Abstract
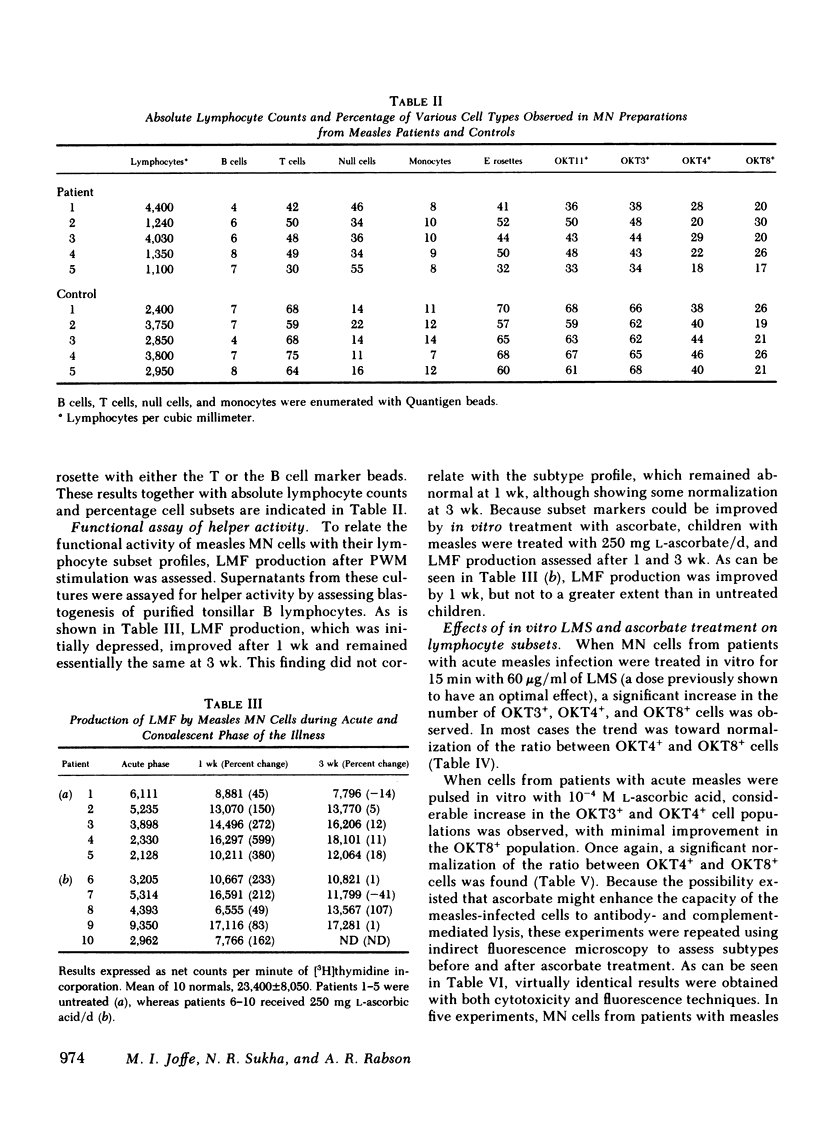
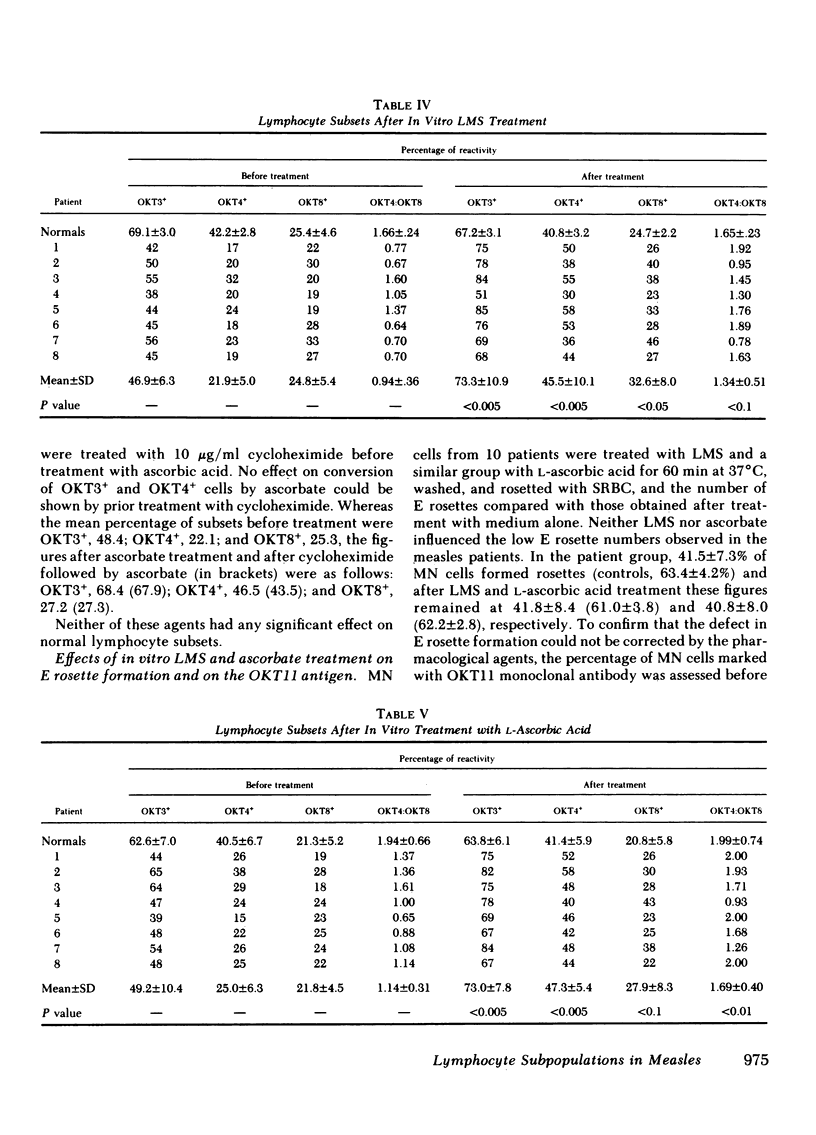
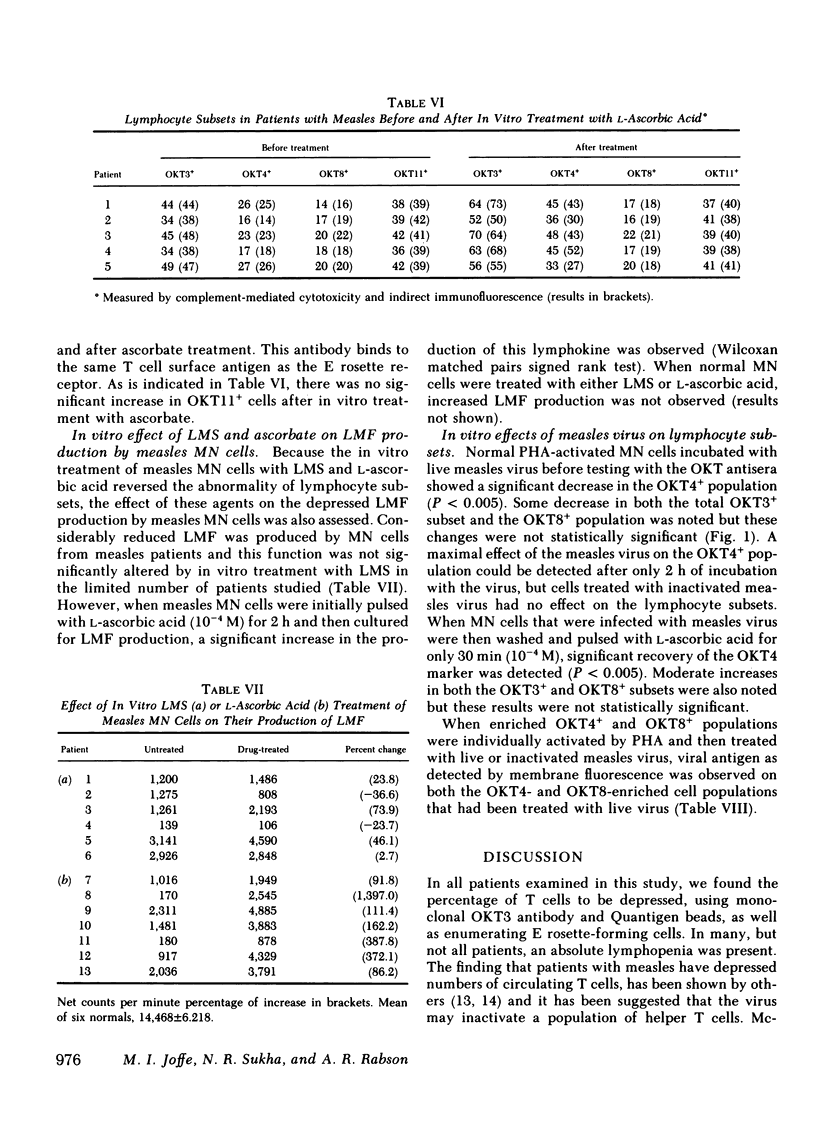
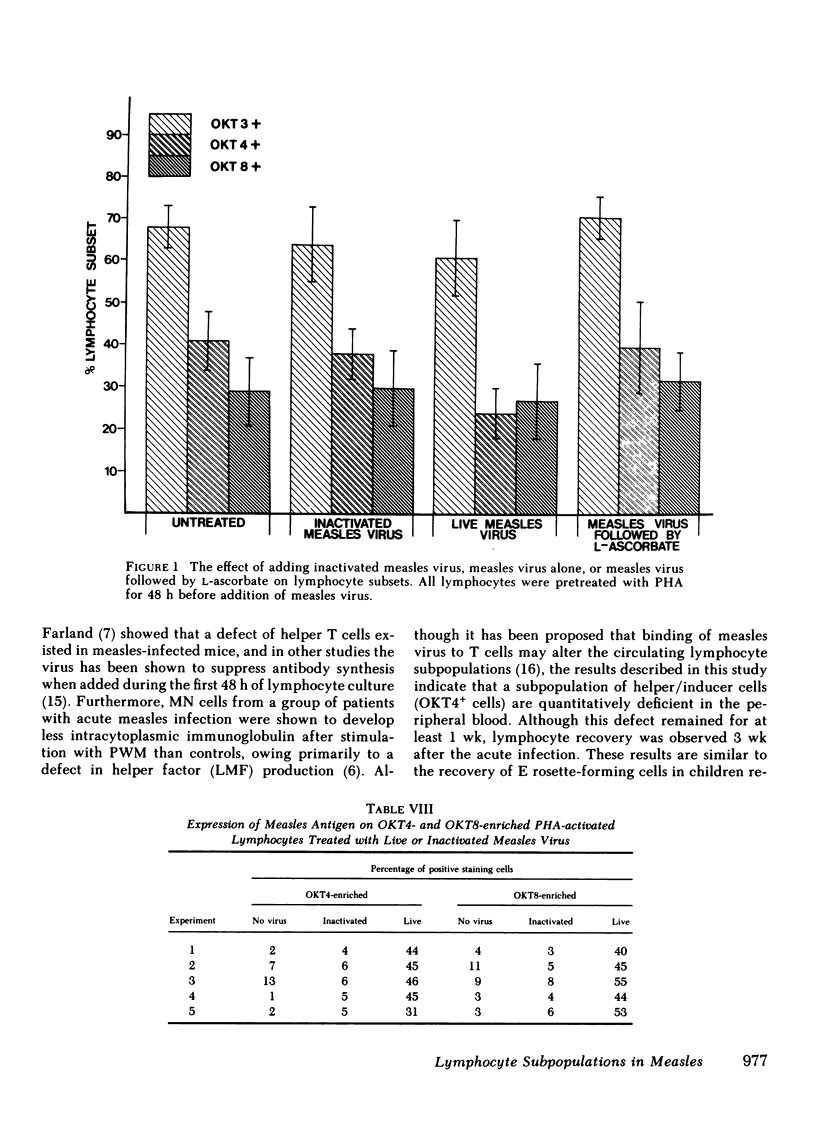
Lymphocyte subsets were assessed in patients with measles using the OKT range of monoclonal antibodies. A significant decrease in cells reacting with the OKT3 and OKT4 monoclonal antibodies was observed. When the tests were repeated 3 wk after the acute infection, significant recovery of these subsets was observed. The abnormality in lymphocyte subsets could be reproduced by treating normal lymphocytes with measles virus in vitro. When lymphocytes from patients with measles or when normal cells infected with measles virus in vitro were treated with either levamisole or L-ascorbic acid for 15 min and then retested with the OKT antisera, restoration of the previously depleted OKT3+ and OKT4+ cell population was observed. Ascorbic-acid treatment also, to a certain extent, reversed the inability of measles mononuclear cells to produce lymphocyte mitogenic factor (helper factor for B cells) after pokeweed mitogen activation. This abnormality, however, could not be reversed by in vitro treatment with levamisole. Measles patients treated with L-ascorbic acid demonstrated no accelerated recovery in either their lymphocyte subset profile or their ability to produce lymphocyte mitogenic factor. Although the cause of the depressed OKT3+ and OKT4+ lymphocyte subpopulations in patients with measles is not clear, the results suggest that the effect is not due to an aberration of protein synthesis, but rather to a blocking or steric change produced by the virus on the cell membrane. It is likely that both ascorbic acid and levamisole have the ability to reverse this effect by virtue of their antioxidant properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Bach J. F. The use of monoclonal anti-T cell antibodies to study T cell imbalances in human diseases. Clin Exp Immunol. 1981 Sep;45(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- Callard R. E., Smith C. M., Worman C., Linch D., Cawley J. C., Beverley P. C. Unusual phenotype and function of an expanded subpopulation of T cells in patients with haemopoietic disorders. Clin Exp Immunol. 1981 Mar;43(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- Denman A. M. Lymphocyte function and virus infections. J Clin Pathol Suppl (R Coll Pathol) 1979;13:39–47. doi: 10.1136/jcp.s3-13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Coffey R. G., Hadden E. M., Lopez-Corrales E., Sunshine G. H. Effects of levamisole and imidazole on lymphocyte proliferation and cyclic nucleotide levels. Cell Immunol. 1975 Nov;20(1):98–103. doi: 10.1016/0008-8749(75)90088-x. [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Sullivan J. L., Albrecht P. Immune responses during measles infection in immunosuppressed Rhesus monkeys. J Immunol. 1977 Oct;119(4):1452–1456. [PubMed] [Google Scholar]
- Huddlestone J. R., Lampert P. W., Oldstone M. B. Virus-lymphocyte interactions: infection of Tg and Tm subsets by measles virus. Clin Immunol Immunopathol. 1980 Mar;15(3):502–509. doi: 10.1016/0090-1229(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Defective helper factor (LMF) production in patients with acute measles infection. Clin Immunol Immunopathol. 1981 Aug;20(2):215–223. doi: 10.1016/0090-1229(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Dissociation of lymphokine production and blastogenesis in children with measles infections. Clin Immunol Immunopathol. 1978 Jul;10(3):335–343. doi: 10.1016/0090-1229(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. P., Roberts N. J., Jr Human macrophage and lymphocyte responses to mitogen stimulation after exposure to influenza virus, ascorbic acid, and hyperthermia. J Immunol. 1979 Nov;123(5):1940–1944. [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. Levamisole increases activated T lymphocytes. Lancet. 1977 Sep 24;2(8039):665–665. doi: 10.1016/s0140-6736(77)92538-7. [DOI] [PubMed] [Google Scholar]
- Salo R. J., Cliver D. O. Inactivation of enteroviruses by ascorbic acid and sodium bisulfite. Appl Environ Microbiol. 1978 Jul;36(1):68–75. doi: 10.1128/aem.36.1.68-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler J. A., Gallin J. I., Vaughan M. Effects of serotonin, carbamylcholine, and ascorbic acid on leukocyte cyclic GMP and chemotaxis. J Cell Biol. 1975 Nov;67(2PT1):480–484. doi: 10.1083/jcb.67.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Albrecht P., Lucas S. J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975 May;114(5):1458–1461. [PubMed] [Google Scholar]
- Symoens J., Rosenthal M. Levamisole in the modulation of the immune response: the current experimental and clinical state. J Reticuloendothel Soc. 1977 Mar;21(3):175–221. [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. IV. Induction of suppressor cells within the OKT4+ population. J Exp Med. 1981 Aug 1;154(2):459–467. doi: 10.1084/jem.154.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]



