Abstract
Background
Shared decision‐making (SDM) has the potential to improve hypertension care quality and equity. However, research lacks diverse representation and evidence about how race and ethnicity affect SDM. Therefore, this study aims to explore SDM in the context of hypertension management.
Methods and Results
Explanatory sequential mixed‐methods design was used. Quantitative data were sourced at baseline and 12‐month follow up from RICH LIFE (Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone) participants (n=1212) with hypertension. Qualitative data were collected from semistructured individual interviews, at 12‐month follow‐up, with participants (n=36) selected based on their SDM scores and blood pressure outcome. Patients were cross‐ categorized based on high or low SDM scores and systolic blood pressure reduction of ≥10 or <10 mm Hg. Multinomial logistic regression analysis showed that predictors of SDM scores and blood pressure outcome were race and ethnicity (relative risk ratio [RRR], 1.64; P=0.029), age (RRR, 1.03; P=0.002), educational level (RRR, 1.87; P=0.016), patient activation (RRR, 0.98; P<0.001; RRR, 0.99; P=0.039), and hypertension knowledge (RRR, 2.2; P<0.001; and RRR, 1.57; P=0.045). Qualitative and mixed‐methods findings highlight that provider–patient communication and relationship influenced SDM, being emphasized both as facilitators and barriers. Other facilitators were patients' understanding of hypertension; clinicians' interest in the patient, and clinicians' personality and attitudes; and barriers included perceived lack of compassion, relationship hierarchy, and time constraints.
Conclusions
Participants with different SDM scores and blood pressure outcomes varied in determinants of decision and descriptions of contextual factors influencing SDM. Results provide actionable information, are novel, and expand our understanding of factors influencing SDM in hypertension.
Keywords: health equity, hypertension, mixed‐methods, shared decision‐making
Subject Categories: Hypertension, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- RICH LIFE
Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone
- SDM
shared decision‐making
Clinical Perspective.
What Is New?
This study found that race and ethnicity are important determinants of shared decision‐making (SDM) and can indicate preferences for treatment options and attitudes toward health care providers, including communication style.
Barriers and facilitators, along with racial and ethnic aspects, experienced by patients from diverse backgrounds, were indicated as factors influencing SDM in the context of hypertension control.
Participants with high SDM scores and positive blood pressure outcomes described the discussion of treatment options, pros, and cons as facilitators of SDM; on the other hand, participants with lower SDM scores and negative blood pressure outcomes reported intimidation and patient–clinician racial and ethnic discordance as factors influencing SDM.
What Are the Clinical Implications?
Results provide actionable information that can be used to facilitate patients' engagement in SDM, emphasizing an effective patient–clinician communication, and providing patients with opportunities to better understand hypertension and treatment.
The barriers and facilitators identified in our study underline the impact of social determinants of health in the relationship between SDM and blood pressure reduction, highlighting an opportunity to address these aspects as part of the SDM process.
Shared decision‐making (SDM) is a process in which clinicians and participants make decisions together using the best available evidence, along with participants' preferences and values. 1 This approach is particularly relevant in situations where there are multiple treatment options with varying risks and benefits, and no single clearly superior option. 1 The US Affordable Care Act promotes SDM, 2 , 3 and the Centers for Medicare and Medicaid Services stipulates it as a prerequisite to reimbursement for certain health conditions. 4 , 5 Several health care professional bodies and guidelines endorse SDM and its potential to significantly transform medical culture and health systems. 6 , 7 , 8 , 9 , 10 In addition, evidence suggests that SDM often reduces costs and improves use of health care. 11 SDM is advocated in the care of chronic health conditions such as hypertension. 9 Considering individual preferences for the risks and benefits of available options, SDM can be particularly important in an asymptomatic condition such as hypertension, due to drug treatment being generally lifelong. 12
In the United States, hypertension contributes to about 1000 deaths a day 13 and to an estimated annual cost of $51 billion. Despite improvements, national hypertension control rates remain inadequate. About 1 in every 2 adults in the United States with hypertension and using antihypertensive pharmacologic treatment have uncontrolled blood pressure (BP) and racial and ethnic disparities persist in this chronic condition. 14 , 15 Hypertension prevalence is higher among Black (57%) and Latino (45%) compared with White (44%) people in the United States. 15 In addition to the prevalence of hypertension being higher among racially minoritized populations, its control is also affected by racial and ethnic disparities. 15 , 16 , 17 Among men using antihypertensive medication, rates of achieving hypertension control are lowest among Latino (44%) followed by non‐Latino Black (52%) and non‐Latino White (59%) men. 18 Self‐reported nonadherence to antihypertensive medication is also higher among Latino (34%) and Black (36%) compared with White (24%) people. 16 This is attributed to inadequate hypertension awareness and treatment among racially minoritized populations. 18 , 19
It has been suggested that SDM has the potential to improve the quality of cardiovascular care and reduce disparities. This issue can be illustrated by the adherence of individuals to therapy and improved provider–patient communication. Together, these aspects could contribute to decreasing BP and improving hypertension symptoms. 10 , 20 Nonetheless, evidence to date does not provide the needed evaluation of effectiveness and implementation of such approaches in the context of hypertension care in minoritized populations. In addition, people who are medically underresourced might benefit from SDM because it recognizes everyone's preferences, values, and barriers to engagement and outcomes improvement. 21 , 22 Nevertheless, there is a lack of representation of individual who are medically underresourced in SDM literature. 23
This explanatory sequential mixed‐methods study's aim was to describe SDM in the hypertension management context in relation to SDM scores and BP levels. We hypothesized that participants with high SDM scores and systolic BP reduction of ≥10 mm Hg from baseline to 12 months would be more likely to emphasize collaborative decision‐making processes and differ in their characteristics and experiences compared with participants who have lower SDM scores and systolic BP reduction <10 mm Hg.
Methods
The data that support the findings of this study are available upon reasonable request from qualified researchers trained in human subject confidentiality protocols and may be sent to the Johns Hopkins Center for Health Equity at healthequity@jhmi.edu.
Study Design
This was an explanatory sequential mixed‐methods design involving 2 distinct phases: quantitative followed by qualitative. 24 This design was chosen to achieve an in‐depth understanding of SDM in the context of hypertension management by exploring quantitative results using a qualitative approach. Groups were formed based on quantitative results and in‐depth interviews were conducted in the qualitative phase.
The quantitative phase assessed the differences in participants’ characteristics based on their SDM scores and systolic BP outcome. Qualitative interviews explored the experiences of participants who had different SDM scores and BP outcomes, focusing specifically on barriers and facilitators to SDM. Furthermore, integration of quantitative and qualitative data amplifies the strengths and reduces the weaknesses of each type of data. 25
Participants and Recruitment
The current study is part of a larger pragmatic trial, the RICH LIFE (Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone) project, that aimed to determine whether a multilevel intervention (encompassing use of care managers, community health workers, and a virtual specialist consultation service), was more effective for reducing disparities and achieving BP control, compared with enhanced standard of care. 26 This project was conducted in partnership with 30 clinical sites, federally qualified health centers, and private clinics across Maryland and Pennsylvania. Individuals' eligibility for the RICH LIFE project was determined based on their electronic medical records and inclusion criteria were (1) age ≥21 years; (2) diagnosis of hypertension with at least 1 other cardiovascular risk factor (coronary heart disease, diabetes, hyperlipidemia, current tobacco smoker, or diagnosis of depression); and (3) self‐identification as a non‐Latino White, non‐Latino Black, or Latino individual. Details on the study protocol and recruitment strategy can be found elsewhere. 26 Eligible individuals received mailed invitations to participate, along with an oral consent paper copy.
Critical terminologies used throughout this article include the terms Latino and non‐Latino, Black and White people. Self‐identification was employed for this categorization. As there are many languages and dialects spoken in Latin American countries (Spanish, Portuguese, English, French, among others), the term Hispanic would not be suitable to refer to the ethnic group examined in this article. Consequently, the term Latino, which means individuals, men and women, of Latin American origin or descent, was used to represent the population included.
The Johns Hopkins Medicine Institutional Review Board approved (IRB00085630) all RICH LIFE project procedures, including those carried out in this study.
Enrollment Procedures
Individuals interested in participating in the RICH LIFE project were further screened, consented, and completed the baseline survey from August 1, 2017, to October 31, 2019, and 12 months follow up survey data were collected between August 7, 2018, and March 31, 2021. RICH LIFE allowed a 6‐month window around the 12‐month follow‐up date to complete the survey. Participants received a $25 incentive for each of these data collection points. Qualitative interviews were also voluntary. A selected group of RICH LIFE participants were approached to participate in a 1‐time semistructured individual interview. After oral consent, interviews were carried out between June and September 2022. Per the parent study's protocol, interview participants received a $60 incentive. A total of 1212 individuals participated in the quantitative phase and of those 36 participated in the semistructured interviews.
Quantitative Study Procedures and Data Collection
Part of the RICH LIFE data were sourced from electronic medical records and part from surveys administered by trained research assistants via phone using a secure electronic Research Electronic Data Capture database. Participants were surveyed in Spanish by bilingual research assistants when this was their preferred language.
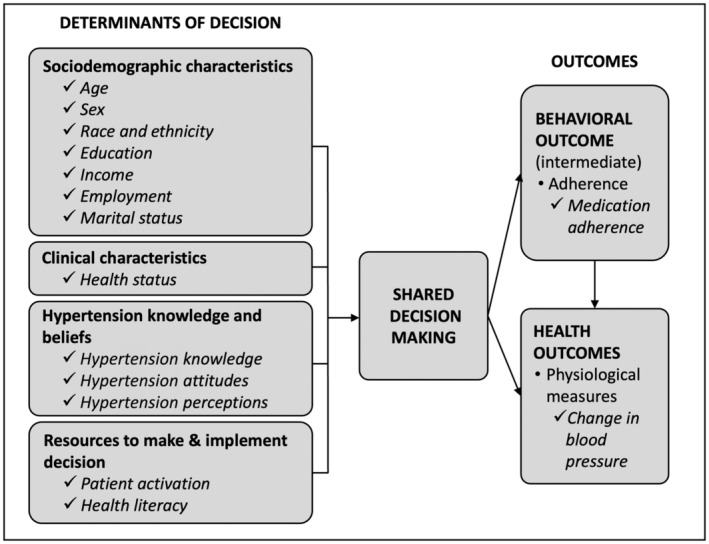
We developed an adaptation of the Ottawa Decision Support Framework, 27 , 28 and used it to guide selection of relevant RICH LIFE clinical and nonclinical variables for inclusion in the analysis. The adapted conceptual framework, shown in Figure 1, guided the selection of relevant patients' clinical and nonclinical determinants for health‐related decisions. Within our adapted conceptual framework, we posited that the determinants of decision influenced the degree to which SDM occurs, which, in turn, may affect health outcomes. Determinants of decision explored at baseline included sociodemographic (age, sex, race and ethnicity, education, income, employment, and marital status) and clinical characteristics (health status), hypertension knowledge and beliefs (hypertension knowledge, attitudes, and perceptions), and resources to make and implement decision (patient activation; health literacy). This framework outlines how the selection of one option over another depends not only on the patients' characteristics but also on other aspects that influence how the decision is perceived as well as personal resources available to make and implement the decision. Given that patients from different racial, ethnic, and cultural backgrounds appraise their decision‐making processes differently, the National Institute on Minority Health and Health Disparities Research Framework was applied to examine possible influencing factors of health disparities related to SDM in the context of hypertension. 29 , 30 Influencing factors examined in this study were selected based on the Ottawa Decision Support Framework as described and included sociodemographic characteristics (age, sex, race and ethnicity, and education), hypertension knowledge and beliefs, and patient activation. For our quantitative aim, the outcome was group membership based on SDM scores and BP.
Figure 1. Adapted conceptual framework.
We assessed SDM during the 12‐month follow‐up survey using CollaboRATE, 31 a 3‐item patient‐reported measure. Participants were asked to rate each CollaboRATE item on a 10‐point scale (0–9, “no effort was made” to “every effort was made”) regarding a recent visit with the primary health care provider where a hypertension‐related decision was made. We used the CollaboRATE top score, which dichotomized the score as =27, and ≤26. The CollaboRATE score, which was developed in collaboration with patients, was previously evaluated as performing well both in English and in Spanish, with strong internal consistency (α=0.91 and α=0.97, respectively). 32 BP was measured at baseline and 12‐month follow‐up by trained staff of the clinics following RICH LIFE standardized measurement protocol. For data quality the RICH LIFE project also implemented the use of a specific BP device, OMRON 907XL, at the practice sites and data collection started only after 3 months of device implementation. BP data were obtained from electronic medical records. We used systolic BP change specifically because the RICH LIFE project's inclusion criteria required that systolic BP be uncontrolled at baseline, that is, systolic BP ≥140 mm Hg. Previously published meta‐analyses reported relative risk reductions proportional to the magnitude of the BP reductions achieved. Results showed that every 10 mm Hg reduction in systolic BP significantly reduced the risk of major cardiovascular disease events, coronary heart disease, stroke, and heart failure, with consequent reduction in all‐cause mortality. 33 Given these findings we established a threshold of systolic BP reduction of ≥10 mm Hg.
Determinants of decision, such as sociodemographic characteristics, were collected during the baseline survey. These included age, sex, race and ethnicity, education, income, employment, and marital status. Hypertension knowledge, perceptions, and attitudes were assessed during baseline survey with a single item each based on previously reported study. 34 Patient activation is the knowledge, skill, and confidence necessary for chronic disease self‐management, and it was assessed at baseline using the Patient Activation Measure‐13 35 a 13‐item 4‐point Likert scale (1–5, “disagree strongly” to “agree strongly”) with scores ranging from 0 to 100, higher scores indicating higher patient activation. Health literacy is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health‐related decisions,” and it was measured in our study with the Screening Questions for Limited Health Literacy. 36 , 37
Power analysis was conducted and unequal sample sizes for the race and ethnicity groups were taken into consideration. Calculations focused on how different the groups needed to be on SDM scores, based on standardized units, that is, Cohen's d effect size. With a sample of 1426, we would be able to detect significant differences in SDM if the effect size was 0.29 or greater when comparing Latino and non‐Latino White participants, and 0.16 for non‐Latino Black versus non‐Latino White participants. Power was set at 0.80 and alpha at 0.05.
Qualitative Study Procedures and Data Collection
Participants who completed the RICH LIFE survey, including the SDM measurement tool, and had data on systolic BP at 12 months were eligible for the qualitative semistructured individual interviews. Given our aim to explore participants' perspectives based on their SDM scores and BP outcomes, a purposive maximum variation sample of 36 participants was selected from the larger study's sample based on the participant's SDM score, BP outcome, and race and ethnicity. The goal was to form 4 groups with 9 individuals each, and 3 people from each racial and ethnic background per group. This approach was used to ensure that multiple perspectives were represented while also achieving data saturation. 38
Interviews, lasting an average of 30 minutes, were carried out via phone call using a Health Insurance Portability and Accountability Act‐compliant password‐protected Zoom account with callout feature. This Zoom feature was used to call participants on their landlines or cellphones. Interviews were audio recorded and real‐time notes were taken and stored on password‐protected cloud storage, encrypted servers. Interviews were transcribed verbatim in the language recorded, and those conducted in Spanish were translated into English by professional translators before coding. Participants provided oral consent before initiation of the interview.
The semistructured interview guide included open‐ended questions and probes based on SDM in hypertension literature 39 and relevant factors included in the Ottawa Decision Support Framework. 27 , 28 The interview guide explored aspects of decision‐making including questions that explored the facilitators and barriers to getting involved in decision‐making and the influence of racial and ethnic factors in SDM. The interview guide was pilot tested and adjusted accordingly and it is included in Data S1.
Statistical Analysis
The analytic approach consisted of 3 phases. We first analyzed quantitative data, then we selected participants for the interviews based on these results, analyzed the qualitative data, and, lastly, we analyzed the combined results. 24 We performed quality assurance checks for the survey and interview data. Deidentified survey and electronic medical records data were imported into Stata (Stata Corp. 2021. Stata Statistical Software: Release 17. StataCorp LLC, College Station, TX) for statistical analyses, and deidentified interview transcripts data were imported into ATLAS.ti version 23.1.0 for qualitative analyses.
Quantitative Data Analysis
We conducted a descriptive analysis of quantitative data for all measures. Data on covariates were largely complete (<1% missingness). Differences were evaluated using ANOVA for continuous variables, and chi‐square (χ2) test for categorical variables. Given that determinants of decision could be closely related, we assessed multicollinearity using the variance inflation factor. Results ranged from 1.04 to 2.34 indicating that multicollinearity was not found in the model. Participants were cross‐categorized in 4 groups based on their CollaboRATE top score (high SDM =27; lower SDM ≤26) and systolic BP (reduction ≥10 mm Hg; reduction <10 mm Hg SBP). We refer to these throughout this paper as the SDM and BP groups. Multinomial logistic regression was used to exam the relative risk ratio (RRR) of SDM and BP group membership controlling for intervention group assignment. ANOVA was employed when group was the independent variable, and we were comparing clinical characteristics across distinct groups. When group was the dependent variable and we were investigating the predictors of SDM scores and BP improvement, a model of multinomial logistic regression was used. Both of these analytical approaches control for the multiple comparison across groups.
Qualitative Data Analysis
We used Miles, Huberman, and Saldaña guidance for our analytical approach. 40 Trained research team members analyzed the transcripts using both a deductive and an inductive approach. Independent coding of 2 transcripts by 2 research team members was carried to ensure agreement among coders. A priori codes were developed based on the interview guide, imported into ATLAS.ti software. Using an iterative process, a priori codes were applied across all interviews' transcripts, and were merged into sublevel codes until a comprehensive list of facilitators and barriers. The sublevel codes were then categorized based on 6 high‐level codes or domains. Throughout this process, we compared the codes with the interview transcripts and reviewed the a priori codes and refined codes with the study team performing the analysis. The team consisted of people from Black, White, Latino, and Asian backgrounds who discussed and reflected upon positionalities and possible influences on the analyses. Biweekly meetings were carried out throughout the coding process to discuss discrepancies, review newly created codes, and reach agreement regarding new codes.
Data Integration and Triangulation
We integrated quantitative and qualitative data at the methods level by using quantitative results to guide purposeful sampling of interview participants and at the interpretation and reporting level by combining numeric and interview data to develop a joint display 24 , 41 and a data matrix table. These displays allowed us to visualize differences across the 4 SDM and BP groups.
Results
Sample Characteristics
Figure 2 shows the distribution of participants in the study by group and respective race and ethnicity of the selected participants.
Figure 2. Study flow chart.
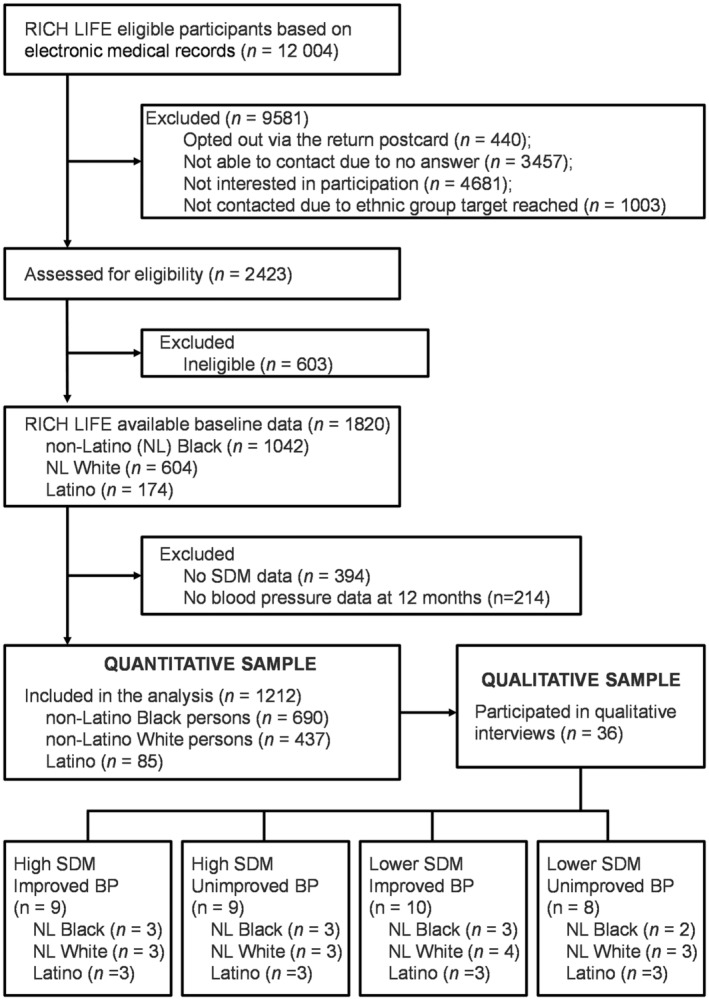
BP indicates blood pressure; RICH LIFE, Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone; and SDM, shared decision‐making.
Table 1 displays sociodemographic and other characteristics for the total (n=1212) and interview samples (n=36), and cross‐categorization of the 4 SDM and BP groups. There were statistically significant differences among these groups for the following variables: race and ethnicity (P=0.001), education (P<0.001), hypertension knowledge (P=0.002), perceptions (P=0.034), attitudes (P=0.004), and patient activation (P=<0.001); systolic and diastolic BP levels (P<0.001) and SDM scores (P<0.001) were also statistically different due to group membership criteria based on these variables.
Table 1.
Sociodemographic and Other Characteristics by SDM and BP Group, Total and Interview Sample
| Characteristics | High SDM | Lower SDM | P value | Total sample | Interview sample | ||
|---|---|---|---|---|---|---|---|
| Reduction in SBP ≥10 mm Hg | Reduction in SBP <10 mm Hg | Reduction in SBP ≥10 mm Hg | Reduction in SBP <10 mm Hg | ||||
| Sample, n (%) | 338 (27.9) | 198 (16.3) | 402 (33.2) | 274 (22.6) | 1212 (100) | 36 (100) | |
| Sociodemographic | |||||||
| Age, y, mean ±SD | 59.8 (11.8) | 62.5 (10.4) | 61.1 (11.9) | 61.6 (11.9) | 0.055 | 61.1 (11.7) | 61 (12.6) |
| Male sex, n (%) | 130 (26.1) | 78 (15.7) | 166 (33.3) | 124 (24.9) | 0.366 | 498 (41.1) | 11 (30.6) |
| Race and ethnicity | 0.001* | ||||||
| NL Black participants | 198 (28.7) | 127 (18.4) | 230 (33.3) | 135 (19.6) | 690 (56.9) | 13 (36.1) | |
| NL White participants | 110 (25.2) | 53 (12.1) | 153 (35.0) | 121 (27.7) | 437 (36.1) | 11 (30.6) | |
| Latino participants | 30 (35.3) | 18 (21.2) | 19 (22.4) | 18 (21.2) | 85 (7.0) | 12 (33.3) | |
| Education, n (%) | <0.001* | ||||||
| <High school | 65 (32.0) | 46 (22.7) | 49 (24.1) | 43 (21.2) | 203 (16.8) | 9 (25.0) | |
| High school | 170 (30.1) | 88 (15.6) | 198 (35.0) | 109 (19.3) | 565 (46.7) | 15 (41.7) | |
| >High school | 102 (23.1) | 63 (14.3) | 155 (35.1) | 121 (27.4) | 441 (36.5) | 12 (33.3) | |
| Income, n (%) | |||||||
| <20k | 71 (30.1) | 39 (16.5) | 78 (33.1) | 48 (20.3) | 0.667 | 236 (19.5) | 5 (13.9) |
| $20k to $39k | 51 (26.3) | 41 (21.1) | 59 (30.4) | 43 (22.2) | 194 (16.0) | 5 (13.9) | |
| ≥40k | 127 (27.0) | 68 (14.5) | 159 (33.8) | 116 (24.7) | 470 (38.8) | 12 (33.3) | |
| Did not report | 89 (28.5) | 50 (16.0) | 106 (34.0) | 67 (21.5) | 312 (25.7) | 14 (38.9) | |
| Employed, n (%) | 133 (27.6) | 75 (15.6) | 160 (33.2) | 114 (23.7) | 0.860 | 482 (39.9) | 12 (33.3) |
| Married, n (%) | 141 (27.8) | 75 (14.8) | 166 (32.7) | 125 (24.7) | 0.423 | 507 (41.9) | 14 (38.9) |
| Have health insurance, n (%) | 335 (27.8) | 197 (16.3) | 401 (33.2) | 274 (22.7) | 0.743 | 1207 (99.8) | 36 (100) |
| Clinical | |||||||
| SBP baseline, mean±SD | 154.7 (13.0) | 148.8 (9.0) | 154.5 (13.0) | 147.4 (8.2) | <0.001* | 152.0 (11.9) | 151.4 (11.0) |
| DBP baseline, mean±SD | 86.2 (12.3) | 82.9 (12.0) | 85.5 (12.0) | 82.3 (11.5) | <0.001* | 84.5 (12.1) | 83.5 (10.3) |
| SBP change, mean±SD | −26.3 (13.6) | 3.1 (12.9) | −25.1 (13.2) | 3.2 (12.5) | −14.4 (19.2) | −13.5 (19.8) | |
| DBP change, mean±SD | −11.5 (11) | 0 (11.4) | −9.6 (10.4) | 0.5 (11.1) | −6.3 (12.1) | −4.14 (11.0) | |
| Health status, mean±SD† | 45.3 (9.2) | 45.0 (9.4) | 44.4 (8.5) | 45.1 (8.7) | 0.535 | 44.9 (8.9) | 44.4 (7.9) |
| Health status, n (%) | 0.744 | ||||||
| Poor | 48 (28.9) | 26 (15.7) | 55 (33.1) | 37 (22.3) | 166 (13.7) | 4 (11.1) | |
| Fair | 63 (22.7) | 50 (18.0) | 99 (35.6) | 66 (23.7) | 278 (22.9) | 9 (25.0) | |
| Good | 110 (29.3) | 57 (15.2) | 127 (33.9) | 81 (21.6) | 375 (30.9) | 13 (36.1) | |
| Very good and excellent | 117 (29.8) | 65 (16.5) | 121 (30.8) | 90 (22.9) | 393 (32.4) | 10 (27.8) | |
| Other | |||||||
| Knows that high BP means hypertension, n (%) | 251 (26.0) | 161 (16.7) | 316 (32.7) | 237 (24.6) | 0.002* | 965 (79.6) | 27 (75.0) |
| High BP is a serious health concern, n (%) | 216 (30.2) | 125 (17.5) | 225 (31.5) | 149 (20.8) | 0.034* | 715 (59.0) | 23 (63.9) |
| Taking BP medication is very important, n (%) | 314 (28.7) | 187 (17.1) | 352 (32.2) | 240 (22.0) | 0.004* | 1093 (90.3) | 34 (94.4) |
| Patient activation, mean±SD‡ | 67.8 (17.2) | 69.0 (17.0) | 63.7 (15.0) | 65.9 (14.9) | <0.001* | 66.2 (16.1) | 62.7 (17.3) |
| Adequate health literacy§ | 236 (27.8) | 141 (16.6) | 280 (32.9) | 193 (22.7) | 0.980 | 850 (70.1) | 24 (66.7) |
| ecision‐making | |||||||
| Shared decision‐making, mean±SD|| | 9 (0) | 9 (0) | 5.8 (2.7) | 5.7 (2.9) | <0.001* | 7.2 (2.6) | 7.6 (2.5) |
BP indicates blood pressure; DBP, diastolic blood pressure; high SDM, CollaboRATE sum score 27; Lower SDM, CollaboRATE sum score ≤26; NL, non‐Latino; SBP, systolic blood pressure; and SDM, shared decision‐making.
Statistically significant, P value <0.05, comparing the 4 SDM and BP groups.
Patient‐Reported Outcomes Measurement Information System Global Physical Health, T‐scores ≤35: poor; 35–42: fair; 42–50: good; 50–58: very good; and >58: excellent.
Patient Activation Measure, scores 0–100, higher scores indicate higher activation.
Screening Questions for Limited Health Literacy.
CollaboRATE score, range from 0 to 9, higher scores indicate greater shared decision‐making. Per high SDM group definition, participants had to have SDM score of 9 (maximum score) to be included.
At baseline, the total sample's average age was 61 years (±12.6), and most participants were non‐Latino Black individuals(n=690, 56.9%). The majority of the participants had high school or lower education (n=768, 63.5%), and adequate health literacy (n=850, 70.1%). A total of 482 (39.9%) participants were employed, and 507 (41.9%) were married. The majority of participants knew that high BP is the same as hypertension (n=965, 79.6%), considered high BP a serious health concern (n=715, 59.0%), and believed that taking BP medication is very important (n=1093, 90.3%). Mean systolic and diastolic BP were 152.0 mm Hg (±11.9) and 84.5 mm Hg (±12.1), respectively. Sociodemographic characteristics of the interview sample are included in the qualitative data results section.
Quantitative Results Comparison Among Shared Decision‐Making and Blood Pressure Groups
Multinomial logistic regression analysis was used to examine key participant characteristics and predictors of SDM scores and BP improvement (see Table 2). Among individuals with high SDM, being a non‐Latino Black individual compared with being a non‐Latino White individual (RRR, 1.64, P=0.029) and increased age (RRR, 1.03, P=0.002) were less likely to be associated with 10 mm Hg SBP reduction. Among those with education beyond high school, individuals have almost double the chances of experiencing lower SDM and SBP reduction ≥10 mm Hg (RRR, 1.87, P=0.016); and participants with knowledge that high BP is the same as hypertension were more likely to have SBP reduction <10 mm Hg regardless of SDM score (RRR, 1.57, P=0.045, and RRR, 2.2, P<0.001). In addition, higher patient activation minimized the probability of lower SDM regardless of BP outcomes (RRR, 0.98, P<0.001, and RRR, 0.99, P=0.039).
Table 2.
Multinomial Logistic Regression Reporting on the Relative Risk Ratio of SDM and BP Group Membership*
| High SDM | Lower SDM | ||||||
|---|---|---|---|---|---|---|---|
| Reduction in SBP ≥10 | Reduction in SBP <10 | Reduction in SBP ≥10 | Reduction in SBP <10 | ||||
| RRR (95% CI) | P value | RRR (95% CI) | P value | RRR (95% CI) | P value | ||
| Sociodemographic characteristics | |||||||
| Age | Ref | 1.03 (1.01,1.04) | 0.002‡ | 1.01 (0.99–1.02) | 0.371 | 1.01 (0.99–1.02) | 0.396 |
| Male sex | Ref | 1.08 (0.75–1.56) | 0.686 | 1.08 (0.79–1.46) | 0.628 | 1.25 (0.90–1.75) | 0.186 |
| Race and ethnicity | |||||||
| NL White participants (437) | Ref | ||||||
| NL Black participants (690) | Ref | 1.64 (1.05–2.54) | 0.029‡ | 1.09 (0.77–1.55) | 0.610 | 0.81 (0.56–1.19) | 0.286 |
| Latino participants (85) | Ref | 1.56 (0.73–3.34) | 0.251 | 0.54 (0.27–1.09) | 0.085 | 0.63 (0.30–1.30) | 0.211 |
| Education | |||||||
| <High school | Ref | ||||||
| High school | Ref | 0.73 (0.44–1.19) | 0.200 | 1.44 (0.91–2.28) | 0.115 | 0.81 (0.50–1.33) | 0.410 |
| >High school | Ref | 0.83 (0.48–1.43) | 0.502 | 1.87 (1.12–3.11) | 0.016‡ | 1.27 (0.75–2.17) | 0.378 |
| Other characteristics | |||||||
| Knowledge that high BP means hypertension | Ref | 1.57 (1.01–2.44) | 0.045‡ | 1.25 (0.87–1.80) | 0.232 | 2.20 (1.41–3.43) | <0.001‡ |
| High BP is a serious health concern | Ref | 0.89 (0.60–1.32) | 0.559 | 0.77 (0.55–1.06) | 0.109 | 0.75 (0.53–1.07) | 0.113 |
| Taking BP medication is very important | Ref | 1.36 (0.62–2.96) | 0.440 | 0.60 (0.35–1.01) | 0.056 | 0.58 (0.32–1.03) | 0.062 |
| Patient activation† | Ref | 1.00 (0.99–1.01) | 0.517 | 0.98 (0.97–0.99) | <0.001‡ | 0.99 (0.98–1.00) | 0.039‡ |
Log likelihood = −1572.7. BP indicates blood pressure; High SDM, CollaboRATE sum score 27; HTN, hypertension; Lower SDM, CollaboRATE sum score ≤26; NL, non‐Latino; RRR, relative risk‐ratio; SBP, systolic blood pressure; and SDM, shared decision‐making.
Adjusted for intervention group assignment.
Patient Activation Measure (PAM‐13).
Statistically significant, P value <0.05.
Qualitative Results From In‐Depth Interviews
A total of 36 individuals participated in the interviews. At baseline, mean age was 61 (±12.6) and about 30.6% were men. Total sample and interview sample were fairly similar, except regarding distribution by race and ethnicity. The interview sample included an equitable representation of non‐Latino Black (n=13, 36%), non‐Latino White (n=11, 31%), and Latino (n=12, 33%) participants.
During interviews participants described barriers and facilitators in the context of SDM. We named high level codes as domains, and sublevel codes as facilitators and barriers. Domains included (1) patient–clinician communication, (2) patient–clinician relationship, (3) patient factors, (4) clinician factors, (5) clinic or health system factors, and (6) influence of participants' race and ethnicity. Table 3 shows high level and sublevel codes and highlighted quotes. Qualitative data results are further expanded in the context of the quantitative data in the mixed‐methods results section.
Table 3.
High and Sublevel Codes and Highlighted Quotes
| High level codes/domains | Sublevel codes/facilitators and barriers | Quotes |
|---|---|---|
| Patient–clinician communication | Two‐way communication; lack of communication; discussion of options, pros, and cons; able to ask questions |
“His [clinician] willingness to discuss my blood pressure, the consequences. He doesn't hold back when he talks to me, and I'm able to discuss my views, and he listens to it, and we make the decision together. … It's something positive.” (Pt. 1041) “Well, it was easy when she was like “Well, you have the option to exercise, or change your eating habits to make your blood pressure more healthier.” So I felt as though that was a option that I was willing to take.” (Pt. 1215) |
| Lack of information provision | “I think that I should've been giving a little more information than I do get. I don't know if I have an actual doctor or a practitioner, but sometimes I do feel like I'm not getting all the information that I would get if I had a black doctor.” (Pt. 4101) | |
| Clinicians’ accent | “I speak English … basically only English speaking person, and if they have a difficult accent to understand, then it's a very difficult conversation. So I don't care what color they are, or what their ethnic background is, if I can't understand them then it's difficult” (Pt. 0017) | |
| Patient–clinician relationship | Trust and respect for the clinician; respect for clinician's education | “I think that is done with the trust you have in your doctor. The trust your doctors give you to treat him. It makes you get involved in the treatments and not to forget how the treatments were indicated.” (Pt. 5011) |
| Hierarchy; patient feeling intimidated | “I think she thought that I didn't understand, and I'm coming from— I'm thinking “I'm the doctor, I know best, I've got the education, I've got the knowhow.” But still, no matter what you have, you can always listen to someone else. You can come to a person and talk to them like you would like to be spoken to if you was the patient, and that person was the doctor.” (Pt. 1238) | |
| Shared history and experiences; mutual understanding; no mutual understanding/different perspectives | [patient–clinician racial discordance] “She is a younger generation person and she's also Asian descent, so they understand stuff differently than how Caucasians understand.” (Pt. 0106) | |
| Different lifestyles | [patient–clinician racial discordance] “It could be. It could be because if we're from different ethnic groups, in the way we eat, and our lifestyle will be different. I think it is.” (Pt. 0076) | |
| Continuity of care | “They knew what we have tried, what's worked, why we are where we are with the meds. So, the fact that they know the background is reassuring to me and then we don't have to start from scratch each time. … a primary physician who has the context of my history and then he or she brings what's the latest in the research, in the medication. I bring how I'm feeling and together, we either affirm what we're doing or we modify it.” (Pt. 0010) | |
| Patient factors | Understanding of hypertension and treatment; lack of knowledge and understanding | “Maybe there are other people who don't understand like me, at the beginning. I didn't understand what high blood pressure implied. If I don't understand, then I don't know how to correct it.” (Pt. 3039) |
| Control over oneself | “It's easy for me [to participate in decision‐making] knowing that I am in control of myself. I'm still of my mind and I know what's right and what's wrong. I'm still in my mind to say I will do this, or I won't do this. Nobody has that privilege over me.” (Pt. 1238) | |
| Lack of regimen compliance | “The most difficult thing is that sometimes I don't take the medication and that has an impact on them not being able to help me like they want to help me.” (Pt. 5294) | |
| Clinician factors | Time constraints | “Well, pressure on the provider to limit time with the patient, and I've seen that happen, when administration says you should see X number of patients in X number of time.” (Pt. 1171) |
| Clinician takes time; clinician shows interest in the patient | “I have a physician that shows interest in me. And so when he sits and he looks at me in the face and we are communicating, it makes it easy for me— and I know he's listening to me. So it makes it easy for me to get involved and express myself regarding my health. And he works along with me. We're on the same page. And so that makes it easy.” (Pt. 1035) | |
| Clinician knowledgeable of health disparities | “I guess my provider understand that because I'm African‐American and, you know, the hypertension affect us more than any other race. And she's Caucasian so she has a very good understanding of what it is that I'm dealing with.” (Pt. 1108) | |
| Clinician's personality and attitudes | “Animosity between the two working together as a team. I mean, by “animosity” I mean him not listening to you or you're not listening to him or vice‐versa on the medical for your best interests. Like me, I catch an attitude when a doctor gives me an attitude. How's that? So that would be detrimental to my health and making decisions.” (Pt. 1041) | |
| Perceived lack of compassion | “She just rarely cares about what I have to say. You know what I mean? She always tells me what she thinks about what I said, and we never come up with decisions about my health. That's why I had to kind of like find another person that was more interested in my well‐being than she was.” (Pt. 1034) | |
| Clinic or health system factors | Time constraints | “Well, pressure on the provider to limit time with the patient, and I've seen that happen, when administration says you should see X number of patients in X number of time.” (Pt. 1171) |
| Having an interpreter | “It doesn't affect me [patient–clinician racial and ethnic discordance] because there's always an interpreter, and she treats everyone equally.” (Pt. 5294) | |
| Limited availability of clinicians | “In rural areas as well yeah. There aren't enough of them [clinicians] and two they're all rushed I mean … I'm always concerned because my provider is over 70 and I know that he's going to retire so. And I think, who's gonna be there when he does, I have no idea.” (Pt. 2013) | |
| Influence of participants' race and ethnicity | Patient's race specific risks | “I'm African‐American and I know that we're at the top of the chart as far as having hypertension. So knowing that fact makes me very well aware of what's going on. And it really vastly impacts decision‐making.” (Pt. 1108) |
| Family history of hypertension | “Well, it's important because my family— a lot of my family members have high blood pressure. So it's very important that I be— it's very important to me.” (Pt. 1193) | |
| Not taken seriously | “No, I will make a decision, but you do feel like maybe you are not being taken serious or understanded all the way through. But, no, because if the doctor is fine, I'm fine with whatever they are.” (Pt. 1025) |
Pt. indicates participant.
Mixed‐Methods Results
Figure 3 shows a joint display with SDM factors discussed by participants in the context of hypertension treatment reported in the first and third columns (qualitative data), and the second and fourth columns are a representation of the data as discussed by each of the 4 SDM and BP groups. Detailed results are reported in the following paragraphs. Exemplary quotes are included in a data matrix in Table S1.
Figure 3. Facilitators, barriers, and corresponding domains related to SDM in the context of hypertension by SDM and BP group.
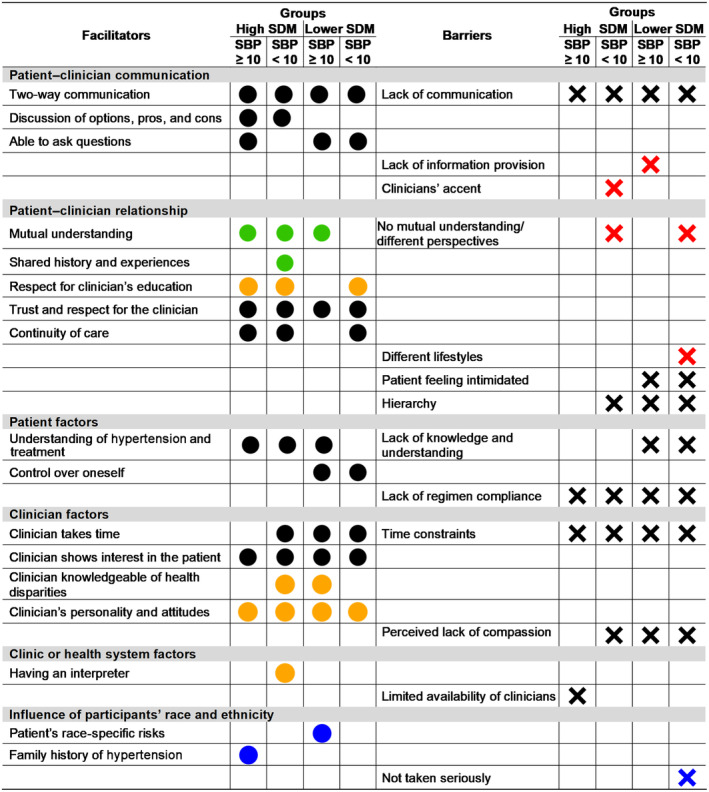
Black circles: Facilitators discussed by the corresponding group. Black cross: Barriers discussed by the corresponding group. Green circles: Facilitators of patient‐clinician racial and ethnic concordance. Red cross: Barriers of patient‐clinician racial and ethnic discordance. Orange circles: Facilitators mentioned as relevant rather than patient–clinician racial and ethnic concordance or discordance. Blue circles: Participants' own race and ethnicity as a facilitator of participation in SDM. Blue cross: Participants' own race and ethnicity as a barrier of participation in SDM. BP indicates blood pressure; High SDM, CollaboRATE sum score 27; Lower SDM, CollaboRATE sum score ≤26; SBP, systolic blood pressure; and SDM, shared decision‐making. SBP ≥10: reduction in SBP of ≥10 mm Hg; and SBP <10: reduction in SBP of <10 mm Hg.
Participants described factors including patient–clinician communication, understanding or knowledge of hypertension, time, and mutual understanding both as barriers and facilitators to SDM based on their presence and absence. Particularly, mutual understanding was discussed in the context of patient–clinician racial and ethnic concordance and discordance, as shown in Figure 3 with green dots.
Facilitators to SDM in the context of hypertension treatment discussed by all SDM and BP groups include trust and respect for the clinician, clinician shows interest in the patient, and clinicians' personality and attitudes. Most groups also discussed being able to ask questions, clinician knowledge of health disparities, continuity of care, and respect for the clinicians' education as facilitators of SDM. In addition, participants with high SDM and systolic BP reduction of 10 mm Hg or more also described discussion of options, pros, and cons as facilitators of SDM. Some of these facilitators were mentioned as being more relevant than patient–clinician racial and ethnic concordance or discordance as represented in Figure 3 with orange dots.
Barriers to SDM discussed by most SDM and BP groups were lack of regimen compliance, hierarchy in the patient–clinician relationship, and perceived lack of compassion. Participants with lower SDM and systolic BP reduction of <10 mm Hg mentioned feeling intimidated, control over oneself, and different lifestyles as factors that influence SDM. Some of the barriers were discussed in the context of patient–clinician racial and ethnic discordance as presented in Figure 3 with red crosses.
Communication both as a facilitator and a barrier was the factor most extensively discussed by participants, followed by facilitators such as patient's understanding of hypertension, clinician's interest in the patient, and clinician's personality, and attitudes; and barriers such as perceived lack of compassion, relationship hierarchy, and time constraints.
We also interviewed participants regarding the influence of their own racial and ethnic background in their participation in SDM as shown in Figure 3 with blue dots. Participants mentioned their race‐specific cardiovascular health risks and their family history of hypertension as motivating factors to engage in SDM. Regarding barriers, participants perceived that due to a patient's racial and ethnic background they might not be taken seriously by the clinician.
Another aspect important to highlight is language. Clinician accent was mentioned as a barrier to SDM with patient–clinician racial and ethnic discordance. However, having an interpreter was discussed as an important facilitator that is more relevant than patient–clinician racial and ethnic concordance or discordance. For instance, all Spanish‐speaking participants (n=10) who had an interpreter mediating their appointments had higher SDM, whereas those who did not have interpreters had lower SDM.
Discussion
This mixed‐methods explanatory sequential study aimed to broaden the current understanding of SDM in the context of hypertension treatment, including barriers, facilitators, and factors related to race and ethnicity, based on participants' SDM scores and BP outcome. Our study found that participants with high and lower SDM scores and systolic BP reduction of ≥10 and <10 mm Hg varied in their characteristics, their outcome predictors, and their descriptions of contextual factors influencing SDM. The barriers and facilitators identified in our study highlight the impact of social determinants of health in the relationship between SDM and BP; some of these determinants include age, race and ethnicity, education, and knowledge. The integration of qualitative and quantitative data poses a challenge to ensure both the richness of qualitative insights and the statistical robustness of quantitative findings to achieve generalizability. 24 Our mixed‐methods approach tries to ease such gap by evaluating quantitative data in light of participants' perspectives.
In the context of race and ethnicity, among those with high SDM being a Black individual compared with being a White individual increased the likelihood of having systolic BP reduction of <10 mm Hg. Comparable issues were observed in the qualitative data. Black people participating in the interviews said that cardiovascular health risks specific to their race and ethnicity, family history of hypertension, and clinician's knowledge of health disparities were motivating factors for engagement in SDM. By incorporating the social context that contributes to health outcomes, SDM has the potential to reduce disparities in cardiovascular health and improve care quality. 10 , 20 , 21 Importantly, the implementation of SDM interventions should take into consideration that having information on race‐specific risk and social context might also improve patients' engagement in SDM. Black participants interviewed by Peek and colleagues mentioned that when they felt well informed about their disease and more capable of self‐management, they had increased self‐efficacy and were more motivated to speak to the clinician about their health issues and participate in SDM. 42 It is important to highlight that in this study a self‐definition of race and ethnicity was considered.
Another important aspect related to race and ethnic disparities that needs to be considered is patients' primary or preferred language. Our participants mentioned that having an interpreter is a more relevant aspect in SDM than patient–clinician racial and ethnicity status per se. In our study, all Latino individuals participating in interviews who reported having an interpreter had high SDM score, whereas those without it had lower SDM score. Althought the goal is to diversify racial and ethnic representation in the health care workforce through national, state, and local initiatives, 43 language enabling resources are crucial for improving SDM uptake and consequently care quality and health outcomes. 44
The quantitative results showed that individuals with education level beyond high school have almost double the chances of experiencing lower SDM. Controversially, Chang and colleagues showed that education level influenced health literacy, which in turn positively affected SDM. 45 Indeed, we propose that high‐level school education might favor the process of SDM due to the linkage of skills, knowledge, and critical thinking abilities acquired through education to the requirements of effective SDM. Nonetheless, the lower SDM seems to have no impact on systolic BP reduction that was ≥10 mm Hg. In agreement with our findings, other studies have indicated that higher levels of formal education were associated with lower BP. 46 We suggest that even though education level beyond high school did not favor SDM, it could have had positive impact on BP reduction ≥10 mm Hg by contributing to a deeper understanding of the hypertension complexity and approaches to control it.
On the other hand, participants who knew that high BP is the same as hypertension had lower BP reduction regardless of the SDM score. In agreement, a previous report suggested that SDM interventions seemed to benefit people with lower literacy more than those with higher literacy. 22 In this scenario, it is important to mention that we considered improvement only as BP reduction of ≥10 mm Hg. Future studies reflecting smaller reductions could aid further understanding regarding this result. Our interview participants shared with us that knowledge and understanding of hypertension and treatment were considered as critical SDM facilitators and barriers that drive patient behavior. We propose that literacy skills alone may not be sufficient to ensure treatment compliance, better outcomes, and effective SDM, and that additional factors and competencies may play a crucial role.
In line with previous statements, the degree to which individuals were actively engaged, informed, and empowered in managing their own health and health care decisions decreases the likelihood of having lower SDM regardless of BP outcomes. Controversially, our interview participants with lower SDM mentioned having control over oneself as a facilitator of SDM. Poon and colleagues also found bidirectionality in the relationship between patient activation and patients' experiences of SDM. 47 Smith and colleagues reported that higher patient activation was associated with greater perceived benefit of SDM across various types of health care related decisions. 48
The qualitative data show emphasis on barriers and facilitators related to communication and the patient–clinician relationship. For instance, all SDM and BP groups described clinician's personality and attitudes, trust and respect for the clinician, clinician shows interest in the patient, 2‐way communication, and discussion of options, pros, and cons as facilitators of SDM. In addition, all our interview participants considered lack of communication, lack of regimen compliance, and time constrains as barriers to SDM. Participants with lower SDM mentioned perceived lack of compassion, hierarchy, and feeling intimidated as barriers. Some of the barriers identified in our study are aligned with those discussed in other studies, such as knowledge, race, education, lack of information, lack of interpersonal skills, 42 and time constraints. 49 The American Heart Association advocates clinicians' education on communication techniques as an effective strategy to promote SDM in cardiovascular health decisions. 10 Zisman‐Ilani and colleagues reported that patient understanding favored by storytelling in communication, to contextualize medical information, is a facilitator of SDM. 50
The implementation of SDM interventions should take into consideration aspects that facilitate patients' engagement in SDM. Evidence from other studies and statement from the American Heart Association support validation of patient experiences, trust in the clinician, and clinician's interpersonal skills as important facilitators of SDM. 10 , 21 , 42 However, patient–clinician relationship factors are not incorporated in most SDM instruments. 44 In this study, a previous adaptation of the Ottawa Decision Support Framework was chosen. It emphasizes that decisions depend not only on the patients' characteristics but also on other aspects that influence how the decision is perceived as well as personal resources available to make and implement the decision. 27 , 28 Therefore, in order to improve patients' activation and engagement in SDM, we ponder that clinicians should be trained in carrying out a 2‐way communication, promote trust, respect and comfort, express empathy, and interest, and to avoid hierarchy and patients' feeling of intimidation. 50
Our findings showed variation in SDM among patients from different racial and ethnic groups. To guide the implementation of high‐quality and achievable SDM, policy should reflect the differences and recommend patient decision aids and other interventions to facilitate SDM that have been tested and proven effective for various racial and ethnic backgrounds. 10 Policy makers and health system leaders should also incentivize the availability of clinicians in areas of limited care access and ensure that patients and clinicians have access to interpreter services and time to engage in SDM. In addition, further studies are needed to evaluate barriers and facilitators, including not only those that pertain to the clinician–patient dyad but also at the clinic, system, and policy levels. 5 , 10 Other variables important in the context of SDM, such as confidence in the decision and decision quality should also be taken into consideration.
The current study has limitations. First, the perspectives of clinicians and participants' family or caregivers were not sought, and we did not investigate participants' perspective regarding decision aids. Second, although surveys and interviews were carried out both in English and Spanish to ensure proper representation of a diverse sample, the interview guide might have not captured aspects relevant to our study.
Conclusions
In conclusion, the results of this explanatory sequential mixed‐methods study are novel and corroborate expanding evidence in the field. Triangulation of quantitative and interview findings uncovered previously obscured factors and allowed some knowledge related to categories of facilitators and barriers experienced by patients from diverse backgrounds. Creating and translating new policies into clinical practice based on well‐defined and established standards are crucial. In that sense, our results provide actionable information that can be used to enhance policy development, establishment of effective clinician and patient training, and creation of decision support interventions such as decision aids.
Appendix
RICH LIFE Project Investigators
Jill A. Marsteller, PhD, MPP; Rexford Ahima, MD, PhD; Carmen Alvarez, PhD, RN, CRNP; Denis G. Antoine 2nd, MD; Gideon Avornu, MS; Jagriti Bhattarai, PhD; Lee Bone, MPH; Romsai T. Boonyasai, MD, MPH; Kathryn A. Carson, ScM; Jeanne Charleston, PhD; Suna Chung, MPH; Marcia Cort, MD, MBA; Deidra C. Crews, MD, ScM; Gail L. Daumit, MD, MHS; Katherine B. Dietz, MPH; Teresa Eyer, LCSW‐C; Demetrius Frazier; Raquel Greer, MD, MHS; Debra Hickman, MDiv; Felicia Hill‐Briggs, PhD; Cheryl R. Dennison Himmelfarb, PhD, RN; Anika Hines, PhD; Tammie Hull, RN; Chidinma A. Ibe, PhD; Lawrence Johnson, MD; Susan Johnson, RN, MPH; Mary Kargbo, MS; Mary Kelleher, MD, MPH; Mariana Lazo, MD, PhD, ScM; Lisa Lubomski, PhD; Lena M. Mathews, MD; Edgar R. Miller 3rd, MD, PhD; Dr. Chiadi E. Ndumele, MD, PhD, MHS; Ruth‐Alma Turkson‐Ocran, PhD, MPH, MSN, RN; Randy Parker; Cassandra Peterson, MBA, LCSW‐C; Tanjala S. Purnell, PhD, MPH; Natalie Spicyn, MD; DeNotta Teagle, PA‐C; Nae‐Yuh Wang, PhD; Marcee White, MD; Hsin‐Chieh Yeh, PhD; Joan Young, and Kimberly L. Zeren, CRNP.
Sources of Funding
The RICH LIFE project was supported by a grant from the National Heart, Lung, and Blood Institute through a partnership with the Patient‐Centered Outcomes Research Institute (UH2/UH3 HL130688). Sabrina Elias was supported by a grant from the National Institute of Nursing Research (F31 NR019523‐01A1).
Disclosures
None.
Supporting information
Data S1
Table S1
Acknowledgments
The investigators would like to acknowledge the following people and organizations for their contributions to patient recruitment, data collection, delivery of the study interventions, and operations of the study: Millie Aquino, Chara Bauer, Deven Brown, Tiffany Campbell, Lisa Carpenter, Maria Collazo, Stacye Cooper, Andria Coryatt, Cynthia Crandall, Keah Crosby, McKenzie Eakin, Dorethia Easley, Lia Escobar‐Acosta, Sabrina Elias, Martha Franz, Kenya Ferguson, Minyun Fogg, Jennifer Halbert, Dairy Jennings, Jon Kawatachi, Mary Krob, Jolene Lambertis, Joseph Landavaso, Gee Eun Lee, Stella Marine, Erika McCannon, Krystle McConnell, Margaret Mejia, Theresa Messer, Nancy Molello, Kandice Oakley, Modupe Oduwole, Chibuzo Opara, Princess Osazuwa, Aidan Ottoni‐Wilhelm, Helen Owhonda, Sherry Perkins, Jodi Peters, Lauren Phillips, Camila Montejo‐Poll, Christine Richardson, Don Ritchie, Denise Saint‐Jean, Abhay Singh, Sheryl Sprague, Anita Stokes, Tricia Taylor, Cleonda Thompson, Jodi Watkowski, Gloria Leonard‐ Witten, Randi Woods, Zehui Zhou, Sisters Together and Reaching, Inc., Johns Hopkins HealthCare, LLC, and i2i Population Health. The authors would also like to thank the members of the Johns Hopkins Center for Health Equity Community Advisory Board for guidance throughout the project and all of the staff, providers, organizational leaders, and patients at participating clinical sites at Berks Community Health Centers, Choptank Community Health System, Johns Hopkins Community Physicians, Park West Health System, and Total Health Care, Inc., for making the completion of this study possible. Lastly, the authors would like to thank the appointed DSMB and Hypertension Disparities Reduction Program Partnership membership for the oversight of this study. Research concept and design: Sabrina Elias, Cheryl R. Himmelfarb; Acquisition of data: Sabrina Elias, Lisa A. Cooper, Jill Marsteller; Data analysis and interpretation: Sabrina Elias, Jennifer Wenzel, Nancy Perrin, Cheryl R. Himmelfarb, Yvonne Commodore‐Mensah; Manuscript draft: Sabrina Elias, Cheryl R. Himmelfarb, Jennifer Wenzel; Qualitative analysis: Sabrina Elias, Jennifer Wenzel, Sarah Slone, Samuel Byiringiro; Statistical expertise: Nancy Perrin, Sabrina Elias; Acquisition of funding: Lisa A. Cooper, Sabrina Elias, Jill Marsteller; Supervision: Cheryl R. Himmelfarb.
This article was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032568
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Sabrina Elias, Email: sdesouz5@jhu.edu.
the RICH LIFE Project Investigators:
Jill A. Marsteller, Rexford Ahima, Carmen Alvarez, Denis G. Antoine, Gideon Avornu, Jagriti Bhattarai, Lee Bone, Romsai T. Boonyasai, Kathryn A. Carson, Jeanne Charleston, Suna Chung, Marcia Cort, Deidra C. Crews, Gail L. Daumit, Katherine B. Dietz, Teresa Eyer, Demetrius Frazier, Raquel Greer, Debra Hickman, Felicia Hill‐Briggs, Cheryl R. Dennison Himmelfarb, Anika Hines, Tammie Hull, Chidinma A. Ibe, Lawrence Johnson, Susan Johnson, Mary Kargbo, Mary Kelleher, Mariana Lazo, Lisa Lubomski, Lena M. Mathews, Edgar R. Miller, Chiadi E. Ndumele, Ruth‐Alma Turkson‐Ocran, Randy Parker, Cassandra Peterson, Tanjala S. Purnell, Natalie Spicyn, DeNotta Teagle, Nae‐Yuh Wang, Marcee White, Hsin‐Chieh Yeh, Joan Young, and Kimberly L. Zeren
References
- 1. Bomhof‐Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-031763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patient Protection and Affordable Care Act (PPACA) ‐ H.R.3590. In: 111th Congress (2009–2010), United States; 2010:119–1024.
- 3. Selby JV, Beal AC, Frank L. The Patient‐Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–1584. doi: 10.1001/jama.2012.500 [DOI] [PubMed] [Google Scholar]
- 4. Alishahi Tabriz A, Neslund‐Dudas C, Turner K, Rivera MP, Reuland DS, Elston LJ. How health‐care organizations implement shared decision‐making when it is required for reimbursement: the case of lung cancer screening. Chest. 2021;159:413–425. doi: 10.1016/j.chest.2020.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spatz ES, Krumholz HM, Moulton BW. Prime time for shared decision making. JAMA. 2017;317:1309–1310. doi: 10.1001/jama.2017.0616 [DOI] [PubMed] [Google Scholar]
- 6. American Medical Association . AMA code of medical ethics opinions on consent, communication & decision making. Code of Medical Ethics of the American Medical Association. Chicago, IL: American Medical Association; 2017. [Google Scholar]
- 7. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677. Epub 2019 Mar 17. Erratum in: Circulation. 2019 Sep 10;140(11):e647‐e648. Erratum in: Circulation. 2020 Jan 28;141(4):e59. Erratum in: Circulation. 2020 Apr 21;141(16):e773. PMID: 30879339; PMCID: PMC8351755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casey DE, Thomas RJ, Bhalla V, Commodore‐Mensah Y, Heidenreich PA, Kolte D, Muntner P, Smith SC, Spertus JA, Windle JR, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure. J Am Coll Cardiol. 2019;74:2661–2706. doi: 10.1016/j.jacc.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alston C, Berger Z, Brownlee S, Elwyn G Jr, Fowler FJ Jr, Hall LK, Montori VM, Moulton B, Paget L, Haviland‐Shebel B, et al. Shared decision‐making strategies for best care: patient decision aids. NAM Perspectives. Discussion Paper. Washington, DC: National Academy of Medicine; 2014. [Google Scholar]
- 10. Dennison Himmelfarb CR, Beckie TM, Allen LA, Commodore‐Mensah Y, Davidson PM, Lin G, Lutz B, Spatz ES. Shared decision‐making and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2023;148:912–931. doi: 10.1161/CIR.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 11. Schmidt T, Valuck T, Riposo J, Patel P, Perkins B, Westrich K, Dubois RW. Impact of shared decision‐making and patient decision aids on health care cost and utilization in the US: a systematic review. J Clin Pathways. 2022;8:33–43. [Google Scholar]
- 12. Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br J Gen Pract. 2003;53:446–453. [PMC free article] [PubMed] [Google Scholar]
- 13. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) ; Division for Heart Disease and Stroke Prevention . High Blood Pressure. Centers for Disease Control and Prevention (CDC); 2020. Accessed February 10, 2022. https://www.cdc.gov/bloodpressure/index.htm [Google Scholar]
- 14. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. doi: 10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888. doi: 10.1161/JAHA.118.008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritchey M, Chang A, Powers C, Loustalot F, Schieb L, Ketcham M, Durthaler J, Hong Y. Vital signs: disparities in antihypertensive medication nonadherence among medicare part D beneficiaries—United States, 2014. Morb Mortal Wkly Rep. 2016;65:967–976. doi: 10.15585/mmwr.mm6536e1 [DOI] [PubMed] [Google Scholar]
- 17. Elias S, Turkson‐Ocran RA, Koirala B, Byiringiro S, Baptiste D, Himmelfarb CR, Commodore‐Mensah Y. Heterogeneity in cardiovascular disease risk factors among Latino immigrant subgroups: evidence from the 2010 to 2018 National Health Interview Survey. J Am Heart Assoc. 2023;12:e027433. doi: 10.1161/JAHA.122.027433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoon SSS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics; 2015:1. [PubMed] [Google Scholar]
- 19. Cooper‐DeHoff RM, Aranda JM, Gaxiola E, Cangiano JL, Garcia‐Barreto D, Conti CR, Hewkin A, Pepine CJ. Blood pressure control and cardiovascular outcomes in high‐risk Hispanic patients—findings from the International Verapamil SR/Trandolapril Study (INVEST). Am Heart J. 2006;151:1072–1079. doi: 10.1016/j.ahj.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 20. Turkson‐Ocran RN, Ogunwole SM, Hines AL, Peterson PN. Shared decision making in cardiovascular patient care to address cardiovascular disease disparities. J Am Heart Assoc. 2021;10:e018183. doi: 10.1161/JAHA.120.018183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enard KR, Hauptman PJ. Heart failure, shared decision‐making, and social determinants of health: an upstream perspective. JAMA Cardiol. 2019;4(7):609–610. doi: 10.1001/jamacardio.2019.1763 [DOI] [PubMed] [Google Scholar]
- 22. Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, Elwyn G. Do interventions designed to support shared decision‐making reduce health inequalities? A systematic review and meta‐analysis. PLoS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perez Jolles M, Richmond J, Thomas KC. Minority patient preferences, barriers, and facilitators for shared decision‐making with health care providers in the USA: a systematic review. Patient Educ Couns. 2019;102:1251–1262. doi: 10.1016/j.pec.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 24. Creswell JW, Plano Clark VL. Designing and Conducting Mixed Methods Research. 3rd ed. Los Angeles: Sage; 2018. [Google Scholar]
- 25. Creswell JW, Klassen AC, Plano Clark VL, Smith KC; Office of Behavioral and Social Sciences Research (OBSSR) . Best Practices for Mixed Methods Research in the Health Sciences. Bethesda, MD: National Institutes of Health (NIH); 2011. [Google Scholar]
- 26. Cooper LA, Marsteller JA, Carson KA, Dietz KB, Boonyasai RT, Alvarez C, Ibe CA, Crews DC, Yeh HC, Miller ER 3rd, et al. The RICH LIFE project: a cluster randomized pragmatic trial comparing the effectiveness of health system only vs. health system plus a collaborative/stepped care intervention to reduce hypertension disparities. Am Heart J. 2020;226:94–113. doi: 10.1016/j.ahj.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stacey D, Legare F, Boland L, Lewis KB, Loiselle MC, Hoefel L, Garvelink M, O'Connor A. 20th anniversary Ottawa decision support framework: part 3 overview of systematic reviews and updated framework. Med Decis Mak. 2020;40:379–398. doi: 10.1177/0272989X20911870 [DOI] [PubMed] [Google Scholar]
- 28. O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, McPherson R, Bunn H, Graham I, Drake E. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33:267–279. doi: 10.1016/s0738-3991(98)00026-3 [DOI] [PubMed] [Google Scholar]
- 29. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The national institute on minority health and health disparities research framework. Am J Public Health. 2019;109:S16–S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hawley ST, Morris AM. Cultural challenges to engaging patients in shared decision making. Patient Educ Couns. 2017;100:18–24. doi: 10.1016/j.pec.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient‐reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102–107. doi: 10.1016/j.pec.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 32. Hurley EA, Bradley‐Ewing A, Bickford C, Lee BR, Myers AL, Newland JG, Goggin K. Measuring shared decision‐making in the pediatric outpatient setting: psychometric performance of the SDM‐Q‐9 and CollaboRATE among English and Spanish speaking parents in the US Midwest. Patient Educ Couns. 2019;102:742–748. doi: 10.1016/j.pec.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 33. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 34. Oliveria SA, Chen RS, McCarthy BD, Davis CC, Hill MN. Hypertension knowledge, awareness, and attitudes in a hypertensive population. J Gen Intern Med. 2005;20:219–225. doi: 10.1111/j.1525-1497.2005.30353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23:561–566. doi: 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Medicine . Health Literacy: A Prescription to End Confusion. Washington DC: National Academic Press; 2004. [Google Scholar]
- 38. Guest G, Bunce A, Johnson L. How many interviews are enough?: an experiment with data saturation and variability. Field Methods. 2006;18:59–82. doi: 10.1177/1525822x05279903 [DOI] [Google Scholar]
- 39. Johnson RA, Huntley A, Hughes RA, Cramer H, Turner KM, Perkins B, Feder G. Interventions to support shared decision making for hypertension: a systematic review of controlled studies. Health Expect. 2018;21:1191–1207. doi: 10.1111/hex.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. Thousand Oaks: Sage; 2019. [Google Scholar]
- 41. Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs‐principles and practices. Health Serv Res. 2013;48:2134–2156. doi: 10.1111/1475-6773.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peek ME, Wilson SC, Gorawara‐Bhat R, Odoms‐Young A, Quinn MT, Chin MH. Barriers and facilitators to shared decision‐making among African‐Americans with diabetes. J Gen Intern Med. 2009;24:1135–1139. doi: 10.1007/s11606-009-1047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stanford FC. The importance of diversity and inclusion in the healthcare workforce. J Natl Med Assoc. 2020;112:247–249. doi: 10.1016/j.jnma.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flores G. The impact of medical interpreter services on the quality of health care: a systematic review. Med Care Res Rev. 2005;62:255–299. doi: 10.1177/1077558705275416 [DOI] [PubMed] [Google Scholar]
- 45. Chang HL, Li FS, Lin CF. Factors influencing implementation of shared medical decision making in patients with cancer. Patient Prefer Adherence. 2019;13:1995–2005. doi: 10.2147/PPA.S217561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu SY, Buka SL, Linkletter CD, Kawachi I, Kubzansky L, Loucks EB. The association between blood pressure and years of schooling versus educational credentials: test of the sheepskin effect. Ann Epidemiol. 2011;21:128–138. doi: 10.1016/j.annepidem.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poon BY, Shortell SM, Rodriguez HP. Patient activation as a pathway to shared decision‐making for adults with diabetes or cardiovascular disease. J Gen Intern Med. 2020;35:732–742. doi: 10.1007/s11606-019-05351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith SG, Pandit A, Rush SR, Wolf MS, Simon CJ. The role of patient activation in preferences for shared decision making: results from a National Survey of U.S. adults. J Health Commun. 2016;21:67–75. doi: 10.1080/10810730.2015.1033115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs (Project Hope). 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078 [DOI] [PubMed] [Google Scholar]
- 50. Zisman‐Ilani Y, Khaikin S, Savoy ML, Paranjape A, Rubin DJ, Jacob R, Wieringa TH, Suarez J, Liu J, Gardiner H, et al. Disparities in shared decision‐making research and practice: the case for black American patients. Ann Fam Med. 2023;21:112–118. doi: 10.1370/afm.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


