Abstract
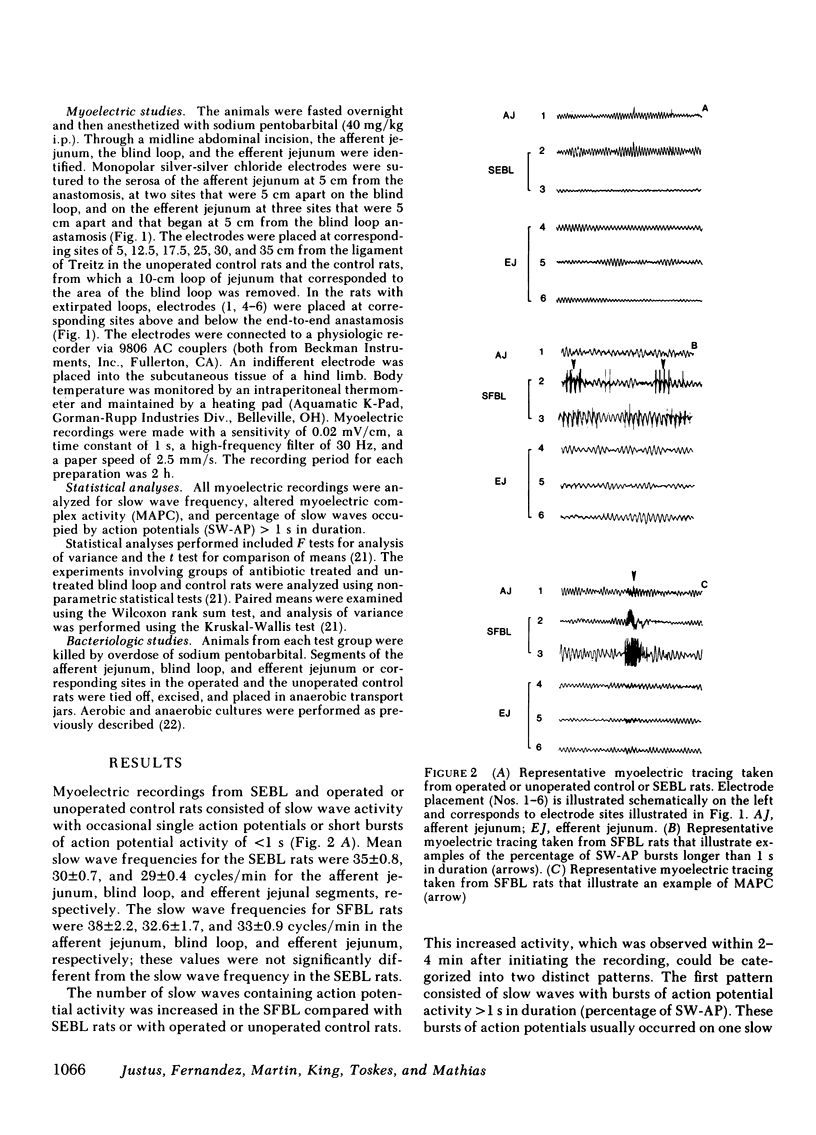
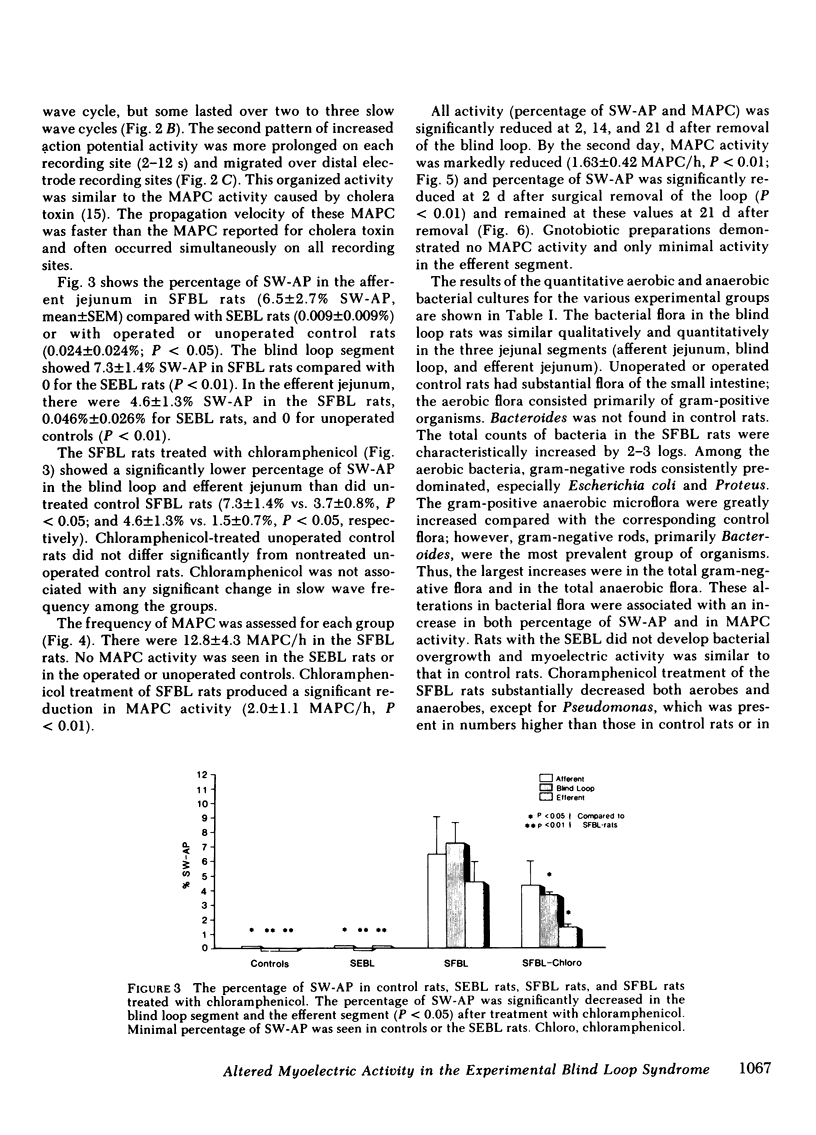
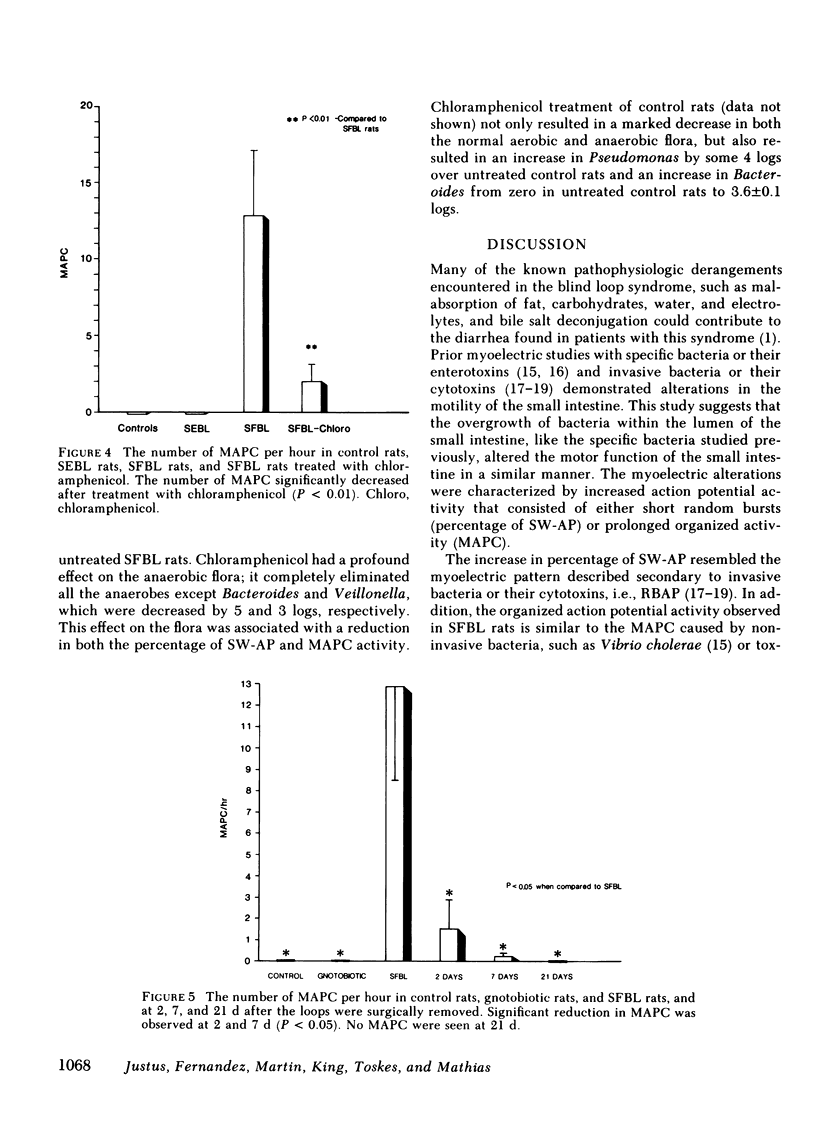
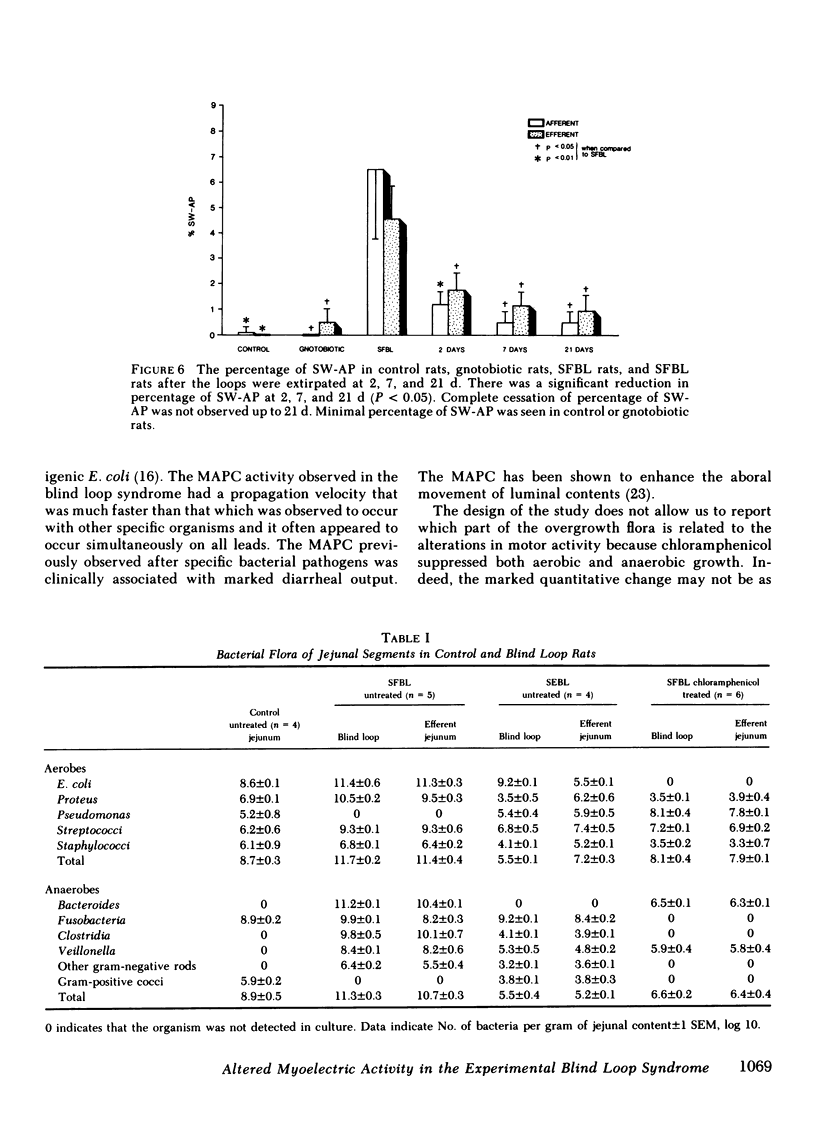
Nutrient malabsorption and diarrhea are characteristic of the blind loop syndrome. Alterations in motility have been implicated as a cause of bacterial overgrowth, but the possibility that altered motility may result from alterations in the flora has not been explored. The purpose of this study was to characterize the myoelectric activity of the small intestine in the blind loop rat model. Eight groups of rats were studied: rats with self-filling blind loops, which develop bacterial overgrowth; rats with self-emptying blind loops, which are surgical controls that do not develop overgrowth; unoperated litter mates; rats with self-filling blind loops and unoperated controls treated with chloramphenicol, 200 mg/d i.p.; rats with surgically removed self-filling blind loops; operated control rats; and gnotobiotic rats with self-filling blind loops. In the untreated rats with self-filling blind loops, there was altered myoelectric activity characterized by an increased percentage of slow waves occupied by action potentials and by organized activity similar to the migrating action potential complex. Migrating action potential complex activity and percentage of slow waves occupied by action potentials were significantly decreased with chloramphenicol therapy; that decrease correlated with a decrease in aerobes and anaerobes. Migrating action potential complex activity was abolished in rats with surgically removed self-filling blind loops; they also showed a significant decrease in percentage of slow waves occupied by action potentials. Gnotobiotic rats with self-filling blind loops showed no alteration in myoelectric activity. These data indicate: (a) bacterial overgrowth is associated with a significant increase in percentage of slow waves occupied by action potentials and migrating action potential complex activity; (b) chloramphenicol significantly reduced both percentage of slow waves occupied by action potentials and migrating action potential complex activity; and (c) surgical removal of the loop reduced the alterations in motor function. This study suggests that the altered myoelectric activity in this model of bacterial overgrowth was due, in part, to the abnormal bacterial flora and supports the concept that alterations in motility may contribute to the diarrhea that is characteristic of the blind loop syndrome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browning G. G., Buchan K. A., Mackay C. The effect of vagotomy and drainage on the small bowel flora. Gut. 1974 Feb;15(2):139–142. doi: 10.1136/gut.15.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns T. W., Mathias J. R., Carlson G. M., Martin J. L., Shields R. P. Effect of toxigenic Escherichia coli on myoelectric activity of small intestine. Am J Physiol. 1978 Sep;235(3):E311–E315. doi: 10.1152/ajpendo.1978.235.3.E311. [DOI] [PubMed] [Google Scholar]
- Burns T. W., Mathias J. R., Martin J. L., Carlson G. M., Shields R. P. Alteration of myoelectric activity of small intestine by invasive Escherichia coli. Am J Physiol. 1980 Jan;238(1):G57–G62. doi: 10.1152/ajpgi.1980.238.1.G57. [DOI] [PubMed] [Google Scholar]
- DONALDSON R. M., Jr Malabsorption of Co60-labeled cyanocobalamin in rats with intestinal diverticula. I. Evaluation of possible mechanisms. Gastroenterology. 1962 Sep;43:271–281. [PubMed] [Google Scholar]
- Donaldson R. M., Jr Studies on the pathogenesis of steatorrhea in the blind loop syndrome. J Clin Invest. 1965 Nov;44(11):1815–1825. doi: 10.1172/JCI105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Toskes P. P. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974 Nov;67(5):965–974. [PubMed] [Google Scholar]
- Goldstein F., Wirts C. W., Kowlessar O. D. Diabetic diarrhea and steatorrhea. Microbiologic and clinical observations. Ann Intern Med. 1970 Feb;72(2):215–218. doi: 10.7326/0003-4819-72-2-215. [DOI] [PubMed] [Google Scholar]
- Greenlee H. B., Gelbart S. M., DeOrio A. J., Francescatti D. S., Paez J., Reinhardt G. F. The influence of gastric surgery on the intestinal flora. Am J Clin Nutr. 1977 Nov;30(11):1826–1833. doi: 10.1093/ajcn/30.11.1826. [DOI] [PubMed] [Google Scholar]
- Justus P. G., Mathias J. R., Martin J. L., Carlson G. M., Shields R. P., Formal S. B. Myoelectric activity in the small intestine in response to Clostridium perfringens A enterotoxin: correlation with histologic findings in an in vivo rabbit model. Gastroenterology. 1981 May;80(5 Pt 1):902–906. [PubMed] [Google Scholar]
- King C. E., Toskes P. P. Small intestine bacterial overgrowth. Gastroenterology. 1979 May;76(5 Pt 1):1035–1055. [PubMed] [Google Scholar]
- Mathias J. R., Carlson G. M., DiMarino A. J., Bertiger G., Morton H. E., Cohen S. Intestinal myoelectric activity in response to live Vibrio cholerae and cholera enterotoxin. J Clin Invest. 1976 Jul;58(1):91–96. doi: 10.1172/JCI108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias J. R., Carlson G. M., Martin J. L., Shields R. P., Formal S. Shigella dysenteriae I enterotoxin: proposed role in pathogenesis of shigellosis. Am J Physiol. 1980 Nov;239(5):G382–G386. doi: 10.1152/ajpgi.1980.239.5.G382. [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Martin J. L., Burns T. W., Carlson G. M., Shields R. P. Ricinoleic acid effect on the electrical activity of the small intestine in rabbits. J Clin Invest. 1978 Mar;61(3):640–644. doi: 10.1172/JCI108975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson A. J., Brzechwa-Ajdukiewicz A., McCarthy C. F. Intestinal pseudo-obstruction with bacterial overgrowth in the small intestine. Am J Dig Dis. 1969 Mar;14(3):200–205. doi: 10.1007/BF02235883. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. A migrating electric complex of canine small intestine. Am J Physiol. 1969 Dec;217(6):1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Toskes P. P., King C. E., Spivey J. C., Lorenz E. Xylose catabolism in the experimental rat blind loop syndrome: studies, including use of a newly developed d-[14C]xylose breath test. Gastroenterology. 1978 Apr;74(4):691–697. [PubMed] [Google Scholar]
- Vantrappen G., Janssens J., Hellemans J., Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977 Jun;59(6):1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Toskes P. P., Baer H. Importance of anaerobic bacteria in the cobalamin malabsorption of the experimental rat blind loop syndrome. Gastroenterology. 1981 Feb;80(2):313–320. [PubMed] [Google Scholar]


