Abstract
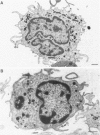
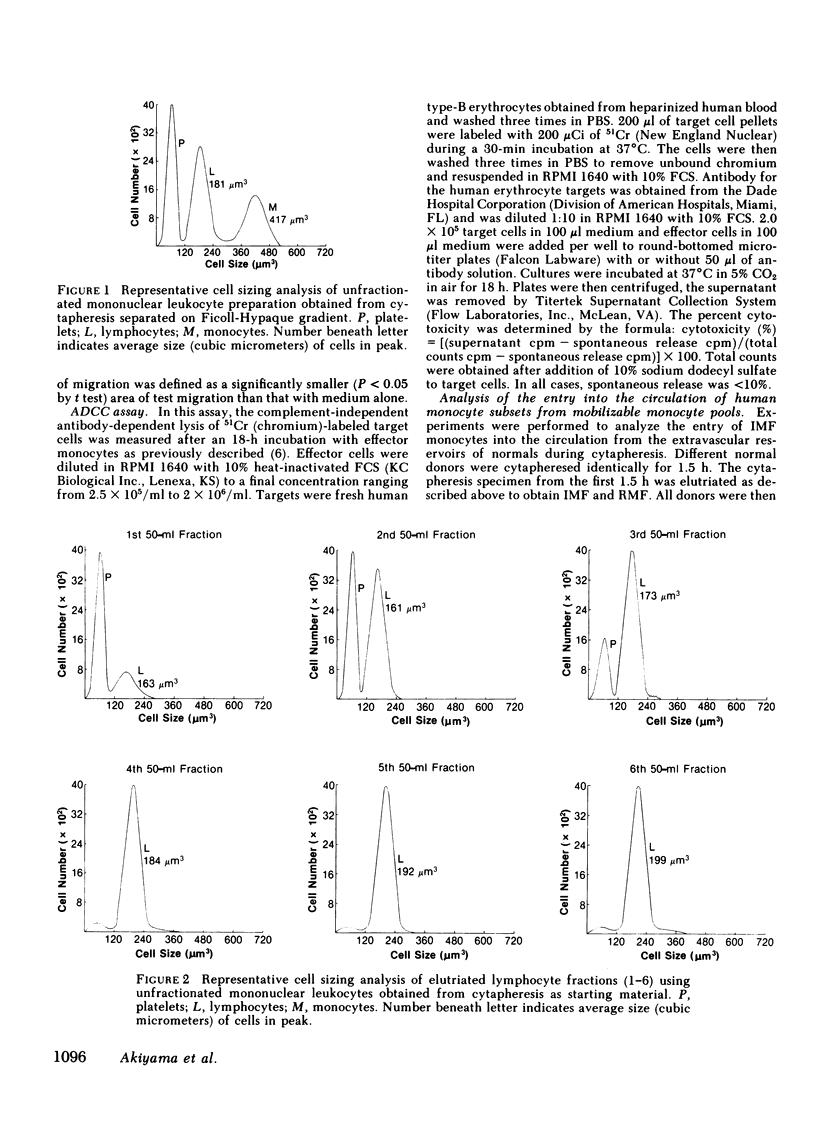
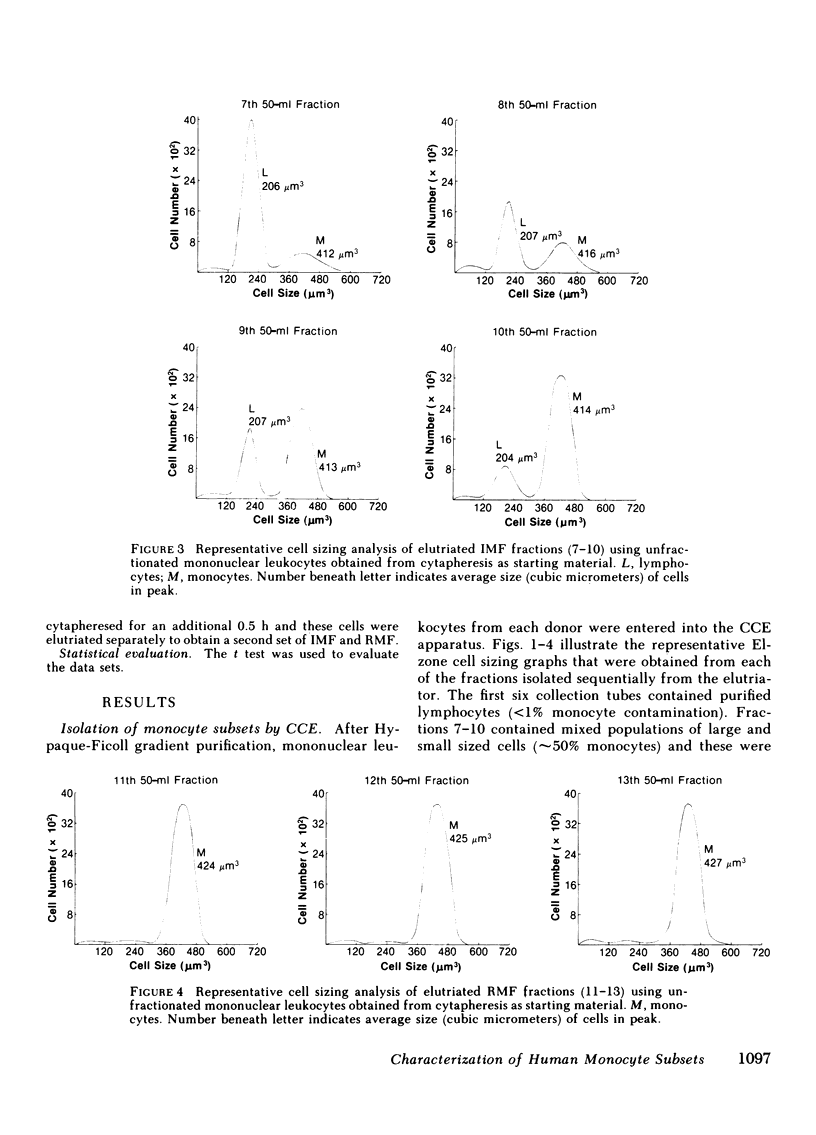
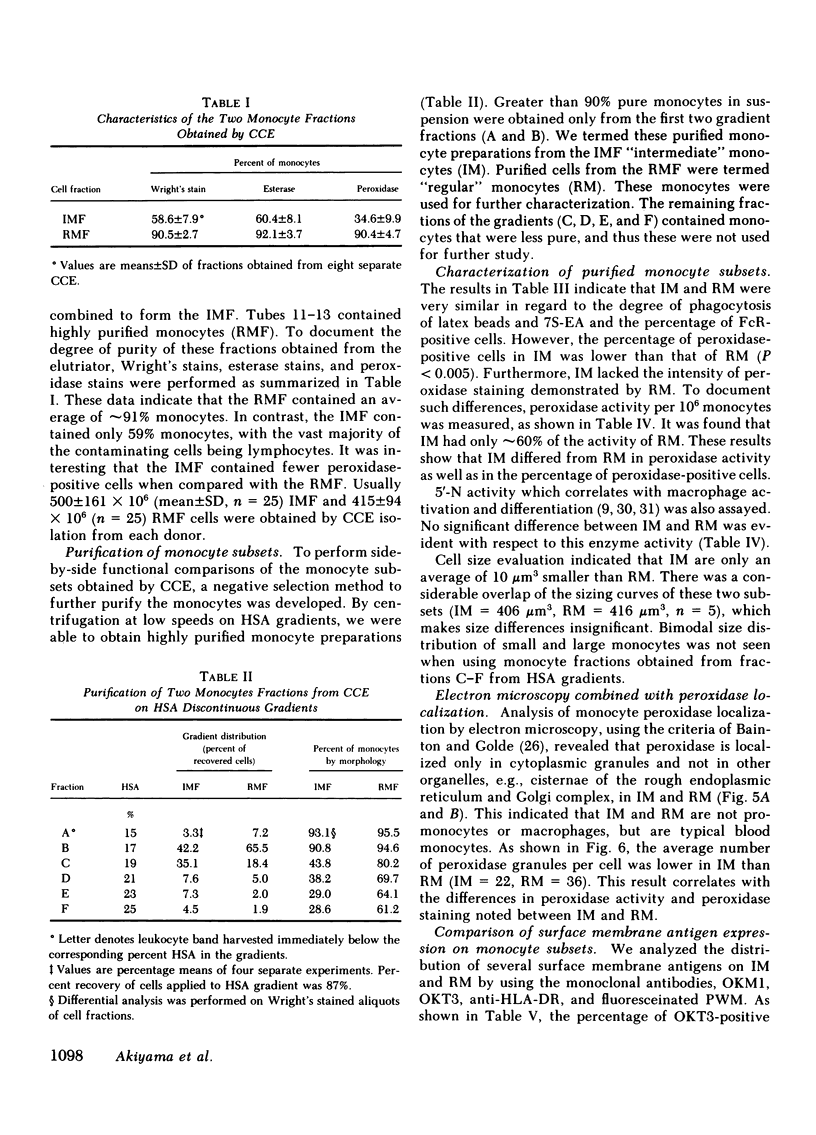
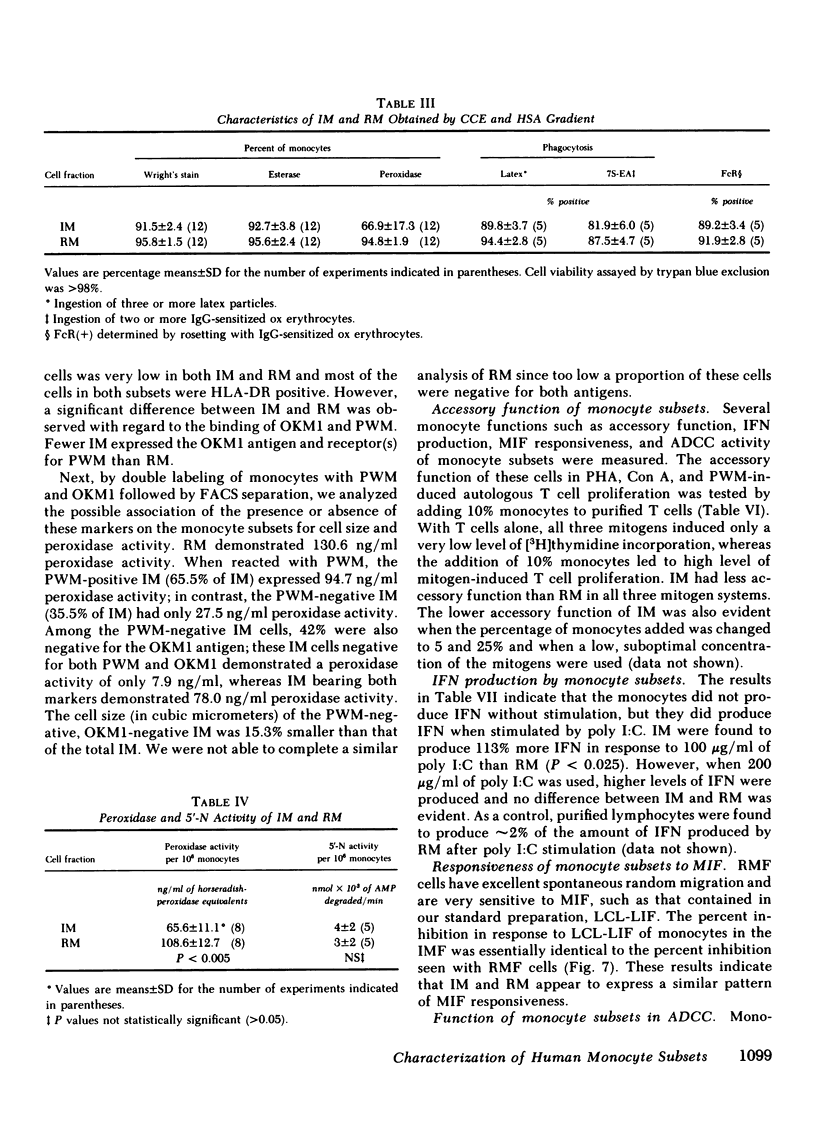
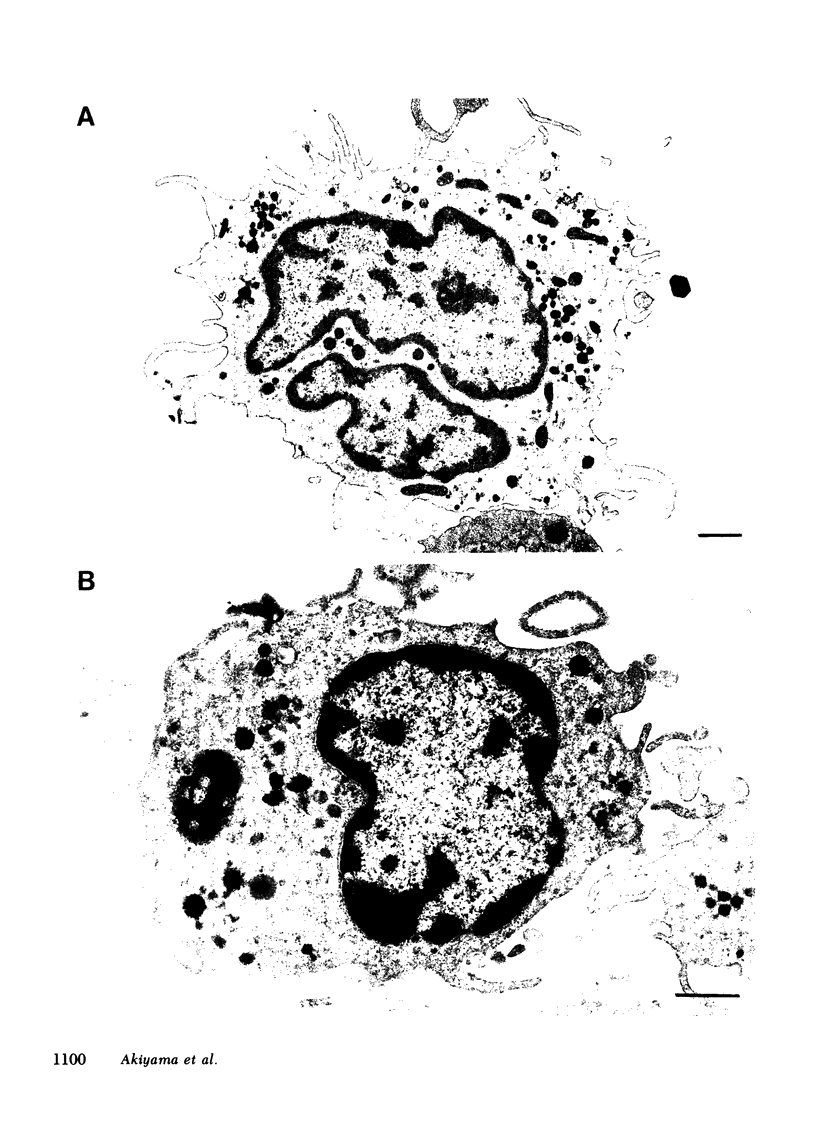
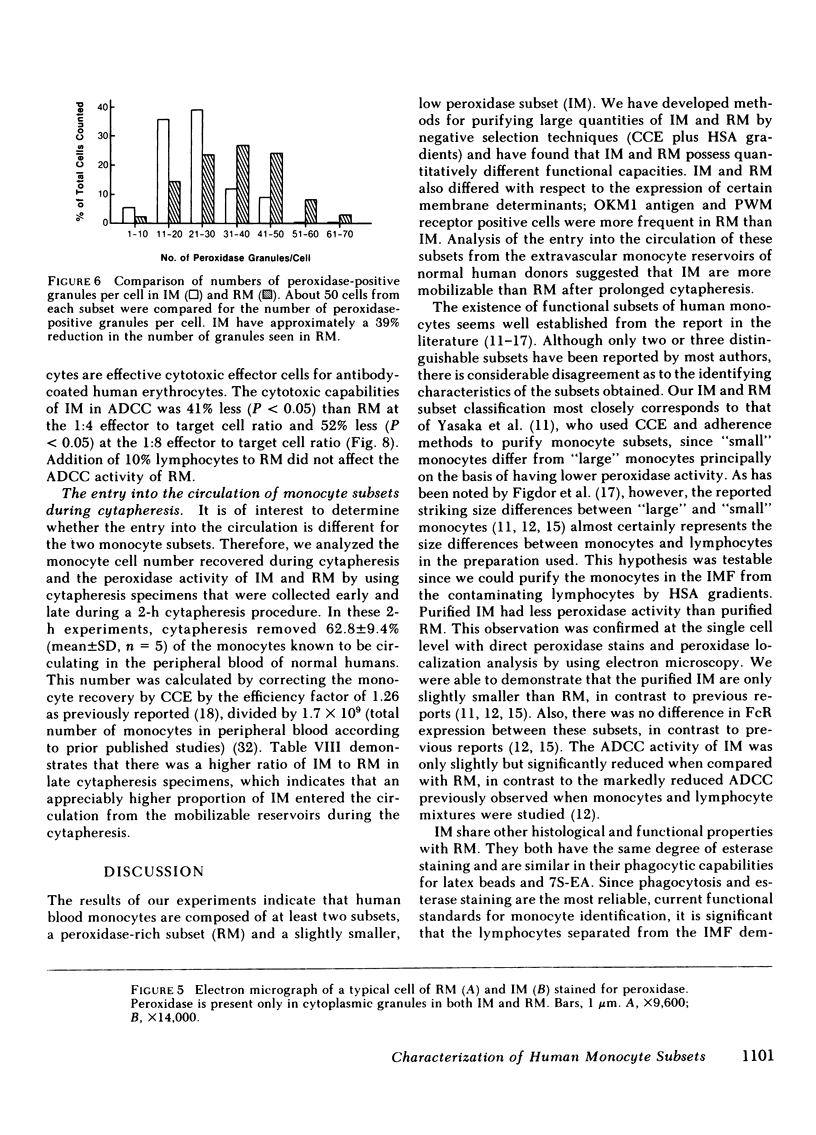
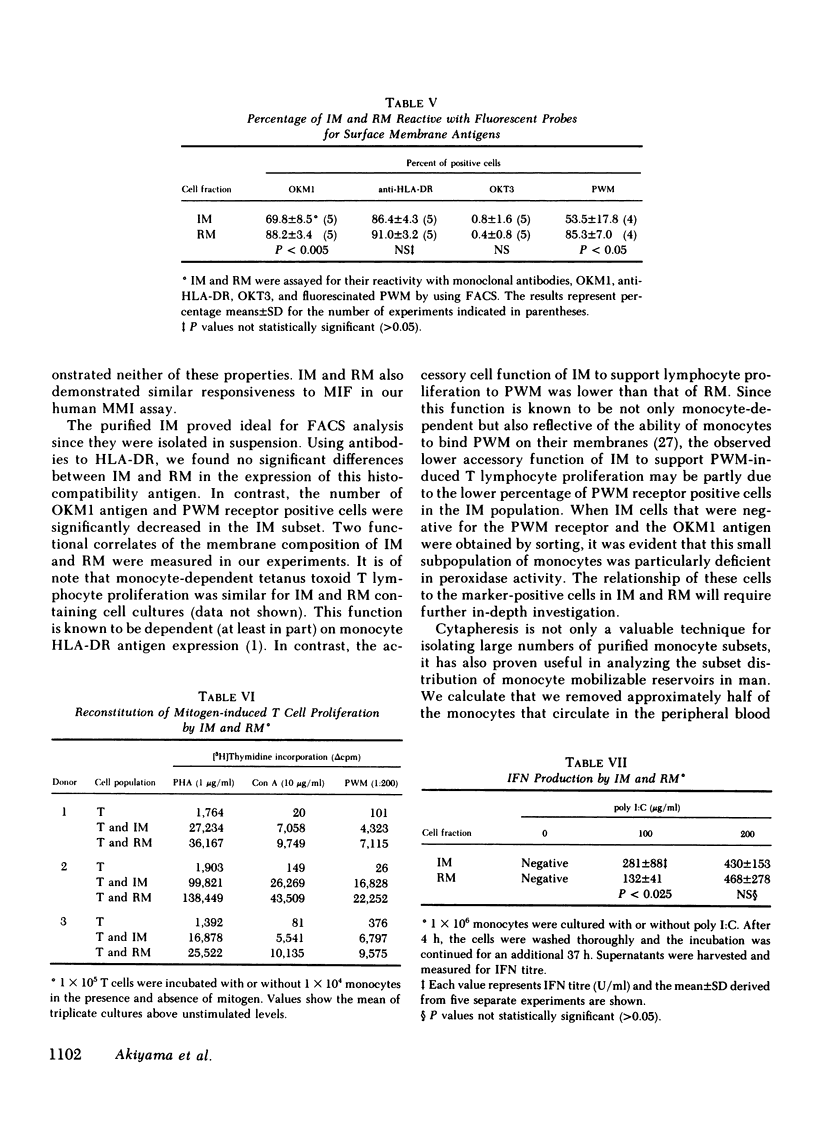
Two human monocyte subsets from the peripheral blood of healthy donors have been isolated in greater than 90% purity by countercurrent centrifugal elutration and human serum albumin gradients and their functional capabilities have been assessed. We have demonstrated that one subset ("regular" monocytes, RM) showed intense cytoplasmic peroxidase staining and contained substantial peroxidase activity. In contrast, another subset ("intermediate" monocytes, IM) stained poorly for peroxidase and had low peroxidase activity. By electron microscopic analysis combined with peroxidase localization, it was found that IM had fewer peroxidase-positive granules per cell than did RM. IM coelutriated with some lymphocytes and by cell sizing analysis were shown to be slightly smaller than RM. Functional and cytochemical analysis of these subsets indicated that IM had less activity than RM in assays such as accessory cell function for mitogen-induced T lymphocyte proliferation and antibody-dependent cellular cytotoxicity, and that fewer IM expressed OKM1 antigen and pokeweed mitogen (PWM) receptors on their membranes than did RM. The subset of IM not bearing either the PWM receptor or the OKM1 antigen had very low peroxidase activity. IM also were found to have a greater sensitivity to polyriboinosinic and polyribocytidilic acid (100 micrograms/ml)-induced secretion of interferon. There was no significant difference in the phagocytic capability, the percentage of Fc receptor-positive cells, 5'-nucleotidase activity, DR antigen expression, or the responsiveness to migration inhibitory factor of IM as compared with RM. Furthermore, it was found that the ratio of IM to RM increased after prolonged cytapheresis, which suggests that IM are more mobilizable than RM from the extravascular reservoirs of human monocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenson E. B., Jr, Epstein M. B., Seeger R. C. Volumetric and functional heterogeneity of human monocytes. J Clin Invest. 1980 Mar;65(3):613–618. doi: 10.1172/JCI109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. R., Golde D. W. Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J Clin Invest. 1978 Jun;61(6):1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Contreras T. J., Jemionek J. F., Stevenson H. C., Hartwig V. M., Fauci A. S. An improved technique for the negative selection of large numbers of human lymphocytes and monocytes by counterflow centrifugation--elutriation. Cell Immunol. 1980 Aug 15;54(1):215–229. doi: 10.1016/0008-8749(80)90203-8. [DOI] [PubMed] [Google Scholar]
- Figdor C. G., Bont W. S., Touw I., de Roos J., Roosnek E. E., de Vries J. E. Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood. 1982 Jul;60(1):46–53. [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Kedar E., Ortiz de Landazuri M., Fahey J. L. Comparative studies of immunoglobulin receptors and antibody-dependent cell cytotoxicity (ADCC) in rat lymphoid organs. J Immunol. 1974 Jan;112(1):37–46. [PubMed] [Google Scholar]
- Kávai M., Bodolay E., Szöllösi J. Characterization of human monocyte subpopulations by flow cytometry. Immunology. 1982 Oct;47(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- McPherson T. A., Tan Y. H. Phase I pharmacotoxicology study of human fibroblast interferon in human cancers. J Natl Cancer Inst. 1980 Jul;65(1):75–79. [PubMed] [Google Scholar]
- Meuret G., Batara E., Fürste H. O. Monocytopoiesis in normal man: pool size, proliferation activity and DNA synthesis time of promonocytes. Acta Haematol. 1975;54(5):261–270. doi: 10.1159/000208084. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Neubauer R. H., Briggs C. J., Noer K. B., Rabin H. Identification of normal and transformed lymphocyte subsets of nonhuman primates with monoclonal antibodies to human lymphocytes. J Immunol. 1983 Mar;130(3):1323–1329. [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Raff H. V., Goldyne M. E., Stobo J. D. Metabolic heterogeneity among human monocytes and its modulation by PGE2. J Immunol. 1980 Jun;124(6):2557–2562. [PubMed] [Google Scholar]
- Poplack D. G., Bonnard G. D., Holiman B. J., Blaese R. M. Monocyte-mediated antibody-dependent cellular cytotoxicity: a clinical test of monocyte function. Blood. 1976 Dec;48(6):809–816. [PubMed] [Google Scholar]
- Priestman T. J. Initial evaluation of human lymphoblastoid interferon in patients with advanced malignant disease. Lancet. 1980 Jul 19;2(8186):113–118. doi: 10.1016/s0140-6736(80)90004-5. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Picker L. J., Stobo J. D. Macrophage heterogeneity in man. A subpopulation of HLA-DR-bearing macrophages required for antigen-induced T cell activation also contains stimulators for autologous-reactive T cells. J Exp Med. 1980 Sep 1;152(3):581–593. doi: 10.1084/jem.152.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaman G. H., Levin N., Muchmore A., Holiman B. J., Poplack D. G. Diminished lymphoblast 5'-nucleotidase activity in acute lymphoblastic leukemia with T-cell characteristics. N Engl J Med. 1979 Jun 14;300(24):1374–1377. doi: 10.1056/NEJM197906143002406. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S. Regulation of the immune response--role of the macrophage. N Engl J Med. 1980 Nov 13;303(20):1153–1156. doi: 10.1056/NEJM198011133032005. [DOI] [PubMed] [Google Scholar]
- Shenoy T. S., Clifford A. J. Adenine nucleotide metabolism in relation to purine enzymes in liver, erythrocytes and cultured fibroblasts. Biochim Biophys Acta. 1975 Nov 10;411(1):133–143. doi: 10.1016/0304-4165(75)90292-5. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Knost J. A., Fein S., Abrams P. G., Foon K. A., Ochs J. J., Schoenberger C., Maluish A. E., Oldham R. K. A multiple-dose phase I trial of recombinant leukocyte A interferon in cancer patients. JAMA. 1982 Nov 19;248(19):2461–2466. [PubMed] [Google Scholar]
- Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981 Sep;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Whisler R. L., Newhouse Y. G., Lachman L. B. Heterogeneity of human monocyte subsets in the promotion of B cell colonies and the role of interleukin 1. J Immunol. 1982 Aug;129(2):455–460. [PubMed] [Google Scholar]
- Yasaka T., Mantich N. M., Boxer L. A., Baehner R. L. Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J Immunol. 1981 Oct;127(4):1515–1518. [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]