Abstract
Case Description—
In April 2012, Salmonella enterica serotype Infantis was detected in an unopened bag of dry dog food collected during routine retail surveillance. PulseNet, a national bacterial subtyping network, identified humans with Salmonella Infantis infection with the same genetic fingerprint as the dog food sample.
Clinical Findings—
An outbreak investigation identified 53 ill humans infected with the outbreak strain during January 1 to July 5, 2012, in 21 states and 2 provinces in Canada; 20 (38%) were children ≤ 2 years old, and 12 of 37 (32%) were hospitalized. Of 21 ill people who remembered the dog food brand, 12 (57%) reported a brand produced at a plant in Gaston, SC. Traceback investigations also identified that plant. The outbreak strain was isolated from bags of dry dog food and fecal specimens obtained from dogs that lived with ill people and that ate the implicated dry dog food.
Treatment and Outcome—
The plant was closed temporarily for cleaning and disinfection. Sixteen brands involving > 27,000 metric tons (> 30,000 tons) of dry dog and cat food were recalled. Thirty-one ill dogs linked to recalled products were reported through the FDA consumer complaint system.
Clinical Relevance—
A one-health collaborative effort on epidemiological, laboratory, and traceback investigations linked dry dog foods produced at a plant to illnesses in dogs and humans. More efforts are needed to increase awareness among pet owners, health-care professionals, and the pet food industry on the risk of illness in pets and their owners associated with dry pet foods and treats.
On April 2, 2012, personnel from the Michigan Department of Agriculture and Rural Development detected Salmonella enterica serotype Infantis in an unopened bag of dry dog fooda collected during routine surveillance of retail samples. The dog food had been produced at a plant in Gaston, SC. The plant, which was 1 of 4 plants owned by the production firm, manufactured > 30 brands of dry food products for dogs, cats, and ferrets under the firm’s own brands and brands for other companies. A voluntary recall for the dog food product was issued by the production firm on April 6, 2012. Because of the isolation of Salmonella spp from that dry food product, personnel from the South Carolina Department of Agriculture inspected the plant in Gaston on April 9, 2012.
Molecular subtyping of pathogens plays a critical role in detection and investigation of disease outbreaks. In the United States, isolates of strains of Salmonella spp obtained from clinically affected humans are routinely forwarded to state public health laboratories for serotyping and PFGE. The PFGE subtyping patterns are then electronically submitted to PulseNet, the national molecular subtyping network for foodborne disease surveillance.1,2 Several other countries, including Canada, have similar surveillance networks. The PFGE patterns of some nonhuman isolates (eg, isolates obtained from food, domestic animals, and the environment), which are typically collected as a part of routine surveillance, are also uploaded to PulseNet. Therefore, the PFGE pattern of the Salmonella isolate for the dog food was submitted to PulseNet.
The PFGE pattern was uncommon. On April 10, 2012, the PulseNet database was searched for recent human infections of Salmonella Infantis with a PFGE pattern indistinguishable from the isolate found in the dog food sample. A human case was initially defined as an illness in a person with an onset after January 1, 2012, for which culture results yielded Salmonella Infantis with PFGE XbaI pattern JFXX01.0164. That search identified 8 human cases from 6 states reported between February 1 and April 10, 2012. This appeared to be an excessively large number of human cases for that isolate during that time span because typically only 0 to 3 human clinical isolates with that PFGE pattern were uploaded to PulseNet each month. Therefore, a multistate outbreak investigation coordinated by the CDC was initiated on April 10, 2012, to determine the source of the illnesses in humans attributable to that strain and to prevent additional illnesses.
On April 11, 2012, personnel at the plant were informed that there were humans with salmonellosis caused by the same strain of Salmonella spp that had been found in the contaminated pet food product. The plant stopped production of pet foods that evening, and the plant was thoroughly cleaned and disinfected. During April 12 to 20, personnel from the South Carolina Department of Agriculture and the US FDA inspected the plant and collected environmental, ingredient, and finished product samples. Environmental samples were collected from various production (eg, preparation lines and extrusion lines) and nonproduction (eg, carts and floors) surfaces in the plant. Samples were not obtained from plant workers because they were not considered a likely source of contamination. The outbreak strain of Salmonella Infantis was isolated from samples of a finished product, but environmental and ingredient samples collected during this inspection yielded negative results for Salmonella spp. Deficiencies detected during the inspection included lack of assurance (eg, microbial analysis) that pathogens were not introduced into the production line and failure to maintain equipment to protect against contamination or to facilitate cleaning.3 The ultimate source of the contamination and the events leading to the contamination were not determined. The plant resumed production of pet foods on April 18, 2012. On the basis of inspection observations, recommendations were made to increase product testing for detection of contamination with Salmonella spp and address facility protective measures to prevent contamination.
The multistate outbreak investigation of salmonellosis in humans initiated on April 10, 2012, involved personnel from state and local health departments who conducted interviews of case patients using routine enteric disease questionnaires that contained questions about foods, restaurants, travel, and animal contact during the week before each case patient’s illness began. Within a few days after initiation of the investigation, additional human cases were identified through PulseNet. Information obtained during interviews with 9 case patients revealed that 6 had contact with a dog in the week before they became ill.
Because of the high rate of exposure to dogs reported in initial interviews, combined with the Salmonella isolate from the retail sample of dry dog food in Michigan, it was hypothesized that these human cases could have been associated with dry pet food manufactured at the production plant in Gaston, SC, where the bag of dog food that yielded the initial Salmonella isolate was produced. The CDC requested that state and local officials reinterview case patients using a CDC questionnaire focused on various pet and pet food exposures. Of 5 case patients who initially remembered the type of dog food with which they had contact, 4 identified 4 brands of dry dog food that had been produced at the Gaston plant. Two case patients provided production code information from packages of 2 dog food products that they had purchased. Those production codes yielded information on the production date, plant, and production line. This information indicated that the 2 products were manufactured at the Gaston plant. On the basis of this information, it was determined that the outbreak of salmonellosis was most likely attributable to the pet food produced at the plant in Gaston.
On April 20, 2012, public health and agriculture officials in Ohio isolated the outbreak strain of Salmonella Infantis from a sample of dry dog food collected at the home of a case patient. The package that contained the dog food was not available, but the case patient indicated the food was a brand produced at the Gaston plant. Subsequently, the outbreak strain was isolated from an unopened bag of the same brand of dry dog food manufactured at the Gaston plant; the bag was obtained from a retail store. A sample of another product collected by the US FDA during an inspection at the Gaston plant on April 30, 2013, also yielded the Salmonella strain involved in the outbreak.
On April 26, 2012, the FDA Coordinated Outbreak and Response Evaluation Network began coordinating regulatory activities. Information collected from case patients and results of product testing were reviewed. Consumer complaints submitted through the FDA consumer complaint system4 were also examined for information about product exposure, production codes, and animals with clinical signs (eg, diarrhea, vomiting, and fever) suggestive of salmonellosis. Production codes from dry dog food linked to human cases and animal illnesses were traced back to pet food produced between late December 2011 and early April 2012 on 2 production lines at the Gaston plant. A voluntary recall was issued on April 26, 2012, for the product from which the outbreak strain was detected in the opened and unopened bags in Ohio.5,6 Another voluntarily recall was issued on April 30, 2012, for the product from which the outbreak strain was detected in the sample collected at the Gaston plant.5,6 Because of investigation findings and growing public health concerns, a recall expansion was issued by the production firm on May 4, 2012, that included all dry food products for dogs and cats that had been manufactured on the 2 implicated production lines between late December 2011 and early April 2012. Other companies opted for inclusion in the voluntary recall expansion or issued individual recalls. These voluntary recalls were issued from May 4 to 8, 2012.6
The recalled products included 16 brands of dry foods for dogs and cats manufactured on the implicated production lines between December 9, 2011, and April 7, 2012. Although no confirmed human illness linked to contaminated cat food was reported, dry food for cats manufactured on the same production lines was recalled out of an abundance of caution. The recalled products had been shipped to 40 states and 26 countries, including Canada, and involved > 27,000 metric tons (> 30,000 tons) of dry foods for dogs and cats.
As more dog food samples were tested, Salmonella Infantis with a second PFGE pattern (XbaI JFXX01.0070), which was also uncommonly reported to PulseNet, was isolated from retail samples of dry dog food produced at the Gaston plant. The case definition was revised to include the second PFGE pattern and to include humans with an illness between January 1 and July 5, 2012, for which culture results yielded Salmonella Infantis with either of the PFGE patterns.
The expanded case definition helped in the identification of additional human cases. Ultimately, the investigation identified 53 individuals from 21 states (n = 51) and Canada (2) who met the case definition (Figures 1 and 2). Eighteen (34%) human cases had the second PFGE pattern. The median age of case patients was 19.5 years (range, < 1 to 82 years); 20 (38%) were children < 2 years of age, and 30 (57%) were female. Of 37 case patients for whom information was available, 12 (32%) were hospitalized; none of the 37 died. Regardless of whether they owned a dog, 28 (76%) reported contact with a dog (in the home or at another location) during the week before the case patients became ill. Of the 21 case patients who remembered the dog food brand, 12 (57%) reported a brand produced at the Gaston plant.
Figure 1—
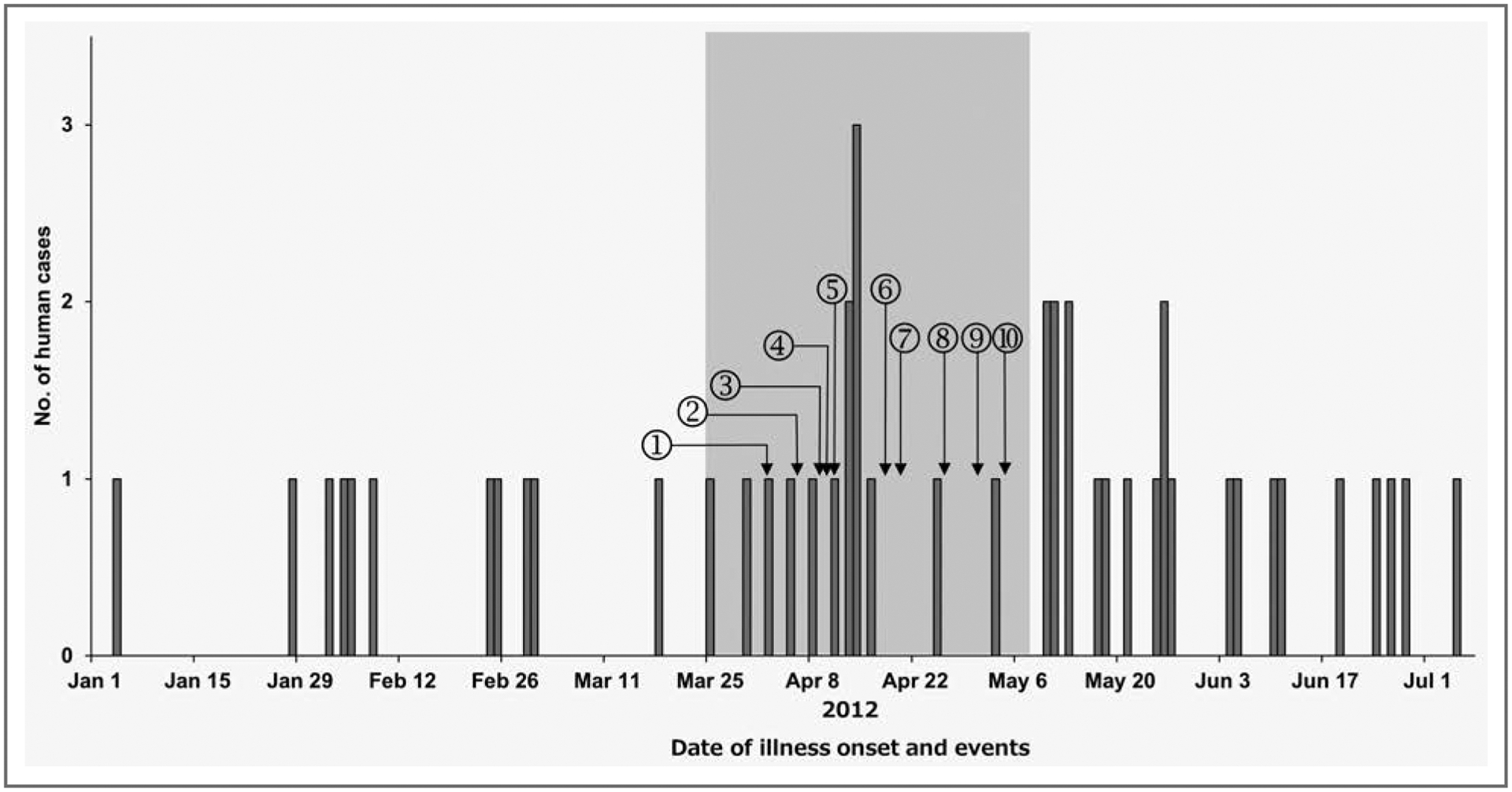
Infections attributable to the outbreak strain of Salmonella enterica serotype Infantis in humans during January 1 to July 5, 2012, by date of illness onset. A human case was defined as an illness in a person during January 1 to July 5, 2012, whose culture results yielded Salmonella Infantis with PFGE XbaI pattern JFXX01.0164 or XbaI JFXX01.0070. Illness in dogs (canine case) were detected during the period indicated by the gray shading; a canine case was defined as a gastrointestinal illness in a dog after consuming recalled pet food products reported to the FDA consumer complaint system during the period between December 1, 2011, and June 30, 2012. 1 = Outbreak strain of Salmonella Infantis isolated from dry dog food in Michigan. 2 = Voluntary recall issued for the dry dog food product. 3 = Manufacturing plant inspected by the South Carolina Department of Agriculture. 4 = Human cases initially identified by the PFGE pattern in PulseNet. 5 = Manufacturing plant cleaned and disinfected. 6 = Plant resumed operations but with increased testing procedures. 7 = Outbreak strain of Salmonella Infantis isolated from dog food obtained from case patient home and bag of dog food collected from retail in Ohio. 8 = The FDA initiates response coordination; product recall issued because of the isolates in Ohio. 9 = Outbreak strain of Salmonella Infantis isolated from dog food sample collected during plant inspection; product recall issued because of this isolate. 10 = Product recall expanded to include all pet food products manufactured on the implicated lines at the plant.
Figure 2—
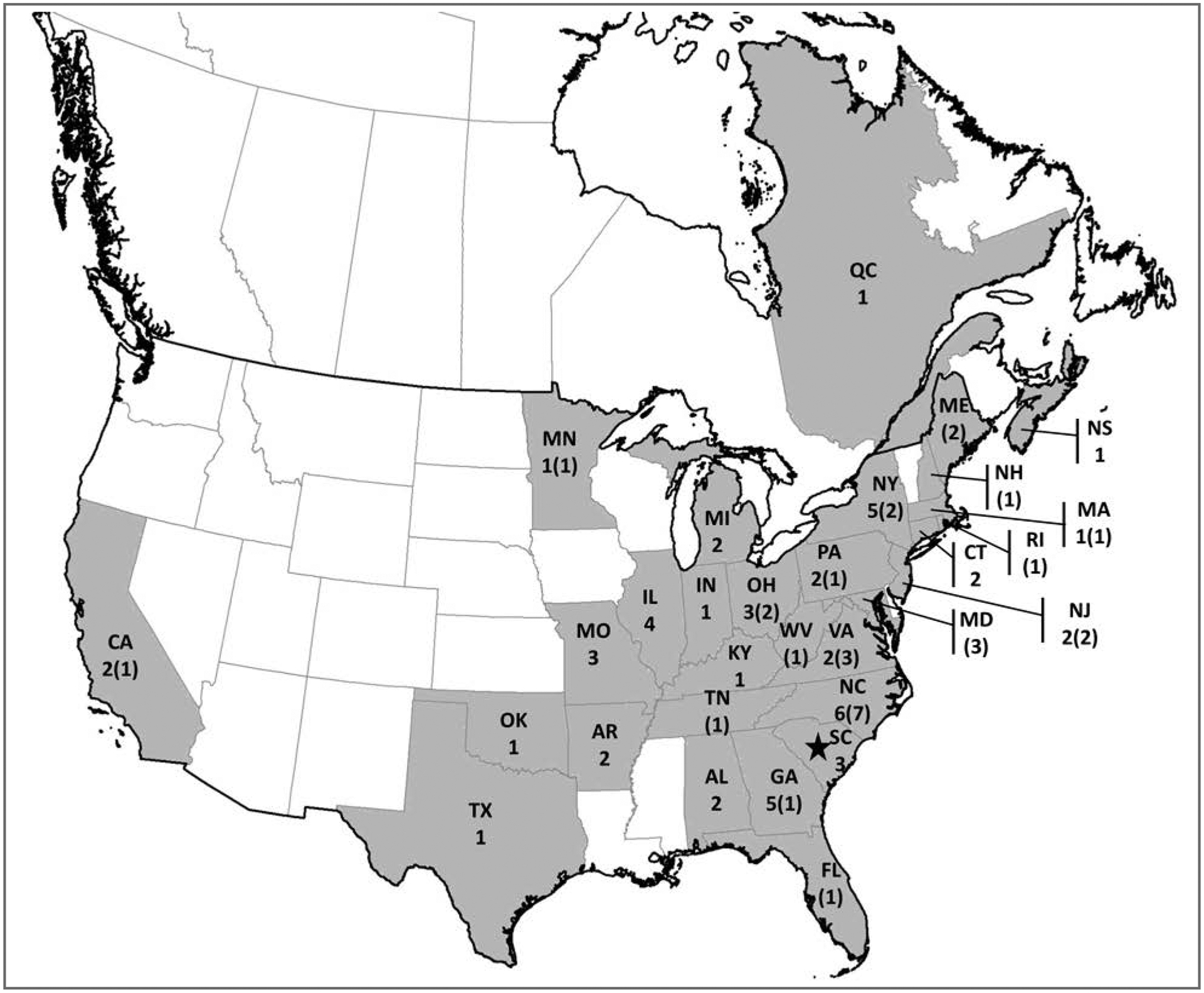
Illness in humans (n = 53) and dogs (31) detected in an outbreak of Salmonella Infantis infection during January 1 to July 5, 2012, in the United States and Canada. For each state or province, the values reported are number of human cases (number of canine cases). In Canada, there is no equivalent to the FDA consumer complaint system. The star indicates the location of the plant that manufactured the dry dog food implicated in the outbreak. See Figure 1 for remainder of key.
Follow-up inspection by personnel from the US FDA and South Carolina Department of Agriculture during September 10 to 19, 2012, revealed violations similar to those detected during the inspection performed in April 2012. Recommendations for corrective action were provided, and compliance with those recommendations was to be reevaluated during future inspections. Multiple Salmonella strains, but not the outbreak strain, were isolated from environmental samples. No outbreaks of salmonellosis in humans attributable to the strains isolated during the September inspection were identified.
Personnel at the US FDA and state agencies continued analysis of consumer complaints and surveillance of retail samples to determine whether additional recalls or expansion of existing recalls might be required. No additional reports of Salmonella spp isolated from people, pets, or dry pet food manufactured at the Gaston plant had been received from public health and agricultural departments, the FDA consumer complaints system, or other sources as of December 10, 2013.
To identify any illness in pets associated with this outbreak, case patients were interviewed, fecal samples collected from dogs and cats that lived with case patients were tested, and the FDA consumer complaint system was monitored. Of 14 case patients who reported the illness status of their pets, 3 reported that at least 1 dog or cat had been ill.
A number of state and provincial health departments, provincial animal health laboratories, and the FDA Vet-LIRN,7 which is a network of collaborating veterinary diagnostic laboratories, offered to test samples obtained from dogs and cats that resided in households of case patients, with no cost to the owners or attending veterinarians. To our knowledge, animals living in the households of only 4 case patients (2 in the United States and 2 in Canada) were tested (Figure 3).
Figure 3—
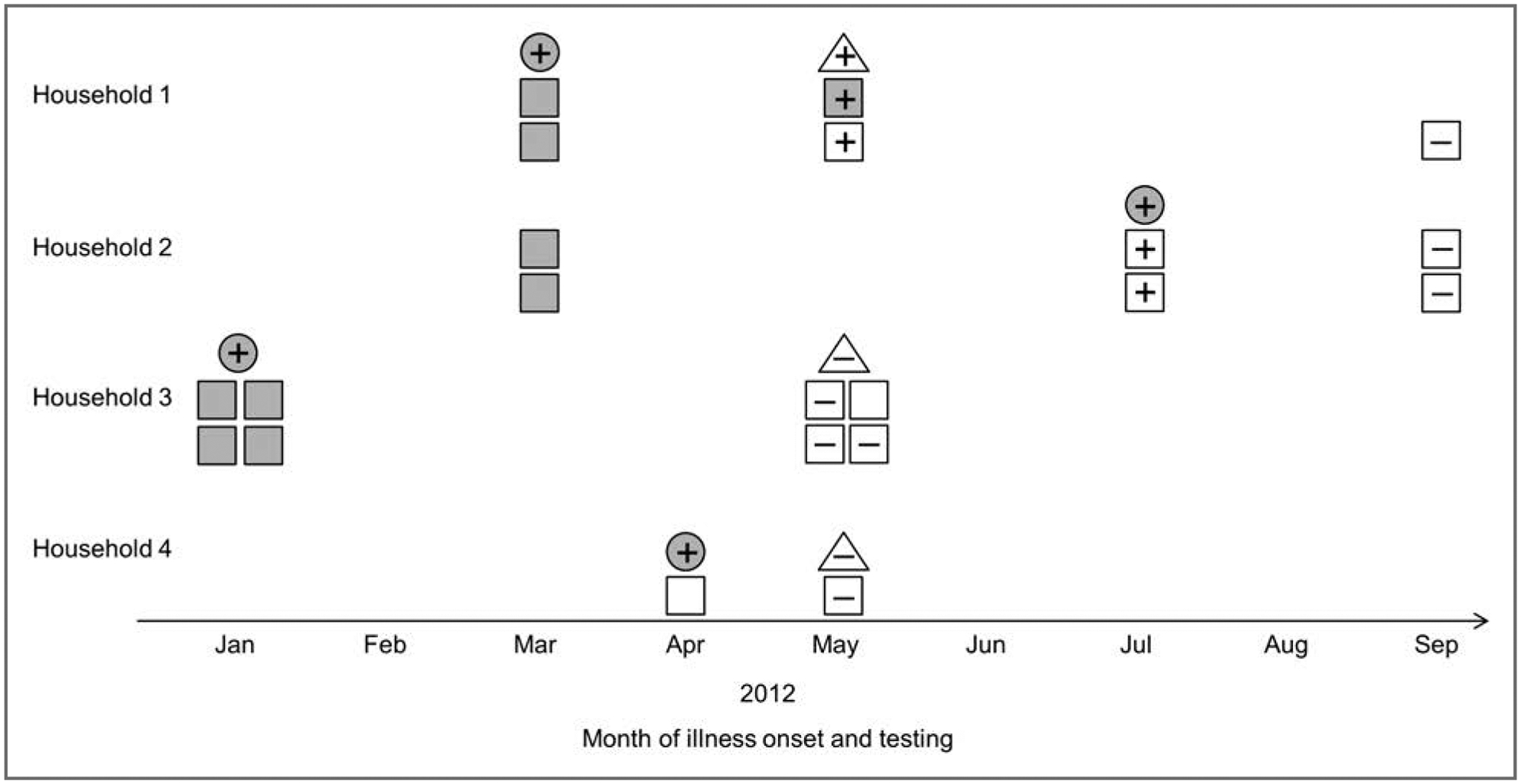
Results of testing to detect Salmonella Infantis in ill humans (gray circles), ill dogs or cats (gray squares), apparently healthy dogs or cats (white squares), and dry pet food (white triangles) in 4 households with human cases between January and September 2012. Households 1 and 2 were in the United States, and households 3 and 4 were in Canada. + = Specimen obtained and tested; outbreak strain isolated. − = Specimen obtained and tested; outbreak strain not isolated. See Figure 1 for remainder of key.
Two dogs from one of the US households developed diarrhea (loose or runny feces) in March 2012 on approximately the same day as the onset of diarrheal illness in the case patient, a 5-month-old infant. The family was notified about the outbreak in early May 2012. Samples were collected from the household, and the outbreak strain was isolated from the feces of both dogs and from samples collected from an open bag of a brand of dry dog food manufactured at the Gaston plant. The condition of one of these dogs, a 12-year-old dog that was receiving treatment for cancer of the urinary bladder, deteriorated, and the dog was euthanized. The other dog recovered from the episode of diarrhea; follow-up testing of that dog in September 2012 did not yield Salmonella spp.
The other US household had 2 dogs with vomiting, lethargy, fever, and bloody diarrhea during March 2012. Both dogs had consumed a brand of dry dog food manufactured at the Gaston plant. There was improvement in the clinical signs when the dogs were fed a different dog food in early April 2012, and both dogs subsequently had complete resolution of clinical signs. The owners discarded the dry food that may have been produced at the Gaston plant. In early July 2012, approximately 3 months after the illness in the dogs resolved, an infection attributable to the outbreak strain of Salmonella Infantis was diagnosed in the owner’s 10-day-old infant. The mother of the infant reportedly had diarrhea 1 day before onset of illness in the infant. The outbreak strain was isolated from fecal samples collected from both dogs a few days after the infant’s illness as well as 2 weeks later while the dogs were receiving a 2-week course of ampicillin. In August 2012, a 1-month course of ampicillin was prescribed for both dogs, and fecal samples collected from the dogs in September, October, and December 2012 did not yield Salmonella spp.
One of the Canadian households had 2 dogs and 2 cats. The owners reportedly fed a brand of dry food for dogs and cats manufactured at the Gaston plant. The case patient was a 1-year-old child with illness onset in January 2012. The animals reportedly had vomiting in January 2012 and occasionally had soft feces from January to May 2012; however, Salmonella spp were not isolated from fecal specimens collected from 2 dogs and 1 cat in May 2012. The pet food that had been fed in January was not available in May; however, a sample of the same brand of pet food was collected from the household in May but did not yield Salmonella spp.
The case patient in the other household in Canada was an adult with illness onset in April 2012. The dog in that household was fed dry food manufactured at the plant in Gaston, but the dog had no signs of illness. A fecal specimen obtained from the dog and a sample of the dry food collected from the household in May 2012 did not yield Salmonella spp.
Additional surveillance for animal illnesses linked to the outbreak was performed. In the United States, the FDA consumer complaint system was reviewed for reports of illnesses in animals. An animal case was defined as a gastrointestinal illness (vomiting or diarrhea) in an animal that consumed recalled pet food products that was reported to the FDA consumer complaint system between December 1, 2011, and June 30, 2012. Recall of pet food products was determined on the basis of the production code on the packaging included in the reports. During the period from December 2011 to March 2012, only 1 complaint of a product manufactured at the implicated plant was submitted, but the signs of illness in that animal were not consistent with salmonellosis. During the period from April to June 2012, 167 consumer complaints on brands of products manufactured at the Gaston plant were reported, of which 113 had incomplete or missing production codes and were excluded from further analysis. Of the remaining 54 consumer complaints about products manufactured at the plant, 31 (57%) matched the case definition; those complaints involved 7 brands of recalled product. All 31 cases were illnesses in dogs. Date of illness onset ranged from March 26 to May 7, 2012. Vomiting was reported for 22 (71%) dogs, and diarrhea was reported for 30 (97%) dogs (diarrhea was bloody in 13/30 dogs). The geographic distribution and reported dates of illness onset of these canine cases were similar to those for the human cases (Figures 1 and 2). No illness in cats was linked to recalled products. Follow-up efforts (including testing of fecal and food specimens) were focused on reports of animal illness associated with products manufactured at the Gaston plant outside the recall period as well as products manufactured at other facilities. No additional outbreak-related cases of salmonellosis in animals were identified.
Discussion
Epidemiological, laboratory, traceback, and environmental investigations linked dry dog foods manufactured at a production plant in Gaston, SC, to illnesses caused by Salmonella Infantis infections in humans and dogs in multiple states and 2 provinces in Canada, which led to a recall of > 27,000 metric tons of dry foods for dogs and cats. Because many people with salmonellosis do not seek medical care or are not tested, it is likely that there are more cases of illness than those that are reported.8 Similarly, the number of animals affected during this outbreak is unknown, but it likely was more than the number reported in this investigation.
It is estimated that S enterica causes 1.2 million human illnesses, 19,000 hospitalizations, and 400 deaths in the United States annually.8 Salmonellosis is a zoonotic bacterial illness frequently associated with the consumption of contaminated foods such as meat, poultry, eggs, dairy, produce, and processed foods.9–12 Recently, 11% of cases of domestically acquired salmonellosis in humans were estimated to be attributable to contact with animals.13 People can become infected through direct and indirect contact with animals or animal environments, and it is often challenging to establish the route of transmission. In the outbreak described here, human illnesses could have been attributable to direct contact with contaminated pet foods, exposure to human food items and related implements (eg, baby bottles) cross-contaminated by the implicated pet foods, or direct or indirect contact with infected animals (both clinically and subclinically affected animals) or animal environments. Feeding pets in the kitchen has been linked with salmonellosis in humans, which suggests that cross-contamination in the kitchen is an important source of illness in humans.14
Most persons infected with Salmonella spp develop diarrhea, fever, and abdominal cramps. Children < 5 years old, older adults, and people with compromised immune systems are more likely to have severe illness, including sepsis, joint infections, and meningitis. In contrast, many domestic animals exposed to Salmonella spp become subclinically affected carriers, although they may also develop fever, anorexia, vomiting, abdominal cramps, diarrhea, and sepsis.15,16 The estimated prevalence of Salmonella spp in pets ranges between 0% and 36% in healthy dogs and 1% and 18% in healthy cats.15,17,18 The prevalence is substantially higher in dogs that eat raw food diets.18,19 Currently, the FDA Vet-LIRN is conducting a study to more accurately estimate the prevalence of Salmonella spp in dogs and cats in the United States.7,20 Fecal shedding of Salmonella spp (often intermittent) has been reported for 3 to 6 weeks after infection in most cases.18 In the investigation described here, the outbreak strain was isolated from 2 dogs approximately 3 months after their last likely exposure to contaminated dry food, which supports the findings in prior studies that indicate dogs can become subclinically affected carriers and suggests that shedding duration may be considerably prolonged in some cases. Similar to salmonellosis in humans, it is widely accepted that antimicrobial treatment is not warranted for uncomplicated salmonellosis in domestic animals when the animals have no or only mild clinical signs.16
It is estimated that 43 million (approx 36%) US households own dogs and 36 million (approx 30%) own cats,21 and most households feed their pets a dry food. Because many pet foods, treats, and supplement-type products (eg, vitamins) contain food ingredients of animal origin, these items are at risk for contamination with Salmonella spp. During production of dry pet foods, extrusion is a component of the manufacturing process, whereby the combined ingredients are heated (a step intended to kill pathogens) and formed into the final product of various shapes and sizes. Typically, flavor enhancers, which are animal digest (material resulting from hydrolysis of animal tissue) and fat, are added after extrusion, but the food does not subsequently undergo additional steps that would destroy pathogens.22 Salmonella spp was isolated from 6.1% of pet foods and treats and 7.1% of supplement-type pet products collected from manufacturers, distributors, wholesalers, or retailers during surveillance sampling in 2007 to 2009.23 In a more recent study,24 only 1 of 670 dry pet foods or treats had positive results for Salmonella spp, in contrast to 15 of 196 commercial raw pet foods that had Salmonella spp. Human illnesses associated with contaminated pet foods and treats have been reported.14,25,26
In the United States, the US FDA regulates pet foods, treats, and nutritional products. In Canada, the Canadian Food Inspection Agency and Health Canada have legislative authorities applicable to pet foods. When a pet food or treat is contaminated with Salmonella spp, the product is considered adulterated and subject to product recalls by regulatory agencies. From 2008 to 2012 in the United States, there were 54 recalls of pet foods (335 products) because of possible contamination with Salmonella spp, with 139 (41%) products being pet treats, as determined by one of the authors (DHW; unpublished data). The US FDA works closely with their state regulatory counterparts to investigate pet food products from which Salmonella spp have been isolated; these efforts include facility inspections. In the investigation of the outbreak attributable to Salmonella Infantis described here, Salmonella spp were not isolated from environmental or ingredient samples collected at the Gaston plant during the inspection in April 2012, which was immediately after a thorough cleaning and disinfection of the plant. However, multiple strains of Salmonella spp were isolated during a follow-up inspection conducted in September 2012, which suggested that there was continuous introduction of bacteria into the plant environment. Environmental monitoring and characterization of isolates to ensure the absence of persistent bacteria are crucial components for assessing sanitation and control measures in plants. In accordance with the 2011 FDA Food Safety Modernization Act, rules to improve safety of food for animals (including pet food) and to prevent illnesses in animals and humans have been proposed; the US FDA held 3 public comment sessions and is scheduled to finalize the rules in 2014.27,28
Pet owners often rely on veterinarians for advice during disease outbreaks that might affect their pets; thus, it is important that veterinarians understand how outbreak investigations are conducted, the history or information that is gathered, and the specimens that are collected from pets or pet foods for testing. Veterinarians also play an important role in educating pet owners on zoonotic conditions, including salmonellosis, which can be transmitted from contact with pets and other animals or animal products, including pet foods and treats.14,29–32
During the outbreak described here, information on how to report animal illnesses to the US FDA was provided on the CDC’s outbreak website.33 The CDC also collaborated with the American Association of Veterinary Laboratory Diagnosticians and USDA National Veterinary Services Laboratories to provide real-time information on testing of animals and pet food during the outbreak. Veterinary organizations, including the AVMA and Canadian Veterinary Medical Association, were involved in outreach to the practicing veterinary medical community. Many pet owners are not able to test their animals for Salmonella spp because of the expense of the clinic visit and laboratory costs. To address this issue in the United States, the CDC partnered with Vet-LIRN to encourage testing of fecal samples from pets of case patients. Monitoring the status of these pets provided information to the owners so that they could take appropriate hygienic measures to reduce further exposure, especially for individuals at high risk for salmonellosis.
Pet owners, veterinarians, animal shelter personnel, pet store personnel, people who sell pet food, and health-care professionals (including pediatricians, family practitioners, internists, infectious disease specialists, state and federal regulatory officials, and health department staff) should be aware that dry pet food, treats, and supplement-type products can be contaminated with pathogens such as Salmonella spp, which poses public health risks to both humans and other animals. When examining an animal with diarrhea or an animal that has consumed pet food recalled for possible contamination with Salmonella spp, veterinarians should consider the possibility of salmonellosis and educate pet owners about zoonotic diseases. Veterinarians should ask owners of ill pets about possible sources of infection, such as recent introduction of new bags of pet food or treats and other ill animals in the household. Veterinarians may also submit fecal samples for bacterial culture and further typing of isolates, such as by PFGE. Public health officials rely on veterinarians at the frontlines of patient care to report suspicious illnesses that may be associated with commercial products. Any animal illnesses suspected to be caused by contaminated pet food products should be reported (by use of the product code) to the FDA consumer complaint system by veterinarians or pet owners.4 Because the same brand of pet food can be manufactured at multiple production plants, a production code is frequently required to enable FDA personnel to identify the facility at which the implicated product was manufactured. Suspected disease outbreaks should be reported to local or state health departments or to a state public health veterinarian.
During evaluations of humans with gastrointestinal illnesses suggestive of salmonellosis, health-care providers should ask about contact with animals and the animals’ health status. Good hand hygiene practices should be recommended. Pediatricians and other health-care professionals should counsel patients and their families on sources of salmonellosis (eg, farm animals, live poultry, reptiles, amphibians, rodents, and ill animals) that may infect young children.14 If a patient has a persistent Salmonella infection, physicians should consider recommending testing of household animals by a veterinarian because an animal could be the source of the recurrent infections.
Public health information, including recommendations, for preventing human salmonellosis associated with pet food and treats is available.15,33,34 Recommendations have been formulated.15,33–35 Briefly, those recommendations include the following:
Wash hands with soap and water for at least 20 seconds after touching pets; food, toys, or waste of pets; or a pet’s environment. It is important to wash hands before preparing or serving foods to humans, before eating, and especially before preparing baby bottles.
Buy bags of pet food that have no visible signs of damage to the packaging, such as tears or discoloration.
Avoid feeding pets a raw diet because such diets can harbor pathogens such as Salmonella spp, Campylobacter spp, and Listeria spp.22
Do not store bags of pet food in the kitchen or other areas where human food is stored or prepared.
Store dry pet food (in its original bag) inside a clean plastic container with a lid. The top of the bag should be folded or closed.
Feed pets in areas other than the kitchen.
Do not allow pets to eat from the same food dishes as humans to prevent cross-contamination with human food.
When feeding a pet a wet (cans or pouches) food, promptly cover and refrigerate any wet pet food that remains in the container. Throw away any wet pet food that the pet did not eat during that meal.
Wash the food and water dishes of pets and any utensils used for feeding of pets regularly.
Prevent cross contamination. Do not wash food and water dishes of pets in the kitchen sink or bathtub. If there is no alternative, promptly clean and disinfect the sink (or bathtub) after washing a pet’s food items.
Do not allow pets to hunt prey, to scavenge food, or to drink unchlorinated water.
Clean up after pets. Pick up and discard feces of dogs in a tightly sealed plastic bag. Scoop litter material of cats daily and discard in a tightly sealed plastic bag. Place all waste (in the tightly sealed plastic bags) into a closed trash can. Keep young children away from pet feces.
Do not allow children < 5 years old to touch or eat pet foods, treats, or supplement-type products.
Specific recommendations should be provided to families with young children. Twenty of 53 (38%) case patients in the outbreak described here and 38 of 79 (48%) case patients in an outbreak of salmonellosis in humans linked to use of dry foods for dogs and cats in 2006 to 200814 were children ≤ 2 years old. Young children are at high risk for illness because their immune systems are still developing and because they are more likely to put their fingers or other items into their mouths. Children ≤ 5 years old should not be allowed to touch or eat pet foods, treats, or supplement-type products or to clean up feces of pets. In addition, they should be kept away from pet-feeding areas to prevent illness and injury.
If salmonellosis is confirmed or suspected in a pet, or if a pet consumed pet food recalled for possible contamination with Salmonella spp, owners should closely adhere to public health advice. In addition, the owners should bathe the pet and thoroughly clean and disinfect the pet’s environment. Pet owners can be made aware of resources that provide guidelines for safe animal interactions and handling of pet foods; these resources include the CDC Gastrointestinal (Enteric) Zoonoses website32 and other CDC materials.34 In addition, educational brochures and posters (available in multiple languages and sizes)32,36 can be displayed in veterinary clinics, animal shelters, pet stores, and other locations where pet food is sold.
The present report described a one-health collaboration of an outbreak investigation that linked contaminated dry dog foods to illnesses in humans and dogs and led to the largest recall of dry foods for dogs and cats because of human illness. Testing of retail products and use of PulseNet were vital for detecting the outbreak and highlight the importance of routine surveillance of retail products. The investigation revealed that dry pet food may be contaminated with Salmonella spp and can be a source of illnesses in humans (especially young children) and, less frequently, pets. Furthermore, the investigation confirmed that dogs that consume contaminated products can become subclinically affected carriers of Salmonella spp. More efforts are needed to increase awareness about potential illness associated with pet foods and treats and to promote safe practices for handling of pet foods.
Acknowledgments
The authors thank Beverly Billard, Annamaria Castiglia, Geneviève Côté, Kathryn Dalton, April Hodges, Kari Irvin, Marie-Andrée Leblanc, Sherri McGarry, Sarah Nemser, Johnson Nsubuga, Brittany Orlando, Anne Parkinson, Jeshua Pringle, Cassan Pulaski, Marianne Ross, and Stelios Viazis for technical assistance.
Abbreviations
- PFGE
Pulsed-field gel electrophoresis
- Vet-LIRN
Veterinary Laboratory Investigation and Response Network
Footnotes
Diamond Pet Foods, Meta, Mo.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or other represented agencies.
References
- 1.Gerner-Smidt P, Hise K, Kincaid J, et al. PulseNet USA: a five-year update. Foodborne Pathog Dis 2006;3:9–19. [DOI] [PubMed] [Google Scholar]
- 2.CDC. PulseNet Available at: www.cdc.gov/pulsenet. Accessed Nov 25, 2013.
- 3.FDA. Form FDA 483. Available at: www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM304252.pdf. Accessed Nov 25, 2013. [Google Scholar]
- 4.FDA. How to report a pet food complaint. Available at: www.fda.gov/AnimalVeterinary/SafetyHealth/ReportaProblem/ucm182403.htm. Accessed Nov 25, 2013.
- 5.FDA. Investigation of multistate outbreak of human infections linked to dry pet food. Available at: www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm302904.htm. Accessed Nov 25, 2013.
- 6.FDA. Animal and veterinary. Recalls and withdrawals. Available at www.fda.gov/animalVeterinary/safetyhealth/recallswithdrawals/default.htm. Accessed Dec 12, 2013. [Google Scholar]
- 7.FDA. Veterinary Laboratory Investigation and Response Network. Available at: www.fda.gov/AnimalVeterinary/ScienceResearch/ucm247334.htm. Accessed Nov 25, 2013.
- 8.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011;17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Reports of selected Salmonella outbreak investigations. Available at: www.cdc.gov/salmonella/outbreaks.html. Accessed Nov 25, 2013.
- 10.Barton Behravesh C, Mody RK, Jungk J, et al. 2008 outbreak of Salmonella Saintpaul infections associated with raw produce. N Engl J Med 2011;364:918–927. [DOI] [PubMed] [Google Scholar]
- 11.Cavallaro E, Date K, Medus C, et al. Salmonella Typhimurium infections associated with peanut products. N Engl J Med 2011;365:601–610. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Surveillance for foodborne disease outbreaks—United States, 2009–2010. MMWR Morb Mortal Wkly Rep 2013;62:41–47. [PMC free article] [PubMed] [Google Scholar]
- 13.Hale CR, Scallan E, Cronquist AB, et al. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis 2012;54(suppl 5):S472–S479. [DOI] [PubMed] [Google Scholar]
- 14.Barton Behravesh C, Ferraro A, Deasy M, et al. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 2010;126:477–483. [DOI] [PubMed] [Google Scholar]
- 15.Carter ME, Quinn PJ. Salmonella infections in dogs and cats. In: Wray C, Wray A, eds. Salmonella in domestic animals. Wallingford, Oxfordshire, England: CAB International, 2000;231–244. [Google Scholar]
- 16.Marks SL, Rankin SC, Byrne BA, et al. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J Vet Intern Med 2011;25:1195–1208. [DOI] [PubMed] [Google Scholar]
- 17.Tupler T, Levy JK, Sabshin SJ, et al. Enteropathogens identified in dogs entering a Florida animal shelter with normal feces or diarrhea. J Am Vet Med Assoc 2012;241:338–343. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez S, Hofacre CL, Lee MD, et al. Animal sources of salmonellosis in humans. J Am Vet Med Assoc 2002;221:492–497. [DOI] [PubMed] [Google Scholar]
- 19.Finley R, Reid-Smith R, Weese JS. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin Infect Dis 2006;42:686–691. [DOI] [PubMed] [Google Scholar]
- 20.NIH. Grants and funding. Evaluation of Salmonella in symptomatic and asymptomatic pets: study for the Vet-LRN program. Available at: www.grants.nih.gov/grants/guide/rfa-files/RFA-FD-11-010.html. Accessed Dec 12, 2013. [Google Scholar]
- 21.AVMA. 2012 US pet ownership and demographics sourcebook. Schaumburg, Ill: AVMA, 2012. [Google Scholar]
- 22.Thompson A Ingredients: where pet food starts. Top Companion Anim Med 2008;23:127–132. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Bethune LA, Jia Y, et al. Surveillance of Salmonella prevalence in animal feeds and characterization of the Salmonella isolates by serotyping and antimicrobial susceptibility. Foodborne Pathog Dis 2012;9:692–698. [DOI] [PubMed] [Google Scholar]
- 24.FDA. Get the facts! Raw pet food diets can be dangerous to you and your pet. Available at: www.fda.gov/animalveterinary/resourcesforyou/animalhealthliteracy/ucm373757.htm. Accessed Nov 21, 2013.
- 25.Clark C, Cunningham J, Ahmed R, et al. Characterization of Salmonella associated with pig ear dog treats in Canada. J Clin Microbiol 2001;39:3962–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Human salmonellosis associated with animal-derived pet treats—United States and Canada, 2005. MMWR Morb Mortal Wkly Rep 2006;55:702–705. [PubMed] [Google Scholar]
- 27.FDA. FDA proposed rule to establish current good manufacturing practice and hazard analysis and risk-based preventive controls for food for animals. Available at: www.fda.gov/Food/GuidanceRegulation/FSMA/ucm366510.htm. Accessed Nov 21, 2013.
- 28.FDA. FDA issues proposed rule under FSMA to improve safety of food for animals. Available at: www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm372128.htm. Accessed Nov 21, 2013.
- 29.Gaffga NH, Barton Behravesh C, Ettestad PJ, et al. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N Engl J Med 2012;366:2065–2073. [DOI] [PubMed] [Google Scholar]
- 30.Harris JR, Bergmire-Sweat D, Schlegel JH, et al. Multistate outbreak of Salmonella infections associated with small turtle exposure, 2007–2008. Pediatrics 2009;124:1388–1394. [DOI] [PubMed] [Google Scholar]
- 31.Mettee Zarecki SL, Bennett SD, Hall J, et al. US outbreak of human Salmonella infections associated with aquatic frogs, 2008–2011. Pediatrics 2013;131:724–731. [DOI] [PubMed] [Google Scholar]
- 32.CDC. Gastrointestinal (enteric) diseases from animals. Available at: www.cdc.gov/zoonotic/gi/. Accessed Nov 25, 2013.
- 33.CDC. Multistate outbreak of human Salmonella Infantis infections linked to dry dog food. Available at: www.cdc.gov/salmonella/dog-food-05-12/index.html. Accessed Nov 25, 2013.
- 34.CDC. CDC Features. Pet food and treats—tips for keeping people and pets healthy and safe from Salmonella. Available at: www.cdc.gov/features/salmonelladrypetfood. Accessed Nov 25, 2013. [Google Scholar]
- 35.Kukanich KS. Update on Salmonella spp contamination of pet food, treats, and nutritional products and safe feeding recommendations. J Am Vet Med Assoc 2011;238:1430–1434. [DOI] [PubMed] [Google Scholar]
- 36.CDC. Tips for keeping people and pets healthy and safe from germs in pet food (poster). Available at: www.cdc.gov/zoonotic/gi/education.html. Accessed Nov 25, 2013.


