules that are released upon tissue damage and activate the immune system), leading toward chronic inflammatory events.17 After endogenous HMGB1 is released by the necrotic cells at the site of injury, it can remain in tissues for about 72 h, inducing inflammatory or healing responses.52 Previous in vitro studies showed that HMGB1 was not detectable in the cell medium after 48 h.53 In vivo studies also demonstrated that HMGB1 reached peak levels in serum between 2 and 6 h post-injury, indicating that HMGB1 is important in the early inflammatory response.54 Compared to our exogenous isoforms, we believe that the protein remained less than 48 h, considering that we only observed HMGB1+ staining in the cell nuclei and cytoplasm (the cellular compartments for endogenous HMGB1). However, it is reasonable to hypothesize that the addition of exogenous HMGB1 may have induced a higher expression of endogenous HMGB1 and thus may have influenced the results of HMGB1 staining (Figure 4) and gene expression (Figure 5) in the groups with isoforms.
Conversely during resolution, all treated samples had a similar trend in positive staining of the anti-inflammatory M2 marker CD163 (Figure 3) and the HMGB1 receptor CXCR4 (Figure 4) in comparison to Ti-IonL-DS samples, demonstrating the lowest/slowest shift toward resolution for Ti-IonL-DS samples at 2 days (Figure 3). CXCR4 is a chemokine receptor that is expressed in multiple cell types with migratory and/or repair function, including monocytes/macrophages (i.e., M2 phenotypes).55,56 Necrotic cells release HMGB1 in a fully reduced isoform (FR) that binds to CXCL12, forming a heterodimer that then binds to CXCR4 present in stem cells and certain immune cells, such as monocytes/macrophages.17 Previous observations have shown that the systemic inhibition of FR impaired osseointegration in mice by affecting macrophage polarization and stem cell migration.16 Other studies demonstrated that the direct exogenous administration of FR and 3S to the osteotomy sites of mice accelerated bone healing by improving cell homing and proliferation of mesenchymal stem cells and immune cells through the chemotactic HMGB1/CXCL12/CXCR4 axis.17,57 In our studies, we observed a significant increase in blood vessels seen in Ti-IonL-3S samples at 14 days, compared to all other treatment groups (Figure 2). Similarly, there was also a significant increase in Cd163 and Cxcl12 expression at 14 days (Figure 5). It is important to note that angiogenesis allows for the delivery of oxygen and nutrients, which could positively impact healing.58 Although the fold change in expression for M2 markers was not consistent with immunohistochemical results at 2 days, it is important to note that there was an upregulation of Cd163 and Cxcl12 seen for Ti-IonL-3S samples at 14 days compared to other samples (Figure 5), suggesting a resolution of inflammation.50,59,60
As with any in vivo study, this study had limitations. This model employs subcutaneous tissues as implantation sites in rats, creating a large surface area in contact with the host tissues, which is effective in assessing biocompatibility and inflammatory responses.43 Ti implants are typically used in oral or osseous environments, so it is important to consider that the inflammatory microenvironment can be negatively affected by surface properties, corrosion, implant instability, infection, or biological factors (age, gender, underlying conditions, bacteria, etc.).30,61,62 However, the biological role of an HMGB1 coating itself could have similar inflammatory responses in subcutaneous and osseous tissues.16 Within the limitations of the present study, this study demonstrated the feasibility that both Ti-IonL-3S and Ti-IonL-FR coatings can trigger inflammation that led toward resolution and healing. Although the coatings resulted in similar effects to Ti, it is important to note that there was an increase in blood vessels for Ti-IonL-3S treated tissues at 14 days (Figure 2), which becomes important for cell homing and differentiation. Additionally, there was an increase in the area density % of CD163+ (Figure 3) and CXCR4+ (Figure 4) for tissues treated with Ti-IonL-FR and Ti-IonL-3S at 2 days, which demonstrated signs of resolution. Furthermore, the increased fold change expression for Cd163 and Cxcl12 (Figure 5) at 14 days further implies that both Ti-IonL-3S and Ti-IonL-FR coatings can trigger inflammation that led toward resolution and healing. Taking into account the failure rates of Ti orthopedic implants in diabetic patients, it is imperative that different approaches be considered for this population. With the known beneficial potential of HMGB1, it is predicted that these formulations could be valuable in a diabetic environment where healing is impaired. Future studies should focus on a more relevant osseointegration model to assess its effectiveness in promoting a constructive healing response.
5. CONCLUSIONS
Our findings suggest that HMGB1 isoforms led to different healing responses when combined with our IonL coating. While DS-HMGB1 resulted in a detrimental effect, the Ti-IonL-3S and Ti-IonL-FR coatings presented a modulatory response toward healing, with promising results when compared to pure Ti implants. Based on FR and 3S-IonL coating results, future studies are necessary to investigate the beneficial effects on environments where inflammation resolution is challenging or in conditions where there is a lack of signaling molecules for CXCR4-positive cells. In summary, our study shows the feasibility and potential application of FR and 3S-IonL coatings to improve early implant integration.
■. ACKNOWLEDGMENTS
The authors would like to acknowledge the support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK/NIH) F31 fellowship number F31DK121483–01. This project was also supported by the University of Texas at Dallas (UTD) Office of Research through a seed grant, Collaborative Biomedical Research Award (CoBRA).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acsbiomaterials.3c00367
Notes
The authors declare no competing financial interest.
Contributor Information
Alexandra Arteaga, Department of Bioengineering, The University of Texas at Dallas, Richardson 75080-3021 Texas, United States.
Claudia Cristina Biguetti, Department of Surgery and Biomechanics, School of Podiatric Medicine, The University of Texas Rio Grande Valley, Harlingen 78539 Texas, United States.
Bhuvana Lakkasetter Chandrashekar, Department of Bioengineering, The University of Texas at Dallas, Richardson 75080-3021 Texas, United States.
Jimena Mora, Department of Bioengineering, The University of Texas at Dallas, Richardson 75080-3021 Texas, United States.
Adeena Qureshi, Department of Bioengineering, The University of Texas at Dallas, Richardson 75080-3021 Texas, United States.
Danieli C. Rodrigues, Department of Bioengineering, The University of Texas at Dallas, Richardson 75080-3021 Texas, United States
■ REFERENCES
- (1).Arteaga A; Qu J; Haynes S; Webb BG; LaFontaine J; Rodrigues DC Diabetes as a Risk Factor for Orthopedic Implant Surface Performance: A Retrieval and In Vitro Study. J. Bio Tribocorros. 2021, 7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mihalko WM Retrieval Studies in Orthopaedic Surgery: Editorial Comment: Learning Every Implant’s Story. Clin. Orthop. Relat. Res. 2012, 470, 1803–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Weinstein Allan; Horowitz Emanuel; Ruff Arthur W. Retrieval and Analysis of Orthopaedic Implants. DOI: 10.6028/NBS.SP.472. [DOI] [Google Scholar]
- (4).Eliaz N Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ciampolini J; Hubble MJW Early Failure of Total Hip Replacements Implanted at Distant Hospitals to Reduce Waiting Lists. Ann. R. Coll. Surg. Engl. 2005, 87, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wukich DK Diabetes and Its Negative Impact on Outcomes in Orthopaedic Surgery. World J. Orthop. 2015, 6, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shibuya N; Humphers JM; Fluhman BL; Jupiter DC Factors Associated with Nonunion, Delayed Union, and Malunion in Foot and Ankle Surgery in Diabetic Patients. J. Foot Ankle Surg. 2013, 52, 207–211. [DOI] [PubMed] [Google Scholar]
- (8).Kashiwagi K; Tsuji T; Shiba K Directional BMP-2 for Functionalization of Titanium Surfaces. Biomaterials 2009, 30, 1166–1175. [DOI] [PubMed] [Google Scholar]
- (9).Wang J-J; Xue Q; Wang Y-J; Zhang M; Chen Y-J; Zhang Q Engineered Chimeric Peptides with IGF-1 and Titanium-Binding Functions to Enhance Osteogenic Differentiation In Vitro under T2DM Condition. Materials 2022, 15, 3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Thevenot P; Hu W; Tang L Surface Chemistry Influences Implant Biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Gindri IM; Siddiqui DA; Bhardwaj P; Rodriguez LC; Palmer KL; Frizzo CP; Martins MAP; Rodrigues DC Dicationic Imidazolium-Based Ionic Liquids: A New Strategy for Non-Toxic and Antimicrobial Materials. RSC Adv. 2014, 6, 62594. [Google Scholar]
- (12).Sandhu PPK; Gindri IM; Siddiqui DA; Rodrigues DC Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization. J. Funct. Biomater. 2017, 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Gindri IM; Siddiqui DA; Frizzo CP; Martins MAP; Rodrigues DC Improvement of Tribological and Anti-Corrosive Performance of Titanium Surfaces Coated with Dicationic Imidazolium-Based Ionic Liquids. RSC Adv. 2016, 6, 78795–78802. [Google Scholar]
- (14).Wheelis SE; Biguetti CC; Natarajan S; Guida L; Hedden B; Garlet GP; Rodrigues DC Investigation of the Early Healing Response to Dicationic Imidazolium-Based Ionic Liquids: A Biocompatible Coating for Titanium Implants. ACS Biomater. Sci. Eng. 2020, 6, 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Arteaga A; Ranathunga DTS; Qu J; Biguetti CC; Nielsen SO; Rodrigues DC Exogenous Protein Delivery of Ionic Liquid-Mediated HMGB1 Coating on Titanium Implants. Langmuir 2023, 6, 2204. [DOI] [PubMed] [Google Scholar]
- (16).Biguetti CC; Cavalla F; Silveira EV; Tabanez AP; Francisconi CF; Taga R; Campanelli AP; Trombone APF; Rodrigues DC; Garlet GP HGMB1 and RAGE as Essential Components of Ti Osseointegration Process in Mice. Front. Immunol. 2019, 10, 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lee G; Espirito Santo AI; Zwingenberger S; Cai L; Vogl T; Feldmann M; Horwood NJ; Chan JK; Nanchahal J Fully Reduced HMGB1 Accelerates the Regeneration of Multiple Tissues by Transitioning Stem Cells to GAlert. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E4463–E4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Yu Y; Tang D; Kang R Oxidative Stress-Mediated HMGB1 Biology. Front. Physiol. 2015, 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Alfadda AA; Sallam RM Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, No. 936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Biscetti F; Rando MM; Nardella E; Cecchini AL; Pecorini G; Landolfi R; Flex A High Mobility Group Box-1 and Diabetes Mellitus Complications: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 6258. MDPI AG [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Venereau E; Casalgrandi M; Schiraldi M; Antoine DJ; Cattaneo A; de Marchis F; Liu J; Antonelli A; Preti A; Raeli L; Shams SS; Yang H; Varani L; Andersson U; Tracey KJ; Bachi A; Uguccioni M; Bianchi ME Mutually Exclusive Redox Forms of HMGB1 Promote Cell Recruitment or Proinflammatory Cytokine Release. J. Exp. Med. 2012, 209, 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fukumoto K; Yoshizawa M; Ohno H Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [DOI] [PubMed] [Google Scholar]
- (23).Shirota H; Mandai T; Fukazawa H; Kato T Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar]
- (24).de Mello Gindri I Design of Multifunctional Ionic Liquids for Surface Modification of Dental Implants; ProQuest Dissertations and Theses, 2016; p 261. [Google Scholar]
- (25).Sandhu PPK; Gindri IM; Siddiqui DA; Rodrigues DC Functional Biomaterials Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization. J. Funct. Biomater. 2017, 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Gindri IM; Siddiqui DA; Frizzo CP; Martins MAP; Rodrigues DC Ionic Liquid Coatings for Titanium Surfaces: Effect of IL Structure on Coating Profile. ACS Appl. Mater. Interfaces 2015, 7, 27421–27431. [DOI] [PubMed] [Google Scholar]
- (27).Siddiqui DA; Gindri IM; Rodrigues DC Corrosion and Wear Performance of Titanium and Cobalt Chromium Molybdenum Alloys Coated with Dicationic Imidazolium-Based Ionic Liquids. J. Bio Tribocorros. 2016, 2, 27. [Google Scholar]
- (28).Gindri IM; Palmer KL; Siddiqui DA; Aghyarian S; Frizzo CP; Martins MAP; Rodrigues DC Evaluation of Mammalian and Bacterial Cell Activity on Titanium Surface Coated with Dicationic Imidazolium-Based Ionic Liquids †. RSC Adv. 2016, 6, 36475–36483. [Google Scholar]
- (29).Gindri IM; Frizzo CP; Bender CR; Tier AZ; Martins MAP; Villetti MA; MacHado G; Rodriguez LC; Rodrigues DC Preparation of TiO2 Nanoparticles Coated with Ionic Liquids: A Supramolecular Approach. ACS Appl. Mater. Interfaces 2014, 6, 11536–11543. [DOI] [PubMed] [Google Scholar]
- (30).Biguetti CC; Cavalla F; Fonseca AC; Tabanez AP; Siddiqui DA; Wheelis SE; Taga R; Fakhouri WD; Silva RM; Rodrigues DC; Garlet GP Effects of Titanium Corrosion Products on In Vivo Biological Response: A Basis for the Understanding of Osseointegration Failures Mechanisms. Front. Mater. 2021, 8, 159. [Google Scholar]
- (31).Coulter FB; Levey RE; Robinson ST; Dolan EB; Deotti S; Monaghan M; Dockery P; Coulter BS; Burke LP; Lowery AJ; Beatty R; Paetzold R; Prendergast JJ; Bellavia G; Straino S; Cianfarani F; Salamone M; Bruno CM; Moerman KM; Ghersi G; Duffy GP; OCearbhaill ED Additive Manufacturing of Multi-Scale Porous Soft Tissue Implants That Encourage Vascularization and Tissue Ingrowth. Adv. Healthcare Mater. 2021, 10, No. e2100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dockery P; Khalid J; Sarani SA; Bulut HE; Warren MA; Li TC; Cooke ID Changes in Basement Membrane Thickness in the Human Endometrium during the Luteal Phase of the Menstrual Cycle. Hum. Reprod. Update 1998, 4, 486. [DOI] [PubMed] [Google Scholar]
- (33).Aoyagi H; Yamashiro K; Hirata-Yoshihara C; Ideguchi H; Yamasaki M; Kawamura M; Yamamoto T; Kochi S; Wake H; Nishibori M; Takashiba S HMGB1-Induced Inflammatory Response Promotes Bone Healing in Murine Tooth Extraction Socket. J. Cell. Biochem. 2018, 119, 5481–5490. [DOI] [PubMed] [Google Scholar]
- (34).Lewallen EA; Riester SM; Bonin CA; Kremers HM; Dudakovic A; Kakar S; Cohen RC; Westendorf JJ; Lewallen DG; van Wijnen AJ Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng., Part B 2015, 21, 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Biguetti CC; Cavalla F; Silveira EM; Fonseca AC; Vieira AE; Tabanez AP; Rodrigues DC; Trombone APF; Garlet GP Oral Implant Osseointegration Model in C57Bl/6 Mice: Microtomographic, Histological, Histomorphometric and Molecular Characterization. J. Appl. Oral Sci. 2018, 26, No. e20170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wheelis SE; Biguetti CC; Natarajan S; Arteaga A; El Allami J; Lakkasettar Chandrashekar B; Garlet GP; Rodrigues DC Cellular and Molecular Dynamics during Early Oral Osseointegration: A Comprehensive Characterization in the Lewis Rat. ACS Biomater. Sci. Eng. 2021, 7, 2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Shirazi S; Ravindran S; Cooper LF Topography-Mediated Immunomodulation in Osseointegration; Ally or Enemy. Biomaterials 2022, 291, No. 121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Civantos A; Martínez-Campos E; Ramos V; Elvira C; Gallardo A; Abarrategi A Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [DOI] [PubMed] [Google Scholar]
- (39).Lewis JS; Roy K; Keselowsky BG Materials That Harness and Modulate the Immune System. MRS Bull. 2014, 39, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wheelis SE; Natarajan SA; Lakkasetter Chandrashekar B; Arteaga A; Garlet GP; Rodrigues DC Effects of Dicationic Imidazolium-Based Ionic Liquids on Oral Osseointegration of Titanium Implants: An in Vivo Biocompatibility Study in Multiple Rat Demographics. Genes 2022, 13, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ranathunga D; Arteaga A; Biguetti CC; Rodrigues DC; Nielsen SO Molecular-Level Understanding of the Influence of Ions and Water on HMGB1 Adsorption Induced by Surface Hydroxylation of Titanium Implants. Langmuir 2021, 37, 10100–10114. [DOI] [PubMed] [Google Scholar]
- (42).Schindeler A; Mills RJ; Bobyn JD; Little DG Preclinical Models for Orthopedic Research and Bone Tissue Engineering. J. Orthop. Res. 2017, 36, 832–840. [DOI] [PubMed] [Google Scholar]
- (43).Kastellorizios M; Tipnis N; Burgess DJ Foreign Body Reaction to Subcutaneous Implants; Springer: Cham, 2015; pp 93–108. [DOI] [PubMed] [Google Scholar]
- (44).Ferrara M; Chialli G; Ferreira LM; Ruggieri E; Careccia G; Preti A; Piccirillo R; Bianchi ME; Sitia G; Venereau E Oxidation of HMGB1 Is a Dynamically Regulated Process in Physiological and Pathological Conditions. Front. Immunol. 2020, 11, 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Forrester SJ; Kikuchi DS; Hernandes MS; Xu Q; Griendling KK Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wautier M-P; Chappey O; Corda S; Stern DM; Schmidt AM; Wautier J-L Activation of NADPH Oxidase by AGE Links Oxidant Stress to Altered Gene Expression via RAGE. Am. J. Physiol.: Endocrinol. Metab. 2001, 280, E685. [DOI] [PubMed] [Google Scholar]
- (47).Kwak MS; Kim HS; Lee B; Kim YH; Son M; Shin JS Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Tirone M; Tran NL; Ceriotti C; Gorzanelli A; Canepari M; Bottinelli R; Raucci A; Di Maggio S; Santiago C; Mellado M; Saclier M; François S; Careccia G; He M; De Marchis F; Conti V; Ben Larbi S; Cuvellier S; Casalgrandi M; Preti A; Chazaud B; Al-Abed Y; Messina G; Sitia G; Brunelli S; Bianchi ME; Vénéreau E High Mobility Group Box 1 Orchestrates Tissue Regeneration via CXCR4. J. Exp. Med. 2018, 215, 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lu D; Xu Y; Liu Q; Zhang Q Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Schiraldi M; Raucci A; Muñoz LM; Livoti E; Celona B; Venereau E; Apuzzo T; de Marchis F; Pedotti M; Bachi A; Thelen M; Varani L; Mellado M; Proudfoot A; Bianchi ME; Uguccioni M HMGB1 Promotes Recruitment of Inflammatory Cells to Damaged Tissues by Forming a Complex with CXCL12 and Signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Alfaro S; Acuña V; Ceriani R; Cavieres MF; Weinstein-Oppenheimer CR; Campos-Estrada C Involvement of Inflammation and Its Resolution in Disease and Therapeutics. Int. J. Mol. Sci. 2022, 23, 10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ito T; Kawahara K; Nakamura T; Yamada S; Nakamura T; Abeyama K; Hashiguchi T; Maruyama I High-Mobility Group Box 1 Protein Promotes Development of Microvascular Thrombosis in Rats. J. Thromb. Haemostasis 2007, 5, 109–116. [DOI] [PubMed] [Google Scholar]
- (53).Palumbo R; Sampaolesi M; de Marchis F; Tonlorenzi R; Colombetti S; Mondino A; Cossu G; Bianchi ME Extracellular HMGB1, a Signal of Tissue Damage, Induces Mesoangioblast Migration and Proliferation. J Cell Biol. 2004, 164, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Peltz ED; Moore EE; Eckels PC; Damle SS; Tsuruta Y; Johnson JL; Sauaia A; Silliman CC; Banerjee A; Abraham E HMGB1 Is Markedly Elevated within 6 Hours of Mechanical Trauma in Humans. Shock 2009, 32, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Li X; Bu W; Meng L; Liu X; Wang S; Jiang L; Ren M; Fan Y; Sun H CXCL12/CXCR4 Pathway Orchestrates CSC-like Properties by CAF Recruited Tumor Associated Macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [DOI] [PubMed] [Google Scholar]
- (56).Brown JM; Recht L; Strober S The Promise of Targeting Macrophages in Cancer Therapy. Clin. Cancer Res. 2017, 23, 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Yellowley CE; Toupadakis CA; Vapniarsky N; Wong A Circulating Progenitor Cells and the Expression of Cxcl12, Cxcr4 and Angiopoietin-like 4 during Wound Healing in the Murine Ear. PLoS One 2019, 14, No. e0222462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Johnson KE; Wilgus TA Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Etzerodt A; Moestrup SK CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid. Redox Signal. 2013, 18, 2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Isles HM; Herman KD; Robertson AL; Loynes CA; Prince LR; Elks PM; Renshaw SA The CXCL12/CXCR4 Signaling Axis Retains Neutrophils at Inflammatory Sites in Zebrafish. Front. Immunol. 2019, 10, 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Huber M; Reinisch G; Trettenhahn G; Zweymüller K; Lintner F Presence of Corrosion Products and Hypersensitivity-Associated Reactions in Periprosthetic Tissue after Aseptic Loosening of Total Hip Replacements with Metal Bearing Surfaces. Acta Biomater. 2009, 5, 172–180. [DOI] [PubMed] [Google Scholar]
- (62).Hanawa T Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]


