Abstract
Objectives:
To determine transmitted drug resistance (TDR) and HIV-1 genetic diversity in Bulgaria.
Methods:
The prevalence of TDR and HIV-1 subtypes was determined in 305/1446 (21.1%) persons newly diagnosed with HIV/AIDS from 1988 to 2011. TDR mutations (TDRMs) in protease and reverse transcriptase were defined using the WHO HIV drug mutation list. Phylogenetic analysis was used to infer polymerase (pol) genotype.
Results:
TDRMs were found in 16/305 (5.2%) persons, 11 (3.6%) with resistance to NRTIs, 5 (1.6%) with resistance to NNRTIs and 3 (0.9%) with resistance to PIs. Dual-class TDRMs were found in three (1.0%) patients and one statistically supported cluster of TDRMs comprising two individuals with subtype B infection. TDRMs were found in 10 heterosexuals, 4 MSM and two intravenous drug users. Phylogenetic analyses identified high HIV-1 diversity consisting of mostly subtype B (44.6%), subtype C (3.3%), sub-subtype A1 (2.6%), sub-subtype F1 (2.3%), sub-subtype A-like (3.6%), subtype G (0.3%), CRF14_BG (1.6%), CRF05_DF (1.3%), CRF03_AB (0.3%) and unique recombinant forms (1.3%).
Conclusions:
We found a low prevalence of TDR against a background of high HIV-1 genetic diversity among antiretroviral-naive patients in Bulgaria. Our results provide baseline data on TDR and support continued surveillance of high-risk populations in Bulgaria to better target treatment and prevention efforts.
Keywords: antiretroviral mutations, surveillance, viral heterogeneity, eastern Europe
Introduction
HAART has decreased morbidity and mortality in HIV-1 infections and reduces HIV-1 transmission in some risk groups, including newborns and sexual partners.1 Nonetheless, ART may select drug-resistant strains that can be transmitted from person to person. Infection with drug-resistant HIV may negatively impact first-line antiretroviral regimens.2 Therefore, the International AIDS Society–USA and European guidelines recommend HIV drug resistance testing for drug-naive patients before beginning ART.3,4 The highest rates of transmitted drug resistance (TDR) mutations (TDRMs) have been reported in North America (14.6%), followed by Europe (10.9%), Latin America (6.3%), Africa (4.7%) and Asia (4.2%), likely correlating with the historic availability of treatment in these countries.5–7 TDR varies widely in some Balkan countries, with 21.6% reported in Croatia, 14.75% in Romania, 12.5% in northern Greece, 4.7% in Slovenia and 8.8% in Serbia.8–12
Although ART was initiated in Bulgaria in 1987 with zidovudine monotherapy, followed by the addition of lamivudine in 1998 and inclusion of PIs in the regimen in 1999, very little is known about HIV-1 TDR in Bulgaria. In a preliminary study in 2008, we found genotypic evidence of TDRMs in 9.1% (2/22) of drug-naive patients.13 Following these findings, we implemented the European guidelines for resistance testing of antiretroviral-naive patients to better monitor HIV-1 TDRMs in Bulgaria. Our current study aims to further investigate TDRM prevalence and to expand our molecular epidemiological surveillance to better explore the evolutionary history of HIV-1 in Bulgaria.
Methods
Ethics statement
All patients provided written informed consent to participate in this study, which was approved by the Ethics Committee at the National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria (NCIPD) institutional review board (IRB) 00006384. The CDC IRB determined that participant consent was not required for the analysis of sequences in this study.
Study design and specimen preparation
Blood samples were collected from 305 ART-naive persons out of 1446 patients diagnosed with HIV/AIDS in Bulgaria between 1998 and 2011 at the National HIV Reference Laboratory and/or in the clinics responsible for the management of patients with HIV in Sofia, Plovdiv and Varna. Patients were from 29 different cities and various risk groups, including heterosexual persons (HETs), MSM, intravenous drug users (IDUs) and patients with other sexually transmitted infections (STIs).
Plasma samples were prepared at the National HIV Reference Laboratory as previously described and stored at ‒80°C.14 Specimens were linked to demographic and clinical data through an anonymous numerical code in accordance with the ethics standards of Bulgaria.
Sequence analysis
Plasma viral RNA was extracted using the MagCor Nucleic Acid Extraction Kit (RBC Bioscience, Taiwan). Protease and reverse transcriptase sequences of the HIV-1 pol gene were generated using the TruGene DNA Sequencing System (Siemens Healthcare, USA) following the manufacturer’s protocol.14 HIV-1 drug resistance mutations were determined according to the WHO 2009 Surveillance Drug Resistance Mutations (SDRM) list15 using the current Calibrated Population Resistance tool v5.0 of the Stanford University HIV Drug Resistance Database (http://cpr.stanford.edu/cpr.cgi). Nucleotide substitution models and alignments for phylogenetic analyses were prepared using MEGA516 and contained the Bulgarian pol sequences along with reference sequences from the Los Alamos HIV database.17 All 23 resistance mutation codons were manually removed from the alignment to exclude the possibility of convergent evolution.
Phylogenetic relationships and subtypes were inferred using Bayesian analysis with BEAST v1.8.0.18 Two independent 100×106 Markov chain Monte Carlo (MCMC) generations were used with sampling every 10000th generation. Statistical support was assessed with posterior probabilities. MCMC convergence was assessed by effective sampling size .200 using Tracer v1.6. The maximum clade credibility tree was chosen from the posterior distribution of 10000 sampled trees after burning in the first 1001 sampled trees with the program TreeAnnotator v1.8.0. HIV-1 subtypes were also inferred using the internet-based tools REGA and COMET as described in our previous study.14
Potential epidemiological clusters were defined using a stringent set of criteria and included those sequences grouping together with posterior probabilities ≥0.97 and sharing .90% nucleotide identity per total sampling period between related sequences analysed.
Recombination was investigated using bootscan analysis in the program SimPlot v3.5 with an F84 nucleotide substitution model, a 200 bp sliding window, a 40 bp step and the transition/transversion ratio determined empirically.19
GenBank accession numbers
JQ259060, JQ259075, JQ259077, JQ259078, JQ259085, JQ259088, JQ259089, JQ259092-JQ259094, JQ259097-JQ259100, JQ259103, JQ259104, JQ259109, JQ259112-JQ259117, JQ259119, JQ259120, JQ259122-JQ259129, JQ259131-JQ259147, JQ259149-JQ259153, JQ259155, JQ259157-JQ259184 and KJ765390-KJ765610.
Results
Low prevalence of genotypic TDRMs in Bulgaria
Three hundred and five of 1446 (21.1%) HIV-1-infected persons naive to ART participated in our study. The majority of persons (79.3%) were male and the potential infection routes included HET (42.6%), IDU (27.5%), MSM (26.9%), mother-to-child (1.6%), MSM/IDU (1.0%) and blood transfusion recipients (0.3%) (Table 1). Infection was also distributed across other groups, including previous prisoners (12.8%), sex workers (3.0%), persons with other STIs (2.0%), pregnant women (3.3%) and blood donors (6.2%). The majority of patients (89.8%) reported likely acquiring infection in Bulgaria.
Table 1.
Study population characteristicsa
| Characteristic | Prevalence in 16 naive patients with TDRMs (%) | Prevalence in 305 HAART-naive patients (%) | Total HIV-1-positive patients (%) | P b |
|---|---|---|---|---|
| Gender | ||||
| male | 13 (81.3) | 13/242 (5.4) | 1085 (75.0) | |
| female | 3 (18.8) | 3/63 (4.8) | 361 (25.0) | >0.05 |
| Likely route of HIV infection | ||||
| HET | 10 (62.5) | 10/130 (7.7) | 851 (58.9) | >0.05 |
| IDU | 2 (12.5) | 2/84 (2.4) | 342 (2.7) | >0.05 |
| MSM | 4 (25.0) | 4/82 (4.9) | 203 (14.0) | <0.001 |
| mother to child | 0 | 5 | 14 (1.0) | >0.05 |
| MSM+IDU | 0 | 3 | 13 (0.9) | >0.05 |
| HET+IDU | 0 | 1 | 4 (0.3) | >0.05 |
| blood transfusion | 0 | 0 | 19 (1.3) | |
| Likely country of infection | ||||
| Bulgaria | 12 (75.0) | 12/274 (4.4) | 1219 (84.3) | |
| other | 4 (25.0) | 4/31 (12.9) | 227 (15.7) | <0.05 |
| Other epidemiological data | ||||
| individuals reporting incarceration | 2 (12.5) | 2/39 (5.1) | 149 (10.3) | >0.05 |
| sex worker | 0 | 9 | 41 (2.8) | >0.05 |
| STI patient | 0 | 6 | 40 (2.8) | >0.05 |
| pregnant women | 0 | 10 | 48 (3.3) | >0.05 |
| blood donors | 1 (6.3) | 1/19 (5.3) | 94 (6.5) | >0.05 |
Prevalence of each population characteristic is provided for 16 patients with TDRMs and HAART-naive patients separately.
P value for differences between the total HIV-1 population (n=1446) and the studied patient group (n=305), concerning the frequency of basic demographic and epidemiological characteristics (χ2 test, GraphPad Prism 4.0).
The overall rate of TDRMs in this population was 5.2% (16/305); those with TDRMs comprised 13 (4.3%) men and 3 (0.9%) women (Table 2). Eleven of the 305 (3.6%) had resistance to NRTIs, 5/305 (1.6%) had resistance to NNRTIs and 3/305 (0.9%) had resistance to PIs. The most prevalent mutations were T215C/D/S, M41L, K219Q and F77L for NRTIs; Y181C, K103N, V106M and G190E for NNRTIs, and D30N, N88D and M46L for PIs.
Table 2.
Characteristics of HIV-1 patients with genotypic TDRMs
| Patient code | Gender | Subtype | Reported country of infection | Likely route of infection | Year of diagnosis | Year of specimen collection | Antiretroviral mutations | ||
|---|---|---|---|---|---|---|---|---|---|
| NRTI | NNRTI | PI | |||||||
| 08BG460 | M | B | Bulgaria | HET | 2004 | 2008 | M41L, T215D | — | D30N, N88D |
| 09BG534 | M | 14_BG | Spain | HET | 2005 | 2009 | — | Y181C | — |
| 09BG651 | M | B | Bulgaria | HET | 2006 | 2008 | M41L | — | — |
| 09BG862 | F | A1 | Bulgaria | HET | 2008 | 2009 | K219Q | — | — |
| 11BG892 | M | 01_AE | Bulgaria | IDU | 2008 | 2011 | — | G190E | — |
| 08BG893 | M | B | Germany | MSM | 2008 | 2008 | M41L | — | — |
| 08BG914 | M | B | Dominican Republic | HET | 2008 | 2008 | F77L | — | — |
| 09BG944 | M | 01_AE | Bulgaria | HET | 2009 | 2009 | T215S | — | — |
| 10BG1100 | F | A-like | Bulgaria | HET | 2009 | 2010 | — | — | M46L |
| 10BG1101 | M | C | not reported | HET | 2010 | 2010 | — | V106M | — |
| 10BG1109 | M | A1 | Turkey | MSM | 2010 | 2010 | F77L, T215C | Y181C | — |
| 11BG1119 | M | B | Bulgaria | MSM | 2010 | 2011 | M41L, T215D | — | D30N, N88D |
| 11BG1318 | F | 01_AE | Bulgaria | HET | 2011 | 2011 | K219Q | — | — |
| 11BG1362 | M | 02_AG | Bulgaria | IDU | 2011 | 2011 | — | K103N | — |
| 11BG1373 | M | B | Bulgaria | MSM | 2011 | 2011 | T215D | — | — |
| 11BG1429 | M | 01_AE | Bulgaria | HET | 2011 | 2011 | V75M | — | — |
M, male; F, female.
Thirteen of the 16 patients (81.3%) with TDRMs had single-class TDRMs, while dual-class TDRMs were identified in 3/16 (18.8%); all three were men, two of whom were MSM and one was HET. Two patients had both NRTI and PI mutations and one had NRTI and NNRTI resistance mutations (Table 2). TDRM prevalence across risk groups was highest among HETs (10/130, 7.7%), followed by MSM (4/82, 4.9%) and IDUs (2/84, 2.4%). TDRMs were found in 5/31 (16.1%) patients who reported likely HIV-1 infection while travelling or living abroad and in persons with different non-B HIV-1 subtypes (Table 2). TDRMs were not detected in samples from sex workers, blood transfusion recipients, STI patients, pregnant women or HIV-1-positive newborns.
High HIV-1 diversity in drug-naive patients
Phylogenetic analysis showed broad HIV-1 diversity in drug-naive patients in Bulgaria. One hundred and thirty-six infections (44.6%) were subtype B, 61 (20.0%) CRF01_AE, 57 (18.7%) CRF02_AG, 11 (3.6%) subtype A-like, 10 (3.3%) subtype C, 8 (2.6%) sub-subtype A1, 5 (1.6%) CRF14_BG, 7 (2.3%) sub-subtype F1, 4 (1.3%) CRF05_DF, 4 (1.3%) unique recombinant forms (URFs), 1 (0.3%) CRF03_AB and 1 (0.3%) subtype G (Figure 1 and Table 3). Of the 11 A-like sub-subtypes, 10 were found in the current study and one sequence was previously reported.14 Partial polymerase sequences from this subtype were analysed in detail previously and will require further characterization using complete genomes.14
Figure 1.
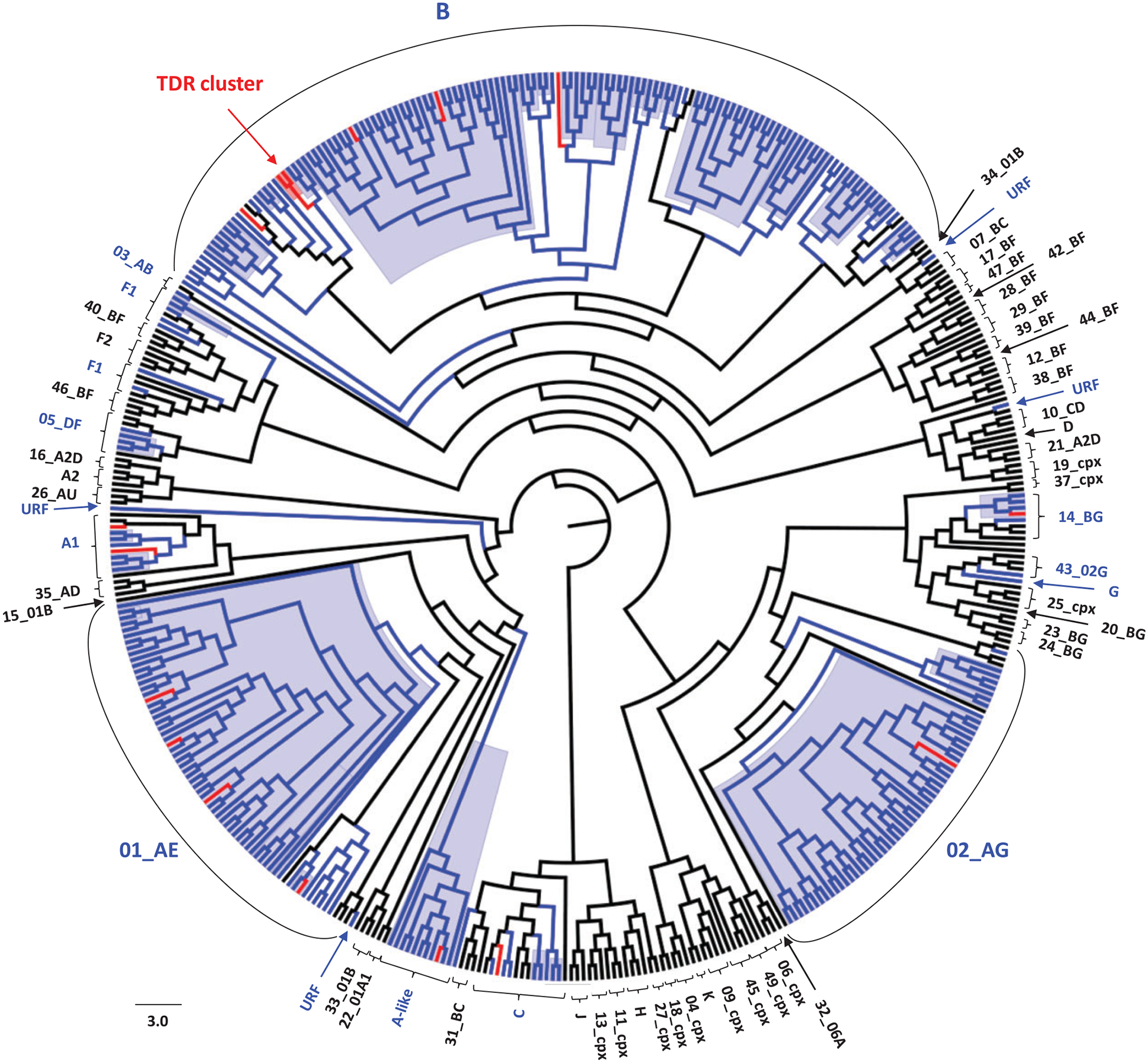
Inferred subtypes and phylogenetic relationships of Bulgarian HIV-1 polymerase sequences. The 715 bp alignment was composed of 305 HIV-1 sequences from Bulgaria and 157 reference sequences of all HIV-1 subtypes and complex recombinant forms (CRFs) available in the Los Alamos HIV 2010 database. Bayesian inference was performed using BEAST v1.8.0. The general time-reversible model with discrete gamma and invariant among-site rate variation, a lognormal relaxed clock model and a constant coalescent tree prior was used. Two independent 100×106 Markov chain Monte Carlo (MCMC) generations were used with sampling every 10000th generation. Statistical support for the best inferred trees was assessed with posterior probabilities. Black, blue and red branches indicate reference, Bulgarian and Bulgarian sequences with TDRMs, respectively. Blue background indicates phylogenetic clusters with posterior probabilities ≥0.97 and within-clade nucleotide identities >90%.
Table 3.
HIV-1 diversity in 305 antiretroviral-naive Bulgarian patients
| Subtype | Number (%) | Male (%) | Female (%) | HET (%) | MSM (%) | IDU (%) | Newborn, MSM + IDU, HET + IDU (%) | Infected in Bulgaria (%) | Likely infected abroada | |
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | country | |||||||||
| B | 136 (44.6) | 120 (88.2) | 16 (11.8) | 52 (38.2) | 76 (55.9) | 7 (5.1) | 1 (0.7) | 122 (89.7) | 14 (10.3) | Dominican Republic, England, Republic of Macedonia, Germany, Italy, Spain, Slovakia, USA, unknown |
| 01_AE | 61 (20) | 48 (78.7) | 13 (21.3) | 23 (37.7) | 1 (1.6) | 34 (55.7) | 3 (4.9) | 60 (98.4) | 1 (1.6) | Germany |
| 02_AG | 57 (18.7) | 45 (78.9) | 12 (21.1) | 15 (26.3) | 0 | 40 (70.2) | 2 (3.5) | 53 (93) | 4 (7) | Libya, Cyprus, China |
| A-like | 11 (3.6) | 3 (27.3) | 8 (72.7) | 11 (100) | 0 | 0 | 0 | 8 (72.7) | 3 (27) | Moldova, Russia |
| C | 10 (3.3) | 6 (60) | 4 (40) | 9 (90) | 0 | 0 | 1 (10) | 8 (80) | 2 (20) | South Africa, unknown |
| A1 | 8 (2.6) | 7 (87.5) | 1 (12.5) | 5 (62.5) | 3 (37.5) | 0 | 0 | 7 (87.5) | 1 (12.5) | Turkey |
| 14_BG | 5 (1.6) | 4 (80) | 1 (20) | 1 (20) | 0 | 2 (40) | 1 (20) | 3 (60) | 2 (40) | Spain |
| F1 | 7 (2.3) | 5 (71.4) | 2 (28.6) | 6 (85.7) | 0 | 1 (14.3) | 0 | 6 (85.7) | 1 (14.3) | unknown (Africa) |
| 05_DF | 4 (1.3) | 0 | 4 (100) | 3 (75) | 0 | 0 | 1 (25) | 3 (75) | 1 (25) | unknown |
| URF | 4 (1.3) | 2 (50) | 2 (50) | 3 (75) | 1 (25) | 0 | 0 | 3 (75) | 1 (25) | Nigeria |
| 03_AB | 1 (0.3) | 1 (100) | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 | |
| G | 1 (0.3) | 1 (100) | 0 | 1 (100) | 0 | 0 | 0 | 0 | 1 (100) | Nigeria |
| Totals | 305 | 242 | 63 | 130 | 82 | 84 | 9 | 274 | 31 | |
Self-reported country of HIV-1 acquisition.
We also found broad genotypic diversity in persons with TDRMs, including 6/16 (37.5%) subtype B, 4/16 (25%) CRF01_AE, 2/16 (12.5%) subtype A1, 1/16 (6.3%) subtype A-like, 1/16 (6.3%) CRF02_AG, 1/16 (6.3%) CRF14_BG and 1/16 (6.3%) subtype C (Figure 1 and Table 2).
Identification of subtype and TDRM clusters
Phylogenetic analysis inferred 32 strongly supported clusters (Figure 1); the largest consisted of 52 CRF01_AE sequences, most of which were from IDUs living in Sofia. More than a third of the patients in the large CRF01_AE cluster reported previous imprisonment. We also identified two clusters of CRF02_AG sequences, most of which were from IDUs living in Plovdiv. Almost a third of the patients in the CRF02_AG cluster also reported a history of imprisonment. Fifteen subtype B clusters were found, mostly from MSM living in the capital city of Sofia. HET patients were also present in two of these MSM clusters.
Of sixteen sequences with TDRMs, only two subtype B sequences (patients 08BG460 and 11BG1119) clustered together with strong phylogenetic support (posterior probability=1.0) and 99% nucleotide identity, suggesting an epidemiological link with this HET–MSM pair (Figure 1 and Table 2). These two patients had dual-class TDRMs to NRTI (M41L and T215D) and PI (D30N and N88D), which may have lasted for 3 years (Table 2) or have been transmitted by a third person not tested in our study.
Most clusters contained sequences with relatively short branch lengths in the phylogenetic tree, suggestive of a short evolutionary history and relatively recent infection (Figure 1). Although there were no data available for determining the date of infection, most of the patients’ blood specimens were collected shortly after HIV/AIDS diagnosis; 49.8% of the patients’ specimens were analysed the same year as diagnosis, 36.1% between 1 and 3 years after diagnosis, and 14.1% ≥4 years after diagnosis.
Discussion
Although HIV-1 was introduced .28 years ago into Bulgaria, which has one of the highest HIV infection rates per million persons of the Balkan countries, very little is known about the characteristics of the epidemic.20 Assessment of TDRMs and viral diversity is a key factor in monitoring the HIV-1 epidemic and optimizing first-line therapy for long-term management of HIV-1 infection in Bulgaria.21 Here we found that TDRMs and HIV-1 diversity in Bulgaria differ from those in other European and Balkan countries. The 5.2% TDRM prevalence in Bulgaria is about half of that we previously reported13 and that reported across Europe (10.9%),5 and is generally less than that of the majority of neighbouring Balkan countries (4.7%–21.8%).8–12 In Western Europe, a 10% TDRM rate was reported and is higher in MSM,5,7 which may be due to the fact that in Western Europe HIV-1 was initially introduced and transmitted among MSM, who have the longest history of treatment with antiretroviral drugs and who thus typically have more TDRMs. In contrast, the lower TDRM prevalence in Bulgaria may reflect the fact that HIV-1 was first introduced among HETs in Bulgaria from persons travelling mostly in non-Western European countries, who have lower TDRM rates.14 The decrease in TDRM seen in drug-naive patients in our current study compared with that reported in 2008 is a likely result of identifying only 2/22 HAART-naive persons in the initial study. TDRMs were present in about one-third of persons reporting acquiring infection abroad, demonstrating that TDRMs and a variety of subtypes are being imported into Bulgaria. Lately, the HIV-1 epidemic in Bulgaria has seen dramatic prevalence increases in MSM and IDUs, which may cause a future increase in TDRMs in IDUs and MSM with the concomitant introduction of TDRMs into other risk groups. Indeed, in our study we identified one MSM and one HET infected with subtype B with identical dual-class TDRMs, suggestive of possible spillover from MSM into HET risk groups. TDRM clusters in MSM and IDUs have also been reported in outbreaks in neighbouring Balkan countries.9,22–24
As in our previous report,13 most TDRMs concerned RTIs, including those in patients with dual resistance. The majority of patients had non-polymorphic mutations selected by the thymidine analogues zidovudine and stavudine, including M41L and K219Q. The revertant TDRMs T215C/D/S were also found in five patients. These mutations usually occur in individuals primarily infected with strains containing the primary resistance mutation T215Y/F, which can also be transmitted. The common NRTI resistance mutation V75M, which occurs predominantly in CRF01_AE infection, was found in one patient with this subtype. Five patients, each infected with a different subtype, three of whom reported acquiring infection abroad, had NNRTI TDRMs. In this study we found E138G/A/D/K variants that are related to rilpivirine resistance. However, these mutations are also encountered as polymorphisms and are therefore not considered a certain indicator of transmitted resistance according to the definition used by the WHO.15 Three patients had PI TDRMs, although PI TDRMs were not observed in our previous study.13 One individual was infected with an HIV-1 A-like subtype with the M46L mutation selected by various PIs. Sequences from two other patients infected in Bulgaria had the D30N and N88D mutations, both of which are selected by nelfinavir and commonly occur together but have little clinical impact on first-line therapy. Although nelfinavir has not been used in clinical practice for years, these two mutations had already been detected in Bulgaria in our previous study13 and one of these two patients was diagnosed in 2004 when nelfinavir was still in use.
Analysis of the geographical distribution of TDRMs showed that half of the cases were found in two major cities, Sofia and Plovdiv, where the largest number of HIV-1 patients in our study were registered. However, TDRMs were also identified in persons from five cities in remote locations across Bulgaria, demonstrating that TDRMs are widespread in the country, though the overall prevalence is low. In addition, about one-third of TDRM cases were found in individuals reporting they acquired infection abroad, suggesting that some TDRMs are likely being imported into Bulgaria.
As in our prior studies, phylogenetic analyses revealed a high HIV-1 diversity, with over half (54.4%) of the sequences defined as non-B subtypes, compared with most European countries (66.1%),25 including most neighbours in the Balkan region except Romania and Albania, where the most prevalent subtypes are F1 (80.3%) and A1 (56.1%), respectively.9,14,20,26 After subtype B, CRF01_AE and CRF02_AG were the second most prevalent strains, with the highest rate of these two CRFs reported in the Balkans.20 The remaining 16.7% of HIV-1 infections consisted of at least 10 different subtypes. The subtypes were not evenly distributed amongst the various risk groups; nearly 90% of subtype B viruses were found in men and the majority were MSM. These results are consistent with the HIV-1 epidemic in Western Europe5,8,20 and with those from our previous study in Bulgaria.14 In contrast, CRF01_AE and CRF02_AG were found mostly in IDUs. Most likely, recent introduction and rapid dissemination of different HIV-1 strains in IDUs has contributed to the increase in these two subtypes in Bulgaria, as previously observed.27 In contrast, most infections with subtypes A1, F1, C, CRF14_BG and CRF05_DF and URFs were found in HETs, including CRF03_AB, and have had limited spread to date. This is the first report in Bulgaria of this HIV-1 subtype, which circulates in Russia, former Soviet Union countries and neighbouring Turkey.28,29 Interestingly, ~25% of the minor subtypes found in our study were likely acquired outside Bulgaria, suggesting that there have been multiple introductions of rare HIV-1 subtypes from immigrants.
We did not find an association between time from diagnosis and TDR in our study; however, the numbers of resistance mutations found are too low for statistically supported associations.
Our findings are limited by inclusion of persons who were not followed longitudinally to determine whether TDRMs persist or revert to WT virus, which might influence the level of observed TDRMs in persons from whom samples were collected .1 year after diagnosis. This is especially relevant for comparisons with results from Western countries, where genotyping and drug resistance testing occurs at the time of diagnosis. Also, the results from a cross-sectional study design may not be truly representative of the other 80% of reported cases in Bulgaria. For example, a comparison of demographic and epidemiological characteristics of the subset of patients (n=305) studied in the current report and all HIV-1-diagnosed persons (n=1446) in Bulgaria showed that the numbers of TDRMs in MSM and persons acquiring HIV-1 abroad may have been overestimated (Table 1). The effect of this possible sampling error in our analyses may also influence subtype distribution in different populations. The findings may also be limited by the use of only standard population-based sequencing, which may not detect minority TDRMs present at <20% of the viral population in plasma.15 Finally, self-reporting of epidemiological data used in this study could introduce recall biases, especially for those reporting infection abroad or non-MSM, which could affect the subtype prevalence by country of origin or route of HIV-1 transmission.
Conclusions
We found low TDRM prevalence and high viral diversity in treatment-naive, HIV-1-infected persons in Bulgaria. The contribution of TDRMs and genetic diversity acquired outside Bulgaria by migrants and the increasing number and size of local transmission clusters in high-risk groups raises public health concerns. Combined, our findings provide baseline TDRM data and support the need for further surveillance of TDRMs and viral diversity in Bulgaria, especially in high-risk populations such as those involved in the emerging MSM and IDU sub-epidemics.
Funding
This study was funded in part by the Bulgarian Ministry of Education and Science, Project ‘Science and Business’ (grant number BG051PO001-3.3.05-0001), by the European Commission sixth framework supported programs EuropeHIVResistance, grant LSHPCT-2006-518211 and by the Bulgarian Ministry of Health Directorate ‘Management of Specialized Donor-funded Programs’. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Transparency declarations
None to declare.
Disclaimer
Use of trade names is for identification only and does not imply endorsement by the US Department of Health and Human Services, the Public Health Service or the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- 1.Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338: 853–60. [DOI] [PubMed] [Google Scholar]
- 2.Oette M, Kaiser R, Däumer M et al. Primary HIV drug resistance and efficacy of first-line antiretroviral therapy guided by resistance testing. J Acquir Immune Defic Syndr 2006; 41: 573–81. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch MS, Günthard HF, Schapiro JM et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society–USA panel. Clin Infect Dis 2008; 47: 266–85. [DOI] [PubMed] [Google Scholar]
- 4.European AIDS Clinical Society (EACS) 2013. http://www.eacsociety.org/.
- 5.Vercauteren J, Wensing AM, van de Vijver DA et al. Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis 2009; 200: 1503–8. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler WH, Ziebell RA, Zabina H et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, US-2006. AIDS 2010; 24: 1203–12. [DOI] [PubMed] [Google Scholar]
- 7.Bozicevic I, Handanagic S, Lepej S et al. The emerging and re-emerging human immunodeficiency virus epidemics in Europe. Clin Microbiol Infect 2013; 19: 917–29. [DOI] [PubMed] [Google Scholar]
- 8.Grgic I, Lepej SZ, Lunar MM. The prevalence of transmitted drug resistance in newly diagnosed HIV-infected individuals in Croatia: the role of transmission clusters of men who have sex with men carrying the T215S surveillance drug resistance mutation. AIDS Res Hum Retroviruses 2013; 29: 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temereanca A, Ene L, Mehta S et al. Transmitted HIV drug resistance in treatment-naive Romanian patients. J Med Virol 2013; 85: 1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoura L, Metallidis S, Pilalas D et al. High rates of transmitted drug resistance among newly-diagnosed antiretroviral naive HIV patients in Northern Greece, data from 2009–2011. Clin Microbiol Infect 2013; 19: 169–72. [DOI] [PubMed] [Google Scholar]
- 11.Lunar MM,Židovec LS, Abecasis A et al. Prevalence of HIV type 1 transmitted drug resistance in Slovenia: 2005–2010. AIDS Res Hum Retroviruses 2013; 29: 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanojevic M, Siljic M, Salemovic D et al. Ten years survey of primary HIV-1 resistance in Serbia: the occurrence of multiclass resistance. AIDS Res Hum Retroviruses 2014; 30: 634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoro MM, Ciccozzi M, Alteri C et al. Characterization of drug-resistance mutations in HIV type 1 isolates from drug-naive and ARV-treated patients in Bulgaria. AIDS Res Hum Retroviruses 2008; 24: 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov IA, Beshkov D, Shankar A et al. Detailed molecular epidemiologic characterization of HIV-1 infection in Bulgaria reveals broad diversity and evolving phylodynamics. PLoS One 2013; 8: e59666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DE, Camacho RJ, Otelea D et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4: e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28: 2731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Los Alamos HIV Databases. http://www.hiv.lanl.gov.
- 18.Drummond AJ, Suchard MA, Xie D et al. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 2012; 29: 1969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lole KS, Bollinger RC, Paranjape RS et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 1999; 73: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanojevic M, Alexiev I, Beshkov D et al. HIV1 molecular epidemiology in the Balkans—a melting pot for high genetic diversity. AIDS Rev 2012; 14: 28–36. [PubMed] [Google Scholar]
- 21.DHHS Panel. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 12 February 2013. http://aidsinfo.nih.gov/guidelines.
- 22.Antoniadou ZA, Kousiappa I, Skoura L et al. Molecular epidemiology of HIV type 1 infection in northern Greece (2009–2010): evidence of a transmission cluster of HIV type 1 subtype A1 drug-resistant strains among men who have sex with men. AIDS Res Hum Retroviruses 2014; 30: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siljic M, Salemovic D, Jevtovic D et al. Molecular typing of the local HIV-1 epidemic in Serbia. Infect Genet Evol 2013; 19: 378–85. [DOI] [PubMed] [Google Scholar]
- 24.Paraschiv S, Otelea D, Batan I et al. Molecular typing of the recently expanding subtype B HIV-1 epidemic in Romania: evidence for local spread among MSMs in Bucharest area. Infect Genet Evol 2012; 12: 1052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abecasis AB, Wensing AM, Paraskevis D et al. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology 2013; 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salemi M, de Oliveira T, Ciccozzi M et al. High-resolution molecular epidemiology and evolutionary history of HIV-1 subtypes in Albania. PLoS One 2008; 3: e1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salemi M, Goodenow MM, Montieri S et al. The HIV type 1 epidemic in Bulgaria involves multiple subtypes and is sustained by continuous viral inflow from West and East European countries. AIDS Res Hum Retroviruses 2008; 24: 771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liitsola K, Holm K, Bobkov A et al. An AB recombinant and its parental HIV type 1 strains in the area of the former Soviet Union: low requirements for sequence identity in recombination. AIDS Res Hum Retroviruses 2000; 16: 1047–53. [DOI] [PubMed] [Google Scholar]
- 29.Sayan M, Willke A, Ozgunes N et al. HIV-1 subtypes and primary antiretroviral resistance mutations in antiretroviral therapy naive HIV-1 infected individuals in Turkey. Jpn J Infect Dis 2012; 66: 306–11. [DOI] [PubMed] [Google Scholar]


