Abstract
Background
Stroke survivors believe neighborhood resources such as community centers are beneficial; however, little is known about the influence of these resources on stroke outcomes. We evaluated whether residing in neighborhoods with greater resource density is associated with favorable post‐stroke outcomes.
Methods and Results
We included Mexican American and non‐Hispanic White stroke survivors from the Brain Attack Surveillance in Corpus Christi project (2009–2019). The exposure was density of neighborhood resources (eg, community centers, restaurants, stores) within a residential census tract at stroke onset. Outcomes included time to death and recurrence, and at 3 months following stroke: disability (activities of daily living/instrumental activities of daily living), cognition (Modified Mini‐Mental State Exam), depression (Patient Health Questionnaire‐8), and quality of life (abbreviated Stroke‐Specific Quality of Life scale). We fit multivariable Cox regression and mixed linear models. We considered interactions with stroke severity, ethnicity, and sex. Among 1786 stroke survivors, median age was 64 years (interquartile range, 56–73), 55% men, and 62% Mexican American. Resource density was not associated with death, recurrence, or depression. Greater resource density (75th versus 25th percentile) was associated with more favorable cognition (Modified Mini‐Mental State Exam mean difference=0.838, 95% CI=0.092, 1.584) and among moderate–severe stroke survivors, with more favorable functioning (activities of daily living/instrumental activities of daily living=−0.156 [95% CI, −0.284 to 0.027]) and quality of life (abbreviated Stroke‐Specific Quality of Life scale=0.194 [95% CI, 0.029–0.359]).
Conclusions
We observed associations between greater resource density and cognition overall and with functioning and quality of life among moderate–severe stroke survivors. Further research is needed to confirm these findings and determine if neighborhood resources may be a tool for recovery.
Keywords: activities of daily living, census tract, cognition, depression, quality of life, recurrence, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Epidemiology, Quality and Outcomes, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- 3MSE
Modified Mini‐Mental State Examination
- SS‐QoL
abbreviated Stroke‐Specific Quality of Life scale
Research Perspective.
What Is New?
Greater neighborhood resource density was associated with more favorable 3‐month poststroke cognitive scores among stroke survivors.
Greater neighborhood resource density was associated with more favorable 3‐month poststroke functioning and quality‐of‐life scores among moderate–severe stroke survivors.
What Question Should Be Addressed Next?
Future research should confirm these findings in other study populations, investigate potential mechanisms, and consider other neighborhood features that may support poststroke health outcomes.
Based on the Global Burden of Disease Study (2016), roughly 24% of Americans will experience a stroke in their lifetime. 1 Stroke is a leading cause of disability and death, particularly among older adults. 2 Findings from the Health and Retirement Study (1998–2014) indicate a substantial number of healthy years lost due to death or disability among middle‐aged to older adults who suffer a stroke. 3 Due to the impact of stroke (eg, disability, depression), survivors are thought to spend increased time in their neighborhood; thus, we expect neighborhood environments to be important for recovery. 3 , 4 , 5 , 6 Previous research has largely focused on the impact of individual factors on poststroke outcomes, and there is little empirical knowledge on how the neighborhood environments may influence poststroke outcomes.
Individual factors such as the severity of the stroke, sex, and race and ethnicity likely interact with the survivor's environment to influence poststroke outcomes. 7 , 8 , 9 Higher neighborhood socioeconomic status (SES) is associated with more favorable poststroke outcomes of functioning, quality of life (QOL), depression, stroke recurrence, and death, particularly among moderate–severe stroke survivors. 8 , 10 , 11 , 12 , 13 , 14 , 15 One potential explanation is that higher SES neighborhoods may have more resources providing beneficial opportunities for poststroke physical activity, socialization, and cognitive stimulation. 16 , 17 , 18 Qualitative researchers have concluded that stroke survivors and their caregivers believe availability of community centers, places to exercise, eateries, and stores to be beneficial for recovery. 19 , 20 Cross‐sectional studies of survivors of primarily milder strokes have reported conflicting findings on the benefits of neighborhood resources on poststroke physical activity. 17 , 21 , 22 , 23 , 24 A population‐based cohort study reported that neighborhood density of recreation centers may be beneficial to poststroke functioning and QOL among moderate–severe stroke survivors. 9 It remains unclear whether resources, overall, are beneficial to poststroke outcomes.
Our objective was to test the hypothesis that residing in neighborhoods with greater resource density would be favorably associated with all‐cause death, stroke recurrence, and 3‐month poststroke functioning, cognition, QOL, and depressive symptoms, particularly among moderate–severe stroke survivors.
Methods
Because of the sensitive and potentially identifying nature of the data collected for this study, qualified researchers trained in human subject confidentiality protocols may request access to the data set and SAS program codes from the author, Dr Lynda Lisabeth, at llisabet@umich.edu.
Study Population
The study population was acquired from the Brain Attack Surveillance in Corpus Christi project, a population‐based cohort previously described. 25 Active surveillance of emergency department and hospital admissions and passive surveillance of hospital discharge records were conducted to identify all stroke cases among persons aged at least 45 years residing in Nueces County, Texas, a predominantly urban area. 25 , 26 The Brain Attack Surveillance in Corpus Christi project enrolled 3965 persons with incident stroke (ischemic or intracerebral hemorrhage) occurring in 2009 to 2019. Stroke fellowship–trained physicians validated all strokes using source documentation and standard clinical definitions. 26 , 27 The institutional review boards at the University of Michigan and 2 local hospital systems approved the Brain Attack Surveillance in Corpus Christi project. Written informed consent was obtained from participants or their proxy.
We excluded people institutionalized before the stroke (N=167), who did not complete the initial interview (N=1074), who completed by proxy (eg, relative; N=787), and if the residential census tract was unknown (N=9). We excluded 142 stroke survivors who were not Mexican American or non‐Hispanic White (98 non‐Hispanic Black, 9 Hispanic Black, 16 non‐Hispanic American Indian, 6 non‐Hispanic Asian, and 10 Hispanic Asian) stroke survivors due to the small numbers in these racial groups reflecting the Nueces County, Texas, population. 28 This yielded 1786 people for death and stroke recurrence outcomes (Figure 1). For 3‐month poststroke outcomes, we included 1284 survivors after additionally excluding people who died within 90‐days after a stroke (N=39) and those who didn't complete the follow‐up interview (N=426) or completed by proxy (N=37).
Figure 1. Eligibility flow chart.
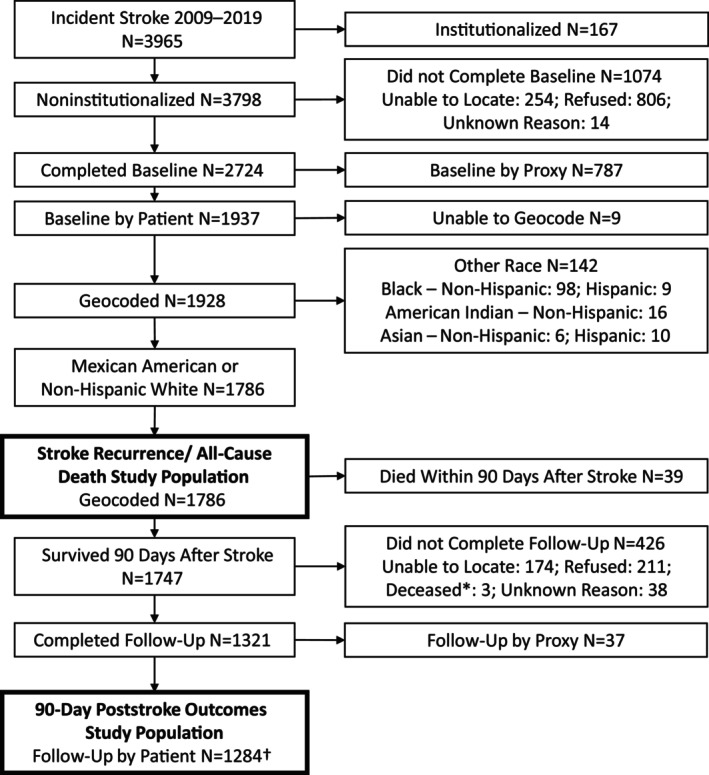
*Survived 90 days after stroke but died before completion of follow‐up interview. †Quality of life collected from 2010 to 2019, N=1189; depression collected from 2011 to 2019, N=1086.
Exposure
Information on resources and square land miles by census tract was available through 2017 from the National Neighborhood Data Archive. 29 , 30 , 31 , 32 Resources were identified using the North American Industry Classification System and included places that have been reported as possible facilitators of physical activity, socialization, or cognitive stimulation outside one's home/work (Table S1). 9 , 33 , 34 , 35 , 36 , 37 , 38 These included stores; entertainment, intellectual, recreation, and religious centers and eating/drinking places. We linked resource density by year of stroke and survivor's residential census tract around the time of hospital admission. Survivors with stroke in 2018 to 2019 were matched to the closest available data (2017).
Outcomes
Stroke recurrences were identified using the surveillance methods described above. We defined recurrence to be the first validated ischemic or hemorrhagic stroke identified after the incident stroke. 39 All‐cause death data were obtained from the Texas Department of State Health Services death certificate database. 39 The Brain Attack Surveillance in Corpus Christi project uses a probabilistic record linkage software to match death certificate data with participants. 39 We censored on date of death (stroke recurrence model) or December 31, 2019, whichever occurred first.
Outcomes of functioning, cognition, depressive symptoms, and QOL were evaluated at 3 months following the incident stroke. Self‐ratings of difficulty on activities of daily living (ADL) and instrumental ADL (IADL) were averaged to assess functioning (total score range, 1–4). 8 , 27 The Modified Mini‐Mental State Examination (3MSE) provided a measure of global cognition (total score range, 0–100). 27 The Patient Health Questionnaire 8 was administered to assess depressive symptoms (total score range, 0–24). 8 , 40 The abbreviated Stroke‐Specific QOL scale (SS‐QoL) was used to measure health‐related QOL (total score range, 1–5). 8 , 41 Lower scores for the ADL/IADL and Patient Health Questionnaire 8 and higher scores on the 3MSE and SS‐QoL are more favorable.
Covariates
We conducted a literature review to identify risk factors for each outcome and created directed acyclic graphs and identified a minimal set of covariates for which to adjust to reduce confounding. 42 , 43 Risk factors identified included key demographics such as age, sex, and race and ethnicity, and socioeconomic status. 44 , 45 , 46 , 47 Also important were prestroke social support (eg, marital status, living with someone, and social network), prestroke health (eg, presence of comorbidities, cognitive status, depression), and health behaviors (eg, smoking, and excessive alcohol use). 44 , 45 , 46 , 47 , 48 , 49 , 50 These individual and interpersonal risk factors for stroke health outcomes are likely to be associated with our exposure (density of neighborhood resources) due to self‐selection into a neighborhood (eg, due to financial means or desire to live somewhere with certain resources) or the aggregate of these characteristics among residents creating a demand for the neighborhood resource. 51 , 52 Additionally, neighborhood‐level characteristics such as neighborhood‐level SES and other attributes like availability of public transportation, sidewalk presence/quality, and perceptions of safety are likely to be associated with neighborhood resources and risk factors for subsequent poststroke health outcomes. 7 , 38 , 53 , 54 , 55 , 56 Below is the minimal set of covariates we ascertained for this study and included in our analysis.
We assessed the following by interview or medical record abstraction:
1. Demographics: age in years (categorized by quantiles), sex (male or female), race and ethnicity (non‐Hispanic White or Mexican American).
2. Individual SES: education (less thanhigh school, high school, greater than high school) and whether insured.
3. Prestroke health: disability (modified Rankin scale), cognition (informant questionnaire on cognitive decline in the elderly), self‐reported depression or antidepressant use, ever smoker, and comorbidity score (includes following comorbidities: amyotrophic lateral sclerosis, atrial fibrillation, cancer, chronic obstructive pulmonary disease, coronary heart disease or myocardial infarction, dementia or Alzheimer disease, diabetes, end‐stage renal disease, epilepsy, excessive alcohol use, heart failure, hyperlipidemia, hypertension, and Parkinson disease). 8
4. Interpersonal factors: marital status (single/never married, married/living with someone, widowed, divorced/separated) and social support score, as previously described. 8
5. Neighborhood SES: disadvantage score and affluence score as previously described (obtained from National Neighborhood Data Archive and linked by residential census tract and year of stroke with closest 5‐year period available: 2008 to 2012 and 2013 to 2017). 32 , 57
6. Stroke characteristics: stroke type (ischemic or intracerebral hemorrhage), initial stroke severity (National Institutes of Health Stroke Scale as abstracted from medical records or by validated algorithm, dichotomized as mild [<5] or moderate–severe [≥5]). 8 , 58 , 59
Statistical Analysis
We conducted analyses to describe the overall population and by resource density quartile. We described categorical variables using frequencies and percentages and continuous variables using means and SDs where normally distributed, otherwise using medians and interquartile range (IQRs). We evaluated differences by resource density quartiles with χ2 or ANOVA tests.
We fit Cox regression models using a shared frailty model accounting for clustering by residential census tract to determine the hazard ratio of recurrence or death associated with an IQR difference in neighborhood resource density (reflects high versus low density). We fit mixed linear models for 3‐month poststroke outcomes allowing for random intercepts by residential census tract. We applied inverse probability weighting to account for potential selection bias and to upweight those in the study who were similar to those excluded from the study. The applied weights were the inverse product of the probabilities generated by logistic regression models for participating in the baseline assessment, completing the baseline assessment by oneself and without a proxy, and for the 3‐month poststroke outcomes only, participating in the follow‐up assessment. We then applied chained multiple imputation to account for selection bias that might have occurred if we excluded people with missing data using all available data from study populations for each outcome. All variables except for prestroke cognition were missing for <5% of participants (Table S2). We sequentially adjusted for the covariates described herein. Additionally, we considered effect modification by stroke severity, race and ethnicity, or sex by including interaction terms. A priori, we considered significance on the basis of coefficient magnitudes, 95% CIs, and P values (main effect, P=0.05; interactions, P=0.15). 8 , 60 , 61 We conducted post hoc analysis to calculate the minimum detectable effect size for an IQR increase in neighborhood resources for each outcome measure and intraclass correlation combination (Table S3). With our sample sizes, we will be able to detect relatively small effect sizes that are in line with previous findings. We calculated Hedges' g to evaluate the effect size of each outcome scale. 61 We conducted sensitivity analyses to consider whether the survivor moved (data available 2014–2019) by excluding people who indicated they moved at follow‐up. We conducted analyses with SAS 9.4 (SAS Institute, Inc., Cary, NC) and adhered to Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines. 62
Results
Among 1786 stroke survivors, 408 deaths and 189 stroke recurrences occurred over a median follow‐up of 1380 days for death and 1222 days for recurrence (Table 1). Median age was 64 years, 55% were men, 51% were married or living with someone, 62% were Mexican American individuals, 70% completed at least high school, and 82% had insurance. Most initial strokes were ischemic (91%) and of mild severity (74%). The stroke survivors resided in 78 census tracts within Nueces County, with a median 1.10 (IQR, 0.66–3.13) square land miles. Median resource density was 39.5 (IQR,15.9–71.7) resources per square land mile. Resources with the greatest density included retail businesses, eating/drinking places, personal care establishments, and religious organizations (Table S1). In census tracts with a greater density of resources, there was a greater proportion of Mexican American survivors, higher median disadvantage score, and lower median affluence score (Table 1). A higher proportion of survivors died during follow‐up among neighborhoods in the upper quartile of resource density. Study population characteristics for the 1284 stroke survivors included in analyses of 3‐month poststroke outcomes were similar to the 1786 survivors presented in Table 1 (data not shown). Median 3‐month poststroke outcomes were 1.8 (IQR, 1.2–2.5) for ADL/IADL, 81 (IQR, 73–85) for 3MSE, 3.8 (IQR, 2.8–4.5) for SSQoL, and 5.0 (IQR, 1.0–11.0) for Patient Health Questionnaire 8.
Table 1.
Study Population Characteristics by Resource Density Among Stroke Survivors 2009–2019 (N=1786)*
| Characteristics | Overall (N=1786) | Resource density | P value† | |||
|---|---|---|---|---|---|---|
| <15.9 (n=452) | 15.9–39.5 (n=441) | 39.5–71.7 (n=449) | >71.7 (n=444) | |||
| Resource density, median (IQR) | 39.5 (15.9–71.7) | 5.8 (3.4–12.0) | 27.5 (22.9–33.0) | 56.5 (48.0–63.5) | 88.8 (78.4–111.5) | <0.0001 |
| Age, y, median (IQR) | 64.0 (56.0–73.0) | 65.0 (57.0–73.0) | 64.0 (56.0–73.0) | 64.0 (56.0–73.0) | 64.0 (56.0–74.0) | 0.5168 |
| Age, y, quartiles, n (%) | 0.6049 | |||||
| <56 | 406 (23) | 99 (22) | 98 (22) | 110 (25) | 99 (22) | |
| 56–64 | 440 (25) | 99 (22) | 121 (27) | 99 (22) | 110 (25) | |
| 64–73 | 460 (26) | 131 (29) | 108 (25) | 131 (29) | 107 (24) | |
| ≥73 | 480 (27) | 123 (27) | 114 (26) | 115 (26) | 128 (29) | |
| Sex, n (%) | 0.3648 | |||||
| Male | 982 (55) | 252 (56) | 238 (54) | 260 (58) | 232 (52) | |
| Female | 804 (45) | 200 (44) | 203 (46) | 189 (42) | 212 (48) | |
| Race and ethnicity, n (%) | <0.0001 | |||||
| Mexican American | 1114 (62) | 266 (59) | 234 (53) | 342 (76) | 272 (61) | |
| Non‐Hispanic White | 672 (38) | 186 (41) | 207 (47) | 107 (24) | 172 (39) | |
| Education attainment, n (%) | 0.3949 | |||||
| Less than high school | 541 (30) | 137 (30) | 117 (27) | 152 (34) | 135 (30) | |
| High school | 535 (30) | 139 (31) | 134 (30) | 127 (28) | 135 (30) | |
| Greater than high school | 710 (40) | 176 (39) | 190 (43) | 170 (38) | 174 (39) | |
| Health insurance status, n (%) | 0.7576 | |||||
| Insured | 1445 (82) | 372 (84) | 358 (82) | 360 (81) | 355 (82) | |
| No insurance | 316 (18) | 73 (16) | 78 (18) | 85 (19) | 80 (18) | |
|
Modified Rankin Scale score, n (%) ‡ |
0.5504 | |||||
| No disability | 622 (35) | 171 (38) | 154 (36) | 157 (35) | 140 (32) | |
| No significant disability | 317 (18) | 69 (16) | 82 (19) | 84 (19) | 82 (19) | |
| Slight disability | 585 (33) | 152 (34) | 135 (31) | 144 (32) | 154 (35) | |
| Moderate disability | 154 (9) | 41 (9) | 39 (9) | 36 (8) | 38 (9) | |
| Moderately severe to severe disability | 84 (5) | 13 (3) | 24 (6) | 25 (6) | 22 (5) | |
| IQCODE, median (IQR) | 3.0 (3.0–3.2) | 3.0 (3.0–3.1) | 3.0 (3.0–3.1) | 3.0 (3.0–3.2) | 3.0 (3.0–3.3) | 0.0030 |
| Depression, n (%)‡ | 0.3318 | |||||
| No depression | 1175 (66) | 314 (70) | 283 (64) | 292 (66) | 286 (65) | |
| Depression diagnosis or antidepressant use | 595 (34) | 136 (30) | 157 (36) | 150 (34) | 152 (35) | |
| Comorbid score, median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 3.0 (1.0–4.0) | 2.0 (1.0–3.0) | 0.5264 |
| Amyotrophic lateral sclerosis, n (%) | 2 (0) | 0 (0) | 2 (0) | 0 (0) | 0 (0) | |
| Atrial fibrillation, n (%) | 180 (10) | 44 (10) | 55 (12) | 37 (8) | 44 (10) | 0.2093 |
| Cancer, n (%) | 189 (11) | 52 (12) | 58 (13) | 30 (7) | 49 (11) | 0.0129 |
| Chronic obstructive pulmonary disease, n (%) | 186 (10) | 45 (10) | 53 (12) | 45 (10) | 43 (10) | 0.6492 |
| Coronary heart disease or myocardial infarction, n (%) | 425 (24) | 116 (26) | 101 (23) | 117 (26) | 91 (21) | 0.1720 |
| Dementia or Alzheimer disease, n (%) | 44 (2) | 8 (2) | 11 (2) | 11 (2) | 14 (3) | 0.6180 |
| Diabetes, n (%)‡ | 816 (46) | 195 (43) | 196 (44) | 224 (50) | 201 (45) | 0.1968 |
| End‐stage renal disease, n (%) | 50 (3) | 7 (2) | 11 (2) | 15 (3) | 17 (4) | 0.1746 |
| Epilepsy, n (%) | 34 (2) | 8 (2) | 8 (2) | 8 (2) | 10 (2) | 0.9429 |
| Heart failure, n (%) | 108 (6) | 29 (6) | 27 (6) | 30 (7) | 22 (5) | 0.7143 |
| Hyperlipidemia, n (%) | 825 (46.2) | 207 (46) | 203 (46) | 224 (50) | 191 (43) | 0.2307 |
| Hypertension, n (%) | 1416 (79) | 366 (81) | 347 (79) | 363 (81) | 340 (77) | 0.3168 |
| Parkinson disease, n (%) | 13 (1) | 2 (0) | 3 (1) | 5 (1) | 3 (1) | |
| Excessive alcohol use, n (%) | 139 (8) | 35 (8) | 37 (8) | 27 (6) | 40 (9) | 0.3740 |
| Smoking status, n (%)‡ | 0.0889 | |||||
| Never smoked | 1017 (57) | 269 (60) | 229 (52) | 259 (58) | 260 (59) | |
| Ever smoker | 767 (43) | 182 (40) | 212 (48) | 190 (42) | 183 (41) | |
| Marital status, n (%)‡ | 0.1506 | |||||
| Single/never married | 162 (9) | 27 (6) | 47 (11) | 41 (9) | 47 (11) | |
| Married/living with someone | 902 (51) | 237 (52) | 225 (51) | 234 (52) | 206 (46) | |
| Widowed | 315 (18) | 81 (18) | 67 (15) | 76 (17) | 91 (21) | |
| Divorced/Separated | 407 (23) | 107 (24) | 102 (23) | 98 (22) | 100 (23) | |
| Social support scale, mean ±SD | 9.2±3.2 | 9.2±3.1 | 9.1±3.2 | 9.2±3.3 | 9.2±3.2 | 0.9401 |
| Neighborhood disadvantage score, mean ±SD | 0.12±0.06 | 0.12±0.08 | 0.11±0.06 | 0.13±0.06 | 0.14±0.05 | <0.0001 |
| Neighborhood affluence score, mean ±SD | 0.26±0.14 | 0.29±0.14 | 0.28±0.15 | 0.23±0.15 | 0.23±0.11 | <0.0001 |
| Stroke type, n (%) | 0.4196 | |||||
| Ischemic | 1623 (91) | 419 (93) | 401 (91) | 403 (90) | 400 (90) | |
| Intracerebral hemorrhage | 163 (9) | 33 (7) | 40 (9) | 46 (10) | 44 (10) | |
| Stroke severity (NIHSS), n (%) | 0.0697 | |||||
| Mild (<5) | 1318 (74) | 351 (78) | 317 (73) | 337 (75) | 313 (71) | |
| Moderate–Severe (≥5) | 456 (26) | 98 (22) | 120 (28) | 110 (25) | 128 (29) | |
| Stroke recurrence, n (%) | 0.3611 | |||||
| Censored | 1581 (89) | 409 (91) | 391 (89) | 396 (88) | 385 (87) | |
| Second stroke | 205 (12) | 43 (10) | 50 (11) | 53 (12) | 59 (13) | |
| Days of follow‐up for recurrence,§ median (IQR) | 1222.0 (502.0–2152.0) | 1164.5 (490.0–2085.0) | 965.0 (468.0–1871.0) | 1068.0 (467.0–1771.0) | 1660.5 (724.0–2715.5) | <0.0001 |
| All‐cause death, n (%) | 0.0431 | |||||
| Censored | 1345 (75) | 355 (79) | 331 (75) | 345 (77) | 314 (71) | |
| Expired | 441 (25) | 97 (22) | 110 (25) | 104 (23) | 130 (29) | |
| Days of follow‐up for death,‖ median (IQR) | 1379.5 (617.0–2294.0) | 1295.0 (600.5–2158.5) | 1129.0 (552.0–2001.0) | 1234.0 (540.0–1903.0) | 1950.0 (970.0–2.847.5) | <0.0001 |
| ADL/IADL score, median (IQR)** | 1.8 (1.2–2.5) | 1.7 (1.2–2.5) | 1.7 (1.2–2.4) | 1.9 (1.3–2.5) | 1.8 (1.2–2.5) | 0.5569 |
| 3MSE score, median (IQR)¶ | 81.0 (73.0–85.0) | 80.0 (73.0–85.0) | 81.0 (74.0–85.0) | 80.0 (70.0–84.0) | 81.0 (73.0–86.0) | 0.1871 |
| SS‐QoL score, median (IQR)# | 3.8 (2.8–4.5) | 3.7 (2.8–4.5) | 3.8 (2.9–4.6) | 3.7 (2.8–4.4) | 3.8 (2.9–4.4) | 0.4486 |
| PHQ‐8 score, median (IQR)** | 5.0 (1.0–11.0) | 5.0 (1.0–10.0) | 4.0 (1.0–10.0) | 6.0 (3.0–12.0) | 5.0 (1.0–11.0) | 0.0788 |
3MSE indicates Modified Mini‐Mental State Examination; ADL/IADL, activities of daily living/instrumental activities of daily living; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; PHQ‐8, Patient Health Questionnaire 8; SS‐QoL, abbreviated Stroke Specific Quality of Life Scale.
Numbers relate to analytic population for death/recurrence, unless otherwise noted. Other populations are similar.
χ2 or ANOVA test. For categorical variables where expected cell count was <5 for >50% of cells, P value associated with χ2 test is not provided as test is likely not valid.
Some participants missing data.
Censored at death or December 31, 2019.
Censored at December 31, 2019.
ADL/IADL and 3MSE population (2009–2019; N=1284).
SS‐QoL population (2010–2019; N=1189).
PHQ‐8 population (2011–2019; N=1086).
We did not observe an increased hazard for death or recurrence associated with density of resources (HR, 1.006 [95% CI, 0.834–1.214]; HR, 0.975 [95% CI, 0.743–1.279], respectively; Table 2, model 7: fully adjusted). Stroke severity, sex, and race and ethnicity did not modify these associations (Table 2, models 8–10). Restricting to survivors of stroke in 2014 to 2019 (N=1111) and excluding people who moved (N=45) yielded similar results.
Table 2.
HR for Stroke Recurrence and All‐Cause Death, 25th Relative to 75th Percentile of Resource Density (N=1786)
| Model* | All‐cause death (event=441) | Stroke recurrence (event=205) | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| 1: Unadjusted | 1.040 (0.865–1.249) | 0.6777 | 1.003 (0.775–1.297) | 0.9832 |
| 2: 1+Demographics | 1.039 (0.865–1.248) | 0.6791 | 0.991 (0.759–1.294) | 0.9483 |
| 3: 2+Individual SES | 1.043 (0.868–1.253) | 0.6543 | 0.990 (0.758–1.293) | 0.9435 |
| 4: 3+Prestroke health and behaviors | 1.019 (0.849–1.223) | 0.8386 | 0.986 (0.755–1.287) | 0.9156 |
| 5: 4+Interpersonal factors | 1.017 (0.847–1.222) | 0.8528 | 0.978 (0.748–1.279) | 0.8701 |
| 6: 5+Neighborhood SES | 1.013 (0.840–1.221) | 0.8918 | 0.968 (0.737–1.270) | 0.8131 |
| 7: 6+Stroke type and severity | 1.006 (0.834–1.214) | 0.9498 | 0.975 (0.743–1.279) | 0.8552 |
|
8: 7+Stroke severity interaction Mild Moderate–severe |
1.063 (0.858–1.317) ‡ 0.859 (0.591–1.248) ‡ |
0.3276 † 0.5777 0.4238 |
1.011 (0.749–1.366) ‡ 0.842 (0.458–1.550) ‡ |
0.5956 † 0.9417 0.5817 |
|
9: 7+Sex interaction Male Female |
0.914 (0.706–1.182) ‡ 1.126 (0.861–1.473) ‡ |
0.2622 † 0.4907 0.3854 |
0.985 (0.694–1.398) ‡ 0.961 (0.629–1.468) ‡ |
0.9296 † 0.9320 0.8535 |
|
10: 7+Race and ethnicity interaction Mexican American Non‐Hispanic White |
1.088 (0.847–1.397) ‡ 0.918 (0.694–1.213) ‡ |
0.3677 † 0.5110 0.5459 |
0.963 (0.693–1.339) ‡ 0.999 (0.632–1.581) ‡ |
0.8962 † 0.8230 0.9981 |
HR indicates hazard ratio; and SES, socioeconomic status.
(1) Unadjusted model sequentially adjusted for (2) age quartile, sex, race and ethnicity; (3) education attainment, insurance; (4) modified Rankin scale, informant questionnaire on cognitive decline in the elderly, depression, comorbidity score, smoking; (5) marital, social support score; (6) neighborhood disadvantage score, neighborhood affluence score; (7) stroke type and severity. Interactions terms applied to model 7 for (8) stroke severity, (9) sex, and (10) race and ethnicity.
Interaction term P value.
HR computed for each stratum of interaction term using coefficients for resource density and interaction terms.
Greater resource density was associated with more favorable cognition among stroke survivors (3MSE mean difference [75th versus 25th percentile] = 0.838 [95% CI, 0.092–1.584]; Table 3, model 7). Stroke severity modified associations with ADL/IADL and SS‐QoL (P=0.0145 and P=0.0779, respectively; Table 3, model 8). Among moderate–severe stroke survivors only, greater resource density was associated with more favorable functioning (ADL/IADL mean difference, 0.156 [95% CI=‐0.284, −0.027]) and QOL (SS‐QoL mean difference, 0.194 [95% CI, 0.029–0.359]; Table 3, model 8). Resource density was not associated with depressive symptoms (Table 3, Patient Health Questionnaire 8). Sex and race and ethnicity did not modify any associations with poststroke outcomes (interaction term for sex: ADL/IADL, P=0.2036; 3MSE, P=0.2963; SS‐QoL, P=0.6459; interaction term for race and ethnicity: ADL/IADL, P=0.4540; 3MSE, P=0.3145; SS‐QoL, P=0.9322; Table 3, models 9 and 10). Table S4 provides the calculated Hedges' g; we note minimal effect sizes for all outcome measures (g < 0.2). 61 After restricting to survivors of stroke in 2014 to 2019 (N=787) and excluding people who moved (N=42), we observed similar associations (Figure 2). For cognition, the association remained when restricting to 2014 to 2019; however, it was attenuated and no longer significant (Figure 2). Additionally, sex appeared to modify this association (P=0.1178), with a larger mean difference among men (Figure 2). Excluding those who moved yielded similar results to the 2014 to 2019 population for functioning, cognition, and QOL (Figure 2). Other than portrayed in Figure 2, stroke severity, sex, and race and ethnicity did not modify these associations (P>0.15).
Table 3.
Difference in 3‐Month Poststroke Outcomes Associated With an IQR Difference in Density of Resources
| Model* | ADL/IADL (N=1284) | 3MSE (N=1284) | ||
|---|---|---|---|---|
| IQR difference (95% CI) | P value | IQR difference (95% CI) | P value | |
| 1: Unadjusted | 0.051 (−0.035 to 0.138) | 0.2441 | 0.343 (−0.841 to 1.526) | 0.5703 |
| 2: 1+Demographics | 0.042 (−0.033 to 0.117) | 0.2734 | 0.319 (−0.665 to 1.304) | 0.5250 |
| 3: 2+Individual SES | 0.043 (−0.029 to 0.114) | 0.2419 | 0.279 (−0.498 to 1.055) | 0.4819 |
| 4: 3+Prestroke health and behaviors | 0.021 (−0.042 to 0.084) | 0.5185 | 0.549 (−0.201 to 1.300) | 0.1514 |
| 5: 4+Interpersonal factors | 0.016 (−0.046 to 0.078) | 0.6156 | 0.608 (0.376 to −0.130) | 0.1065 |
| 6: 5+Neighborhood SES | −0.008 (−0.068 to 0.053) | 0.8077 | 0.776 (0.030 to 1.522) | 0.0415 |
| 7: 6+Stroke type and severity | −0.015 (−0.075 to 0.045) | 0.6207 | 0.838 (0.092 to 1.584) | 0.0277 |
|
8: 7+Stroke severity interaction Mild Moderate–severe |
0.020 (−0.046 to 0.086) ‡ −0.156 (−0.284 to −0.027) ‡ |
0.0145 † 0.5502 0.0176 |
0.613 (−0.216 to 1.443) ‡ 1.714 (−0.004 to 3.433) ‡ |
0.2591 † 0.1474 0.0506 |
|
9: 7+Sex interaction Male Female |
0.019 (−0.061 to 0.098) −0.054 (−0.139 to 0.031) |
0.2036 † 0.6407 0.2154 |
1.217 (0.187 to 2.247) ‡ 0.411 (−0.684 to 1.506) ‡ |
0.2963 † 0.0206 0.4615 |
|
10: 7+Race and ethnicity interaction Mexican American Non‐Hispanic White |
0.003 (−0.074 to 0.081) ‡ −0.041 (−0.131 to 0.049) ‡ |
0.4540 † 0.9321 0.3753 |
1.167 (0.194 to 2.140) ‡ 0.381 (−0.789 to 1.551) |
0.3145 † 0.0188 0.5232 |
| SS‐QoL (N=1189) | PHQ‐8 (N=1086) | |||
|---|---|---|---|---|
| 1: Unadjusted | −0.016 (−0.123 to 0.092) | 0.7759 | 0.114 (−0.529 to 0.757) | 0.7281 |
| 2: 1+Demographics | −0.007 (−0.098 to 0.085) | 0.8861 | 0.097 (−0.440 to 0.634) | 0.7232 |
| 3: 2+Individual SES | −0.003 (−0.088 to 0.081) | 0.9372 | 0.098 (−0.418 to 0.614) | 0.7100 |
| 4: 3+Prestroke health and behaviors | 0.028 (−0.043 to 0.100) | 0.4410 | −0.036 (−0.529 to 0.457) | 0.8854 |
| 5: 4+Interpersonal factors | 0.029 (−0.042 to 0.100) | 0.4187 | −0.033 (−0.529 to 0.463) | 0.8962 |
| 6: 5+Neighborhood SES | 0.051 (−0.019 to 0.121) | 0.1502 | −0.065 (−0.549 to 0.419) | 0.7915 |
| 7: 6+Stroke type and severity | 0.058 (−0.010 to 0.125) | 0.0933 | −0.083 (−0.569 to 0.404) | 0.7385 |
|
8: 7+Stroke severity interaction Mild Moderate–severe |
0.025 (−0.051 to 0.102) 0.194 (0.029 to 0.359) |
0.0779 † 0.5186 0.0214 |
−0.034 (−0.581 to 0.514) −0.294 (−1.461 to 0.872) |
0.6966 † 0.9042 0.6207 |
|
9: 7+Sex interaction Male Female |
0.074 (−0.022 to 0.170) 0.039 (−0.067 to 0.145) |
0.6459 † 0.1293 0.4689 |
−0.420 (−1.093 to 0.253) −0.317 (−0.420 to 1.054) |
0.1583 † 0.2215 0.3990 |
|
10: 7+Race and ethnicity interaction Mexican American Non‐Hispanic White |
0.060 (−0.061 to 0.151) 0.054 (−0.053 to 0.161) |
0.9322 † 0.1941 0.3206 |
0.013 (−0.646 to 0.672) −0.205 (−0.959 to 0.549) |
0.6761 † 0.9701 0.5935 |
3MSE indicates Modified Mini‐Mental State Exam; ADL/IADL, activities of daily living/instrumental activities of daily living; HR, hazard ratio; IQR, interquartile range; PHQ‐8, Patient Health Questionnaire 8; SES, socioeconomic status; and SS‐QoL, abbreviated Stroke‐Specific Quality of Life scale.
(1) Unadjusted model sequentially adjusted for (2) age quartile, sex, race and ethnicity; (3) education attainment, insurance; (4) modified Rankin scale, informant questionnaire on cognitive decline in the elderly, depression, comorbidity score, smoking; (5) marital status, social support score; (6) neighborhood disadvantage score, neighborhood affluence score; (7) stroke type and severity. Interactions terms applied to model 7 for (8) stroke severity, (9) sex, and (10) race and ethnicity.
Interaction term P value.
HRs computed for each stratum of interaction term using coefficients for resource density and interaction terms.
Figure 2. Average difference in 90‐day poststroke outcomes associated with interquartile range difference in density of resources.
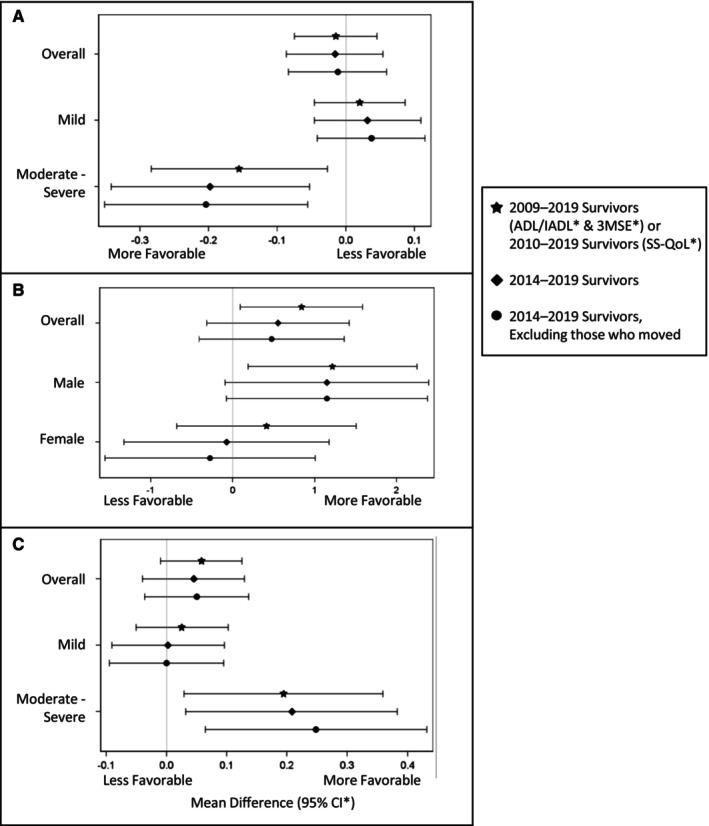
A, Functioning (ADL/IADL)*—overall and by stroke severity. B, Cognition (3MSE)*—overall and by sex. C, Quality of Life (SS‐QoL)*—overall and by stroke severity. 3MSE indicates Modified Mini‐Mental State Exam; ADL/IADL, activities of daily living/instrumental activities of daily living; SS‐QoL, abbreviated Stroke‐Specific Quality of Life scale.
Discussion
Among survivors of a moderate–severe stroke, resource density was associated with more favorable functioning and QOL 3 months after stroke and was associated with better cognition in the general stroke survivor population (did not depend on stroke severity); however, effect sizes were minimal. Resource density wasn't associated with depressive symptoms, all‐cause death, or stroke recurrence. These results suggest that higher neighborhood resource density may be beneficial to stroke survivors, particularly those with moderate–severe strokes.
Our findings contribute to the scarce research on the potential effect of neighborhood environments on poststroke outcomes. We previously reported neighborhood SES and density of recreation centers to have similar favorable associations among those with moderate–severe stroke. 8 , 9 We did not identify any studies considering the exposure of neighborhood resource density with poststroke outcomes; however, we did identify 3 publications evaluating a similar exposure: walk score. 22 , 23 , 24 Walk score is a measure of walkability based on the person's proximity to a variety of neighborhood resources. 22 , 23 , 24 Two of the publications indicated no association between walk score and physical activity, and 1 publication indicated those with greater physical and cognitive ability generally resided in areas with lower walk score (contrary to what we would expect on the basis of our findings). 22 , 23 , 24 These studies were limited by a cross‐sectional design, inclusion of prevalent stroke survivors, and the eligibility criteria (eg, those who could walk and without health conditions impacting mobility or communication ability) likely resulted in a mild‐stroke survivor population and may not be generalizable to those who experience moderate–severe stroke. 22 , 23 , 24 The current study benefited from a longitudinal design using a population‐based cohort and the ability to consider stroke severity/control for many confounders.
There are several potential mechanisms by which neighborhood resources may impact stroke outcomes. Neighborhood resources are posited to provide opportunities for poststroke physical activity, socialization, community engagement, and cognitive stimulation (eg, navigating, conversing, and decision making). 16 , 17 , 18 Among older adults, studies have supported associations between overall neighborhood resources with physical activity, social participation, and health outcomes like cognition, QOL, and depression. 37 , 38 , 55 , 63 Neighborhood resources may be particularly important to stroke survivors who are reported to spend more time in their neighborhood than those of similar age who have not had a stroke. 6 , 45 , 64 , 65 , 66
The potential benefit of neighborhood resources may have implications for rehabilitation for community‐dwelling stroke survivors. One component of poststroke rehabilitation is physical activity. 67 Stroke survivors are often physically inactive despite recommendations. 67 The American Heart Association/American Stroke Association recommends addressing commonly reported environmental barriers, such as lack of availability or awareness of opportunities for physical activity. 67 A qualitative study on integrating the environment into poststroke rehabilitation reported that rehabilitation tended to be generic and include little consideration of the environment beyond the home. 68 Kylen et al highlighted that survivors believed their rehabilitation would benefit from not only considering obstacles in the home but also neighborhood resources. 68 The neighborhood resources we included are purported to be beneficial by stroke survivors, their caregivers, and older adults in general. 9 , 19 , 20 , 33 , 34 , 35 , 36 , 37 , 38 Our findings suggest that neighborhood resources may be beneficial for stroke recovery efforts; however, these benefits may be small and not noticeable by the survivors or their caregivers.
This study has some limitations. The study was conducted in an urban area in Texas, and results may not be generalizable to other regions. This study may not be generalizable to rural areas or regions with different cultures or colder climates. This study may not be generalizable to areas reflecting different racial and ethnic distributions, particularly those racial and ethnic groups we were not able to include; however, we did not identify race and ethnicity as an effect modifier between non‐Hispanic White and Mexican American individuals. 8 , 9 We also note our exposure used resource density within the residential census tract as a proxy for frequenting these resources. 30 , 35 We did not assess whether survivors frequented these resources or other intermediates (eg, physical activity, socialization). Additionally, we defined neighborhood with census tract, which may have resulted in misclassification of resources frequented. 18 , 69 This is likely to be nondifferential and bias findings toward the null and could be a possible reason for the minimal differences observed. Furthermore, we don't know if certain types of resources may be more or less beneficial than other types of resources. Of note, we included fast food places and post offices; however, reported results conflict and these resources may be detrimental. 18 , 19 , 33 , 34 , 35 If resources that are detrimental rather than beneficial are included in our measure of neighborhood resource density, we would expect this to have reduced the observed effect size and biased our results toward the null. There remains potential confounding, particularly due to individual factors related to self‐selection into a neighborhood (eg, prior health status, comorbidities, and health behaviors), although we controlled for many individual‐level factors. 52 Other sources of residual confounding include neighborhood factors like sidewalk quality, public transit availability, and safety; these are, in part, controlled by adjustment for neighborhood SES. 19 , 21 , 53 We also acknowledge lack of data on the use of secondary prevention medications. Following a stroke, the use of secondary prevention medications is an important prognostic factor and expected to have a significant impact on stroke outcomes. However, the use of these medications seems unlikely to be associated with our exposure through self‐selection into a neighborhood. Therefore, the use of secondary prevention medications is not likely to confound the association between neighborhood resources and poststroke outcomes.
In conclusion, we observed that greater neighborhood resource density was associated with more favorable cognition among overall stroke survivors and with more favorable functioning and QOL among moderate–severe stroke survivors; however, differences were minimal. Further research is needed to confirm these findings and identify potential mechanisms. We also observed no association between neighborhood resource density and poststroke recurrence, death, or depression. Future research is needed to determine whether certain types of resources or other neighborhood features may have a greater effect on poststroke outcomes and support secondary stroke prevention, poststroke survival, or reduced poststroke depression.
Sources of Funding
Investigator‐initiated grants (R01NS038916, Lynda Lisabeth and Lewis Morgenstern, multiple principal investigators; and R01HL126700, Devin Brown and Lynda Lisabeth, multiple principal investigators) funded by the National Institute of Neurologic Disorders and Stroke and National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke/National Heart, Lung, and Blood Institute/National Institutes of Health.
Disclosures
Dr Delhey reports grants from National Institute of Neurologic Disorders and Stroke/National Institutes of Health (StrokeNet, U24NS107214; R01NS038916), National Heart, Lung, and Blood Institute/National Institutes of Health (R01HL126700) and American Heart Association (23POST1026064). E. Case reports grants from the National Institutes of Health (R01NS038916 and R01HL126700). Dr Springer reports employment by the Neurology Department, University of Michigan, and grants from National Institute of Neurologic Disorders and Stroke/National Institutes of Health (K01NS117555). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Acknowledgments
This study was performed in the Corpus Christi Medical Center and CHRISTUS Spohn hospitals, CHRISTUS Health system, in Corpus Christi, Texas.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
This work was presented as abstracts at the International Stroke Conference (ISC), February 7–9, 2024, in Phoenix, AZ, and at the Interdisciplinary Association for Population Health Sciences Conference, September 10–13, 2024, in St. Louis, MO.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.034308
For Sources of Funding and Disclosures, see page 11.
References
- 1. Collaborators GBDLRoS , Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd‐Allah F, et al. Global, regional, and country‐specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. McGrath R, Al Snih S, Markides K, Hall O, Peterson M. The burden of health conditions for middle‐aged and older adults in the United States: disability‐adjusted life years. BMC Geriatr. 2019;19:100. doi: 10.1186/s12877-019-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang YY, Chen SD, Leng XY, Kuo K, Wang ZT, Cui M, Tan L, Wang K, Dong Q, Yu JT. Post‐stroke cognitive impairment: epidemiology, risk factors, and management. J Alzheimers Dis. 2022;86:983–999. doi: 10.3233/JAD-215644 [DOI] [PubMed] [Google Scholar]
- 5. Guo J, Wang J, Sun W, Liu X. The advances of post‐stroke depression: 2021 update. J Neurol. 2022;269:1236–1249. doi: 10.1007/s00415-021-10597-4 [DOI] [PubMed] [Google Scholar]
- 6. Tsunoda S, Shimizu S, Suzuki Y, Tsunoda A, Yamada R, Shimose R, Kawabata M, Ogura M, Matsunaga A. Longitudinal changes in life‐space mobility and the factors influencing it among chronic community‐dwelling post‐stroke patients. Disabil Rehabil. 2021;44:7872–7876. doi: 10.1080/09638288.2021.2001054 [DOI] [PubMed] [Google Scholar]
- 7. Brenner AB, Burke JF, Skolarus LE. Moving toward an understanding of disability in older U.S. stroke survivors. J Aging Health. 2018;30:75–104. doi: 10.1177/0898264316666125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stulberg EL, Twardzik E, Kim S, Hsu CW, Xu Y, Clarke P, Morgenstern LB, Lisabeth LD. Association of neighborhood socioeconomic status with outcomes in patients surviving stroke. Neurology. 2021;96:e2599–e2610. doi: 10.1212/WNL.0000000000011988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delhey LM, Shi X, Morgenstern LB, Brown DL, Smith MA, Case EC, Springer MV, Lisabeth LD. Association of neighborhood recreation centers and poststroke outcomes in a population‐based cohort. Stroke. 2023;54:2583–2592. doi: 10.1161/STROKEAHA.122.041852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Twardzik E, Clarke P, Elliott MR, Haley WE, Judd S, Colabianchi N. Neighborhood socioeconomic status and trajectories of physical health‐related quality of life among stroke survivors. Stroke. 2019;50:3191–3197. doi: 10.1161/STROKEAHA.119.025874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salwi S, Kelly KA, Patel PD, Fusco MR, Mistry EA, Mistry AM, Chitale RV. Neighborhood socioeconomic status and mechanical thrombectomy outcomes. J Stroke Cerebrovasc Dis. 2021;30:105488. doi: 10.1016/j.jstrokecerebrovasdis.2020.105488 [DOI] [PubMed] [Google Scholar]
- 12. Brown AF, Liang LJ, Vassar SD, Merkin SS, Longstreth WT Jr, Ovbiagele B, Yan T, Escarce JJ. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013;80:520–527. doi: 10.1212/WNL.0b013e31828154ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egan M, Kubina LA, Dubouloz CJ, Kessler D, Kristjansson E, Sawada M. Very low neighbourhood income limits participation post stroke: preliminary evidence from a cohort study. BMC Public Health. 2015;15:528. doi: 10.1186/s12889-015-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osypuk TL, Ehntholt A, Moon JR, Gilsanz P, Glymour MM. Neighborhood differences in post‐stroke mortality. Circ Cardiovasc Qual Outcomes. 2017;10:e002547. doi: 10.1161/CIRCOUTCOMES.116.002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morovatdar N, Thrift AG, Stranges S, Kapral M, Behrouz R, Amiri A, Heshmati A, Ghahremani A, Farzadfard MT, Mokhber N, et al. Socioeconomic status and long‐term stroke mortality, recurrence and disability in Iran: the Mashhad stroke incidence study. Neuroepidemiology. 2019;53:27–31. doi: 10.1159/000494885 [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Lee C, Huang H. Neighborhood built environment associated with cognition and dementia risk among older adults: a systematic literature review. Soc Sci Med. 2022;292:114560. doi: 10.1016/j.socscimed.2021.114560 [DOI] [PubMed] [Google Scholar]
- 17. Twardzik E, Clarke PJ, Lisabeth LL, Brown SH, Hooker SP, Judd SE, Colabianchi N. The relationship between environmental exposures and post‐stroke physical activity. Am J Prev Med. 2022;63:251–261. doi: 10.1016/j.amepre.2022.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finlay J, Esposito M, Langa KM, Judd S, Clarke P. Cognability: an ecological theory of neighborhoods and cognitive aging. Soc Sci Med. 2022;309:115220. doi: 10.1016/j.socscimed.2022.115220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jellema S, van Hees S, Zajec J, van der Sande R, Nijhuis‐van der Sanden MW, Steultjens EM. What environmental factors influence resumption of valued activities post stroke: a systematic review of qualitative and quantitative findings. Clin Rehabil. 2017;31:936–947. doi: 10.1177/0269215516671013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Espernberger KR, Fini NA, Peiris CL. Personal and social factors that influence physical activity levels in community‐dwelling stroke survivors: a systematic review of qualitative literature. Clin Rehabil. 2021;35:1044–1055. doi: 10.1177/0269215521993690 [DOI] [PubMed] [Google Scholar]
- 21. Kanai M, Izawa KP, Kubo H, Nozoe M, Mase K, Koohsari MJ, Oka K, Shimada S. Association of perceived built environment attributes with objectively measured physical activity in community‐dwelling ambulatory patients with stroke. Int J Environ Res Public Health. 2019;16:3908. doi: 10.3390/ijerph16203908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanai M, Izawa KP, Kubo H, Nozoe M, Shimada S. Objectively measured physical activity was not associated with neighborhood walkability attributes in community‐dwelling patients with stroke. Sci Rep. 2022;12:3475. doi: 10.1038/s41598-022-07467-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller A, Pohlig RT, Reisman DS. Social and physical environmental factors in daily stepping activity in those with chronic stroke. Top Stroke Rehabil. 2021;28:161–169. doi: 10.1080/10749357.2020.1803571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller A, Pohlig RT, Wright T, Kim HE, Reisman DS. Beyond physical capacity: factors associated with real‐world walking activity after stroke. Arch Phys Med Rehabil. 2021;102:1880–1887. doi: 10.1016/j.apmr.2021.03.023 [DOI] [PubMed] [Google Scholar]
- 25. Smith MA, Risser JM, Moye LA, Garcia N, Akiwumi O, Uchino K, Morgenstern LB. Designing multi‐ethnic stroke studies: the brain attack surveillance in Corpus Christi (BASIC) project. Ethn Dis. 2004;14:520–526. [PubMed] [Google Scholar]
- 26. Morgenstern LB, Smith MA, Sanchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, et al. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. doi: 10.1002/ana.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lisabeth LD, Sanchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, Brown DL, Morgenstern LB. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke. 2014;45:1096–1101. doi: 10.1161/STROKEAHA.113.003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Datawheel; D . Data USA: Nueces County, TX. 2023. Accessed November 08, 2023. https://datausa.io/profile/geo/nueces‐county‐tx#demographics.
- 29. Esposito M, Li M, Finlay J, Gomez‐Lopez I, Khan A, Clarke P, Chenoweth M. National neighborhood data archive (NaNDA): Personal care services and laundromats by census tract, United States, 2003‐2017 (ICPSR v2) [dataset online]. Ann Arbor, MI: Inter‐University Consortium for Political and Social Research. [distributor], 2020‐11‐23. 10.3886/E115981V2 [DOI] [Google Scholar]
- 30. Finlay J, Li M, Esposito M, Gomez‐Lopez I, Khan A, Clarke P, Chenoweth M. National neighborhood data archive (NaNDA): Religious, civic, and social organizations by Census Tract, United States, 2003‐2017. (ICPSR v2) [dataset online]. Ann Arbor, MI: Inter‐university Consortium for Political and Social Research. [distributor], 2020‐10‐20. 10.3886/E115967V2 [DOI] [Google Scholar]
- 31. Finlay J, Li M, Esposito M, Khan A, Gomez‐Lopez I, Clarke P, Chenoweth M. National neighborhood data archive (NaNDA): Retail establishments by Census Tract, United States, 2003‐2017. (ICPSR v2) [dataset online]. Ann Arbor, MI: Inter‐university Consortium for Political and Social Research. [distributor], 2020‐11‐20. 10.3886/E115972V2 [DOI] [Google Scholar]
- 32. Finlay J, Li M, Esposito M, Gomez‐Lopez I, Khan A, Clarke P, Chenoweth M. National neighborhood data archive (NaNDA): Arts, entertainment, and recreation organizations by Census Tract, United States, 2003‐2017. (ICPSR v2) [dataset online]. Ann Arbor, MI: Inter‐University Consortium for Political and Social Research. [distributor], 2020‐10‐26. 10.3886/E115543V2 [DOI] [Google Scholar]
- 33. Melendez R, Clarke P, Khan A, Gomez‐Lopez I, Li M, Chenoweth M. National neighborhood data archive (NaNDA): Socioeconomic status and demographic characteristics of Census Tracts, United States, 2008‐2017. (ICPSR v2) [dataset online]. Ann Arbor, MI: Inter‐University Consortium for Political and Social Research. [distributor], 2020‐12‐14. 10.3886/E119451V2 [DOI] [Google Scholar]
- 34. Finlay J, Esposito M, Tang S, Gomez‐Lopez I, Sylvers D, Judd S, Clarke P. Fast‐food for thought: retail food environments as resources for cognitive health and wellbeing among aging Americans? Health Place 2020;64:102379. doi: 10.1016/j.healthplace.2020.102379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finlay J, Esposito M, Li M, Colabianchi N, Zhou HJ, Judd S, Clarke P. Neighborhood active aging infrastructure and cognitive function: a mixed‐methods study of older Americans. Prev Med. 2021;150:106669. doi: 10.1016/j.ypmed.2021.106669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clarke PJ, Ailshire JA, House JS, Morenoff JD, King K, Melendez R, Langa KM. Cognitive function in the community setting: the neighbourhood as a source of ‘cognitive reserve’? J Epidemiol Community Health. 2012;66:730–736. doi: 10.1136/jech.2010.128116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levasseur M, Genereux M, Bruneau JF, Vanasse A, Chabot E, Beaulac C, Bedard MM. Importance of proximity to resources, social support, transportation and neighborhood security for mobility and social participation in older adults: results from a scoping study. BMC Public Health. 2015;15:503. doi: 10.1186/s12889-015-1824-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barnett DW, Barnett A, Nathan A, Van Cauwenberg J, Cerin E. Council on E, physical activity—older adults working GBuilt environmental correlates of older adults' total physical activity and walking: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act. 2017;14:103. doi: 10.1186/s12966-017-0558-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sozener CB, Lisabeth LD, Shafie‐Khorassani F, Kim S, Zahuranec DB, Brown DL, Skolarus LE, Burke JF, Kerber KA, Meurer WJ, et al. Trends in stroke recurrence in Mexican Americans and non‐Hispanic whites. Stroke. 2020;51:2428–2434. doi: 10.1161/STROKEAHA.120.029376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 41. Post MW, Boosman H, van Zandvoort MM, Passier PE, Rinkel GJ, Visser‐Meily JM. Development and validation of a short version of the stroke specific quality of life scale. J Neurol Neurosurg Psychiatry. 2011;82:283–286. doi: 10.1136/jnnp.2009.196394 [DOI] [PubMed] [Google Scholar]
- 42. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 44. Matos I, Fernandes A, Maso I, Oliveira‐Filho J, de Jesus PA, Fraga‐Maia H, Pinto EB. Investigating predictors of community integration in individuals after stroke in a residential setting: a longitutinal study. PLoS One. 2020;15:e0233015. doi: 10.1371/journal.pone.0233015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakao M, Izumi S, Yokoshima Y, Matsuba Y, Maeno Y. Prediction of life‐space mobility in patients with stroke 2 months after discharge from rehabilitation: a retrospective cohort study. Disabil Rehabil. 2020;42:2035–2042. doi: 10.1080/09638288.2018.1550533 [DOI] [PubMed] [Google Scholar]
- 46. Thilarajah S, Mentiplay BF, Bower KJ, Tan D, Pua YH, Williams G, Koh G, Clark RA. Factors associated with post‐stroke physical activity: a systematic review and meta‐analysis. Arch Phys Med Rehabil. 2018;99:1876–1889. doi: 10.1016/j.apmr.2017.09.117 [DOI] [PubMed] [Google Scholar]
- 47. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 48. Simic‐Panic D, Boskovic K, Milicevic M, Rabi Zikic T, Cvjetkovic Bosnjak M, Tomasevic‐Todorovic S, Jovicevic M. The impact of comorbidity on rehabilitation outcome after ischemic stroke. Acta Clin Croat. 2018;57:5–15. doi: 10.20471/acc.2018.57.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCann SK, Lawrence CB. Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Sci. 2020;4:e100013. doi: 10.1136/bmjos-2019-100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elloker T, Rhoda AJ. The relationship between social support and participation in stroke: a systematic review. Afr J Disabil. 2018;7:1–9. doi: 10.4102/ajod.v7i0.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zang P, Lu Y, Ma J, Xie B, Wang R, Liu Y. Disentangling residential self‐selection from impacts of built environment characteristics on travel behaviors for older adults. Soc Sci Med. 2019;238:112515. doi: 10.1016/j.socscimed.2019.112515 [DOI] [PubMed] [Google Scholar]
- 52. Lamb KE, Thornton LE, King TL, Ball K, White SR, Bentley R, Coffee NT, Daniel M. Methods for accounting for neighbourhood self‐selection in physical activity and dietary behaviour research: a systematic review. Int J Behav Nutr Phys Act. 2020;17:45. doi: 10.1186/s12966-020-00947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Twardzik E, Clarke P, Judd S, Colabianchi N. Neighborhood participation is less likely among older adults with sidewalk problems. J Aging Health. 2021;33:101–113. doi: 10.1177/0898264320960966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Twardzik E, Judd S, Bennett A, Hooker S, Howard V, Hutto B, Clarke P, Colabianchi N. Walk score and objectively measured physical activity within a national cohort. J Epidemiol Community Health. 2019;73:549–556. doi: 10.1136/jech-2017-210245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barnett A, Zhang CJP, Johnston JM, Cerin E. Relationships between the neighborhood environment and depression in older adults: a systematic review and meta‐analysis. Int Psychogeriatr. 2018;30:1153–1176. doi: 10.1017/S104161021700271X [DOI] [PubMed] [Google Scholar]
- 56. Ruiz LD, Brown M, Li Y, Boots EA, Barnes LL, Jason L, Zenk S, Clarke P, Lamar M. Neighborhood socioeconomic resources and crime‐related psychosocial hazards, stroke risk, and cognition in older adults. Int J Env Res Pub He. 2021;18:5122. doi: 10.3390/ijerph18105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clarke P, Morenoff J, Debbink M, Golberstein E, Elliott MR, Lantz PM. Cumulative exposure to neighborhood context: consequences for health transitions over the adult life course. Res Aging. 2014;36:115–142. doi: 10.1177/0164027512470702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marsh EB, Lawrence E, Gottesman RF, Llinas RH. The NIH stroke scale has limited utility in accurate daily monitoring of neurologic status. Neurohospitalist. 2016;6:97–101. doi: 10.1177/1941874415619964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schlegel D, Kolb SJ, Luciano JM, Tovar JM, Cucchiara BL, Liebeskind DS, Kasner SE. Utility of the NIH stroke scale as a predictor of hospital disposition. Stroke. 2003;34:134–137. doi: 10.1161/01.str.0000048217.44714.02 [DOI] [PubMed] [Google Scholar]
- 60. Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8:E928–E931. doi: 10.21037/jtd.2016.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34:917–928. doi: 10.1093/jpepsy/jsp004 [DOI] [PubMed] [Google Scholar]
- 62. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 63. Townsend BG, Chen JT, Wuthrich VM. Barriers and facilitators to social participation in older adults: a systematic literature review. Clin Gerontol. 2021;44:359–380. doi: 10.1080/07317115.2020.1863890 [DOI] [PubMed] [Google Scholar]
- 64. Allman RM, Baker PS, Maisiak RM, Sims RV, Roseman JM. Racial similarities and differences in predictors of mobility change over eighteen months. J Gen Intern Med. 2004;19:1118–1126. doi: 10.1111/j.1525-1497.2004.30239.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al Snih S, Peek KM, Sawyer P, Markides KS, Allman RM, Ottenbacher KJ. Life‐space mobility in Mexican Americans aged 75 and older. J Am Geriatr Soc. 2012;60:532–537. doi: 10.1111/j.1532-5415.2011.03822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rossler R, Bridenbaugh SA, Engelter ST, Weibel R, Infanger D, Giannouli E, Sofios A, Iendra L, Portegijs E, Rantanen T, et al. Recovery of mobility function and life‐space mobility after ischemic stroke: the MOBITEC‐stroke study protocol. BMC Neurol. 2020;20:348. doi: 10.1186/s12883-020-01920-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay‐Lyons M, Macko RF, Mead GE, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 68. Kylen M, Ytterberg C, von Koch L, Elf M. How is the environment integrated into post‐stroke rehabilitation? A qualitative study among community‐dwelling persons with stroke who receive home rehabilitation in Sweden. Health Soc Care Community. 2022;30:1933–1943. doi: 10.1111/hsc.13572 [DOI] [PubMed] [Google Scholar]
- 69. Sugiyama M, Chau HW, Abe T, Kato Y, Jamei E, Veeroja P, Mori K, Sugiyama T. Third places for older adults' social engagement: a scoping review and research agenda. Gerontologist. 2023;63:1149–1161. doi: 10.1093/geront/gnac180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


