Abstract
Lipomatous pseudohypertrophy of the pancreas (LPH) is a rare disease in which the pancreatic parenchyma is replaced with mature adipose tissue. It is an idiopathic condition whose diagnosis is made based on histopathological analyses. Herein, we report the case of a 50-year-old male patient with a lipomatous mass in the head of the pancreas on computed tomography for close examination of a renal tumor. We suspected liposarcoma, and laparotomy was performed. However, histological analyses revealed LPH. Several imaging findings of LPH can enable a noninvasive diagnosis and help its clinical approach.
Keywords: lipomatous pseudohypertrophy of the pancreas, liposarcoma, computed tomography, magnetic resonance imaging
Introduction
Lipomatous pseudohypertrophy of the pancreas is an extremely rare condition with less than 100 reported cases worldwide. The disease is characterized by the replacement of normal pancreatic tissue by differentiated adipocytes; however, its pathogenesis is not fully elucidated. Herein, we report a case of LPH whose diagnosis was confirmed by histopathology based on our clinical experience.
Case report
The patient was a 50-year-old man with a right renal tumor detected by computed tomography (CT) at a previous hospital. Based on the CT findings, renal cell carcinoma was suspected. In addition, a lipomatous mass in the head of the pancreas was depicted on CT. He was referred to our hospital for surgical treatment of renal cell carcinoma. At the time of admission, he had no complaints of abdominal pain, nausea, or any other abdominal symptoms. Physical examination revealed no specific abnormalities. Serum pancreatic amylase and lipase levels on admission were normal. Routine blood and biochemical tests were normal. The serum tumor markers did not show a significant increase. Carbohydrate antigen 19-9 (CA19-9) 27.4 U/mL, carcinoembryonic antigen (CEA) 2.3 ng/mL, Duke pancreatic monoclonal antigen type 2 (DUPAN-2) <=25 U/mL, s-pancreas antigen type 1 (Span-1) 7.6 U/mL, and neuron-specific enolase (NSE) 9.8 ng/mL.
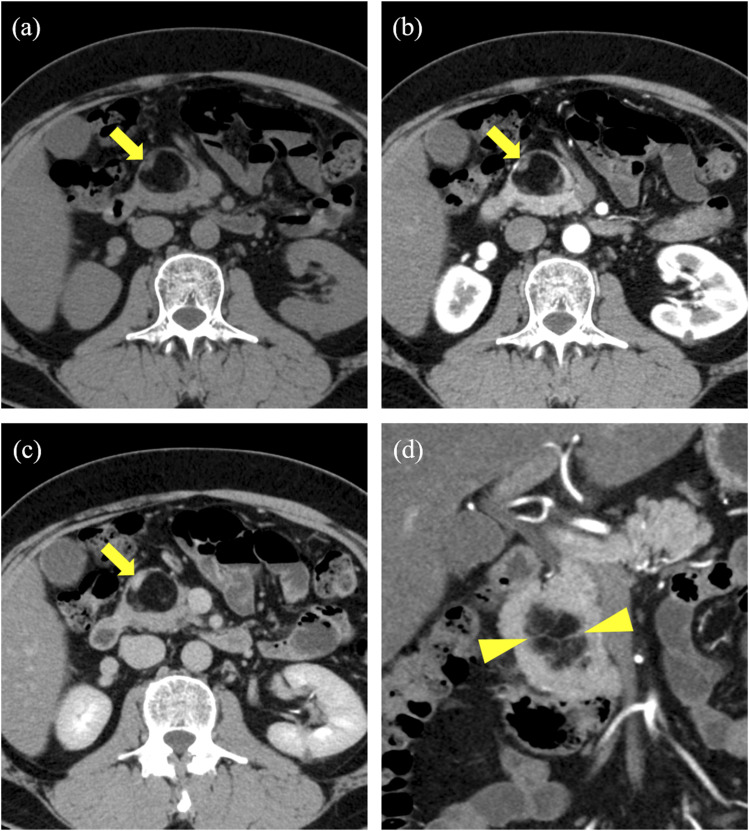
The noncontrast and dynamic enhanced images of the CT scan performed at our hospital indicated a mass measuring 35 × 27 mm with fat density at the head of the pancreas (Fig 1(a)). The original pancreatic parenchyma was dorsally compressed. The boundaries of the mass were mostly clear but partly unclear, and they were observed to be continuous with retroperitoneal fat at the bottom of the mass. A small mural nodule was found within the mass. The nodule had soft tissue density and revealed delayed contrast enhancement (Fig 1(b) and (c)). In addition, some thin septum structures with soft tissue density were found in the mass (Fig 1(d)). We suspected lipoma or liposarcoma and decided to engage in follow-up.
Fig 1.
(a) Precontrast CT showing a fatty mass in the pancreatic head. (b) (c) Postcontrast CT (arterial and equilibrium phases) reveals a slightly contrast-enhanced nodule in the wall (arrows). (d) On coronal images, some thin septum structures were found in the mass (arrowheads).
Surgery was performed for the renal tumor, and the pathological diagnosis was clear cell renal cell carcinoma (RCC). Follow-up CT was performed after 6, 12, and 15 months. There was no RCC recurrence at the postoperative site; however, imaging revealed that the pancreatic mass had gradually increased in size. In 15 months, the mass had enlarged from 35 × 27 mm to 39 × 31 mm; however, the size of the mural nodule had not changed.
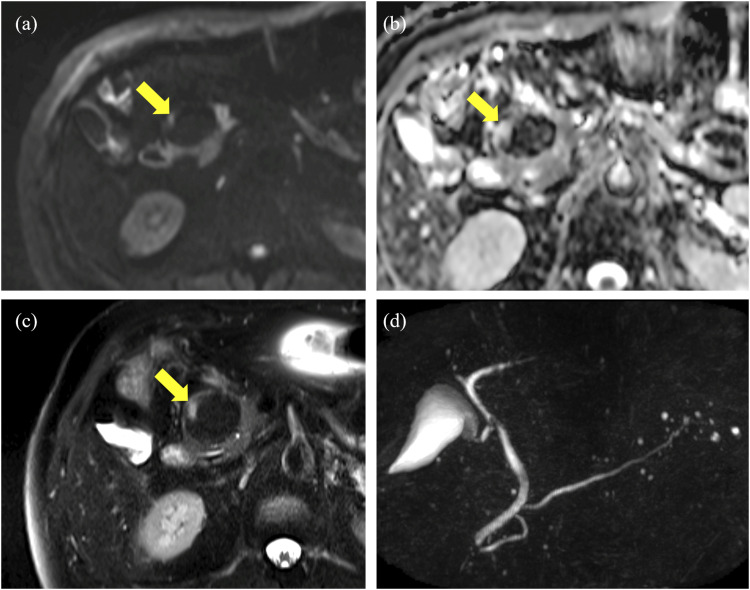
Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) were performed 15 months after surgery. Almost the whole mass had fatty signal intensity of all sequences; however, a mural nodule on CT had mild high signal intensity on T2-weighted images and high signal intensity on diffusion-weighted image (DWI) without low apparent diffusion coefficient (ADC) values, which was different from the intensity of normal pancreatic parenchyma (Fig 2(a)–(c)). MRCP showed that the main pancreatic duct was neither dilated nor obstructed (Fig 2(d)).
Fig 2.
(a) (b) DWI showing the small mural nodule with a high signal without low ADC values (arrows). (c) T2-weighted image showing mild high signal intensity, different from that of normal pancreatic parenchyma (arrow). (d) MRCP showing that the main pancreatic duct was not obstructed.
Because of the increasing size of the mass, we suspected liposarcoma of the pancreas. The mural nodule that had high signal intensity on T2-weighted images was considered to be a nonfatty malignant component such as myxoid degeneration, and the absence of diffusion restriction was not inconsistent. EUS-FNA can sometimes be difficult to distinguish between lipoma and liposarcoma, and there are also risks of complications and dissemination, so we have decided not to perform it. Pylorus-preserving pancreaticoduodenectomy was performed. Intraoperative findings revealed a fatty mass in the pancreatic head that appeared to have partially invaded the transverse mesocolon. Sites suspected of the tumor (including some mesenteric nodes) were extensively resected.
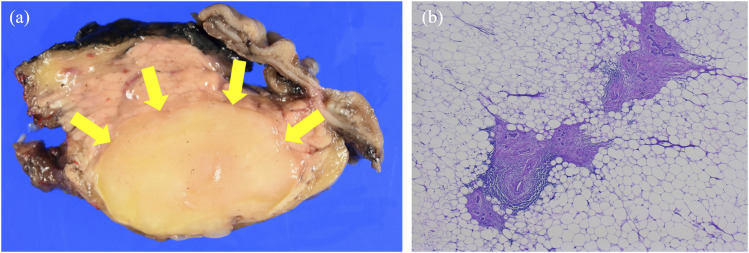
Macroscopically, the surgical specimen was an oval mass well-demarcated with existing pancreatic parenchyma, and the cut surface showed findings consistent with the presence of uniform xanthochromatic adipose tissue (Fig 3(a)). Histopathological features showed the proliferation of mature adipocyte-like cells. No atypical cells or mitotic figures suggestive of malignancy were observed. Existing pancreatic ducts, connective tissue, and acini were also found inside the mass (Fig 3(b)). No capsular structures around the mass (such as those found in lipoma and liposarcoma) were observed. Therefore, the patient was diagnosed with LPH. Although the mural nodule could not be clearly identified, there was degenerated pancreatic parenchyma at the margin of the mass, which may have corresponded to the nodule. The septum structure was also considered to be the pancreatic parenchyma left behind.
Fig 3.
(a)The cut surface of the oval mass showing uniform xanthochromatic adipose tissue findings (arrows). (b) The histopathologic features reveal the proliferation of mature adipocyte-like cells. Existing pancreatic tissues were also found inside the mass, some of them with autolysis.
Discussion
LPH is an extremely rare disease that was first reported by Hantelmann in 1931. It has some characteristic pathological features: (1) increased size and weight of the pancreas, (2) complete replacement of the pancreatic exocrine portion by mature adipose tissue, and (3) the preservation of the duct system and the islets of Langerhans.1,2 The exact etiology of LPH is unknown, although several possibilities have been suggested (including pancreatic parenchymal atrophy by infection, ductal obstruction, toxin exposure, and congenital anomalies).3,4 Several reports suggest an association with chronic liver injury and malignant tumors.5–7 According to reported cases, LPH can occur in people of a wide age range (from 6 days to 80 years) with no sex differences.4,8–10 The clinical manifestations of the condition include discomfort in the epigastric region, diarrhea, and steatorrhea, although asymptomatic patients are also seen. 4 The rarity of LPH means that the duration of the progression of adipose tissue replacement is unknown; however, the condition is believed to develop slowly, taking several years. 4
In more than half of the reported cases, the affected lesions extended throughout the pancreas. Making a mass confined to one segment of the pancreas (such as it was in this case) is rare.4,10 Focal LPH usually mimics lipoma, but differs in that the pancreas is enlarged, the existing pancreatic parenchyma is preserved in the mass, and no capsule is seen.
No specific therapies have been established for LPH, and management with regular follow-up is thought to be sufficient. When the clinical manifestations of pancreatic exocrine functional insufficiency are present, pancreatic enzymes should be administered. However, as in this case, some patients with focal LPH mimicking a tumor were suspected of having liposarcoma preoperatively and underwent surgical resection.4,10
There are some imaging findings that distinguish LPH from liposarcoma, such as the absence of narrowing of the main pancreatic duct and morphological integrity. 11
In this case, because the nodule within the mass showed a different density and signal pattern from the normal pancreatic parenchyma, we suspected liposarcoma. In subsequent pathological examination, the nodule was considered to correspond to degenerated pancreatic tissue. In retrospective view, the nodule did not change despite the growth of the mass over time, indicating that dedifferentiated liposarcoma is unlikely. Furthermore, the absence of main pancreatic duct stenosis caused by the mass was suggestive of LPH. Focusing on these imaging findings might have prevented invasive surgery. Given the paucity of literature on imaging findings of LPH, we believe that the accumulation of future cases will contribute to a more reliable imaging diagnosis.
In conclusion, LPH is considered hard to diagnose based on clinical findings. Understanding the distinctive CT and MRI findings associated with LPH can aid in making treatment decisions.
Acknowledgements
We thank Enago (https://www.enago.com) for the English language review.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Tomomi Kamimura https://orcid.org/0009-0000-8162-1068
References
- 1.Hantelmann W. Fettsucht und Atrophie der Bauch specicheldrüse bei Jungendlichen. Virchows Arch 1931;282:630–642. [Google Scholar]
- 2.Siegler DI. Lipomatous pseudohypertrophy of the pancreas associated with chronic pulmonary suppuration in an adult. Postgrad Med 1974;50:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyer A. Lipomatous pseudohypertrophy of the pancreas with complete absence of exocrine tissue. J Pathol Bacteriol 1949;61:93–100. [Google Scholar]
- 4.Shimada M, Shibahara K, Kitamura H, et al. Lipomatous pseudohypertrophy of the pancreas taking the form of huge massive lesion of the pancreatic head. Case Rep Gastroenterol 2010;4:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki M, Nakanuma Y, Ando H. Lipomatous pseudohypertrophy of the pancreas in a patient with cirrhosis due to chronic hepatitis B. Pathol Int 1998;48:566–568. [DOI] [PubMed] [Google Scholar]
- 6.Jayatunge SP, Mahendra G, Siyabalapitiya S, et al. Total pancreatectomy for cholangiocarcinoma of the distal common bile duct associated with lipomatous pseudohypertrophy of pancreas. Int J Hepatobiliary Pancreat Dis 2015;5:1–34. [Google Scholar]
- 7.Bralet MP, Terris B, Brégeaud L, et al. Squamous cell carcinoma and lipomatous pseudohypertrophy of the pancreas. Virchows Arch 1999;434:569–572. [DOI] [PubMed] [Google Scholar]
- 8.Luu VD, Duc NM, My TT, et al. A rare case of lipomatous pseudohypertrophy of the pancreas. Radiol Case Rep 2021;16:1363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumb G, Beautyman W. Hypoplasia of the exocrine tissue of the pancreas. J Pathol Bacteriol 1952;64:679–686. [DOI] [PubMed] [Google Scholar]
- 10.Altinel D, Basturk O, Sarmiento JM, et al. Lipomatous pseudohypertrophy of the pancreas: a clinicopathologically distinct entity. Pancreas 2010;39:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda M, Niina Y, Uchida M, et al. A case of lipomatous pseudohypertrophy of the pancreas diagnosed by typical imaging. J Pancreas 2010;11:385–388. [PubMed] [Google Scholar]