Abstract
Background:
Hemophilia-associated bleeding and resultant joint pain and mobility restrictions can predispose patients to poor health-related quality of life (HRQoL). Therefore, efficacy of a treatment needs to address more than just annualized bleed rates.
Objectives:
Describe the evolution of HRQoL, pain, and activity in patients with hemophilia A, treated with efmoroctocog alfa prophylaxis.
Design:
A post hoc analysis from Kids A-LONG (NCT01458106), A-LONG (NCT01181128), and long-term extension study ASPIRE (NCT01454739) assessed change in pain and activity-related patient-reported outcomes (PROs).
Methods:
Physical health, pain, and HRQoL were assessed by PROs for a cumulative treatment duration of up to ~6 years. The primary endpoint was change from baseline in EuroQoL (EQ)-5D and Haemophilia Quality of Life Questionnaire (Haem-A-QoL).
Results:
118 adult/adolescents and 71 pediatric patients were included. The proportion of adults and adolescents reporting no problem in the EQ-5D analysis of ‘pain/discomfort’ significantly increased from A-LONG baseline (35.04%; 41/117) to ASPIRE month 30 (44.68%; 21/47; p = 0.024). Mean (standard deviation) Haem-A-QoL subdomain scores for ‘feeling’ and ‘physical health’ at A-LONG baseline improved by −3.24 (15.13; p = 0.018) and −3.85 (23.07; p = 0.047), respectively, at study end. Proportion of pediatric patients reporting no problem on the EQ-5D analysis of ‘pain/discomfort’, significantly increased from A-LONG baseline (75.0%; 42/56) to ASPIRE baseline (95.56%; 43/45; p = 0.046). Satisfaction levels for pediatric patients were high at A-LONG baseline and maintained until study end.
Conclusion:
Long-term efmoroctocog alfa prophylaxis reduces pain and improves HRQoL in adult and adolescent patients with hemophilia A. In pediatric patients, it reduces perceived pain and maintains satisfaction levels.
Trial registration:
Keywords: hemophilia A, pain, patient-reported outcomes measures, quality of life, rFVIIIFc protein
Introduction
Hemophilia is characterized by hemarthrosis that can lead to hemophilic arthropathy and chronic pain, particularly in severe cases. 1 Patients with severe hemophilia have poorer health-related quality of life (HRQoL) compared to the reference population,1,2 and experience more absenteeism and higher unemployment.2–5 While the measurement is subjective, 6 patients report pain levels as ‘intolerable, unbelievable and like being shot’. 7 Yet, the chronic nature of hemophilia can result in patients manifesting coping strategies, leading to normalization of symptom severity, obscuring the full extent of the burden. 6
Bleeding, the resultant hospital visits, and impaired joint health (and associated limitations) predisposes patients to poor mental health and the associated impacts,8–11 such as an increase in uncontrolled pain. 10 Therefore, treatment needs to address more than annualized bleed rates (ABRs).
One approach to assess treatment benefit from the patient’s perspective is patient-reported outcomes (PROs), such as the Haemophilia Quality of Life Questionnaire for Adults (Haem-A-QoL), EuroQoL (EQ)-5D-3L, the hemophilia-specific treatment satisfaction questionnaire (Hemo-Sat), and the Canadian Haemophilia Outcomes – Kids’ Life Assessment Tool (CHO-KLAT). 12 PROs have demonstrated an association between pain and HRQoL, 13 and that increased physical activity improves pain (measured by EQ Visual Analogue Scale), mental health, and reduces bleeding risk.14–16
One bleed can result in hemophilic arthropathy12,17 and prophylaxis with hemostatic agents, ideally initiated before the onset of joint damage, can prevent bleeding and promote physical activity, psychosocial wellbeing, and HRQoL.12,18–20
Efmoroctocog alfa, a recombinant factor VIII (FVIII) Fc fusion protein referred to herein as rFVIIIFc, is an extended half-life FVIII replacement therapy approved for the treatment of bleeding and prophylaxis in patients with hemophilia A.21,22 rFVIIIFc is well tolerated and associated with low ABRs among previously treated adult/adolescent (A-LONG), pediatric (Kids A-LONG) patients,23,24 and previously untreated patients (PUPs A-LONG). 25 Patients from Kids A-LONG and A-LONG were followed in the long-term extension study (ASPIRE), maintaining a low ABR (~6 years of treatment). 26
Here, we present post hoc analysis from Kids A-LONG, A-LONG, and ASPIRE, describing long-term data on the evolution of HRQoL, pain, and physical activity assessed by PROs in adult/adolescents and children with hemophilia A, treated with rFVIIIFc prophylaxis.
Methods
Study design and patient population
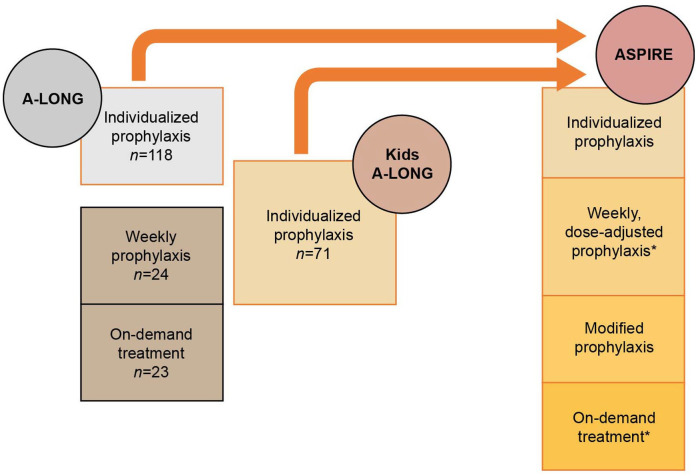
Study designs of Kids A-LONG, A-LONG, and ASPIRE are previously published.23,24,26 Briefly, in Kids A-LONG, previously treated (with any recombinant or plasma-derived FVIII product) children <12 years with severe hemophilia A were given prophylactic rFVIIIFc in twice-weekly infusions [25 IU/kg on day 1 and 50 IU/kg on day 4, with pharmacokinetic-based dose adjustments (maximum 80 IU/kg) and frequency (⩾2 days)]. 24 In A-LONG, previously treated adult/adolescent (⩾12 years) patients with severe hemophilia A were assigned to either: individualized prophylaxis (twice-weekly dosing; 25 IU/kg on day 1 and 50 IU/kg on day 4, followed by 25–65 IU/kg every 3–5 days); weekly prophylaxis (65 IU/kg); or on-demand treatment (10–50 IU/kg, depending on bleeding severity). 23
ASPIRE enrolled patients who completed either A-LONG [⩾12 years; ⩾150 prior exposure days (EDs) to FVIII] or Kids A-LONG (<12 years; ⩾50 prior EDs to FVIII) (Figure 1). Patients were followed for ⩾100 rFVIIIFc EDs across parent and extension trials (⩽4 years or until rFVIIIFc therapy became commercially available). Patients received weekly prophylaxis (⩾12 years; 65 IU/kg every 7 days), individualized prophylaxis (25–65 IU/kg every 3–5 days, or twice weekly at 20–65 IU/kg on day 1 and 40–65 IU/kg on day 4 for ⩾12 years; ⩽80 IU/kg with dosing intervals ⩾2 days for <12 years), modified prophylaxis (dosing modified to meet individual needs), or on-demand (⩾12 years) treatment with rFVIIIFc. Switching treatment regimen was permitted upon enrollment or any time during ASPIRE (⩾12 years) at the investigator’s discretion, to achieve the lowest effective dose resulting in a FVIII trough level of 1–3%, along with dose adjustments to target trough levels up to 5% (or >5% for modified prophylaxis), if warranted. 26
Figure 1.
Study design.
*Adults and adolescents only.
PRO measures
Physical health, pain, and HRQoL were assessed by validated PROs (Table 1). For EQ-5D, ‘usual activities’ and ‘pain/discomfort’ were analyzed. For Haem-A-QoL (adults ⩾18 years), all domains and 10 items relating to ‘sport and leisure, physical health’ and ‘treatment’, were analyzed. For Hemo-Sat, items in the domains ‘ease and convenience’, ‘efficacy’, and ‘general satisfaction’ were analyzed. For CHO-KLAT, items related to physical activity and treatment satisfaction were analyzed.
Table 1.
Study endpoints.
| EQ-5D | Haem-A-QoL | Hemo-Sat | CHO-KLAT |
|---|---|---|---|
| EQ-5D rating of usual activities (discrete) EQ-5D rating of pain/discomfort |
Physical health domain - My swellings hurt - I had pain in my joints - It was painful for me to move - I had difficulty walking as far as I wanted Sport and leisure domain - I had to avoid sports that I like because of my hemophilia - I had to avoid sports like football - I played sports just as much as others - I didn’t have the freedom to travel where I wanted - It was necessary for me to plan everything in advance Treatment domain - I was annoyed about the amount of time spent having the injections All subdomains scores Total score |
Ease and convenience domain - Treatment does not interfere with our everyday life - I am satisfied with the volume of the infusion - I am satisfied with the way that the medication is administered - I am satisfied with how often my son must be infused Efficacy domain - I am satisfied with the activities he is allowed to do with his treatment General satisfaction domain - I am satisfied with his medication |
The child self-report version: - It bothered me that I couldn’t play the sports that I like - I liked playing out with my friends - I felt like I had some control of my life - The treatment I got was okay - Factor injections were a bother - I got upset with my limits in physical activity - Home injections made my life easier The parent proxy report version: - It bothered my son that he couldn’t play the sports that he likes - My son liked playing out with his friends - My son felt like he had some control of his life - The treatment my son got was okay - Factor injections were a bother for my son - My son got upset with his limits in physical activity - Home injections made my son’s life easier |
CHO-KLAT, Canadian Haemophilia Outcomes – Kids’ Life Assessment Tool; EQ-5D, EuroQoL-5D; Haem-A-QoL, Haemophilia Quality of Life Questionnaire; Hemo-Sat, hemophilia-specific treatment satisfaction questionnaire.
Haem-A-QoL responses were grouped into never/rarely/seldom or sometimes/often/all the time; EQ-5D responses were grouped into no problem or some/severe problems; Hemo-Sat responses were grouped into totally agree/somewhat agree or neither agree nor disagree/somewhat disagree/totally disagree; and CHO-KLAT responses were grouped into never/rarely or sometimes/often/always.
Statistical analysis
Patients were followed from Kids A-LONG or A-LONG, into ASPIRE, until study completion (which could be at any timepoint during A-LONG or ASPIRE) or until they switched to modified prophylaxis or discontinued. The main analysis included patients who started individualized rFVIIIFc prophylaxis and completed the Haem-A-QoL and EQ-5D questionnaires in the adult/adolescent population and Hemo-Sat, CHO-KLAT, and EQ-5D in the pediatric population. Sensitivity analysis included all patients who stayed on individualized prophylaxis in ASPIRE for 48 (adults/adolescents) or 36 months (pediatric patients).
Descriptive analyses of categorical variables were reported using number of subjects and percentages; continuous variables using mean, standard deviation, median, percentiles (25% and 75%), minimum, and maximum. Changes over time were assessed by comparing paired values at each timepoint to baseline. For categorical variables, paired McNemar tests were used (a continuity correction was applied when any cell counts were <5). Wilcoxon signed rank test was used for continuous variables.
Results
Patient population
Patient demographics have been previously published.23,24,26 This study included 118 adult/adolescents and 71 pediatric patients; most were previously treated with prophylaxis (73.73% (87/118) adults/adolescents; 88.73% pediatric patients) with the remainder treated on-demand (Table 2). For the sensitivity analysis, patients who did not continue on individualized prophylaxis in ASPIRE for 48 (adults/adolescents; n = 8) or 36 months (pediatric; n = 8), as well as 12 adult/adolescent patients and 1 pediatric patient who switched from individualized prophylaxis during ASPIRE, were excluded. However, as a sensitivity analysis was performed for each endpoint, patient numbers varied.
Table 2.
Patient demographics.
| Adults and adolescents (⩾12 years) | Children (<12 years) | |
|---|---|---|
| N = 118 | N = 71 | |
| Age, years [mean (SD)] | 32.76 (12.88) | 5.87 (2.62) |
| Weight, kg [mean (SD)] | 73.09 (15.15) | 24.82 (10.53) |
| Prior FVIII regimen [n (%)] | ||
| Prophylaxis | 87 (73.73%) | 63 (88.73%) |
| On-demand | 31 (26.27%) | 8 (11.27%) |
| Race | ||
| Asian | 27 (22.88%) | 5 (7.04%) |
| Black or African American | 7 (5.93%) | 9 (12.68%) |
| Other | 5 (4.24%) | 9 (12.68%) |
| White | 79 (66.95%) | 48 (67.61%) |
| Duration of follow-up, months [mean (SD)] | 45.11 (18.52) | 36.04 (17.35) |
FVIII, factor VIII; SD, standard deviation.
The number of patients responding to the questionnaires vary according to subdomain, patient population, and study time point (Supplemental Tables 1–4).
Adults/adolescent patients (⩾12 years)
EQ-5D pain/discomfort and usual activities
Significant and sustained improvements were reported in ‘pain/discomfort’ in the pooled analysis (all patients regardless of previous treatment regimen). At A-LONG baseline, 35.04% (41/117) of patients had no problem, increasing to 45.45% (35/77; p = 0.039) at week 28 and sustained until ASPIRE month 30 (44.68%; 21/47; p = 0.024). This was supported by the sensitivity analysis (data not shown).
For adult/adolescent patients previously treated with prophylaxis, there was no improvement seen in ‘pain/discomfort’, compared with those who were previously treated on-demand. A numerically sustained increase was seen from A-LONG baseline (on-demand: 32.26%; 10/31; prophylaxis: 36.05%; 31/86) to ASPIRE month 30 (on-demand: 47.06%; 8/17; prophylaxis 43.33%; 13/30). The sensitivity analysis gave similar results.
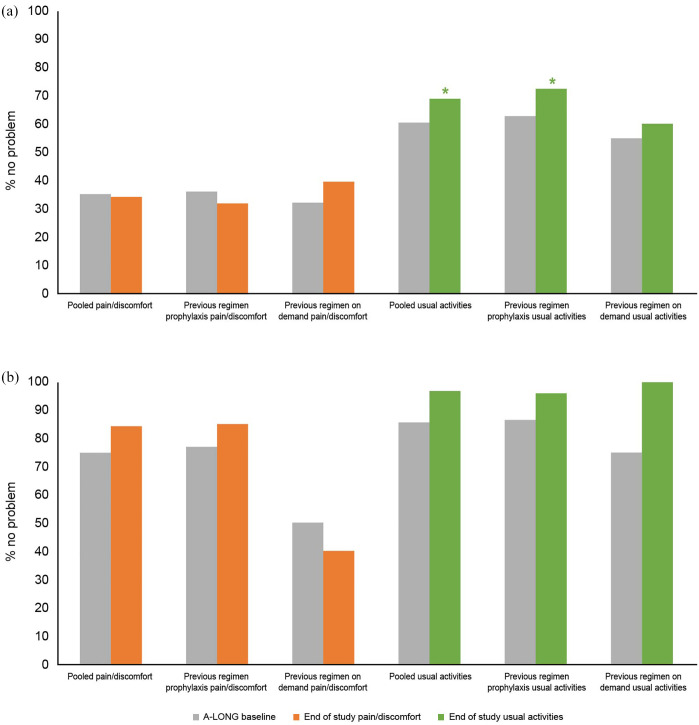
Significant and sustained improvements were reported in ‘usual activities’, with 60.68% (71/117) of patients reporting no problem at A-LONG baseline, increasing to 70.69% (82/116; p = 0.028) at A-LONG study end. This was sustained at study end, which could refer to any timepoint during A-LONG or ASPIRE (69.01%; 49/71; p = 0.027) [Figure 2(a)]. A similar pattern was observed in the sensitivity analysis (data not shown).
Figure 2.
Change from baseline to end of study in ED-5Q response: (a) adults and adolescents and (b) pediatrics.
End of study could refer to any timepoint during A-LONG or ASPIRE, depending on when the patient completed the study. Treatment regimen prior to enrolling in A-LONG.
*Significant results (p value < 0.05 comparison to A-LONG baseline).
In patients previously treated on-demand, a significant improvement from A-LONG baseline (54.84%; 17/31) in ‘usual activities’ was observed at ASPIRE month 18 (78.95%; 15/19; p = 0.023) (sensitivity analysis mirrored this result). There was also a significant increase from A-LONG baseline (62.79%; 54/86) to study end (72.55%; 37/51; p = 0.023) in patients previously treated with prophylaxis (not seen in the sensitivity analysis) [Figure 2(a)].
Haem-A-QoL total and subdomain scores
Significant and sustained improvements from A-LONG baseline were observed for the pooled analysis in Haem-A-QoL total score and the subdomains, ‘feeling’ and ‘physical health’, until ASPIRE month 36 (Supplemental Table 1; Supplemental Figure 1). Significant improvements were observed from baseline to study end for ‘feeling’, −3.24 (15.13; p = 0.018), and ‘physical health’, −3.85 (23.07; p = 0.047) (Figure 3).
Figure 3.
Change from baseline to end of study in Haem-A-QoL domain score for adults.
The change from baseline to end of study in Haem-A-QoL domain score for adults revealed a different pattern of response dependent on the previous treatment regimen. Treatment appeared to be more effective in those previously treated on-demand. End of study could refer to any timepoint during A-LONG or ASPIRE, depending on when the patient completed the study. Treatment regimen prior to enrolling in A-LONG. *Significant results (p value < 0.05 comparison to A-LONG baseline).
Haem-A-QoL, Haemophilia Quality of Life Questionnaire for Adults.
Significant improvements from baseline were observed for the subdomain ‘feeling’ at A-LONG study end, ASPIRE baseline, and ASPIRE month 24 for patients previously treated with prophylaxis. For those previously treated on-demand, there was an initial improvement at A-LONG week 28, then again from ASPIRE month 18 to ASPIRE month 54 (Supplemental Table 1). For the subdomain ‘physical health’, the results when analyzed by previous treatment regimen were similar to the pooled results (Supplemental Table 1).
Haem-A-QoL items
Significant and sustained improvements were found in Haem-A-QoL items ‘my swellings hurt’ in the pooled analysis [Figure 4(a)]. There was a reduction in ‘I didn’t have the freedom to travel where I wanted’ at ASPIRE month 6 (21.67%; 13/60; p = 0.027) versus A-LONG baseline (41.67%; 35/84) [Figure 4(b)], and in ‘I had pain in my joints’ at ASPIRE baseline (56.63%; 47/83; p = 0.016) versus A-LONG baseline (72.63%; 69/95) [Figure 4(c)].
Figure 4.
Response in Haem-A-QoL items for adults: (a) my swellings hurt, (b) I didn’t have the freedom to travel where I wanted, (c) I had pain in my joints, and (d) it was painful for me to move.
Perceived pain, as measured by Haem-A-QoL, was reduced during the study. The most significant reductions were in patients that were previously treated with an on-demand regiment. Large data point markers indicate significant results (p value <0.05 comparison to A-LONG baseline).
Treatment regimen prior to enrolling in A-LONG. Haem-A-QoL, Haemophilia Quality of Life Questionnaire for Adults.
When analyzed by previous treatment regimen, a significant improvement at ASPIRE month 24 (30%; 6/20; p = 0.041) was observed in ‘it was painful for me to move’ for patients previously treated on-demand compared with A-LONG baseline (59.09%; 13/22) [Figure 4(d)].
Pediatric patients (<12 years)
EQ-5D pain/discomfort and usual activities
Significant improvements were observed for the subdomain ‘pain/discomfort’, from A-LONG baseline (75.0%; 42/56) to ASPIRE baseline (95.56%; 43/45; p = 0.046). There were numerical improvements for ‘pain/discomfort’ and ‘usual activities’ from baseline to study end [Supplemental Table 2; Figure 2(b)].
When analyzed by previous treatment regimen, 76.92% (40/52) of patients previously on prophylaxis reported no problem with ‘pain/discomfort’ at A-LONG baseline, increasing to 100% at ASPIRE baseline (38/38; p = 0.041). For patients previously treated on-demand, improvements were observed from baseline (50.00%; 2/4) to 100% (6/6) at ASPIRE month 24.
Hemo-Sat results
A high proportion of patients/carers reported satisfaction in all Hemo-Sat items at A-LONG baseline (Supplemental Table 3). In the pooled analysis, a significant and sustained (all timepoints except ASPIRE month 12) improvement from A-LONG baseline was demonstrated in ‘I am satisfied with how often my son must be infused’. When analyzed by prior regimen, significant increases were reported from A-LONG baseline (70%) to ASPIRE month 30 (83.87%; 26/31; p = 0.023) for patients previously treated with prophylaxis. Numerical improvements were reported from A-LONG baseline (66.67%) to ASPIRE month 30 (100%) for patients previously treated on-demand.
A significant improvement from A-LONG baseline (63.64%; 42/66) was also demonstrated in ‘treatment does not interfere with our everyday life’ at ASPIRE month 30 (83.33%; 30/36; p = 0.01) [Figure 5(a)], and study end (80.00%; 36/45; p = 0.016) (Supplemental Table 3). For patients receiving prior on-demand treatment, numerical improvements were reported at study end [baseline: 50.00% (3/6) versus 85.71% (6/7)], whereas significant improvements were reported for patients receiving prior prophylaxis at study end [baseline: 65.00% (39/60) versus 78.95% (35/38); p = 0.046].
Figure 5.
Response for patients and caregivers for pediatric patients: (a) Hemo-Sat and (b) CHO-KLAT.
There were general improvements associated with treatment burden from the perspective of both the pediatric patient and their caregiver. Large data point markers indicate significant results (p value <0.05 comparison to A-LONG baseline). Treatment regimen prior to enrolling in A-LONG. Hemo-Sat, hemophilia-specific treatment satisfaction questionnaire; CHO-KLAT, Canadian Haemophilia Outcomes – Kids’ Life Assessment Tool; Hemo-Sat, hemophilia-specific treatment satisfaction questionnaire.
There was a significant improvement in ‘I am satisfied with the activities he is allowed to do with his treatment’ from A-LONG baseline (87.69%; 57/65) at ASPIRE month 24 (100.00%; 45/45; p = 0.023). For patients receiving prior on-demand treatment, numerical improvement was observed at ASPIRE month 24, whereas significant improvement was reported in patients receiving prior prophylactic treatment [baseline: 88.14% (52/29) versus 100.00% (39/39); p = 0.041] [Figure 5(a); Supplemental Table 3].
CHO-KLAT results
A significant decrease from A-LONG baseline (51.16%; 22/43) was demonstrated in the child-reported item ‘factor injections were a bother’ at study end (17.07%; 7/41; p = 0.01) in the pooled analysis [Figure 5(b)]. In patients receiving prior on-demand treatment, numerical decreases were observed over study duration whereas significant decreases were observed in patients previously on prophylactic treatment at study end [baseline: 30.00% (10/33) versus 18.92% (7/37); p = 0.027].
A significant decrease from A-LONG baseline (40.32%; 25/62) was demonstrated in the caregiver-reported item ‘factor injections were a bother for my son’ at study end (20.83%; 10/48; p = 0.004) in the pooled analysis, demonstrating a disparity between the child and caregiver-reported outcome (Supplemental Table 4). Significant decreases were observed at ASPIRE month 30 and study end for caregivers of the subgroup of patients who received prior prophylaxis (p = 0.009 and p = 0.003, respectively), with numeric decreases being reported for caregivers of patients who previously received on-demand treatment.
A significant decrease from A-LONG baseline (30.43%; 14/46) was demonstrated in ‘I got upset with my limits in physical activity’ at study end (21.43%; 942; p = 0.041) in the pooled group [Figure 5(b)]. Significant decreases from baseline at ASPIRE months 24, 36, and 42 were reported for patients previously on prophylaxis (p = 0.013, p = 0.023, and p = 0.041, respectively); changes remained similar versus baseline for patients on prior on-demand treatment. A significant decrease from baseline (46.67%; 21/45) was demonstrated in ‘it bothered me that I couldn’t play the sports that I like’ at ASPIRE month 36 (18.52%; 5/27; p = 0.046) in the pooled analysis. When analyzed according to prior treatments, changes over time were small and non-significant regardless of the prior treatment regimen (Supplemental Table 4).
Discussion
In this post hoc analysis of Kids A-LONG, A-LONG, and ASPIRE, PROs assessing HRQoL, pain, and activity demonstrated a maintenance of baseline scores and, in some incidences, a significant and sustained improvement over ~5 years of treatment with rFVIIIFc prophylaxis in patients with hemophilia A. It is noteworthy that, while change in some PROs was small, improvements were often sustained, adding to the published data demonstrating that rFVIIIFc reduces ABRs, is well tolerated, and improves HRQoL, with reduced dosing intervals compared to standard half-life (SHL) FVIII.26–28
EQ-5D results for adults/adolescents showed the proportion of patients reporting problems with ‘pain/discomfort’ and ‘usual activities’ decreased until ASPIRE month 30. It should be noted that patient numbers declined during the course of the study, making it challenging to assess the impact of treatment in later stages and thus being the main limitation of the study.
In adults, significant and sustained Haem-A-QoL improvements were observed in the total score and subdomains, ‘feeling’, ‘physical health’, and ‘work and school’. Along with the EQ-5D results, this reflects the interplay between bleeds, pain, activity, and psychosocial wellbeing, and the need to initiate early prophylaxis to promote good physical and mental health.12,18–20 The Haem-A-QoL improvements observed reflect those of a study by Astermark et al. 29 into the long-term effects of recombinant factor IX Fc fusion protein in adults and adolescents with hemophilia B. In that study, significant and sustained improvements were observed in the overall Haem-A-QoL score, as well as the subdomains of ‘physical health’, ‘sports and leisure’, ‘treatment’, and ‘view of self’. 29 Similarly, a HRQoL study of patients with hemophilia A treated with turoctocog alfa pegol for 6–27 months reported improvements from baseline in both Haem-A-QoL domains ‘physical health’ and ‘feeling’, and improvements in the domain of ‘sport’ in patients previously treated on-demand. 30 These observations are consistent with documented benefits of extended half-life (EHL) factor treatments, namely higher trough levels for clotting factors in patients, resulting in reduced risk of both clinically evident and subclinical bleeds. 12 This can allow patients a greater level of sports participation, improving physical and mental wellbeing. 12
Long-term efficacy and safety analyses of treatments for hemophilia A have been documented,31–35 but long-term HRQoL analyses have only been reported for emicizumab,36,37 and in patients who have switched from SHL to EHL prophylaxis. 38 In a study evaluating long-term (73 weeks) emicizumab prophylaxis, improvements were seen in the total score and the ‘physical health’ and ‘treatment’ domains of Haem-A-QoL, although no other domains or items were reported, and no improvement was observed in EQ-5D-5L. 36 A study evaluating adult/adolescent patients’ perceptions during more than 5 years’ emicizumab treatment found the majority of patients responding to an original questionnaire reported improvements in ‘daily life’ (frequency or amount of daily activities and frequency of going out or exercising) and ‘feelings’ (motivation to learn, work or do housework, and anxiety). 37 However, it should be noted that this was a mixed patient population in regard to inhibitor status, 37 and it is unclear to what extent the improvements seen are driven by patients with a positive inhibitor status. A further study in patients with hemophilia A and B ⩾6 years old compared PROs, including assessment of Haem-A-QoL and SF-36 at baseline and up to 24 months, in those switching from SHL to EHL treatment with those remaining on SHL products. The results indicated that switching from SHL to EHL treatment with rFVIIIFc provided short-term improvement in overall HRQoL in a small proportion of indivduals with hemophilia A. 38
When the EQ-5D and Haem-A-QoL results were analyzed based on treatment regimen prior to enrollment in A-LONG, the greatest improvements were seen in patients previously treated on-demand, and the onset of improvement appeared to be delayed compared with patients previously treated with prophylaxis. The lack of prophylaxis may predispose patients to poorer general health and joint status, and an extended period of prophylaxis may be needed to attain improvements. This was also noted in the emicizumab study, 36 giving further evidence on the benefits of prophylaxis. 12
In pediatric patients, EQ-5D results demonstrated a reduction in pain during Kids A-LONG, which remained numerically lower than baseline throughout the study. This was also observed in the subgroup of patients previously treated with prophylaxis, which could be indicative of increased protection provided by rFVIIIFc, compared with the previous SHL FVIII treatment. The changes were less pronounced than in the adult/adolescent patients, but this was unsurprising given the high level of satisfaction at baseline. Improvement was observed in satisfaction with treatment, which was evident from both the child’s and the parent/carer’s perspective.
Limitations
Limitations of this study included the lack of a comparator, drop-off in patient numbers, and the resulting small sample size and risk of selection bias for subgroups. Reporting of pain and HRQoL is subjective, lacking a constant that can be measured against equally. Moreover, patients often avoid the extreme answers on Likert scales and it cannot differentiate between a patient that had one constant problem and a patient who occasionally had four different problems, hindering a clear and well-defined summary of HRQoL. 39
Conclusion
Long-term rFVIIIFc prophylaxis reduces pain and improves HRQoL in adult/adolescent patients with hemophilia A. In pediatric patients, it reduces pain and sustains levels of satisfaction. These analyses highlight the relevance of pain, physical health, and overall wellbeing as the concepts most sensitive to change with prophylaxis treatment, indicating the potential for improving long-term HRQoL in patients with hemophilia A.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207241257917 for Long-term efmoroctocog alfa prophylaxis improves perceived pain, mental, and physical health in patients with hemophilia A: post hoc analysis of phase III trials using patient-reported outcomes by Priyanka Raheja, Nana Kragh, Linda Bystrická, Daniel Eriksson, Khaoula Aroui, Marwa Mezghani, Sylvaine Barbier and Silvia Linari in Therapeutic Advances in Hematology
Acknowledgments
The Kids A-LONG, A-LONG, and ASPIRE studies (ClinicalTrials.gov identifiers: NCT01458106, NCT01181128, NCT01454739) were sponsored by Sobi and Sanofi. Medical writing and editorial support, funded by Sobi, was provided by Catherine Hoare, PhD, Bioscript Medical Communications, Macclesfield, UK in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Sobi and Sanofi reviewed and provided feedback on the manuscript.
Footnotes
ORCID iD: Daniel Eriksson  https://orcid.org/0000-0001-7699-242X
https://orcid.org/0000-0001-7699-242X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Priyanka Raheja, Haematology Department, Haemophilia Centre, The Royal London Hospital, Barts Health NHS Trust, Whitechapel Road, London E1 1FR, UK.
Nana Kragh, Sobi, Stockholm, Sweden.
Linda Bystrická, Sobi, Stockholm, Sweden.
Daniel Eriksson, Sobi, Stockholm, Sweden.
Khaoula Aroui, Putnam, Les Berges du lac, Tunisia.
Marwa Mezghani, Putnam, Les Berges du lac, Tunisia.
Sylvaine Barbier, Putnam, Lyon, France.
Silvia Linari, Department of Oncology, Center for Bleeding Disorders and Coagulation, Careggi University Hospital, Florence, Italy.
Declarations
Ethics approval and consent to participate: This analysis was based on data from previously published clinical trials. These had been performed in accordance with the Declaration of Helsinki and local regulations. Protocols were approved by the authorities and the ethics committees of the institutions involved. Signed informed consent was obtained from patients or patient guardians prior to participation.
Consent for publication: As this study used anonymized data from the previously published trials, further informed consent was not required.
Author contributions: Priyanka Raheja: Conceptualization; Validation; Writing – review & editing.
Nana Kragh: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Linda Bystrická: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Daniel Eriksson: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Khaoula Aroui: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Marwa Mezghani: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Sylvaine Barbier: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Silvia Linari: Conceptualization; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This analysis was funded by Sobi. The Kids A-LONG, A-LONG, and ASPIRE studies (ClinicalTrials.gov identifiers: NCT01458106, NCT01181128, NCT01454739) were sponsored by Sobi and Sanofi.
PR has received travel support to attend conferences from CSL Behring and Takeda; speaker and/or consultancy honoraria from Pfizer, Sobi, and Idogen. NK, LB, and DE are employees of SOBI AB. SL has received honoraria for speaking and reimbursement for attending symposia or congresses from Roche, Sobi, Novo Nordisk, CSL Behring, Takeda, and Sanofi. KA, SB, and MM have no conflicts to disclose.
Availability of data and materials: Sobi is committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by EMA and/or FDA, while protecting individual participant integrity and compliance with applicable legislation. Data access will be granted in response to qualified research requests. All requests are evaluated by a cross-functional panel of experts within Sobi and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies.
References
- 1. Rodriguez-Merchan EC, Jimenez-Yuste V, Aznar JA, et al. Joint protection in haemophilia. Haemophilia 2011; 17(Suppl. 2): 1–23. [DOI] [PubMed] [Google Scholar]
- 2. Niu J, Ning L, Zhang Q, et al. Health-related quality of life of patients with haemophilia: a cross-sectional survey in the Northeast of China. BMJ Open 2022; 12: e056668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou ZY, Koerper MA, Johnson KA, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ 2015; 18: 457–465. [DOI] [PubMed] [Google Scholar]
- 4. Varekamp I, Smit C, Rosendaal FR, et al. Employment of individuals with haemophilia in The Netherlands. Soc Sci Med 1989; 28: 261–270. [DOI] [PubMed] [Google Scholar]
- 5. Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care 2016; 22: s126–s133. [PubMed] [Google Scholar]
- 6. Auerswald G, Dolan G, Duffy A, et al. Pain and pain management in haemophilia. Blood Coagul Fibrinolysis 2016; 27: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rambod M, Sharif F, Molazem Z, et al. Pain: the voiceless scream in every haemophilia patient’s life. J Haem Pract 2016; 3: 8–13. [Google Scholar]
- 8. O’Hara J, Walsh S, Camp C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes 2018; 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bago M, Butkovic A, Faganel Kotnik B, et al. Health-related quality of life in patients with haemophilia and its association with depressive symptoms: a study in Croatia and Slovenia. Psychiatr Danub 2021; 33: 334–341. [DOI] [PubMed] [Google Scholar]
- 10. Witkop ML, Lambing A, Nichols CD, et al. Interrelationship between depression, anxiety, pain, and treatment adherence in hemophilia: results from a US cross-sectional survey. Patient Prefer Adherence 2019; 13: 1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Huniti A, Reyes Hernandez M, Ten Eyck P, et al. Mental health disorders in haemophilia: systematic literature review and meta-analysis. Haemophilia 2020; 26: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 13. Batt K, Boggio L, Neff A, et al. Patient-reported outcomes and joint status across subgroups of US adults with hemophilia with varying characteristics: results from the Pain, Functional Impairment, and Quality of Life (P-FiQ) study. Eur J Haematol 2018; 100(Suppl. 1): 14–24. [DOI] [PubMed] [Google Scholar]
- 14. Strike K, Mulder K, Michael R. Exercise for haemophilia. Cochrane Database Syst Rev 2016; 12: CD011180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niu X, Poon JL, Riske B, et al. Physical activity and health outcomes in persons with haemophilia B. Haemophilia 2014; 20: 814–821. [DOI] [PubMed] [Google Scholar]
- 16. Biasoli C, Baldacci E, Coppola A, et al. Promoting physical activity in people with haemophilia: the MEMO (Movement for persons with haEMOphilia) expert consensus project. Blood Transfus 2022; 20: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roosendaal G, Jansen NW, Schutgens R, et al. Haemophilic arthropathy: the importance of the earliest haemarthroses and consequences for treatment. Haemophilia 2008; 14(Suppl. 6): 4–10. [DOI] [PubMed] [Google Scholar]
- 18. Castaman G. The benefits of prophylaxis in patients with hemophilia B. Expert Rev Hematol 2018; 11: 673–683. [DOI] [PubMed] [Google Scholar]
- 19. van Vulpen LFD, Thomas S, Keny SA, et al. Synovitis and synovectomy in haemophilia. Haemophilia 2021; 27(Suppl. 3): 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oldenburg J, Zimmermann R, Katsarou O, et al. Controlled, cross-sectional MRI evaluation of joint status in severe haemophilia A patients treated with prophylaxis vs. on demand. Haemophilia 2015; 21: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bioverativ Therapeutics Inc. Eloctate® Prescribing information, https://www.fda.gov/media/88746/download (2020, accessed June 2024).
- 22. Swedish Orphan Biovitrum AB (publ). Elocta® Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf (2019, accessed June 2024).
- 23. Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood 2014; 123: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost 2015; 13: 967–977. [DOI] [PubMed] [Google Scholar]
- 25. Königs C, Ozelo MC, Dunn A, et al. Final results of PUPs A-LONG study: evaluating safety and efficacy of rFVIIIFc in previously untreated patients with haemophilia A. Res Pract Thromb Haemost 2020; 4(Suppl. 1): 8 (Abstract OC 03.02). [Google Scholar]
- 26. Nolan B, Mahlangu J, Pabinger I, et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemophilia 2020; 26: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wyrwich KW, Krishnan S, Auguste P, et al. Changes in health-related quality of life with treatment of longer-acting clotting factors: results in the A-LONG and B-LONG clinical studies. Haemophilia 2016; 22: 866–872. [DOI] [PubMed] [Google Scholar]
- 28. Pasi J, Hermans C, Hakimi Z, et al. Improvement in pain-related quality of life in patients with hemophilia A treated with rFVIIIFc individualized prophylaxis: post hoc analysis from the A-LONG study. Ther Adv Hematol 2022; 13: 20406207221079482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astermark J, Hermans C, Ezzalfani M, et al. Recombinant factor IX Fc prophylaxis reduces pain and increases levels of physical activity, with sustained, long-term improvements in patients with hemophilia B: post hoc analysis of phase III trials using patient-reported outcomes. Ther Adv Hematol 2023; 14: 20406207231170701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kearney S, Raffini LJ, Pham TP, et al. Health-related quality-of-life and treatment satisfaction of individuals with hemophilia A treated with turoctocog alfa pegol (N8-GP): a new recombinant extended half-life FVIII. Patient Prefer Adherence 2019; 13: 497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giangrande P, Abdul Karim F, Nemes L, et al. Long-term safety and efficacy of N8-GP in previously treated adults and adolescents with hemophilia A: final results from pathfinder2. J Thromb Haemost 2020; 18(Suppl. 1): 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Šaulytė Trakymienė S, Economou M, Kenet G, et al. Long-term safety and efficacy of N8-GP in previously treated pediatric patients with hemophilia A: final results from pathfinder5. J Thromb Haemost 2020; 18(Suppl. 1): 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Callaghan MU, Negrier C, Paz-Priel I, et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1–4 studies. Blood 2021; 137: 2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancuso ME, Biss T, Fischer K, et al. PROTECT VIII kids extension study: long-term safety and efficacy of BAY 94-9027 (damoctocog alfa pegol) in children with severe haemophilia A. Haemophilia 2021; 27: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lentz SR, Kavakli K, Klamroth R, et al. Turoctocog alfa pegol (N8-GP) in severe hemophilia A: long-term safety and efficacy in previously treated patients of all ages in the pathfinder8 study. Res Pract Thromb Haemost 2022; 6: e12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skinner MW, Négrier C, Paz-Priel I, et al. The effect of emicizumab prophylaxis on long-term, self-reported physical health in persons with haemophilia A without factor VIII inhibitors in the HAVEN 3 and HAVEN 4 studies. Haemophilia 2021; 27: 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shima M, Nagao A, Taki M, et al. Long-term safety and efficacy of emicizumab for up to 5.8 years and patients’ perceptions of symptoms and daily life: a phase 1/2 study in patients with severe haemophilia A. Haemophilia 2021; 27: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun HL, Yang M, Poon MC, et al. The impact of extended half-life factor concentrates on patient reported health outcome measures in persons with hemophilia A and hemophilia B. Res Pract Thromb Haemost 2021; 5: e12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feldman BM. Issues in the measurement of quality of life in hemophilia. Rev Bras Hematol Hemoter 2013; 35: 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207241257917 for Long-term efmoroctocog alfa prophylaxis improves perceived pain, mental, and physical health in patients with hemophilia A: post hoc analysis of phase III trials using patient-reported outcomes by Priyanka Raheja, Nana Kragh, Linda Bystrická, Daniel Eriksson, Khaoula Aroui, Marwa Mezghani, Sylvaine Barbier and Silvia Linari in Therapeutic Advances in Hematology