Abstract
Apoptosis of virus-infected cells occurs either as a direct response to viral infection or upon recognition of infection by the host immune response. Apoptosis reduces production of new virus from these cells, and therefore viruses have evolved inhibitory mechanisms. We previously showed that laboratory strains of herpes simplex virus type 1 (HSV-1) protect infected cells from apoptosis induced by cytotoxic T lymphocytes or ethanol. We have now evaluated the ability of HSV-1 and HSV-2 laboratory and clinical isolates to inhibit apoptosis induced by anti-Fas antibody or UV irradiation and explored the genetic basis for this inhibition. HSV-1 isolates inhibited apoptosis induced by UV or anti-Fas antibody. In contrast, HSV-2 clinical isolates failed to inhibit apoptosis induced by either stimulus, although the HSV-2 laboratory strain 333 had a partial inhibitory effect on UV-induced apoptosis. Inhibition of apoptosis by HSV was accompanied by marked reduction of caspase-3 and caspase-8 activity. Deletion of the HSV-1 Us3 gene markedly reduced inhibition of UV-induced apoptosis and partially abrogated inhibition of Fas-mediated apoptosis. Conversely, deletion of the HSV-1 Us5 gene markedly reduced protection from Fas-mediated apoptosis and partially abrogated protection from UV. The Us11 and Us12 genes were not necessary for protection from apoptosis induced by either stimulus. The differences between HSV-1 and HSV-2 in the ability to inhibit apoptosis may be factors in the immunobiology of HSV infections.
Successful viruses use multiple mechanisms to alter host cell functions to their advantage. For example, the herpes simplex viruses (HSVs) have evolved mechanisms to inhibit host cell death, or apoptosis. Recently we have demonstrated that HSV type 1 (HSV-1) infection protects target cells from cytotoxic T-lymphocyte (CTL)-induced apoptosis (16). This phenomenon is intuitively beneficial to the virus in that it may protect infected cells from attack by the immune system.
It has been suggested that programmed cell death may have first evolved in unicellular organisms as a primitive population immune system, in which cells infected with a virus would activate their death programs, thus protecting other members of the population (31, 36). In a similar manner, individual cells in multicellular organisms often respond to viral infection by undergoing apoptosis, thus protecting other cells of the organism from infection (32). In addition to our work with CTL-induced apoptosis, Leopardi et al. have shown that HSV-1 can protect cells from apoptosis induced by the virus, through the action of the Us3 gene (19). This presumably allows infected cells to survive long enough to support viral replication.
Recent reports have suggested that viruses may selectively inhibit some but not all portions of the apoptotic machinery. For example, equine herpes virus 8 and molluscum contagiosum virus inhibit apoptosis induced via the Fas receptor, by encoding gene products containing a nonfunctional death domain which interacts with Fas-associated death domain protein (FADD) (6, 33). However, these genes are unable to inhibit apoptosis induced by UV radiation (6). Along similar lines, we have recently shown that HSV-1 inhibits the nuclear events of ethanol-induced apoptosis, such as DNA fragmentation, but has no effect on phosphatidylserine externalization at the cell membrane (16). In contrast, HSV-2 showed no antiapoptotic activity under these conditions.
As a corollary to differences in their relative inhibition of various manifestations of apoptosis, some viruses have evolved multiple apoptosis-inhibitory mechanisms. Herpesviruses, being large, are good candidates to have multiple antiapoptosis genes (5, 32). It has been previously reported that the Us3 gene of HSV-1 is required to inhibit apoptosis induced by the virus (19). However, in our hands, viruses deleted for Us3 retained some antiapoptotic activity. These findings led us to investigate whether HSV-1 encodes additional genes which inhibit apoptosis and whether HSV-2 can also inhibit apoptosis induced by some stimuli. In this report, we demonstrate that HSV-1 encodes at least two antiapoptotic genes, Us5 and Us3, which strongly inhibit apoptosis induced via the Fas receptor or UV radiation. In contrast, at least one HSV-2 strain may modestly inhibit apoptosis induced by UV but does not inhibit apoptosis induced by Fas ligation. Using virus strains with deletions in the HSV-1 genes Us5 and Us3, we demonstrated that these genes cooperate to inhibit apoptosis. The evolution of two HSV genes which inhibit apoptosis suggests that this is an important function for the virus and thus may play a role in the biology and transmission efficiencies of HSV-1 and HSV-2.
MATERIALS AND METHODS
Cell lines.
Vero and Jurkat cells were obtained from the American Type Culture Collection (Manassas, Va.). Cell lines were screened for mycoplasma contamination in an in situ hybridization assay (Geneprobe, San Diego, Calif.).
Viruses.
Clinical isolates of HSV-1 (F28700, H61146, and M36562) and HSV-2 (F43031 and T75419) were obtained from the University of Washington diagnostic virology laboratory. Laboratory strains were E115 (HSV-1) and 333 (HSV-2). Viral stocks were grown in Vero cells, and titers were determined by standard plaque assays. The Us3 deletion and rescue viruses, R7041 and 7306, were a gift from Bernard Roizman (26). The Us5 deletion and rescue viruses, RAS116 and RAS137, are described elsewhere (30).
Morphological assay using acridine orange and ethidium bromide.
Morphological changes of apoptosis were observed by ethidium bromide-acridine orange staining and fluorescence microscopy (Zeiss model 9901). Jurkat cells were split into log phase growth and incubated at 37°C for 12 h. Cells were then infected with virus at 10 PFU/cell, or mock infected, and incubated at 37°C for 5 h. Infected cells were induced into apoptosis by either UV light (30-W UV bulb at 20 cm for 30 s) or anti-Fas antibody APO-1-3 (Alexis, San Diego, Calif.) or CH-11 (Tanvera, Madison, Wis.) at 1,000 ng/ml and incubated at 37C for 4 h. In general, antibody CH-11 induced apoptosis more strongly than did APO-1-3. A minimum of 200 cells were counted from each sample by a blinded observer and categorized into one of three groups by morphological characteristics: live (cells showing no characteristics of apoptosis), apoptotic (cells showing shrinkage, membrane blebbing, and/or nuclear fragmentation), or necrotic (cells that did not show apoptotic morphology but allowed entry of ethidium bromide). Results were analyzed with the two-tailed Student’s t test, using the statistical package in Microsoft Excel (Microsoft Corp., Redmond, Wash.), and are presented as mean and standard error of the mean (SEM).
Fluorometric assay for caspase-3 or caspase-8 activation.
Jurkat cells (106) were infected with HSV at 10 PFU/cell or mock infected and incubated at 37°C for 5 h. Cells were then induced into apoptosis with UV irradiation or anti-Fas antibody and incubated for 4 h at 37°C. The cells were harvested, and lysates were evaluated for caspase activity by using ApoAlert caspase-3 and caspase-8 assay kits (Clontech, Palo Alto, Calif.) according to the manufacturer’s instructions. Fluorescence was measured at 0, 30, and 60 min after addition of substrate on a CytoFluor II fluorescence plate reader (PerSeptive Biosystems, Framingham, Mass.). To allow comparison between separate experiments which may have different maximal relative fluorescence unit (RFU) measurements, the RFU value for control (uninfected) cells induced into apoptosis as measured at 60 min was converted to a relative activity value of 100, and other RFU values within the same experiment were normalized to obtain a relative (percent) activity. Results were analyzed with the two-tailed Student’s t test, using the statistical package in Microsoft Excel.
Effect of apoptosis on viral titer.
Jurkat cells were split into log-phase growth overnight and then infected with HSV at an 5 PFU/cell; 2 or 5 h later the cells were exposed to UV as described above and incubated at 37°C. Virus was harvested from the cells at various time points by sonication, and yield was determined in a standard plaque assay.
RESULTS
Effects of isolates of HSV-1 and HSV-2 on UV-induced apoptosis.
We previously showed that infection with laboratory strains of HSV-1 rendered cells resistant to apoptosis induced by either ethanol or CTL (16). Because CTL use multiple pathways to induce apoptosis in their targets, we investigated the ability of HSV-1 to inhibit apoptosis induced by the Fas receptor, a well-defined pathway involved in both CTL killing and immune regulation (12). To determine whether the ability of HSV-1 to inhibit apoptosis varies depending on the inducing stimulus, we also examined the ability of HSV-1 to inhibit apoptosis induced by UV irradiation. UV-induced apoptosis is mediated in part by a p53-dependent mechanism (13, 35) and partially through other pathways (1, 34). To confirm that our previous findings for laboratory strains of HSV were generally applicable to field strains, we also used several low (three or fewer)-passage clinical isolates.
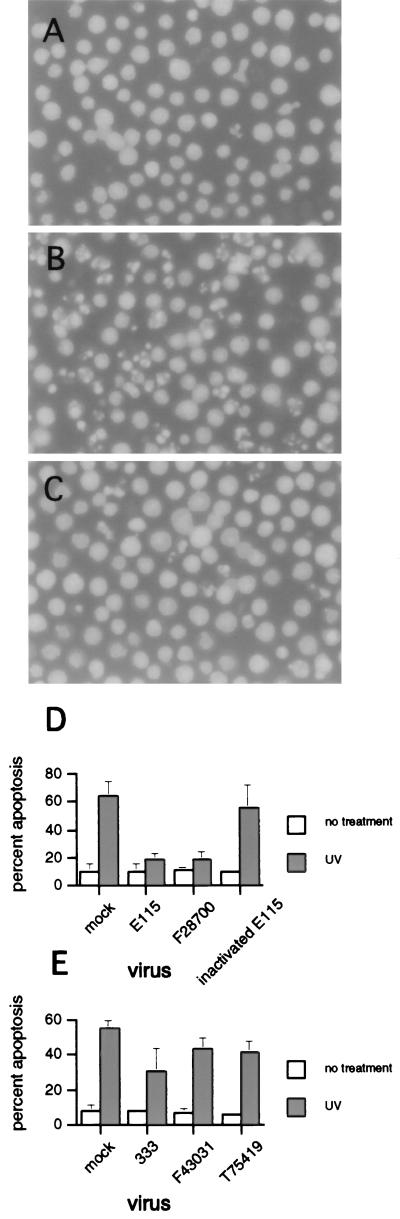
Jurkat cells were infected with an isolate of HSV-1 or mock infected and then incubated for 5 h to allow full expression of the antiapoptotic effect (16). The cells were then UV or mock treated and incubated 4 h before morphologic evaluation by a blinded observer. A representative experiment is shown in Fig. 1. Uninfected, UV-irradiated cells showed clear apoptotic morphology, with markedly shrunken and fragmented nuclei (Fig. 1B). In contrast, cells which were preinfected with a clinical isolate of HSV-1 (strain H61146) were markedly protected from UV-induced apoptosis (Fig. 1C) and had a morphology almost indistinguishable from that of cells which had been left untreated (Fig. 1A). In a blinded manner, cells with healthy, apoptotic, or necrotic morphology were counted in a hemacytometer, which allowed quantification of the antiapoptotic effect of each HSV-1 strain (Fig. 1D). Both the laboratory strain E115 and the clinical isolate F28700 showed strong inhibition of UV-induced apoptosis (P < 0.002 and <0.001, respectively). In contrast, inactivated virus failed to inhibit apoptosis.
FIG. 1.
Clinical and laboratory strains of HSV-1 and HSV-2 inhibit UV-induced apoptosis. Jurkat cells were mock or HSV infected for 5 h prior to induction of apoptosis with UV radiation. The morphological changes of apoptosis were assessed by a blinded observer using fluorescence microscopy. (A) Uninfected cells without UV irradiation; (B) uninfected cells after UV irradiation; (C) HSV-1-infected cells (strain H61146) after treatment with UV; (D and E) percent apoptosis after infection with a clinical (F28700, F43031, and T75419) or laboratory (E115 and 333) strains of HSV-1 (D) and HSV-2 (E). Shown is mean + SEM of two to five independent experiments. In some instances, error bars are too small to be seen.
We next tested the ability of strains of HSV-2 to inhibit UV-induced apoptosis. We previously showed that HSV-2 failed to inhibit apoptosis induced by CTL or ethanol (16). In agreement with those results, the clinical isolates of HSV-2 did not show an inhibitory effect on UV-induced apoptosis (Fig. 1E). The laboratory HSV-2 strain 333 appeared to show a modest inhibitory effect; however, this did not reach statistical significance.
Effects of clinical isolates of HSV-1 and HSV-2 on Fas-mediated apoptosis.
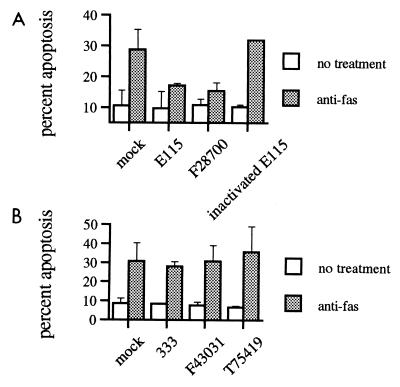
Because our previous studies demonstrated that laboratory strains of HSV-1, but not HSV-2, could inhibit apoptosis induced by CTL (16), we examined whether clinical or laboratory isolates of these viruses might inhibit apoptosis mediated through the Fas receptor. The interaction of Fas ligand on CTL with the Fas receptor on target cells is one of the mechanisms by which CTL induce apoptosis in their targets and is especially important in immune regulation. Consistent with our previous results with CTL-induced apoptosis, strains of HSV-1 showed inhibition of Fas-mediated apoptosis (Fig. 2A, P < 0.01). Again, the antiapoptotic ability was lost after inactivation of virus.
FIG. 2.
Strains of HSV-1, but not HSV-2, inhibit Fas-mediated apoptosis. Percent apoptosis by anti-Fas antibody APO-1-3 after infection with clinical (F28700, F43031, and T75419) or laboratory (E115 and 333) strains of HSV-1 (A) or HSV-2 (B), as determined by a blinded observer using fluorescence microscopy. Shown is mean + SEM of two to five independent experiments. In some instances, error bars are too small to be seen.
In contrast to the inhibition seen with HSV-1, none of the HSV-2 strains were able to inhibit Fas-mediated apoptosis (Fig. 2B). Even the laboratory strain 333, which showed a possible modest inhibitory effect on UV-induced apoptosis, failed to inhibit Fas-mediated apoptosis.
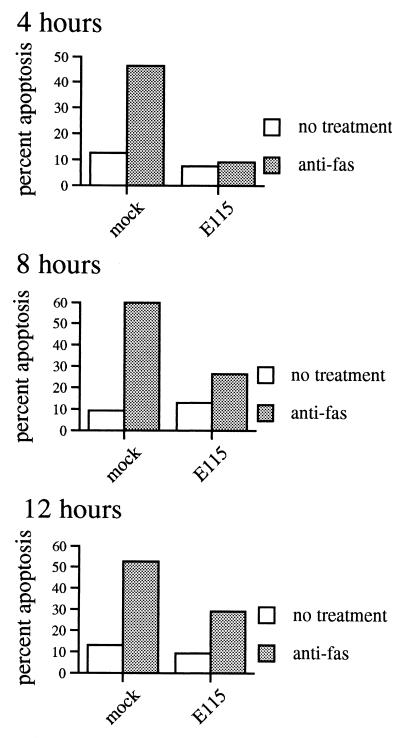
Although HSV-1 protected Jurkat cells from apoptosis at 4 h after treatment with anti-Fas, many previous studies of Fas-mediated cell death in Jurkat cells have used longer incubation times (typically 12 h) to allow full expression of Fas-mediated apoptosis. We therefore performed a time course experiment to evaluate whether HSV could protect Jurkat cells from Fas-mediated death at these later time points. Jurkat cells were mock or HSV-1 infected and 5 h later induced into apoptosis with anti-Fas antibody CH11. Protection from apoptosis was then determined 4, 8, and 12 h after addition of anti-Fas antibody (Fig. 3). Even 12 h posttreatment, HSV-1-infected cells were markedly (>50%) protected from Fas-mediated cell death. The degree of protection was stronger at early time points and decreased somewhat over time, in agreement with our earlier observation that HSV-1 protection from anti-Fas-antibody-induced apoptosis is not necessarily complete. Nevertheless, even at relatively late time points, HSV-1 still strongly protected cells from Fas-mediated death. We evaluated the cultures at a still later time point (24 h after addition of anti-Fas), but extensive viral cytopathic effect in the infected cells precluded evaluation of apoptosis. These observations are consistent with our hypothesis that HSV-1 need only inhibit (or delay) apoptosis until the viral replication cycle can complete.
FIG. 3.
Time course of HSV inhibition of Fas-mediated apoptosis. Jurkat cells were mock or HSV-1 infected, incubated for 5 h, and treated with 1,000 ng of anti-Fas antibody CH11 per ml. Apoptosis was evaluated at the indicated times after anti-Fas treatment by a blinded observer using fluorescence microscopy. Cells could not be evaluated at 24 h due to extensive viral cytopathic effect. Shown is mean of two independent experiments.
Inhibition of caspase activation by HSV-1 and HSV-2.
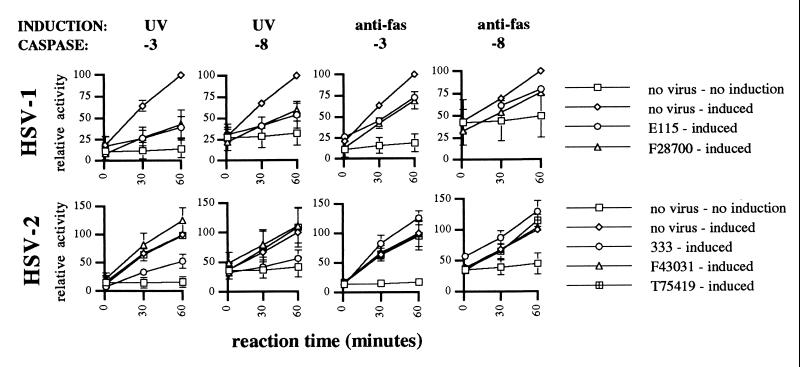
Morphologic examination of cells for apoptosis by a blinded observer is a well accepted and reproducible method of evaluating apoptosis (22), but it provides limited insights into the mechanisms by which inhibition of apoptosis might occur. We therefore investigated whether HSV infection resulted in inhibition of caspase activation during apoptosis. Caspases are cytoplasmic proteins which normally exist in an inactive proenzyme form (20). Upon cleavage, typically by another caspase or apoptosis regulatory protein, caspases are activated and cleave downstream caspases or other apoptotic proteins. Infection with HSV-1 inhibited the activation of caspase-3 by 70 to 75% after UV induction of apoptosis (Fig. 4, P < 0.001). Caspase-3 activation after Fas ligation was also inhibited by HSV-1, although only 30 to 35% (Fig. 4, P < 0.002). Similarly, HSV-1 inhibited caspase-8 (FLICE) activation about 70% after UV treatment (P = 0.003) and 30% after Fas ligation (P < 0.001). HSV-1 infection alone did not result in activation of caspases (not shown) and in fact led to a consistent slight decrease in the level of background caspase-3 and caspase-8 activity. These findings are in agreement with the substantial inhibition of the morphologic changes of apoptosis after infection with HSV-1 and the apparently stronger inhibition of UV- than Fas-mediated apoptosis.
FIG. 4.
Inhibition of caspase activity after UV or anti-Fas treatment by HSV-1 and HSV-2. Jurkat cells were mock or HSV infected for 5 h prior to induction of apoptosis with UV radiation or anti-Fas antibody. Caspase-3 (-8) or caspase-8 (-8) activity was measured in cell lysates made 4 h later. Virus infection alone with HSV-1 or HSV-2 did not lead to caspase-3 or caspase-8 activation (not shown). Shown is mean ± SEM of from two to five independent experiments. In some instances, error bars are too small to be seen.
In agreement with their inability to inhibit the morphologic changes of apoptosis, none of the clinical isolates of HSV-2 showed any inhibitory effect on caspase-3 or caspase-8 activation after apoptosis induction by UV or anti-Fas (Fig. 4). Again, infection with HSV-2 alone did not lead to activation of caspase-3 or caspase-8 (not shown). The HSV-2 laboratory strain 333 also failed to show inhibition of caspase activation after Fas ligation. However, 333 behaved differently after UV induction of apoptosis, showing inhibitory activity against both caspase-3 and caspase-8 (Fig. 4). Caspase-3 activity after UV was inhibited 50 to 60% (P = 0.01), while caspase-8 activity was inhibited 70 to 80% (P < 0.05). Again, this finding is in agreement with the modest inhibition of the morphologic changes of apoptosis seen with strain 333.
The HSV-1 genes Us3 and Us5 are required for the antiapoptotic effect.
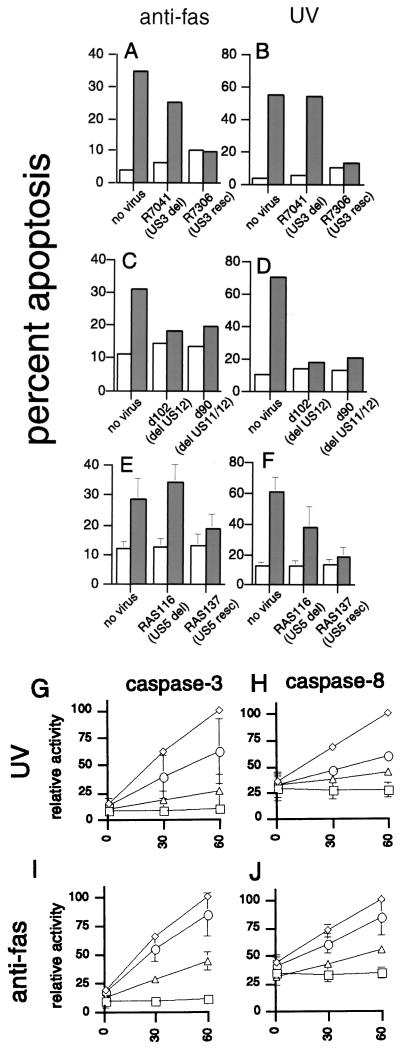
To investigate the mechanisms used by the virus to inhibit apoptosis, we used mutant virus with deletions of individual antiapoptosis genes. The HSV-1 gene Us3 encodes a serine/threonine kinase which has been shown to prevent apoptosis from occurring as a direct result of viral infection (19). To determine whether this gene could also function to protect infected cells from apoptosis induced by other stimuli, we infected Jurkat cells with either a mutant HSV-1 containing a deletion of Us3 (R7041) or the Us3 rescue virus R7306. R7306 has had the Us3 gene restored and thus would be expected to behave as wild-type virus. Inclusion of rescue virus controls are important to exclude possible second mutations in deletion viruses (19). Deletion of Us3 (R7041) completely abrogated the ability of the virus to prevent apoptosis induced by UV (Fig. 5B) and greatly reduced its ability to inhibit apoptosis induced by Fas ligation (Fig. 5A). The rescue virus, R7306, which has had Us3 function restored (19), regained full inhibitory activity after either stimulus. These results suggest that the Us3 gene product not only inhibits apoptosis induced by the virus but also contributes to protection from UV- or anti-Fas-induced apoptosis. However, the partial protection from Fas-induced apoptosis even by the Us3 deletion virus suggested that additional antiapoptosis genes might be present.
FIG. 5.
Inhibition of Fas-mediated or UV-induced apoptosis by deletion mutants of HSV-1. Jurkat cells were infected with the indicated mutant virus at 10 PFU/cell for 5 h, and apoptosis was induced by anti-Fas antibody (A, C, and E) or UV irradiation (B, D, and F). Open bars, untreated cells; shaded bars, treated cells. Percent apoptosis was determined by a blinded observer using fluorescence microscopy. Shown is the mean + SEM of two to four independent experiments. (G to J) Inhibition of caspase activity by HSV-1 deletion mutant RAS116 (○) and rescue mutant RAS137 (▵). Jurkat cells were mock infected (◊) or HSV infected for 5 h prior to induction of apoptosis with UV radiation or anti-Fas antibody. Caspase-3 or caspase-8 activity was measured in cell lysates made 4 h. Shown is mean ± SEM of two to five independent experiments. In some instances, error bars are too small to be seen. □, uninduced mock-infected cells.
Because viruses with deletion of the Us3 gene still retained some antiapoptotic ability, we sought other HSV genes which might mediate the antiapoptotic effect. Our previous results of assays using inhibitors of late gene expression suggested that the antiapoptotic effect was most likely encoded by an immediate-early or early gene (16), so we initially focused our attention on one of the immediate-early genes of HSV, Us12. Us12 has been shown to function in immune evasion by inhibiting CTL recognition of infected cells through prevention of peptide loading of major histocompatibility complex class I (8, 14). Since another mechanism by which HSV can evade CTL is through inhibition of apoptosis (16), we tested whether Us12 might also have an antiapoptosis function. A mutant strain of HSV, d102, which is deleted for Us12, still showed strong inhibitory ability for both anti-Fas and UV-induced apoptosis (Fig. 5C and D), suggesting that this gene is not involved in the suppression of apoptosis. Similarly, another mutant HSV-1, d90, which has mutations of both Us12 and an adjacent gene, Us11, showed strong antiapoptotic ability (Fig. 5C and D). These results demonstrate that neither Us11 nor Us12 is required for the antiapoptotic activity of HSV.
Since HSV-1 deleted for Us3 still showed partial inhibition against Fas-induced apoptosis, we postulated that the other antiapoptotic function might be mediated through a protein targeted to the membrane, where Fas and its associated molecules are found. HSV has several glycoproteins which are transported to cytoplasmic membranes of the cell and are later incorporated into the viral envelope during envelopment (28). These glycoproteins have a variety of functions (28) and are commonly involved in viral entry or spread. However, one small HSV gene, Us5, encodes a glycoprotein (gJ) with no known function (11). HSV strains deleted for Us5 have been reported to be phenotypically normal, including normal plaque formation and cell-to-cell spread of the virus (4, 7). Since these properties do not exclude a role in modulation of apoptosis, we tested whether this gene might be involved in HSV inhibition of apoptosis. In contrast to the lack of effect on antiapoptotic function by deletions of Us11 and Us12, deletion of the HSV Us5 gene profoundly affected the antiapoptotic ability of the virus. The Us5 deletion virus, RAS116, was completely unable to inhibit Fas-mediated apoptosis (Fig. 5E) and had also lost much of its ability to inhibit UV-induced apoptosis (Fig. 5F). Rescue of the Us5 gene (virus RAS137) restored the ability of the virus to fully inhibit anti-Fas- or UV-induced apoptosis. The role of Us5 in the inhibition of DNA fragmentation during apoptosis was confirmed by agarose gel electrophoresis (not shown). Cells infected with RAS116 showed DNA fragmentation after UV or anti-Fas treatment very similar to that of uninfected cells. In contrast, cells infected with RAS137 showed no detectable DNA fragmentation. These results suggest that the Us5 gene product, like Us3, acts to block portions of the apoptosis pathway involved in both anti-Fas- and UV-induced apoptosis. However, the absolute requirement for Us5 is for the inhibition of Fas-mediated apoptosis, while partial inhibition of UV-induced apoptosis is possible without this gene.
To investigate the effect of the Us5 gene on the apoptosis pathways involved in Fas- or UV-induced apoptosis, we evaluated caspase-3 and caspase-8 activation in cells infected with the Us5 deletion or rescue virus. As expected, deletion of Us5 (RAS116) almost completely abrogated the ability of the virus to inhibit caspase-3 or caspase-8 activation after Fas ligation (Fig. 5I and J). Rescue of the Us5 gene (RAS137) restored the ability of the virus to inhibit activation of these caspases. As also predicted by the morphology experiments, deletion of Us5 significantly reduced the ability of the virus to inhibit caspase-3 or caspase-8 activation after UV irradiation (Fig. 5G and H), although to a lesser extent than after anti-Fas treatment. Rescue of the Us5 gene restored the ability of the virus to inhibit activation of both caspases after UV irradiation.
DISCUSSION
These experiments provide several novel insights into the ability of HSV-1 and HSV-2 to inhibit apoptosis of infected cells. First, we have shown for the first time that the HSV-1 gene Us5 is required for protection from apoptosis induced by certain stimuli. Second, our results demonstrate that HSV infection of cells has significant consequences on regulatory aspects of the apoptosis machinery, such as caspase activation. Third, we have shown that the previously reported (16) significant differences between HSV-1 and HSV-2 in the ability to inhibit CTL-induced apoptosis extend to apoptosis induced by UV irradiation or Fas ligation. Finally, our results are consistent with the hypothesis that inhibition of apoptosis is an adaptive strategy of HSV which might serve to augment viral yields in the presence of proapoptotic stimuli.
Previous studies suggested that the antiapoptotic effect of HSV-1 was mediated by an immediate-early or early gene. This conclusion was based either on time course studies of the expression of the antiapoptotic effect (16, 18) or on pharmacologic inhibition of HSV late gene expression (16). Our studies are consistent with a previous report by Leopardi et al. demonstrating that the Us3 gene of HSV-1 was necessary to prevent apoptosis induced by the virus (19). Our results extend these findings and establish that Us3 also contributes to protection of infected cells from UV- or anti-Fas-mediated apoptosis. However, our finding that partial inhibition can occur in the absence of Us3 also suggests that other potentially complementary mechanisms are operative.
Several authors have suggested that the antiapoptotic function of HSV may be encoded by more than one gene. Nevertheless, we were somewhat surprised to find that the HSV-1 gene Us5 contributed to the inhibition of Fas- and UV-induced apoptosis. The Us5 gene contains a small open reading frame predicted to encode a small, membrane-associated glycoprotein, gJ (23). Recent work by Ghiasi et al. has confirmed the expression and localization of gJ to the surface of infected cells (11). Nevertheless, no function for this protein has been found, and viruses deleted for Us5 have been reported to be phenotypically normal, including normal plaque formation and cell-to-cell spread of the virus (4, 7). We have been unable to detect homology between Us5 and other known viral or cellular antiapoptosis genes. Since Us5 is predicted to localize to the cell membrane and appears especially important for the inhibition of fas-mediated apoptosis, it may act directly upon signaling via the Fas receptor. For example, Us5 might interfere with trimerization of Fas after ligation (2), or it may interfere with recruitment of Fas-associated molecules to the death-inducing signaling complex (24, 25, 29). However, Us5 does not appear to contain a death domain as do the viral FLICE-inhibitory proteins (v-FLIPs) (6, 33), and so its mechanism of action is likely different from that of the v-FLIPs. Inhibitors of death receptors can be bypassed by many proapoptotic stimuli, including perforin/granzyme B (17), which might explain the coevolution of other antiapoptosis functions such as that encoded by Us3. Experiments are under way to determine the exact mechanism of Us5 function.
Since Us5 appears to be more critical for the inhibition of Fas-mediated apoptosis than is Us3, these genes may function in somewhat complementary roles. There may also be additional antiapoptotic genes within the HSV genome. For example, a recent report suggests that ICP27 may also contribute to inhibition of apoptosis by HSV-1 (3). Experiments evaluating the role of other immediate-early and early genes in the inhibition of apoptosis by HSV are under way. It is possible that certain HSV genes are antiapoptotic only in certain experimental systems, since some aspects of the antiapoptotic effect appear to be cell type specific (10). HSV late genes may also be involved in inhibition of apoptosis, and transactivation of these genes is a possible mechanism of action for immediate-early or early genes with regulatory functions.
We have previously reported that HSV-1, but not HSV-2, can protect infected cells from apoptosis induced by ethanol or CTL (16). Our results here demonstrate that HSV-2 does not inhibit Fas-mediated apoptosis but that the laboratory strain 333 may have a modest inhibitory effect on UV-induced apoptosis. This is in contrast to clinical isolates of HSV-2, which fail to inhibit apoptosis induced by either stimulus. It is possible that the inhibition of apoptosis by 333 relates to its adaptation to laboratory growth. We are presently testing additional clinical isolates to determine whether any show antiapoptotic function. Since HSV-2 strain 333 inhibits caspase-3 and caspase-8 activation more strongly than it inhibits the morphologic changes of apoptosis, the apoptosis-regulatory pathways may potentially bypass the block of these caspases by HSV-2 and induce the terminal changes of apoptosis through other mechanisms. Our finding that two HSV genes, Us3 and Us5, have different and somewhat complementary antiapoptosis functions may shed some light on the different inhibitory abilities of HSV-1 and HSV-2. The Us3 gene product is well conserved between HSV-1 and HSV-2, with 75% identity. In contrast, the Us5 gene is less conserved between HSV-1 and HSV-2, with only 43% identity. Deletion of either Us3 or Us5 from HSV-1 markedly degraded the ability of the virus to inhibit Fas- or UV-induced apoptosis, and so the sequence differences of HSV-2 relative to HSV-1 may be sufficient to eliminate the antiapoptosis effect. The modest inhibitory effect of HSV-2 strain 333 against UV-induced apoptosis may reflect the closer conservation of the Us3 sequence, which appears to be more critical than Us5 for this function. It is also possible, and perhaps likely, that additional antiapoptosis genes exist in HSV-1 or HSV-2 and may help to explain the differences seen between these viruses. Further work defining the sequences responsible for these effects is in progress and may help resolve these issues.
In addition to the role of Us3 and Us5 in the inhibition of Fas- or UV-induced apoptosis demonstrated here, Leopardi et al. have previously shown that Us3 acts to prevent apoptosis from being induced by the virus (19). Apoptosis induced by HSV is not mediated though the Fas receptor (15), and so these results taken together argue that HSV-1 has an antiapoptosis function which acts at a more central aspect of the apoptosis-regulatory pathway than specific Fas receptor inhibitors such as the v-FLIPs (6, 33). Recent data suggesting an inhibitory activity of HSV-1 which acts downstream of caspase-3 (9) support this hypothesis. The ability of HSV-1 to inhibit both caspase-8 and caspase-3 activation after UV- or anti-Fas antibody would be compatible with a second function acting upon Fas or its accessory molecules, also known as the death-inducing signaling complex (24, 25, 29). There is significant feedback between caspases, and caspase-8 can be activated by UV irradiation as well as Fas-Fas ligand interaction (27). In our experiments, HSV-1 appears to inhibit caspase-8 activation even more effectively after UV irradiation than after Fas ligation. If caspase-8 activation during apoptosis induced by mechanisms such as UV acts as an amplification mechanism for activation of other caspases, use of such a strategy may explain the cooperative inhibitory functions of Us3 and Us5 and the partial loss of inhibitory activity toward UV-induced apoptosis seen with viruses deleted for Us5.
One of the purposes of this study was to determine whether some of the differences in antiapoptotic ability between HSV-1 and HSV-2 seen in our previous work might be due to the use of high-passage laboratory strains of virus. The results presented here demonstrate that the differences in inhibitory ability seen between HSV-1 and HSV-2 hold for clinical isolates as well as laboratory strains. Nevertheless, there are intrastrain differences in antiapoptosis potency. For example, the HSV-2 laboratory strain 333 modestly inhibited UV-induced apoptosis whereas the clinical isolates did not (Fig. 1 and 4). Ongoing sequencing studies suggest that variations in the antiapoptosis genes may be responsible for these differences. In addition, differences in the temporal expression of antiapoptotic genes may explain some of these differences.
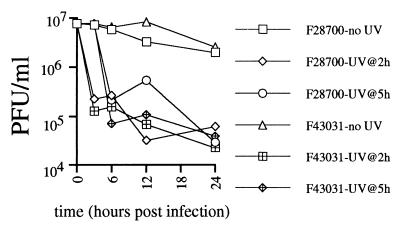
Finally, it has been suggested that inhibition of apoptosis by HSV is a helpful adaptation to the virus, resulting in increased viral yield in the presence of proapoptotic stimuli such as cellular responses to infection or immune pressure. The protection from apoptosis even at time points near the completion of the viral life cycle (when cytopathic effect becomes apparent) would support this suggestion. Koyama and Miwa showed that induction of apoptosis with sorbitol in infected HEp-2 cells modestly retarded virus multiplication (18). However, the virus used in those experiments strongly inhibited apoptosis, and thus the importance of inhibition of apoptosis in the maintenance of viral yield could not be evaluated. The inability of HSV-2 to inhibit UV-induced apoptosis compared to HSV-1, and the development of HSV-1 strains with deletions of the antiapoptosis genes, should provide the opportunity to directly test the importance of inhibition of apoptosis in the presence of external proapoptotic stimuli. We attempted to address this question in the Jurkat apoptosis system, through a one-step growth curve in the presence or absence of proapoptotic stimuli. Jurkat cells are clearly infectable by HSV, as demonstrated by protection from apoptosis, and obvious cytopathic effect at 24 h. However, there was no evidence of a viral eclipse in the Jurkat system (Fig. 6), and viral concentration never exceeded the levels of input virus (5 PFU/cell). On the contrary, viral titers slowly decayed over time, suggesting that viral replication in Jurkat cells is a relatively inefficient process. Treatment of cultures at 2 or 5 h postinfection with UV caused a rapid 2-log decrease in viral titer, possibly due to inactivation of unabsorbed, cell-free virus. The lack of efficient replication of HSV in Jurkat cells was evident in unirradiated cells as well, and viral titers slowly decayed in these cells over time. Thus, the poor replication of HSV in Jurkat cells precludes conclusions regarding the effect of inhibition of apoptosis on viral replication. We are currently pursuing similar time course experiments using cells better suited for replication of virus, such as the HEp-2 cells used by Koyama and Miwa (18), and also primary keratinocytes. These studies should allow determination of whether the inhibition of apoptosis has a positive effect on viral replication. Additional studies using single and multiple gene deletions will be required to evaluate the roles of Us3 and Us5 in the maintenance of viral yield in the presence of proapoptotic insults to the infected cell. Such studies may reveal an intricate system of redundant or complementary genes in HSV-1. Similar studies using more physiologic proapoptotic stimuli may also establish whether inhibition of apoptosis is an adaptive strategy that can be used by HSV-2 as well.
FIG. 6.
One-step growth curve of HSV in Jurkat cells. Jurkat cells were infected with HSV-1 (F28700) or HSV-2 (F43031) at 5 PFU/cell. Infected cultures were either left unirradiated or UV-irradiated at 2 or 5 h postinfection. Viral titers at the indicated time points were determined by a standard plaque assay.
ACKNOWLEDGMENTS
This work was supported by NIH grants K08-AI-01504 to K.R.J. and R01-AI-30731 to L.C.
We thank David Koelle, Jonathan Tait, and Nelson Fausto for useful discussions.
REFERENCES
- 1.Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger T A, Schwartz T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Aubert M, Blaho J A. The herpes simplex type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 5.Barry M, McFadden G. Apoptosis regulators from DNA viruses. Curr Opin Immunol. 1998;10:422–430. doi: 10.1016/s0952-7915(98)80116-7. [DOI] [PubMed] [Google Scholar]
- 6.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G-H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruh K, Ahn K, Djaballah H, Sempé P, van Endert P M, Tampé R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 9.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiasi H, Nesburn A B, Cai S, Wechsler S L. The Us5 open reading frame of herpes simplex virus type 1 does encode a glycoprotein (gJ) Intervirology. 1998;41:91–97. doi: 10.1159/000024919. [DOI] [PubMed] [Google Scholar]
- 12.Golstein P. Fas-based T cell-mediated cytotoxicity. Curr Top Microbiol Immunol. 1995;198:25–37. doi: 10.1007/978-3-642-79414-8_2. [DOI] [PubMed] [Google Scholar]
- 13.Henseleit U, Zhang J, Wanner R, Haase I, Kolde G, Rosenbach T. Role of p53 in UVB-induced apoptosis in human HaCaT keratinocytes. J Investig Dermatol. 1997;109:722–777. doi: 10.1111/1523-1747.ep12340708. [DOI] [PubMed] [Google Scholar]
- 14.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Koide W, Watanabe M, Kamiya H, Sakuri M. Apoptosis of cord blood T lymphocytes by herpes simplex virus type 1. J Gen Virol. 1997;78:1971–1975. doi: 10.1099/0022-1317-78-8-1971. [DOI] [PubMed] [Google Scholar]
- 16.Jerome K R, Tait J F, Koelle D M, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich C J, Tschopp J. FLIP inhibits apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- 18.Koyama A H, Miwa Y. Suppression of apoptotic DNA fragmentation in herpes simplex type 1-infected cells. J Virol. 1997;71:2567–2571. doi: 10.1128/jvi.71.3.2567-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin S J, Green D R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 21.Martz E, Gamble S R. How do CTL control virus infections? Evidence for prelytic halt of herpes simplex. Viral Immunol. 1992;5:81–91. doi: 10.1089/vim.1992.5.81. [DOI] [PubMed] [Google Scholar]
- 22.McGahon A J, Martin S J, Bissonnette R P, Mahboubi A, Shi Y, Mogil R J, Nishioka W K, Green D R. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 1995;46:153–185. doi: 10.1016/s0091-679x(08)61929-9. [DOI] [PubMed] [Google Scholar]
- 23.McGeoch D J. Evolutionary relationships of virion glycoprotein genes in the S regions of alphaherpesvirus genomes. J Gen Virol. 1990;71:2361–2367. doi: 10.1099/0022-1317-71-10-2361. [DOI] [PubMed] [Google Scholar]
- 24.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter M E, Kischel F C, Scheurerpflug C G, Medema J P, Debatin K-M, Krammer P H. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase-8) to the CD95 death-inducing signaling complex. Eur J Immunol. 1997;27:1207–1212. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
- 26.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehemtulla A, Hamilton C A, Chinnaiyan A M, Dixit V M. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 28.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 29.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sears, A. E., and H.-Y. Lee. 1999. Unpublished data.
- 31.Shub D A. Bacterial viruses. Bacterial altruism? Curr Biol. 1994;4:555–556. doi: 10.1016/s0960-9822(00)00124-x. . (Review.) [DOI] [PubMed] [Google Scholar]
- 32.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 34.Vile G F. Active oxygen species mediate the solar ultraviolet radiation-dependent increase in the tumour suppressor protein p53 in human skin fibroblasts. FEBS Lett. 1997;412:70–74. doi: 10.1016/s0014-5793(97)00748-5. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Levine A J. Differential regulation of the p21/WAF and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol Med. 1997;3:441–451. [PMC free article] [PubMed] [Google Scholar]
- 36.Yarmolinsky M B. Programmed cell death in bacterial populations. Science. 1995;267:836–837. doi: 10.1126/science.7846528. [DOI] [PubMed] [Google Scholar]