Abstract
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign pulmonary tumour, most reported cases of PSP are from Eastern Asia, with a female to male ratio of 5:1, and average age at diagnosis in the 5th decade. We present the case of a 63-year-old Caucasian woman diagnosed with PSP who underwent a left lower lobe basal segmentectomy with systematic nodal dissection, performed via video assisted thoracic surgery (VATS).
Keywords: Cardiothoracic surgery < surgery < clinical, histopathology < pathology < clinical
Introduction
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign pulmonary tumour thought to arise from type II pneumocytes of the pulmonary epithelium. Although described as a benign lesion, the potential for nodal metastasis of PSP has been reported. 1 PSP is defined histologically by two cell types, cuboidal surface cells and round stromal cells, arranged into four distinct patterns: papillary, solid, sclerosing and haemorrhagic/angiomatoid. 2 Most reported cases are from Eastern Asia, with a female-to-male ratio of 5:1, and average age at diagnosis in the fifth decade.1,3 Patients with PSP are typically asymptomatic. 1 Diagnosis requires histological and immunohistochemical investigation. Surgical resection is the recommended definitive management.
Due to the relatively rare occurrence of PSP in Western populations, we present an unusual case of PSP in a 63-year-old Caucasian woman.
Case report
A 63-year-old Caucasian woman, previously fit and well, was referred to our hospital due to a lesion detected in her left lung on chest radiography. She presented with a dry cough and wheeze for several months, and previously had a hysterectomy for uterine fibroids. She took no regular medications and was a non-smoker.
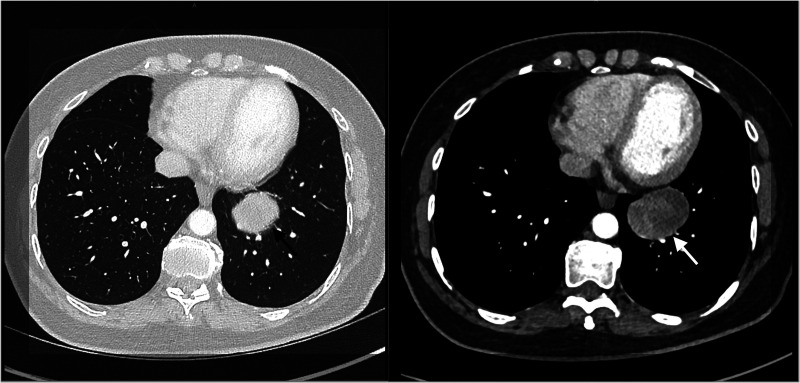
A CT scan of the thorax (Figure 1) showed a well circumscribed peribronchial lesion in the left lower lobe close to the hilum, measuring 48 mm in diameter. A PET-CT scan showed increased FDG uptake by the lesion (SUVmax 3.4) with no further FDG avid nodal disease or metastasis. Incidental uptake at the right tonsil was deemed non-malignant by an Ear, nose and throat specialist.
Figure 1.
CT scan of thorax demonstrates a solid lesion, highlighted by the arrows, in the left lower lobe, measuring 48×33 mm.
CT-guided biopsy of the lesion revealed an interstitial proliferation of atypical epithelioid cells in a fibrous stroma with coexistent hemosiderin deposition, suggestive of PSP. Immunohistochemistry revealed endomysial antibody (EMA), thyroid transcription factor-1 (TTF-1) and pancytokeratin (MNF116) positive epithelioid cells and EMA and TTF-1 positive stromal cells. Both were CD56, chromogranin, synaptophysin and P40 negative.
Left lower lobe basal segmentectomy with systematic nodal dissection was performed via video-assisted thoracic surgery with no complications. The chest drain was removed the following day.
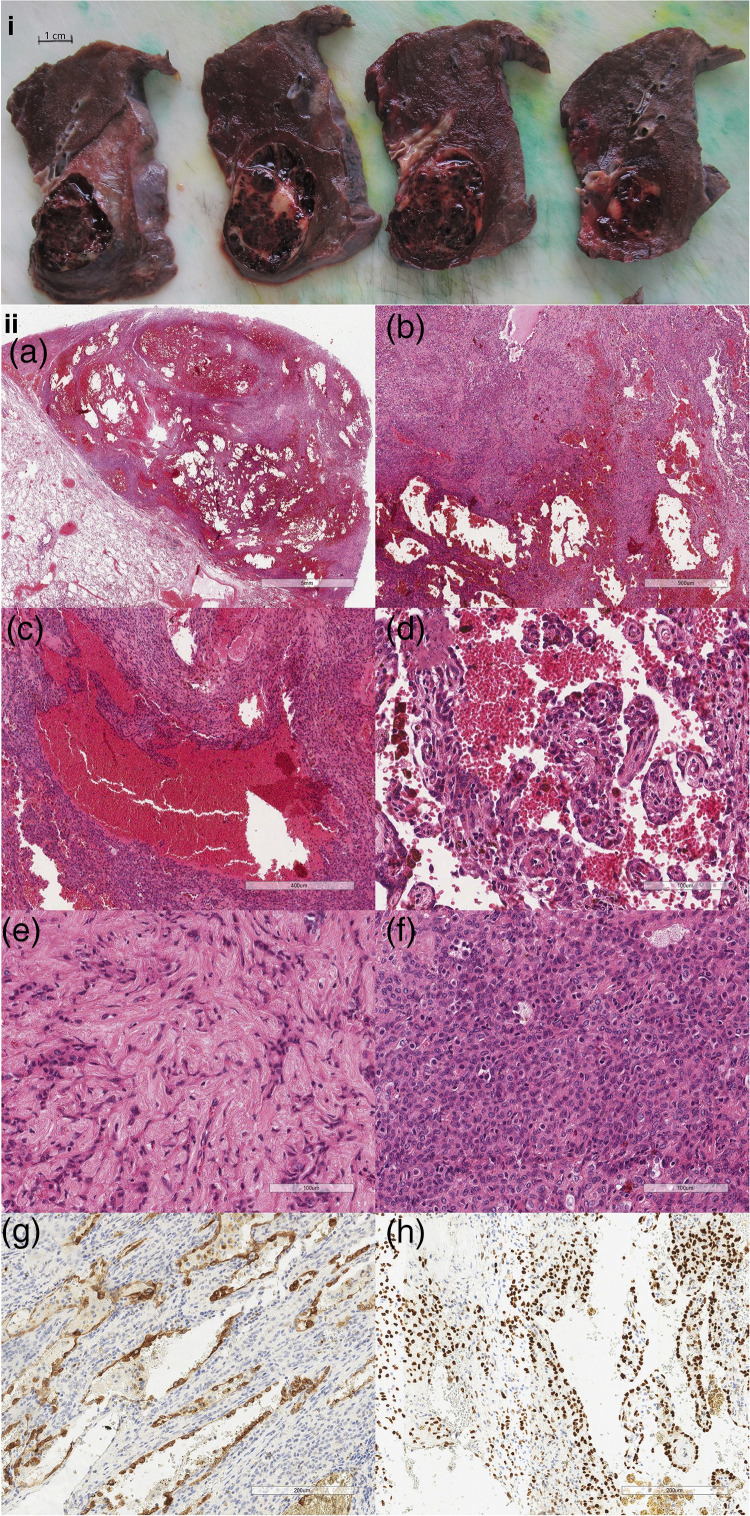
Macroscopic examination of the resected segment (Figure 2i) found a well-circumscribed, haemorrhagic, partially cystic tumour with a minor solid component, immediately distal to the left lower lobar bronchus.
Figure 2.
(i) Macroscopic examination reveals a central well-circumscribed haemorrhagic and partially cystic tumour with a minor solid component, arising immediately distal to the left lower lobar bronchus. (ii) Histopathological features (a) the tumour is well demarcated from the adjacent lung parenchyma. Multiple blood-filled cystic spaces are discernible at low power. (b) In between the cystic spaces is a proliferation of neoplastic cells with four growth patterns present. (c) Haemorrhagic component with irregular blood-filled spaces lined by a single layer of inconspicuous cuboidal surface cells. (d) Papillary component: the papillary structures are covered by cuboidal surface cells. (e) Sclerotic component, comprising single and cords of epithelioid cells, set in densely collagenous stroma. (f) Solid component, in which there are intermingled chronic inflammatory cells within the sheets of round stromal cells. The degree of nuclear atypia is minimal. (g) The cuboidal cyst-lining cells are positive for pancytokeratin (MNF116) while the round stromal cells are negative. (h) Both the surface cells and stromal cells are positive for thyroid transcription factor-1 (TTF-1).
Histological haematoxylin and eosin staining revealed a tumour well demarcated from the adjacent lung parenchyma (Figure 2ii(a) and (b)). The tumour consisted of proliferations of neoplastic cells interspersed with multiple blood-filled cystic spaces. The neoplastic proliferations demonstrated four distinct growth patterns: haemorrhagic (Figure 2ii(c)), papillary (Figure 2ii(d)), sclerotic (Figure 2ii(e)) and solid (Figure 2ii(f)). Two neoplastic cell populations are described: cuboidal surface cells and round stromal cells.
Immunohistochemistry performed found TTF-1 and MNF116 positive cuboidal surface cells and TTF-1 positive and MNF116 negative round stromal cells. The Ki-67 index was less than 1% (Figure 2ii(g) and (h)).
Examination of the dissected lymph nodes revealed no malignancy or granuloma formation.
The patient was reviewed in clinic two months post-operation and discharged to her local team following a full recovery.
Discussion
There is no recorded incidence rate of PSP. Reported cases are predominantly women, with a female- to-male ratio of 5:1. 1 Cases have been reported across a wide age range but are most common in the fifth decade. 3 PSP is rare in Western populations, but in Eastern Asians incidence is higher.
Cases are usually detected incidentally as most patients are asymptomatic.1,3 Typical symptoms include cough, haemoptysis and chest pain, 1 as demonstrated by our patient.
PSPs typically appear on CT as oval-shaped lesions with smooth boundaries and diameter in the range 2.5–51.5 mm. 4 The nodule in our case (Figure 1) was a well-circumscribed lesion measuring 48 mm in diameter.
PSP lesions are often hypometabolic on PET-CT, with an SUVmax lower than the standard tissue SUVmax of 2.9.4,5 The lesion in our case had a SUVmax of 3.4, which is greater than the reported mean. This result, alongside a lesion diameter greater than the reported mean, supports a suggested correlation between diameter and SUVmax of PSP lesions. 5
The lesion in our case (Figure 2i) fit the classic macroscopic description of PSPs, as well-circumscribed, non-encapsulated tumours with solid and haemorrhagic components. 6 Histologically PSPs contain two neoplastic cell populations: cuboidal surface cells and round stromal cells. These cells are arranged in four distinct patterns: papillary, solid, sclerosing and haemorrhagic/angiomatoid. 2 All four patterns aren’t always present, but there tends to be at least three found with one or two being dominant. 6 All these patterns were described in our case (Figure 2ii(a–f)).
Immunohistochemistry reveals a standard pattern of staining in PSP. Epithelioid cells are positive for TTF-1, EMA and MNF116. Stromal cells are also positive for TTF-1 and EMA, but negative for MNF116.2,3,7 This pattern was seen in our case (Figure 2ii(g) and (h)).
The Ki-67 index demonstrates cellular proliferation within a tissue. In our lesion, this was 0.4%, a value higher than the reported mean of 0.19%. However, the Ki-67 index in both our case and the literature is much lower than that of adenocarcinoma at 9.25%. 8 Although PSP is defined as a benign lesion, the potential for nodal metastasis has been reported. 3 There have been no cases of haematogenous spread noted in the literature. 9 Nodal dissection in our patient revealed no metastatic disease.
Surgical resection is curative in non-metastatic disease. Given the low rate of recurrence, limited resection is preferable. 1 Wedge resection, as applied in our case, and segmentectomy have better clinical outcomes than lobectomy with faster recovery and greater lung function preservation. 6 Cases have been successfully managed through radiological monitoring, challenging the need for surgery. Non-surgical management is possible due to the low risk of recurrence and nodal metastasis. This relies on a diagnosis through CT-guided percutaneous biopsy, which isn’t often possible due to insufficient tissue at biopsy site. 10 Surgical management is preferred in patients who are symptomatic or have reduced lung function, and those who can’t be diagnosed by CT-guided percutaneous biopsy.
Footnotes
Declarations:
None declared.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: This case report was determined not to require NHS Research Ethics Committee review by the NHS Research Ethics Committee Decision Tool. Informed consent was obtained prior to the production of this case report.
Guarantor: Mr Simon Jordan.
Contributorship: S Jordan, S Buderi, A AlShammari and K Wassilew provided medical care for the patient. Y Zhang produced the figures and figure legends for the case report. W Gorman, M Shehata, M Bashir and A AlShammari contributed to the production of the manuscript.
Provenance: Not commissioned; peer-reviewed by Philemon Gukop.
ORCID iDs: William Gorman https://orcid.org/0000-0003-3509-7650
Yu Zhi Zhang https://orcid.org/0000-0002-6134-980X
Mohamed Ryan Bashirhttps://orcid.org/0000-0002-1005-6433
Key message
Pulmonary sclerosing pneumocytoma is a rare pulmonary tumour. We present a case of this condition in an unusual patient demographic
References
- 1.Devouassoux–Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000; 24(7): 906–916. [DOI] [PubMed] [Google Scholar]
- 2.Lovrenski A, Vasilijević M, Panjković M, et al. Sclerosing pneumocytoma: a ten-year experience at a Western Balkan University Hospital. Medicina (Kaunas) 2019; 55(2): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo KT, Hsu WH, Wu YC, Huang MH, Li WY. Sclerosing haemangioma of the lung: an analysis of 44 cases. J Chin Med Assoc 2003; 66(1): 33–38. [PubMed] [Google Scholar]
- 4.Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015; 94(4): e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Dang H, Wang R, Yao S, Wu Y, Xu B. Analysis of the F-18 FDG PET/CT features of pulmonary sclerosing pneumocytoma. Nucl Med Commun 2021; 42(6): 665–671. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Q, Zhou J, Li G, et al. Pulmonary sclerosing pneumocytoma: clinical features and prognosis. World J Surg Oncol 2022; 20(1): 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing haemangioma of the lung. Arch Pathol Lab Med 2001; 125(10): 1335–1339. [DOI] [PubMed] [Google Scholar]
- 8.Iyoda A, Hiroshima K, Shiba Met al. Clinicopathological analysis of pulmonary sclerosing hemangioma. Ann Thorac Surg 2004; 78(6): 1928–1931. [DOI] [PubMed] [Google Scholar]
- 9.Hu AM, Zhao D, Zheng H, Wang QH, Lyu Y, Li BL. Preoperative diagnosis in 46 cases of pulmonary sclerosing hemangioma. Chin Med J 2016; 129(11): 1377–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang H, Fang H, Liu Y, Huang X, Zhen W, Ren L. Pulmonary sclerosing haemangioma: report of two cases. World J Surg Oncol 2012; 10(1): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]