Abstract
Background
From a large observational acute coronary syndrome registry in Côte d'Ivoire, we aimed to assess incidence, clinical presentation, management, and in‐hospital outcomes for type 2 myocardial infarction (T2MI) compared with type 1 MI.
Methods and Results
We conducted a cross‐sectional monocentric study using data from REACTIV (Registre des Infarctus de Côte d'Ivoire) at the Abidjan Heart Institute. All patients hospitalized with MI between 2018 and 2022 who underwent coronary angiography were included. For each patient, sociodemographic data, cardiovascular risk factors and history, and clinical and paraclinical presentation were collected at admission. In‐hospital outcomes, including major adverse cardiovascular events and mortality, were reported. Among 541 consecutive patients hospitalized with MI, 441 met the definition of type 1 MI or T2MI. T2MI accounted for 14.1% of cases. Patients with T2MI showed a trend toward slightly younger age (54 versus 58 years, P=0.09). Patients with T2MI seemed to have less severe coronary artery disease, with less frequent multivessel disease (P<0.001). Main triggering factors for T2MI were coronary embolism (24.2%), severe hypertension with or without left ventricular hypertrophy (22.6%), and tachyarrhythmia (16.1%).
In‐hospital event rates were low in both MI types. Although the difference was nonsignificant, death rates for patients with type 1 MI tended to be higher than for patients with T2MI, as well as occurrence of major adverse cardiovascular events.
Conclusions
Our study revealed disparities in clinical characteristics, angiographic features, cause, and in‐hospital outcomes in T2MI in our population compared with Western populations. These results suggest the heterogeneity of T2MI and the potential causative and demographic variability depending on geographical area.
Keywords: coronary artery disease, sub‐Saharan Africa, type 1 myocardial infarction, type 2 myocardial infarction
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- REACTIV
Registre des Infarctus de Côte d'Ivoire
- RICO
Observatoire des Infarctus de Côte d'Or
- T1MI
type 1 myocardial infarction
- T2MI
type 2 myocardial infarction
Clinical Perspective.
What Is New?
This original paper addresses the knowledge gap for type 2 myocardial infarction in sub‐Saharan Africa populations.
When compared with type 2 myocardial infarction in Western countries, our study showed disparities in clinical characteristics (younger age), angiographic features (less severe coronary artery disease), causative framework (more frequently coronary embolism and hypertension), and in‐hospital outcomes.
What Are the Clinical Implications?
These observations emphasize the potential heterogeneity of this multifaceted disease and challenge the hypothesis that it is a geriatric pathology.
Myocardial infarction (MI) is the most common acute presentation of ischemic heart disease, which is the leading cause of death worldwide. 1 In sub‐Saharan Africa, acute MI has recently emerged as a major public health issue in association with the adoption of Western lifestyles. Accordingly, there has been a sharp rise in MI incidence, and high mortality rates have been reported among various studies. 2 Over the past 2 decades, in Côte d'Ivoire, hospital admissions for acute MI increased dramatically from 7.3% to 22.6%, with an estimated in‐hospital mortality rate of 10.4%. 3
MI has been defined by successive universal definitions (ie, rise of cardiac troponin level above the 99th percentile upper reference limit and with at least 1 of the following: symptoms of ischemia, new significant ST changes or new bundle branch block, development of pathological Q waves, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or identification of a coronary thrombus by angiography or by autopsy). 4 , 5 , 6 The last version of the universal classification of MI, which is the fourth universal definition of MI, was updated in 2018. 4 , 5 , 6 The most frequent type of MI is type 1 MI (T1MI), related to an atherothrombotic mechanism, as a consequence of coronary atherosclerosis, associated with “classical” risk factors (including hypertension, smoking, dyslipidemia, diabetes, and obesity). Type 2 MI (T2MI), including a wide range of acute coronary events, is defined as MI with evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute coronary atherothrombosis. T2MI can have various triggers: vasomotor abnormalities (including vasospasm or microvascular dysfunction), coronary embolism, spontaneous coronary artery dissection, sustained tachyarrhythmia, severe bradyarrhythmia, severe hypertension with or without left ventricular hypertrophy, respiratory failure with severe hypoxemia, anemia, and hypotension/shock. 6 Coronary angiography is important for the diagnosis of T2MI because it confirms that there is no visible coronary thrombosis. 7
The wide range of clinical presentations and multiple underlying mechanisms makes T2MI a diagnostic and therapeutic challenge, requiring tailored management. In Western populations, T2MI is common and characterized by specific sociodemographic, angiographic, and outcomes profiles. Patients with T2MI are older and have more comorbidities when compared with those with T1MI. 8 , 9 , 10 T2MI is usually associated with more severe coronary artery disease at angiography. 10 , 11 Moreover, patients with T2MI have dramatically increased in‐hospital 12 , 13 and long‐term mortality. 9
In sub‐Saharan African countries, to the best of our knowledge, the epidemiology of MI types are unknown and no prospective study has specifically addressed T2MI. Côte d'Ivoire, classified as a lower‐ to middle‐income country by the World Bank, is the largest economy in the West African Economic and Monetary Union and is experiencing one of the fastest sustained economic growth rates in sub‐Saharan Africa in nearly a decade. 14 It has a very young and active population and is currently undergoing major demographic growth. Similar to other sub‐Saharan African countries, the epidemiological transition has been marked by the recent emergence of cardiovascular diseases, particularly acute MI, in a context of urbanization and lifestyle changes. 15 In Côte d'Ivoire, as in most of sub‐Saharan Africa, there is limited access to heart centers where acute MI can be effectively managed. Only a few facilities, such as the Abidjan Heart Institute, are equipped with intensive care units and cardiac catheterization laboratories. 16 , 17
The main purpose of the study was to address the incidence, clinical features, management, and in‐hospital outcomes of patients with T2MI compared with patients with T1MI based on data from a prospective study conducted in Côte d'Ivoire.
Methods
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Study Design and Patient Criteria
The study was cross‐sectional and monocentric, using data from REACTIV (Registre des Infarctus de Côte d'IVoire), a prospective survey including patients older than 18 years hospitalized for MI in the intensive care unit of the Abidjan Heart Institute. 3 It is the largest facility in Côte d'Ivoire and the national referral center for the management of acute coronary syndromes, open 24 hours a day, 7 days a week. The center includes an emergency department, an intensive care unit, wards, a department for noninvasive exploration (including echocardiography laboratories and other cardiac diagnostic tests), a pediatric cardiology department, operating rooms for cardiovascular and thoracic surgery, an interventional cardiology laboratory, and a cardiac rehabilitation department. We used the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) cohort checklist when writing our report. 18
In this study, all patients with MI included between 2018 and 2022 who underwent coronary angiography were eligible. MI cases were adjudicated into T1MI and T2MI, based on criteria defined in the fourth universal definition of MI, 6 and variables were collected with standard definitions in agreement with the RICO (Observatoire des Infarctus de Côte d'Or) study 10 in the context of a collaborative project (Dijon/Abidjan Cardiovascular Network). Two cardiologists thoroughly reviewed and adjudicated each MI file. Disagreements (34 cases [7.7%]) were resolved by consensus, and, if necessary, by a third cardiologist. Briefly, T1MI was defined as acute coronary obstruction or reduction of coronary blood flow precipitated by atherosclerotic plaque rupture or erosion. T2MI was defined as MI with context of mismatch between oxygen supply and demand, triggered by an acute stressor. These precipitating factors have been shown in the literature: coronary spasm, 5 coronary embolism, 19 (retained in the cases of definite [≥2 major criteria or 1 major criterion plus ≥2 minor criteria or 3 minor criteria] or probable coronary embolism [1 major criterion plus 1 minor criterion or 2 minor criteria] according to Shibata et al), 20 spontaneous coronary artery dissection, 21 sustained tachyarrhythmia (especially atrial fibrillation [AF] or flutter), 19 severe hypertension, 19 severe bradyarrhythmia, 19 acute respiratory failure, 22 severe anemia, 19 and hypotension or shock.
MI types other than T1MI and T2MI were excluded (see flow chart).
Data Collection
Sociodemographic and anthropometric data (age, sex, body mass index), clinical data (cardiovascular risk factors and history, presentation), and ECG were collected at admission. Variables were collected with standard definitions from the RICO study protocol. 15
Cardiovascular risk factors were defined as follows:
Hypertension: systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or treatment of previously diagnosed hypertension.
Diabetes: fasting hyperglycemia ≥1.26 g/L twice or hyperglycemia ≥2 g/L at any time of day or glycated hemoglobin ≥6.5% or treatment of previously diagnosed diabetes.
Hypercholesterolemia: total cholesterol concentration >2.40 g/L with or without a low‐density lipoprotein cholesterol concentration >1.60 g/L and a high‐density lipoprotein cholesterol concentration <0.40 g/L in men or <0.50 g/L in women.
Family history of coronary artery disease (CAD): premature MI or sudden cardiac death (aged younger than 55 years in the patient's father or in a first‐degree male relative, or aged younger than 65 years in the patient's mother or in a first‐degree female relative).
Obesity: body mass index ≥30 kg/m2.
Left ventricular ejection fraction using transthoracic echocardiography and biplane Simpson method, as well as blood tests were obtained on admission (hemoglobin, C‐reactive protein, N‐terminal pro‐B‐type natriuretic peptide, creatinine). Peak cardiac troponin I was determined by daily blood testing during the first 3 days after admission. Glomerular filtration rate was evaluated by the Modification of Diet in Renal Disease formula.
Coronary angiography and percutaneous coronary interventions were performed based on current guidelines and local clinical practice. Coronary angiography was considered normal when the angiographic images did not show any visible atheromatous plaque or spastic phenomena. Significant CAD was defined as the narrowing of at least 1 coronary vessel ≥50%, and CAD severity was assessed by the number of epicardial vessels involved (1‐vessel, 2‐vessel, or 3‐vessel/left main CAD). Treatment data (revascularization procedures, drugs prescribed during hospitalization) were collected. In‐hospital outcomes were recorded, including heart failure (maximum Killip ≥2), 23 stroke, sustained ventricular arrhythmia, high‐degree atrioventricular block, AF/flutter, severe bleeding (defined as BARC [Bleeding Academic Research Consortium] type 3b), 24 all‐cause/cardiovascular death, and major adverse cardiac events (defined as cardiovascular death, reinfarction, or stroke). 25
Ethics
This study was conducted in accordance with the Declaration of Helsinki and was authorized by the ethics committee of the Abidjan Heart Institute. Each patient gave prior informed consent.
Statistical Analysis
Qualitative variables are expressed as headcounts and percentages and compared using the χ2 test or Fisher exact test when expected values were ≤5. A Kolmogorov–Smirnov test was conducted to analyze the normality of variables. Quantitative variables are expressed as medians and interquartile ranges and compared using the Student t test or the nonparametric Mann–Whitney test, when appropriate. The concordance between the 2 independent reviewers was assessed using the Cohen κ statistic. 26
Kaplan–Meier in‐hospital survival curves were performed and compared by log‐rank test. SPSS software version 26.0.0.0 (IBM) was used for statistical analysis, with statistical significance defined as P<0.05.
Results
Characteristics, Risk Factors, and Management
During the study period, 561 patients with MI were admitted to the Abidjan Heart Institute. Twenty patients (18 T1MI and 2 T2MI) were excluded from the analysis for missing data. Among the remaining 541 patients, 87 did not undergo coronary angiography. Finally, 441 patients met the definition of T1MI or T2MI in the study population. T2MI accounted for 14.1% of cases (62 of 441). The flow chart is shown in Figure 1.
Figure 1. Flow chart of the study.
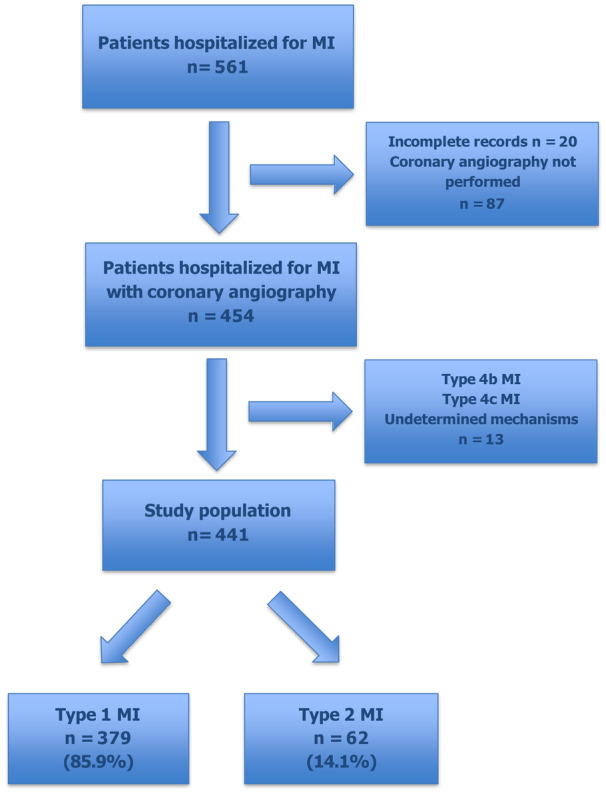
MI indicates myocardial infarction.
Kappa statistics (κ, 0.791 [95% CI, 0.707–0.875]) showed a high degree of agreement in the assessment of MI cases (Table S1).
Albeit without statistical difference, patients with T2MI appeared to be slightly younger (54 years versus 58 years) and more often female than patients with T1MI. Diabetes (P=0.044) and high body mass index were more frequent in patients with T1MI (Table 1). Other classic cardiovascular risk factors (hypertension, hypercholesterolemia, and smoking) showed a nonsignificant trend toward higher rate in patients with T2MI. Prior atherosclerotic cardiovascular diseases including CAD, stroke, and peripheral arterial disease seemed to be similar for both types of MI. Patients with T2MI had a more frequent history of heart failure or left ventricular dysfunction (8.1% versus 2.1%, P=0.025). Prior AF/flutter (8.1%) or AF/flutter on admission ECG (16.1%) (Table 2) were much more common in patients with T2MI (P<0.001). CAD characteristics on coronary angiography are presented in Figure 2. Compared with patients with T1MI, patients with T2MI had less severe CAD with less frequent multivessel disease (37.2% versus 8.1%, P<0.001). However, although younger, almost 42% of patients with T2MI (26 of 62) had significant coronary stenosis. P2Y12 inhibitors were more likely to be prescribed in patients with T1MI (91.0% versus 72.6%, P<0.001). By contrast, vitamin K antagonists were used only in patients with T2MI (8.1%, P<0.001) (Table 3).
Table 1.
Risk Factors, Comorbidities, and Cardiovascular History
| Parameters | T1MI, n=379 | T2MI, n=62 | P value |
|---|---|---|---|
| Risk factors and comorbidities | |||
| Age, y | 58 [50–65] | 54 [43–64] | 0.091 |
| Women | 75 (19.8) | 20 (32.3) | 0.031 |
| Hypertension | 232 (61.2) | 36 (58.1) | 0.675 |
| Diabetes | 110 (29.0) | 10 (16.1) | 0.044 |
| Hypercholesterolemia | 111 (29.3) | 14 (22.5) | 0.362 |
| Smoking | 92 (24.3) | 9 (14.5) | 0.090 |
| Family history of CAD | 17 (4.5) | 0 (0) | 0.147 |
| BMI, kg/m2 | 27.4 [23.9–30.4] | 25.7 [22.1–28.3] | 0.045 |
| Obesity | 87 (22.9) | 10 (16.1) | 0.229 |
| Cardiovascular history | |||
| MI | 32 (8.4) | 2 (3.2) | 0.202 |
| PCI | 14 (3.7) | 0 (0) | 0.235 |
| Stroke | 21 (5.5) | 3 (4.8) | 0.556 |
| PAD | 12 (3.2) | 1 (1.6) | 1.000 |
| Heart failure/LV dysfunction | 8 (2.1) | 5 (8.1) | 0.025 |
| AF/flutter | 1 (0.3) | 5 (8.1) | <0.001 |
Values are expressed as number (percentage) or median [interquartile range].
AF indicates atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; LV, left ventricular; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; T1MI, type 1 myocardial infarction; and T2MI, type 2 myocardial infarction.
Table 2.
Clinical and Paraclinical Data
| Parameters | T1MI, n=379 | T2MI, n=62 | P value |
|---|---|---|---|
| Clinical data | |||
| Onset of symptoms—admission, h | 20 [7–72] | 26 [5–93] | 0.593 |
| Killip admission ≥2 | 79 (20.8) | 12 (19.4) | 0.788 |
| SBP, mm Hg | 140 [127–161] | 135 [112–158] | 0.055 |
| DBP, mm Hg | 90 [80–102] | 87 [71–100] | 0.190 |
| HR, beats per min | 83 [71–99] | 84 [71–97] | 0.722 |
| ECG at admission | |||
| STEMI | 256 (67.5) | 38 (61.3) | 0.332 |
| Anterior wall MI | 195 (51.4) | 22 (35.5) | 0.020 |
| AF/flutter | 4 (1.1) | 10 (16.1) | <0.001 |
| Echocardiography | |||
| LVEF, % | 53 [44–61] | 55 [42–60] | 0.793 |
| LVEF ≤40% | 59 (15.6) | 14 (22.6) | 0.168 |
| Biology | |||
| Hemoglobin, g/100 mL | 13.7 [12.3–15.0] | 13.6 [12.2–15.0] | 0.756 |
| CRP, mg/L | 23.3 [5.4–89.0] | 15.3 [4.5–75.0] | 0.189 |
| CRP ≥6 mg/L | 119 (31.4) | 24 (38.7) | 0.254 |
| Creatinine, mg/L | 12.1 [10.0–14.7] | 12.4 [9.7–15.9] | 0.868 |
| eGFR, mL/min per 1.73 m2 | 74.9 [57.9–93.7] | 69.0 [52.7–89.6] | 0.624 |
| High‐sensitivity cardiac troponin I peak, ng/L | 3416 [552–25 470] | 2113 [2210–18 348] | 0.084 |
| NT‐proBNP, pg/mL | 1663 [518–5013] | 1559 [491–5055] | 0.956 |
| Length of stay in ICU, d | 3 [2–4] | 3 [2–4] | 0.530 |
Values are expressed as number (percentage) or median [interquartile range]. AF indicates atrial fibrillation; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure; STEMI, ST‐segment–elevation myocardial infarction; T1MI, type 1 myocardial infarction; and T2MI, type 2 myocardial infarction.
Figure 2. Severity of CAD according to T1MI and T2MI.
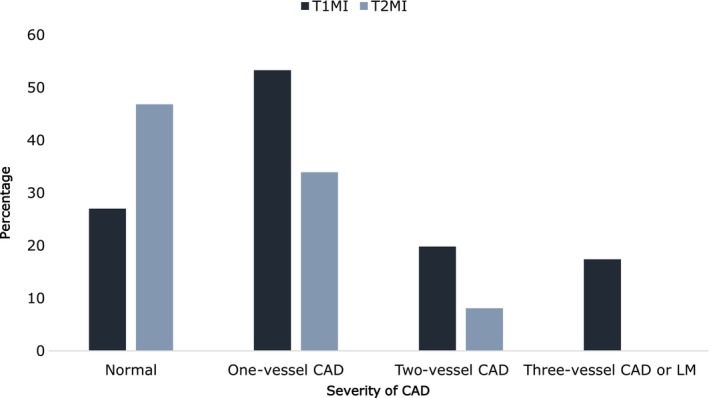
CAD indicates coronary artery disease; LM, left main; T1MI, type 1 myocardial infarction; and T2MI, type 2 myocardial infarction.
Table 3.
In‐Hospital Medications and Revascularization Procedures
| Acute treatments | T1MI, n=379 | T2MI, n=62 | P value |
|---|---|---|---|
| Thrombolysis | 40 (10.5) | 9 (14.5) | 0.357 |
| PCI | 168 (44.3) | 12 (19.3) | <0.001 |
| Aspirin | 356 (93.9) | 54 (87.1) | 0.061 |
| P2Y12 inhibitors | 345 (91.0) | 45 (72.6) | <0.001 |
| Unfractioned heparin | 28 (7.4) | 13 (21.0) | <0.001 |
| LMWH | 308 (81.3) | 40 (64.5) | 0.002 |
| β‐Blockers | 273 (72.0) | 40 (64.5) | 0.226 |
| Statins | 287 (75.7) | 45 (72.6) | 0.594 |
| ACEI/ARB | 271 (71.5) | 44 (71.0) | 0.931 |
| Diuretics | 96 (25.3) | 20 (32.2) | 0.250 |
| DOA | 0 (0) | 1 (1.6) | 0.140 |
| VKA | 0 (0) | 5 (8.1) | <0.001 |
Values are expressed as number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; DOA, direct oral anticoagulant; LMWH, low‐molecular‐weight heparin; PCI, percutaneous coronary intervention; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction; and VKA, vitamin K antagonist.
Acute Stressors for T2MI
Acute or chronic causative factors involved in T2MI pathophysiology are shown in Figure 3. Coronary embolism (24.2%), severe hypertension with or without left ventricular hypertrophy (22.6%), and tachyarrhythmia (16.1%) emerged as the main triggering factors. Anemia was infrequently involved (6.4%).
Figure 3. Pathophysiological mechanisms of type 2 myocardial infarction.
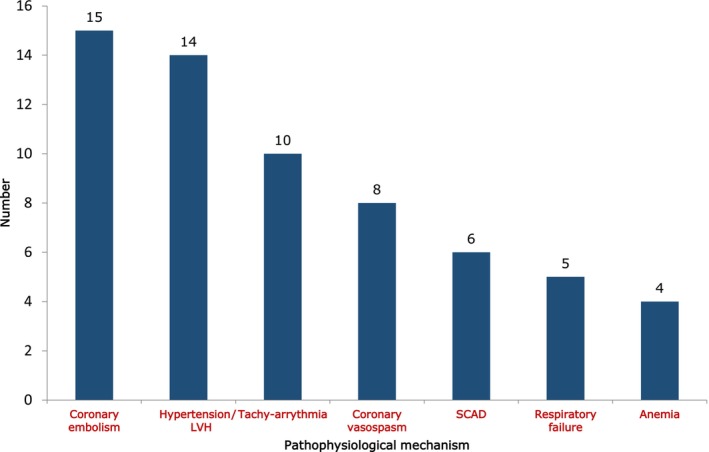
LVH indicates left ventricular hypertrophy; and SCAD, spontaneous coronary artery dissection.
In‐Hospital Outcomes
In‐hospital event rates were low in both MI types. In agreement with history and ECG findings, AF/flutter and stroke showed only a nonsignificant trend toward a higher rate in patients with T2MI. Overall hospital mortality was 2.3%. All deaths were from cardiovascular causes. Although the difference was nonsignificant, death rates in patients with T1MI tended to be higher than in patients with T2MI, as well as occurrence of major adverse cardiovascular events (Table 4). In univariate analysis, the results suggested that the occurrence of heart failure during hospitalization might be the only parameter associated with in‐hospital mortality (P=0.044). Anterior wall MI showed almost a significant trend (P=0.059) (Table 5). Kaplan–Meier analyses did not show significant trends considering occurrence of heart failure (P=0.162) or anterior wall MI (P=0.09) (Figure 4).
Table 4.
In‐Hospital Events
| Variables | T1MI, n=379 | T2MI, n=62 | P value |
|---|---|---|---|
| Heart failure | 15 (3.9) | 3 (4.8) | 0.728 |
| Sustained VT | 12 (3.2) | 3 (4.8) | 0.453 |
| AF/flutter | 2 (0.5) | 1 (1.6) | 0.093 |
| Transfusion or major bleeding | 4 (1.0) | 3 (4.8) | 0.061 |
| Stroke | 3 (0.8) | 2 (3.2) | 0.147 |
| Death | 9 (2.4) | 1 (1.6) | 0.104 |
| MACE | 18 (4.7) | 2 (3.2) | 1.000 |
Values are expressed as number (percentage). AF indicates atrial fibrillation; MACE, major adverse cardiac events; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction; and VT, ventricular tachycardia.
Table 5.
In‐Hospital Mortality: Univariable Analysis
| Parameters | Alive, n=431 | Deceased, n=10 | P value |
|---|---|---|---|
| Age, y | 66 [50–69] | 58 [49–65] | 0.076 |
| Men | 337 (78.2) | 9 (90.0) | 0.697 |
| Heart failure | 91 (21.1) | 5 (50.0) | 0.044 |
| STEMI | 285 (66.1) | 9 (90.0) | 0.176 |
| Anterior wall MI | 209 (48.5) | 8 (80.0) | 0.059 |
| Type 2 MI | 61 (14.2) | 1 (10.0) | 1.000 |
| PCI | 176 (40.8) | 4 (40.0) | 1.000 |
Values are expressed as number (percentage) or median [interquartile range]. MI indicates myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Figure 4. Kaplan–Meier survival curves for in‐hospital mortality.
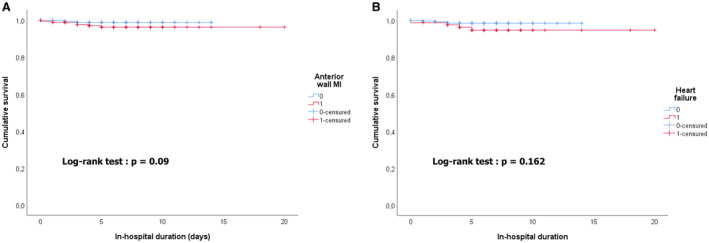
Kaplan–Meier survival curves for in‐hospital mortality (A) considering anterior wall MI and (B) considering occurrence of heart failure. MI indicates myocardial infarction.
Discussion
To the best of our knowledge, this study is the first to investigate the characteristics and in‐hospital outcomes of T2MI compared with T1MI in a sub‐Saharan African population. This study is of particular interest for the global perspective of MI and T2MI because there are still many blind spots, particularly for Black Africans. We found that the prevalence of T2MI was not exceedingly low (14.1%). In the literature, T2MI incidence varies widely across studies, ranging from 1.6% to 73.8% of patients with MI. 27 Retrospective adjudication of MI cases and the recent interest in this topic may explain this relatively low rate. It should also be underscored that differentiating between T1MI, T2MI, and even myocardial injury is challenging in daily clinical practice considering the various clinical conditions associated with cardiac troponin I elevation, potentially leading to misdiagnosis of T2MI. 28 The heterogeneous prevalence of T2MI may also be due to the difference in the thresholds used, acute triggers, and the definition of oxygen supply/demand mismatch (eg, supraventricular tachyarrhythmia and anemia). 27
Almost all Western studies report that patients with T2MI are older than those with T1MI. The age and comorbidities frequently observed in patients with T2MI have resulted in the emergence of a geriatric pathology that includes CAD and additional harmful conditions, which are key determinants of the poor outcomes in these patients. 29 , 30 Unexpectedly, albeit without statistical difference, patients with T2MI in our series appeared to be slightly younger (by roughly 4 years) than patients with T1MI. In addition, main cardiovascular risk factors (apart from diabetes) and comorbidities showed a nonsignificant trend toward higher rate among patients with T2MI. Female predominance was consistent with previous studies. 27 These observations suggest a potential heterogeneity of this multifaceted disease. However, there are explanations for some of these differences. As opposed to Western countries, the sub‐Saharan population, and particularly in Côte d'Ivoire, is young as a result of dynamic population growth and lower life expectancy. 31 Patients with MI are therefore relatively younger than in developed countries, with a 10‐year difference. 2 Patients from Côte d'Ivoire included in REACTIV had a mean age of 56 years in the 2010 to 2016 period (765 patients). 3 This young population is also characterized by a low rate of comorbidities and cardiovascular risk factors, which gradually increase with age. Nevertheless, this result must be carefully interpreted considering potential variability in sampling, as our study population was small. These interesting observations need to be refined and confirmed by multicenter studies on larger populations in sub‐Saharan Africa. Moreover, ethnicity issues concerning T2MI have already been reported. In the largest study conducted in the United States based on the National Inpatient Sample database over 2 years (2018–2019), African Americans were the only race with a significantly higher rate of hospitalization for T2MI (15.9% versus 11.6%; P<0.001), ahead of White and Hispanic populations. 32 These results suggest racial disparities in the epidemiology of T2MI, which could be even greater in sub‐Saharan Africa.
The key issues in T2MI that are highlighted in our series are AF/flutter and heart failure, in relation to 2 predominant triggers (ie, coronary embolism and AF/flutter). In a systematic review by Wang et al, 9 of the 14 studies identified arrhythmia, especially tachyarrhythmia, as the main trigger in T2MI. 33 AF is still a diagnostic and therapeutic challenge in sub‐Saharan Africa, where the use of anticoagulation and therapeutic efficacy are still below standard in daily practice. 34 Unfortunately, in our study, information about chronic treatments, including AF medications, was not available. Hypertension and subsequent left ventricular hypertrophy are also important T2MI triggers. In the multicenter INTERHEART Africa study, hypertension was the most important risk factor associated with a first episode of MI in Black Africans (50.4%; odds ratio, 6.99 [95% CI, 4.23–11.55]). 35 Over the past decade, sub‐Saharan Africa has experienced the highest increase of the rate of hypertension, compared with developed countries. 36 Anemia, which is consistent with older age and frequent comorbidity in the West, 19 , 37 was rarely encountered in our series.
Coronary angiography, performed in all 62 patients with T2MI, highlighted that significant CAD was common in patients with T2MI (41.9%). The rate seen here is lower than in other recent studies, which estimated the prevalence of CAD at >50% among patients with T2MI. 11 , 38 , 39 In contrast with studies in Western countries, multivessel CAD (2‐ or 3‐vessel CAD) was less frequent in T2MI compared with T1MI. 11 , 40 CAD has emerged as a key prognostic issue in T2MI, and recent data suggest that categorizing T2MI based on the presence or absence of significant CAD 41 , 42 would improve the characterization of T2MI as a clinical entity. This classification is supported by the strong prognostic value of CAD in T2MI. 38 , 43 Recent findings from the French RICO survey demonstrated that 1‐year all‐cause mortality has increased significantly, by 40%, in patients with T2MI with significant CAD compared with patients with T1MI, after adjustment for confounding factors (hazard ratio, 1.362 [95% CI, 1.029–1.802]). 39
In our study, while in‐hospital mortality was low overall, it may be lower in patients with T2MI (1.6%) than in patients with T1MI (2.4%). Data on short‐term mortality in patients with T2MI are controversial. Although T2MI is often associated with higher in‐hospital cardiovascular and all‐cause mortality, some studies found no significant difference between T2MI and T1MI. 44 , 45 Hospital deaths in T2MI could relate to the underlying mechanism of onset, as anemia, hypoxia, or hypotension are associated with a worse prognosis. 46 These causative factors were rarely seen in our population.
Study Strength and Limitations
Our study has some limitations. First, our sample size was relatively small compared with some Western T2MI registries. As only patients admitted to an intensive care unit were included, selection bias may be induced. Some findings may be due to sample fluctuations, particularly coronary embolism in the pathophysiological mechanisms of T2MI, so caution may be applied to the results. Only patients who underwent coronary angiography were included, and the relatively high proportion of excluded patients (87 of 541 [16.1%]) may have led to bias in the prevalence of T1MI and T2MI and the related data. In the absence of coronary imaging in some T1MI cases (17.1%), the atherothrombotic mechanism defining T1MI could not be confirmed. The distinction between T1MI, T2MI, and myocardial injury is a clinical challenge. Only patients with MI diagnosed on the basis of clinical, imaging, or ECG signs, as defined by the current fourth universal definition of MI, were included in this study. Despite this challenge, herein, the T2MI diagnosis was based on current guidelines and was made by 2 experienced cardiologists with a good interobserver agreement. Cutoffs used to identify acute triggers for supply–demand oxygen mismatch in T2MI are not standardized to date, and therefore cannot be generalized, given the high variability of individual ischemic thresholds. The management of MI in patients is still a challenge in Cote d'Ivoire, as there is a wide gap between quality of care in sub‐Saharan Africa and Western countries, mainly demonstrated by prolonged time to admission and scarcity of specialized cardiology units.
Some specific explorations were not performed. Spasm provocation test with ergonovine was not performed to confirm coronary vasospasm, nor was the index of microcirculatory resistance to identify microvascular dysfunction. In patients with MI with normal coronary arteries or nonsignificant CAD, magnetic resonance imaging is of particular interest, but this test was not performed in our study.
Finally, the small number of patients with major cardiac events (n=20) and the limited duration of follow‐up (hospital phase) did not provide favorable conditions for performing further analyses such as Cox regression.
Nevertheless, to our knowledge, this study is the first to assess, in a sub‐Saharan African population, characteristics and in‐hospital outcomes of patients with T2MI compared with those with T1MI. It therefore adds key data in the understanding of T2MI.
Conclusion
The current study, conducted over a 5‐year period at the Abidjan Heart Institute, showed disparities in the clinical characteristics, angiographic features, causative framework, and in‐hospital outcomes of T2MI compared with Western studies. These results suggest a potential heterogeneity in the causative characteristics of T2MI according to the epidemiological profile, risk factors, and health access of a population. Given the young age of patients with T2MI in sub‐Saharan Africa, these novel findings challenge the hypothesis of a geriatric pathology. There is thus an urgent need for prospective multicentric studies in this region to confirm our preliminary findings and to assess long‐term prognosis.
Sources of Funding
This work was supported by grants from the University of Burgundy (collaborative project) (Dijon/Abidjan Cardiovascular Network) and the Dijon Bourgogne University Hospital.
Disclosures
Dr Cottin reports having received consultant or speaking fees for Bayer, BMS/Pfizer, Boehringer Ingelheim, Novartis, Sanofi, and Servier. M. Zeller declares research grants from Amarin Corp. The remaining authors have no disclosures to report.
Supporting information
Table S1
Acknowledgments
The authors thank Suzanne Rankin for English review. Author contributions: H.Y.: writing—original draft, formal analysis; C.T.: data curation, visualization; A.E.: validation, E.S.: investigation; Y.C.: methodology; M.Z.: conceptualization, funding; A.P.: writing—review and editing; R.N.: supervision, project administration.
This work was presented in part at the Journées Européennes de la Société Française de Cardiologie (JESFC) conference, January 17–19, 2024, in Paris, France.
This article was sent to Mahasin S. Mujahid, PhD, MS, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032149
For Sources of Funding and Disclosures, see page 9.
References
- 1. World Health Organization . The top 10 causes of death . Accessed September 16, 2022, https://www.who.int/fr/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death.
- 2. Yao H, Ekou A, Niamkey T, Hounhoui Gan S, Kouamé I, Afassinou Y, Ehouman E, Touré C, Zeller M, Cottin Y, et al. Acute coronary syndromes in sub‐Saharan Africa: a 10‐year systematic review. J Am Heart Assoc. 2022;11:e021107. doi: 10.1161/JAHA.120.021107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao H, Ekou A, Brou I, Niamkey T, Koffi F, Tano S, Kouamé I, N'Guetta R. Evolution of epidemiology and management of acute coronary syndromes in Abidjan: a cross‐sectional study of 1011 patients. Ann Cardiol Angeiol. 2022;71:130–135. doi: 10.1016/j.ancard.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 4. Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Joint ESC/ACCF/AHA/WHF task force for universal definition of myocardial infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 6. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Executive group on behalf of the joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/world heart federation (WHF) task force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e652.30571520 [Google Scholar]
- 7. Sandoval Y, Thygesen K, Jaffe AS. The universal definition of myocardial infarction: present and future. Circulation. 2020;141:1434–1436. doi: 10.1161/CIRCULATIONAHA.120.045708 [DOI] [PubMed] [Google Scholar]
- 8. Eggers KM, Baron T, Chapman AR, Gard A, Lindahl B. Management and outcome trends in type 2 myocardial infarction: an investigation from the SWEDEHEART registry. Sci Rep. 2023;13:7194. doi: 10.1038/s41598-023-34312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White K, Kinarivala M, Scott I. Diagnostic features, management and prognosis of type 2 myocardial infarction compared to type 1 myocardial infarction: a systematic review and meta‐analysis. BMJ Open. 2022;12:e055755. doi: 10.1136/bmjopen-2021-055755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Putot A, Jeanmichel M, Chague F, Manckoundia P, Cottin Y, Zeller M. Type 2 myocardial infarction: a geriatric population‐based model of pathogenesis. Aging Dis. 2020;11:108–117. doi: 10.14336/AD.2019.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaggin HK, Liu Y, Lyass A, van Kimmenade RR, Motiwala SR, Kelly NP, Mallick A, Gandhi PU, Ibrahim NE, Simon ML, et al. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation. 2017;135:116–127. doi: 10.1161/CIRCULATIONAHA.116.023052 [DOI] [PubMed] [Google Scholar]
- 12. Putot A, Derrida SB, Zeller M, Avondo A, Ray P, Manckoundia P, Cottin Y. Short‐term prognosis of myocardial injury, type 1, and type 2 myocardial infarction in the emergency unit. Am J Med. 2018;131:1209–1219. doi: 10.1016/j.amjmed.2018.04.032 [DOI] [PubMed] [Google Scholar]
- 13. McCarthy CP, Kolte D, Kennedy KF, Vaduganathan M, Wasfy JH, Januzzi JL. Patient characteristics and clinical outcomes of type 1 versus type 2 myocardial infarction. J Am Coll Cardiol. 2021;77:848–857. doi: 10.1016/j.jacc.2020.12.034 [DOI] [PubMed] [Google Scholar]
- 14. Côte d'Ivoire . World Bank. Accessed February 13, 2023. https://www.worldbank.org/en/country/cotedivoire.
- 15. Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular diseases in sub‐Saharan Africa compared to high‐income countries: an epidemiological perspective. Glob Heart. 2020;15:15. doi: 10.5334/gh.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. N'Guetta R, Ekou A, Yao H, Anzouan‐Kacou JB, Gérardin B, Pillière R, Adoh AM, Seka R. Percutaneous coronary intervention in the management of acute coronary syndromes in Ivory Coast: challenges and outcomes. Ann Cardiol Angeiol. 2018;67:244–249. [DOI] [PubMed] [Google Scholar]
- 17. Ekou A, Yao H, Kouamé I, Boni RY, Ehouman E, N'Guetta R. Primary PCI in the management of STEMI in sub‐Saharan Africa: insights from Abidjan heart institute catheterisation laboratory. Cardiovasc J Afr. 2020;31:201–204. doi: 10.5830/CVJA-2020-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC. Vandenbroucke JP; for the STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 19. Saaby L, Poulsen TS, Hosbond S, Larsen TB, Pyndt Diederichsen AC, Hallas J, Thygesen K, Mickley H. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126:789–797. doi: 10.1016/j.amjmed.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 20. Shibata T, Kawakami S, Noguchi T, Tanaka T, Asaumi Y, Kanaya T, Nagai T, Nakao K, Fujino M, Nagatsuka K, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132:241–250. doi: 10.1161/CIRCULATIONAHA.114.015134 [DOI] [PubMed] [Google Scholar]
- 21. Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. doi: 10.1016/j.jacc.2016.05.034 [DOI] [PubMed] [Google Scholar]
- 22. Celli BR, MacNee W; ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 23. Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9 [DOI] [PubMed] [Google Scholar]
- 24. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 25. Sharma A, Pagidipati NJ, Califf RM, McGuire DK, Green JB, Demets D, George JT, Gerstein HC, Hobbs T, Holman RR. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose‐lowering therapies to treat type 2 diabetes mellitus: lessons learned and future directions. Circulation. 2020;141:843–862. doi: 10.1161/CIRCULATIONAHA.119.041022 [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104 [DOI] [Google Scholar]
- 27. Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1846–1860. doi: 10.1016/j.jacc.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 28. Alpert JS, Thygesen KA, White HD, Jaffe AS. Diagnostic and therapeutic implications of type 2 myocardial infarction: review and commentary. Am J Med. 2014;127:105–108. doi: 10.1016/j.amjmed.2013.09.031 [DOI] [PubMed] [Google Scholar]
- 29. Putot A, Putot S, Chagué F, Cottin Y, Zeller M, Manckoundia P. New horizons in type 2 myocardial infarction: pathogenesis, assessment and management of an emerging geriatric disease. Age Ageing. 2022;51:afac085. doi: 10.1093/ageing/afac085 [DOI] [PubMed] [Google Scholar]
- 30. Curcio F, Gerundo G, Sasso G, Panicara V, Liguori I, Testa G, Della‐Morte D, Gargiulo G, Galizia G, Ungar A, et al. Type 2 myocardial infarction: is it a geriatric syndrome? Aging Clin Exp Res. 2020;32:759–768. doi: 10.1007/s40520-019-01452-8 [DOI] [PubMed] [Google Scholar]
- 31. Population Pyramids of the World from 1950 to 2100 . Accessed June 12, 2023. https://www.populationpyramid.net/2023/.
- 32. Rogers E, Torres C, Rao SV, Donatelle M, Beohar N. Clinical characteristics, outcomes, and epidemiological trends of patients admitted with type 2 myocardial infarction. Journal of the Society for Cardiovascular Angiography & Interventions. 2022;1:100395. doi: 10.1016/j.jscai.2022.100395 [DOI] [Google Scholar]
- 33. Wang G, Zhao N, Zhong S, Li J. A systematic review on the triggers and clinical features of type 2 myocardial infarction. Clin Cardiol. 2019;42:1019–1027. doi: 10.1002/clc.23230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuyun MF, Bonny A, Ng GA, Sliwa K, Kengne AP, Chin A, Mocumbi AO, Ngantcha M, Ajijola OA, Bukhman G. A systematic review of the Spectrum of cardiac arrhythmias in sub‐Saharan Africa. Glob Heart. 2020;15:37. doi: 10.5334/gh.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steyn K, Sliwa K, Hawken S, Commerford P, Onen C, Damasceno A, Ounpuu S, Yusuf S. INTERHEART investigators in Africa. Risk factors associated with myocardial infarction in Africa: the INTERHEART Africa study. Circulation. 2005;112:3554–3561. doi: 10.1161/CIRCULATIONAHA.105.563452 [DOI] [PubMed] [Google Scholar]
- 36. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19·1 million participants. Lancet. 2017;2017(389):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stein GY, Herscovici G, Korenfeld R, Matetzky S, Gottlieb S, Alon D, Gevrielov‐Yusim N, Iakobishvili Z, Fuchs S. Type‐II myocardial infarction—patient characteristics, management and outcomes. PLoS One. 2014;9:e84285. doi: 10.1371/journal.pone.0084285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baron T, Hambraeus K, Sundström J, Erlinge D, Jernberg T, Lindahl B; TOTAL‐AMI study group . Impact on long‐term mortality of presence of obstructive coronary artery disease and classification of myocardial infarction. Am J Med. 2016;129:398–406. doi: 10.1016/j.amjmed.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 39. Yao H, Cottin Y, Chagué F, Maza M, Bichat F, Zeller M, Putot A. Diagnostic and prognostic impact of new pathophysiology‐based categorization of type 1 and type 2 myocardial infarction: data from the French RICO survey. Am Heart J. 2023;266:86–97. doi: 10.1016/j.ahj.2023.09.001 [DOI] [PubMed] [Google Scholar]
- 40. Putot A, Jeanmichel M, Chagué F, Avondo A, Ray P, Manckoundia P, Zeller M, Cottin Y. Type 1 or type 2 myocardial infarction in patients with a history of coronary artery disease: data from the emergency department. J Clin Med. 2019;8:2100. doi: 10.3390/jcm8122100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Lemos JA, Newby LK, Mills NL. A proposal for modest revision of the definition of type 1 and type 2 myocardial infarction. Circulation. 2019;140:1773–1775. doi: 10.1161/CIRCULATIONAHA.119.042157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White HD. Zooming in on the enigmas of type 2 myocardial infarction. Circulation. 2022;145:1201–1204. doi: 10.1161/CIRCULATIONAHA.122.059454 [DOI] [PubMed] [Google Scholar]
- 43. Chapman AR, Shah AS, Lee KK, Anand A, Francis O, Adamson P, McAllister DA, Strachan FE, Newby DE, Mills NL. Long‐term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baron T, Hambraeus K, Sundström J, Erlinge D, Jernberg T, Lindahl B. Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106. doi: 10.1136/heartjnl-2014-306093 [DOI] [PubMed] [Google Scholar]
- 45. Javed U, Aftab W, Ambrose JA, Wessel RJ, Mouanoutoua M, Huang G, Barua RS, Weilert M, Sy F, Thatai D. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol. 2009;104:9–13. doi: 10.1016/j.amjcard.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 46. Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, Rihal CS, Gersh BJ, Lewis B, Lennon RJ, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation. 2020;141:454–463. doi: 10.1161/CIRCULATIONAHA.119.043100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.


