Abstract
Background
Bleeding risk brought by intensive lipid‐lowering therapy and low low‐density lipoprotein cholesterol is concerning, while evidence regarding the relationship between remnant cholesterol and bleeding is frightening. This study aimed to investigate the association between remnant cholesterol at admission and an in‐hospital bleeding event after acute ischemic stroke or transient ischemic attack (TIA).
Methods and Results
A total of 3222 eligible patients admitted to Shanghai Huashan Hospital between 2015 and 2021 with complete lipid data were analyzed. Patients were classified into low (<20.0 mg/dL), moderate (20.0–29.9 mg/dL), and high (≥30 mg/dL) groups by remnant cholesterol. The mean age of patients was 63.0± 13.1 years, including 2301 (71.4%) men and 651 (20.2%) with TIA. The median (interquartile range) of remnant cholesterol was 18.6 (13.5–25.9) mg/dL. After adjustment for confounding variables, patients with low remnant cholesterol had a higher risk of bleeding events (odds ratio, 2.56 [95% CI, 1.12–6.67]) than those with moderate remnant cholesterol. The high remnant cholesterol group was not significantly associated with bleeding risk. Combined assessment of low‐density lipoprotein cholesterol and remnant cholesterol further identified patients with the highest risk of bleeding events.
Conclusions
Low remnant cholesterol levels were associated with bleeding events during the acute stage of ischemic stroke and TIA. The assessment of remnant cholesterol could inform the bleeding risk during hospitalization both for patients and physicians in clinical practice.
Keywords: bleeding, ischemic stroke, remnant cholesterol, transient ischemic attack (TIA)
Subject Categories: Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
- TST
Treat Stroke to Target
Clinical Perspective.
What Is New?
Low remnant cholesterol is associated with an increased risk of bleeding events during the acute stage of ischemic stroke or transient ischemic attack.
Combined evaluation of remnant cholesterol and low‐density lipoprotein cholesterol further stratifies the bleeding risk in acute ischemic stroke.
What Are the Clinical Implications?
Assessment of remnant cholesterol level at admission should be emphasized among patients of ischemic stroke to inform the risk of an in‐hospital bleeding event in advance.
Stroke is the leading cause of death and disability in China, and China bears the largest stroke burden in the world. 1 Ischemic stroke and transient ischemic attack (TIA) are the most common cerebrovascular disorders. 2 Despite improvement and adherence in guideline‐based secondary prevention of risk factors, there are still residual risks of adverse outcomes and death from ischemic stroke. 3 , 4 Patients with stroke are also at a high risk of bleeding. 5 Thus, the identification of novel prognostic factors is of great importance to avoid adverse events for stroke. 6 , 7
Atherosclerosis is the major pathological basis of ischemic stroke, which is closely related to dyslipidemia. 8 , 9 Atherogenic dyslipidemia is a promising modifiable target for secondary stroke prevention, with low‐density lipoprotein (LDL) cholesterol as the primary target in clinical practice. 10 , 11 , 12 , 13 , 14 , 15 In recent decades, the potent atherogenic effect of remnant cholesterol on cardiovascular diseases has been gradually recognized. 16 , 17 Remnant cholesterol encompasses the cholesterol content of triglyceride‐rich lipoproteins, that is, intermediate density lipoprotein, very‐low‐density lipoprotein and chylomicron remnants. 18 Previous epidemiological studies have confirmed the association of remnant cholesterol with incident ischemic stroke in the general population. 19 , 20 , 21 The fact is that the pathophysiological features of patients in the acute stage are different from those in the chronic stage. However, few studies have described the pattern and magnitude of the association between remnant cholesterol and the in‐hospital outcomes of ischemic stroke in the acute phase given the closely linked cholesterol metabolism pathway.
Thus, this study aimed to quantify the association of remnant cholesterol at admission with in‐hospital bleeding and death in patients with ischemic stroke or TIA. We also evaluated whether combined assessment of remnant cholesterol could further stratify the risk of bleeding or death beyond LDL cholesterol.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Source and Study Design
This study was a hospital‐based cross‐sectional study that enrolled inpatients with ischemic stroke in Huashan Hospital (Shanghai, China) from January 2015 to December 2021. Patients in this study met the following eligibility criteria: (1) age ≥18 years; (2) diagnosis of ischemic stroke or TIA at admission; (3) direct hospital admission from emergency departments or physician clinics; and (4) complete data of standard lipid measurements. Among the 3495 inpatients admitted due to ischemic stroke, a total of 273 patients lacking lipid data at admission were excluded.
Each participant provided written informed consent before participating in this study. The protocol and data collection of the study were performed in accordance with the guidelines from the Helsinki Declaration and were approved by the Ethics Committee of Edith Cowan University (No 2021‐03164‐WU).
Data Collection and Definition
Data at admission, including demographics, smoking history, medical history (prior stroke, myocardial infarction, atrial fibrillation, hypertension, diabetes, lipid‐lowering medication) and National Institutes of Health Stroke Scale score at admission, were collected by trained physicians via face‐to‐face interviews. Body mass index (BMI) was calculated as weight (in kilograms)/height (in meters squared). Obesity was defined as BMI ≥28.0 kg/m2 for the Chinese population.
Blood samples were obtained within the first 24 hours of admission for the purpose of routine laboratory tests, including serum creatinine, triglycerides, total cholesterol, LDL cholesterol, and high‐density lipoprotein (HDL) cholesterol. Remnant cholesterol was calculated as subtracting the sum of directly measured LDL cholesterol and HDL cholesterol from total cholesterol. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (2009) serum creatinine equation. 22
All patients were treated using standardized procedures according to clinical guidelines. 23 Information during hospitalization was extracted from medical records, including stroke subtypes by TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria and lipid‐lowering therapy.
Outcomes
The in‐hospital outcomes in this study included bleeding event and all‐cause death. The bleeding event was the composite of intracranial bleeding and major extracranial bleeding, which was confirmed by clinical symptoms and laboratory and radiographic evidence. Intracranial bleeding was defined according to the location identified on imaging (ie, basal ganglionic, lobar, brainstem, or other) or as subarachnoid, subdural, or extradural. Such bleeding was needed to have led to hospitalization, prolonged hospitalization, surgery, or death. 24 Major extracranial bleeding events included fatal bleeding, bleeding in vital organs/locations (intracranial, spinal canal, retroperitoneal, pericardial, and intraocular with compromised vision), gastrointestinal bleeding, bleeding requiring clinical intervention (requiring pressors, surgery, or intravenous vasoactive agents), hemoglobin drop related to bleeding (the admission level minus the nadir level), and bleeding requiring blood transfusion and the total amount of transfusion. All reported events were reviewed and confirmed by a central adjudication committee that was blinded to the laboratory lipid data.
Statistical Analysis
Normally distributed continuous variables are presented as the mean with SD; skewed continuous variables are presented as the median with interquartile range, and categorical variables are presented as the frequency and percentage. The distribution of remnant cholesterol was presented using a density plot and box plot stratified by in‐hospital outcomes. The differences between different remnant cholesterol groups were compared using ANOVA or the Kruskal–Wallis test as appropriate for continuous variables and χ2 test for categorical variables.
Clinically relevant cutoff points of 70, 100, and 130 mg/dL were chosen for LDL cholesterol from the world guideline recommendations. 25 For remnant cholesterol, patients were classified into low (<20.0 mg/dL), moderate (20.0–29.9 mg/dL), and high (≥30 mg/dL) groups according to the variable distribution and previous studies. 20 , 26 The nonlinear relationship was evaluated by using restricted cubic splines in logistic regression models using the 5th, 25th, 50th, 75th, and 95th percentiles as 5 knots for spline. We further estimated the associations between remnant cholesterol groups and in‐hospital outcomes in the following 2 models with reference to previous studies 27 , 28 : (1) age (continuous) and sex adjusted; (2) age (continuous), sex, BMI (continuous), smoking history (yes/no), hypertension (yes/no), diabetes (yes/no), myocardial infarction (yes/no), atrial fibrillation (yes/no), prior stroke (yes/no), estimated glomerular filtration rate (continuous), TOAST subtype (categorical), lipid‐lowering before admission (yes/no), intravenous thrombolytic therapy (yes/no), mechanical thrombectomy (yes/no), LDL cholesterol (continuous), and HDL cholesterol (continuous). Adjusted odds ratio with 95% CI were reported, with the moderate group of remnant cholesterol set as the reference group. We used marginal effect models to examine the adjusted risk difference of in‐hospital outcomes among remnant cholesterol groups. We performed multiple sensitivity analyses. We performed the analyses using 5‐iteration imputed data sets by the Markov chain Monte Carlo method and pooled the results. There were 756 participants lacking BMI data and 45 participants lacking estimated glomerular filtration rate data. We also repeated the analyses after additionally adjusting for National Institutes of Health Stroke Scale (categorical), restricted to 2883 patients using lipid‐lowering therapy during hospitalization, and among a subset of 2571 patients with ischemic stroke.
We further evaluated the rates of in‐hospital outcomes after patients were stratified by both remnant cholesterol and LDL cholesterol. Patients were classified into 6 groups considering the clinical cutoff values, variable distribution and a previous study 29 : (1) LDL cholesterol <70 mg/dL and remnant cholesterol <20 mg/dL; (2) LDL cholesterol <70 mg/dL and remnant cholesterol ≥20 mg/dL; (3) LDL cholesterol 70–129.9 mg/dL and remnant cholesterol <20 mg/dL; (4) LDL cholesterol 70–129.9 mg/dL and remnant cholesterol ≥20 mg/dL; (5) LDL cholesterol ≥130 mg/dL and remnant cholesterol <20 mg/dL; and (6) LDL cholesterol ≥130 mg/dL and remnant cholesterol ≥20 mg/dL.
A 2‐sided P<0.05 was considered statistically significant. The adjusted risk difference was estimated using adjrr in Stata software version 14 (StataCorp, College Station, TX). All other statistical analyses were performed with R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics
A total of 3495 consecutive patients were admitted between 2015 and 2021 due to ischemic stroke or TIA. After excluding 273 patients with missing lipid data, a total of 3222 patients were included in the present analysis. There was no significant difference between those included and excluded in the final analysis as shown in Table S1. The mean age of the patients was 63.0±13.1 years, including 2301 (71.4%) men. Table 1 shows the detailed demographic and clinical characteristics of the included participants. Among the patients, 190 (6.0%) had a prior history of any stroke events; 2571 (79.8%) had an index event of ischemic stroke, and 651 (20.2%) had a TIA. The median (interquartile range) of remnant cholesterol was 18.6 (13.5–25.9) mg/dL. There were 1804 (56.0%), 849 (26.4%) and 569 (17.6%) patients with a remnant cholesterol <20, 20–29.9 and ≥30 mg/dL, respectively. Of note, patients in the low remnant cholesterol group were more likely to be older, have a lower BMI, and have a history of atrial fibrillation and stroke and less likely to have hypertension and diabetes.
Table 1.
Baseline and Clinical Characteristics of 3222 Patients According to Remnant Cholesterol Level at Admission
| Overall | Remnant cholesterol | P value | |||
|---|---|---|---|---|---|
| Low | Middle | High | |||
| Participants, n | 3222 | 1804 | 849 | 569 | |
| Sex, male | 2301 (71.4) | 1291 (71.6) | 596 (70.2) | 414 (72.8) | 0.566 |
| Age, y, mean±SD | 63±13 | 64.29±13.6 | 62.33±12.4 | 59.98±11.8 | <0.001 |
| BMI, kg/m2 (756 missing) | 24.6 (3.5) | 24.11 (3.61) | 24.96 (3.40) | 25.36 (3.26) | <0.001 |
| Smoking history | 2454 (76.2) | 1344 (74.5) | 661 (77.9) | 449 (78.9) | 0.04 |
| Medical history | |||||
| Hypertension | 2075 (64.4) | 1113 (61.7) | 565 (66.5) | 397 (69.8) | 0.001 |
| Diabetes | 1052 (32.7) | 500 (27.7) | 312 (36.7) | 240 (42.2) | <0.001 |
| Myocardial infarction | 73 (2.6) | 49 (3.1) | 17 (2.3) | 7 (1.4) | 0.095 |
| Atrial fibrillation | 402 (12.5) | 284 (15.7) | 76 (9.0) | 42 (7.4) | <0.001 |
| Prior stroke | 190 (6.0) | 123 (6.9) | 42 (5.0) | 25 (4.4) | 0.042 |
| Lipid‐lowering medication before admission | 380 (11.8) | 224 (12.4) | 95 (11.2) | 61 (10.7) | 0.486 |
| Lipid‐lowering therapy in hospital | 2883 (89.5) | 1603 (88.9) | 763 (89.9) | 517 (90.9) | 0.362 |
| Thrombolytic therapy | 484 (15.0) | 267 (14.8) | 125 (14.7) | 92 (16.2) | 0.699 |
| Mechanical thrombectomy | 130 (4.0) | 94 (5.2) | 27 (3.2) | 9 (1.6) | <0.001 |
| TOAST subtype | <0.001 | ||||
| Large‐artery atherosclerosis | 1304 (40.5) | 665 (36.9) | 401 (47.2) | 238 (41.8) | |
| Small‐vessel occlusion | 799 (24.8) | 413 (22.9) | 205 (24.1) | 181 (31.8) | |
| Cardioembolic stroke | 406 (12.6) | 292 (16.2) | 76 (9.0) | 38 (6.7) | |
| Other determined pathogenesis | 127 (3.9) | 79 (4.4) | 24 (2.8) | 24 (4.2) | |
| Undetermined pathogenesis | 586 (18.2) | 355 (19.7) | 143 (16.8) | 88 (15.5) | |
| NIHSS at admission | 0.006 | ||||
| NIHSS mild: 0–4 | 1817 (56.4) | 981 (54.4) | 492 (58.0) | 344 (60.5) | |
| NIHSS moderate: 5–15 | 1156 (35.9) | 659 (36.5) | 303 (35.7) | 194 (34.1) | |
| NIHSS severe: 16–42 | 249 (7.7) | 164 (9.1) | 54 (6.4) | 31 (5.4) | |
Data are presented as the mean±SD, median (25th–75th percentile) or number (%). Low remnant cholesterol group refers to <20 mg/dL, middle group refers to 20–29.9 mg/dL, and high group refers to ≥30 mg/dL. Differences among the 3 groups were compared using the χ2 test for categorical variables, ANOVA for normally distributed variables, and Kruskal–Wallis test for skewed variables. BMI indicates body mass index; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; and TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Association of Remnant Cholesterol and In‐Hospital Outcomes
The distributions of remnant cholesterol by bleeding and death event are shown in Figure 1. Patients with bleeding or death tended to have lower remnant cholesterol levels. Figure 2 and Figure S1 present the concentration‐dependent relationships of remnant cholesterol with bleeding events and death in terms of the relative risk and absolute risk. There shows a relatively linear association between remnant cholesterol and the risk of bleeding event (nonlinear P value=0.085), and a U‐shaped relation in terms of death (nonlinear P value=0.049). Table 2 shows the association of remnant cholesterol with the risk of bleeding and death among patients with acute ischemic stroke or TIA. After adjusting for confounding factors, including LDL cholesterol, patients in the low remnant cholesterol group had a significantly increased risk of bleeding events during hospitalization. The adjusted odds ratios were 1.49 (95% CI, 1.11–2.02) and 2.56 (95% CI, 1.12–6.67), and the adjusted risk differences were 7.3% (95% CI, 2.0%–12.7%) and 2.5% (95% CI, 0.5%–4.4%) compared with the moderate group, respectively. The effect of remnant cholesterol on death was insignificant. Alternatively, using 20 mg/dL as the cutoff point, patients in the low remnant cholesterol group were significantly associated with an increased risk of bleeding events in the hospital (Table S2).
Figure 1. Distribution of remnant cholesterol according to death and bleeding event during hospitalization.
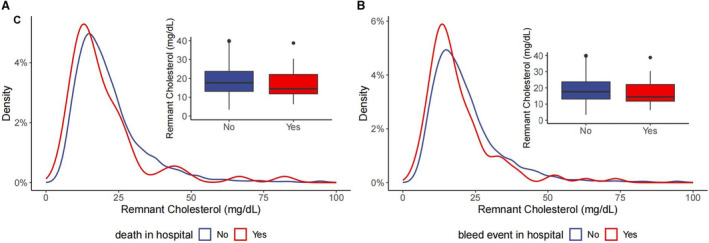
(A) Remnant cholesterol distribution by death; (B) remnant cholesterol distribution by bleeding event.
Figure 2. Dose–response relationship of remnant cholesterol at admission with risk of bleeding and death during acute ischemic stroke or TIA.
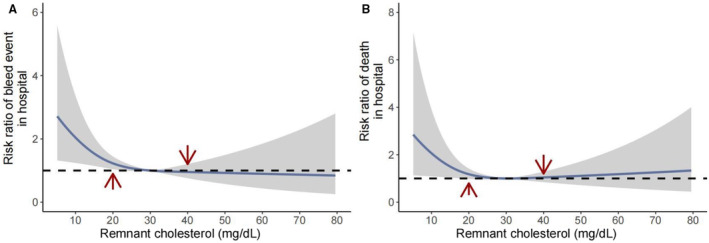
(A) Remaining cholesterol and bleeding risk in the hospital; (B) remaining cholesterol and death risk in the hospital.
Table 2.
Association of Remnant Cholesterol Levels at Admission With Risk of Bleeding and Death
| Model 1 | Model 2 | Risk difference (95% CI), % | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | Model 1 | Model 2 | |
| Bleeding event | ||||||||
| Continuous, mg/dL | 0.979 | 0.958–0.997 | 0.034 | 0.980 | 0.960–0.998 | 0.048 | … | … |
| 20–29.9 mg/dL | Reference | |||||||
| <20 mg/dL | 1.891 | 1.130–3.344 | 0.021 | 2.564 | 1.121–6.668 | 0.036 | 1.7 (0.4 to 3.0) | 2.5 (0.5 to 4.4) |
| ≥30 mg/dL | 1.272 | 0.612–2.603 | 0.511 | 1.170 | 0.338–3.769 | 0.794 | 0.5 (−1.1 to 2.2) | 0.3 (−1.8 to 2.4) |
| Death in hospital | ||||||||
| Continuous, mg/dL | ||||||||
| 20–29.9 mg/dL | 0.987 | 0.962–1.007 | 0.275 | 0.999 | 0.976–1.017 | 0.935 | … | … |
| <20 mg/dL | 1.293 | 0.687–2.612 | 0.446 | 1.763 | 0.516–7.368 | 0.393 | 0.4 (−0.6 to 1.5) | 1.0 (−1.1 to 3.1) |
| ≥30 mg/dL | 1.051 | 0.387–2.651 | 0.917 | 0.868 | 0.042–6.906 | 0.905 | 0.1 (−1.4 to 1.5) | −0.2 (−3.3 to 2.9) |
Model 1 was adjusted for age and sex; model 2 was further adjusted for BMI, smoking, hypertension, diabetes, myocardial infarction, atrial fibrillation, prior stroke, eGFR, TOAST subtype, lipid‐lowering treatment before admission, intravenous thrombolytic therapy and mechanical thrombectomy, LDL cholesterol and HDL cholesterol. BMI indicates body mass index; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; OR, odds ratio; and TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
The results remained consistent after additionally adjusting for National Institutes of Health Stroke Scale (Table S3), repeating the analyses among the imputed data sets (Table S4), restricted to those using lipid‐lowering therapy during hospitalization (Table S5), and among 2571 patients with ischemic stroke (Table 3). Table S6 summarizes the association between low remnant cholesterol and bleeding risk when stratified by smoking status and obesity, and there is no significant interaction effect observed.
Table 3.
Adjusted OR of Remnant Cholesterol Levels at Admission for In‐Hospital Outcomes Among 2571 Patients With Ischemic Stroke
| β | OR | 95% CI | P value | |
|---|---|---|---|---|
| Bleeding event | ||||
| Continuous, mg/dL | −0.020 | 0.977 | 0.958–0.996 | 0.033 |
| 20–29.9 mg/dL | Ref | |||
| <20 mg/dL | 0.588 | 1.800 | 1.039–3.317 | 0.045 |
| ≥30 mg/dL | 0.205 | 1.228 | 0.558–2.647 | 0.602 |
| Death in hospital | ||||
| Continuous, mg/dL | −0.011 | 0.981 | 0.952–1.012 | 0.269 |
| 20–29.9 mg/dL | Ref | |||
| <20 mg/dL | 0.532 | 1.703 | 0.494–7.154 | 0.425 |
| ≥30 mg/dL | −0.074 | 0.928 | 0.044–7.443 | 0.950 |
Analyses were adjusted for age, sex, BMI, smoking, hypertension, diabetes, myocardial infarction, atrial fibrillation, prior stroke, eGFR, TOAST subtype, lipid‐lowering before admission, intravenous thrombolytic therapy and mechanical thrombectomy, LDL cholesterol, and HDL cholesterol. BMI indicates body mass index; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; OR, odds ratio; and TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Combined Assessment of Remnant Cholesterol and LDL Cholesterol
The highest rate of bleeding events was observed among those with low LDL cholesterol and low remnant cholesterol, and the lowest rate of death events was observed in the moderate LDL cholesterol/high remnant cholesterol group as shown in Figure 3. After adjusting for confounders (Table S7), patients with low LDL cholesterol/low remnant cholesterol were associated with a significantly higher risk of bleeding events (odds ratio, 7.03; 95% CI, 2.57–22.57) than the moderate LDL cholesterol/high remnant cholesterol group. When patients were stratified by LDL cholesterol, we found that low remnant cholesterol was still significantly associated with a higher bleeding risk among those with LDL cholesterol <70 mg/dL (Table S8).
Figure 3. Rates of bleeding and death events by remnant cholesterol and LDL cholesterol levels at admission.
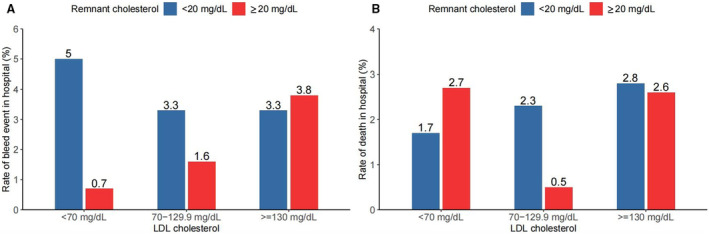
(A) Rates of bleeding event; (B) rates of death. LDL indicates low‐density lipoprotein.
Discussion
In this hospital‐based analysis, we found that a reduced remnant cholesterol level at admission was associated with an increased risk of bleeding events during the acute phase of ischemic stroke or TIA. The association was not confounded by lipid‐lowering therapy before or during hospitalization. When jointly assessing LDL cholesterol, the highest risk of bleeding events was observed among patients in the low LDL cholesterol/low remnant cholesterol group.
Atherosclerosis plays an important role in acute ischemic stroke. 30 , 31 Elevated LDL cholesterol is a well‐established risk factor for both the primary and secondary prevention of ischemic stroke. 13 , 14 , 32 The long‐term effect of LDL cholesterol–lowering therapy has been confirmed in the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial and TST (Treat Stroke to Target) trial in patients with ischemic stroke. 10 , 11 Elevated untreated baseline LDL cholesterol levels were associated with an increased short‐term risk of ischemic stroke among patients with minor ischemic stroke or TIA in a real‐world clinical setting. 12 Our study also observed that a high LDL cholesterol level at admission was significantly associated with a higher risk of death during hospitalization. However, the evidence regarding patients during the acute phase of ischemic stroke is still limited.
Of note, a meta‐analysis of 21 randomized clinical trials indicated that significant heterogeneity existed, and the absolute benefits of statins may not be strongly mediated through the degree of LDL cholesterol reduction. 33 Mounting evidence suggests that other lipid parameters might be additional therapeutic targets or predict adverse outcomes of stroke, 34 such as non‐HDL cholesterol 35 and triglyceride/HDL cholesterol ratio. 36
Our study is the first to investigate the association between remnant cholesterol and in‐hospital outcomes among patients with ischemic stroke or TIA during the acute stage. The strong atherogenic effect of remnant cholesterol is gradually recognized in epidemiological and genetic studies. 29 , 37 , 38 In contrast, no study has investigated the acute effect of remnant cholesterol among patients with stroke. We found that a reduced remnant cholesterol level (<20 mg/dL) at admission was associated with an increased risk of bleeding, even among patients taking lipid‐lowering medication during hospitalization. Evidence about the effect of remnant cholesterol on the secondary prevention of cardiovascular risk is still limited. Among individuals with a diagnosis of myocardial infarction or ischemic stroke, a lower remnant cholesterol was estimated to reduce major cardiovascular events by 20% in secondary prevention. 39 A post hoc analysis among patients receiving statin therapy in the TNT trial reported that increased remnant cholesterol levels were associated with an increased cardiovascular risk among patients with coronary heart disease. 40 Similar results were also reported in other cohorts. 41 , 42 , 43 On the other hand, another prospective cohort study reported that very‐low‐density lipoprotein cholesterol was not associated with recurrent major cardiovascular events among patients with prior cardiovascular disease. 44 The inconsistent results could be due to the heterogeneous effects of cholesterol metabolism between coronary heart disease and stroke. 45
We found that a reduced remnant cholesterol level (<20 mg/dL) at admission was associated with a higher bleeding risk but not death among patients with ischemic stroke or TIA, while the U‐shaped relationship between LDL cholesterol and death during the acute stage of ischemic stroke was previously reported. 46 Furthermore, we found that patients with low LDL cholesterol/low remnant cholesterol had the highest risk of bleeding events during hospitalization, which means the joint assessments of LDL cholesterol and remnant cholesterol potentially identify the patients in need of the extensive clinical attention. Patients with high LDL cholesterol/low remnant cholesterol were marginally associated with a higher risk of bleeding events, which is possibly due to the limited sample size. In addition, we found that low remnant cholesterol is still associated with a higher risk of bleeding event among patients of low LDL cholesterol, suggesting the clinical perspectives of remnant cholesterol independent of LDL cholesterol. As the relative and absolute risk of the low‐remnant‐cholesterol group was significantly higher, the assessment of the remnant cholesterol level could inform the risk of bleeding events in clinical practice, although the overall rate of bleeding events was relatively low (3.1%, as shown in Table 1). Given the threatening consequences of bleeding events and death, it is still of importance to recognize patients at high risk in advance even if the event rate is low. Moreover, the rate of bleeding events could be up to 5.0% among those with both low remnant cholesterol and LDL cholesterol. These findings focused only on the acute stage of ischemic stroke and did not contradict the long‐term benefits of statin therapy. It is also noteworthy that the aggressive or rapid lowering of cholesterol should be applied with caution during the acute stage of ischemic stroke or TIA. In addition, we did not observe a significantly increased risk of death with high LDL cholesterol levels, possibly because LDL cholesterol reflects unfavorable lipid management in the long run. 46 Additionally, the insufficient sample size of death cases is another possible reason, as we observed a trend of higher death risk associated with low remnant cholesterol.
There is a lack of clear biological mechanisms and explanations given the acute stage of stroke. Emerging evidence indicates a potential mechanism between cholesterol metabolism and platelet responsiveness. 47 Low LDL cholesterol levels (<70 mg/dL) were associated with in‐hospital bleeding among patients with acute coronary syndrome. 48 A data‐driven analysis showed that low cholesterol levels were an independent predictor of major bleeding at 30 days after percutaneous coronary intervention. 49 In terms of stroke, a meta‐analysis of 11 randomized clinical trials showed that more intensive LDL cholesterol–lowering statin‐based therapies were correlated with an increased risk of hemorrhagic stroke compared with less intensive therapies among patients with ischemic stroke. 13 , 50 In clinical studies, low cholesterol levels were shown to be associated with a higher risk of symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. 51 , 52 Our study pointed out that the joint assessment of LDL cholesterol and remnant cholesterol at admission potentially informs physicians of the highest risk of bleeding risk facing patients with ischemic stroke or TIA. Although LDL cholesterol and remnant cholesterol are closely related, there are distinct differences between remnant cholesterol and LDL cholesterol in terms of the composition, metabolism, and physiology. Given the clinical significance, remnant cholesterol is associated with bleeding risk among patients with acute ischemic stroke or TIA independent of LDL cholesterol in our study. Data suggested that the combined assessment of remnant cholesterol and LDL cholesterol could identify those with the highest risk of bleeding at the acute stage of ischemic stroke or TIA. Moreover, systemic immunodepression during the acute stage of stroke is another potential mechanism linking low cholesterol levels and inflammation, 53 which could drive bleeding events due to high neutrophil levels by releasing reactive oxygen species and matrix metalloprotein‐9. 54 , 55 , 56 In addition, low cholesterol levels are metabolically associated with low proprotein convertase subtilisin/Kexin 9 concentrations, which could enhance platelet activation by binding to platelet CD36 and lead to impaired platelet aggregation. 57 Furthermore, cholesterol is a key component of the platelet membrane and plays a role in platelet signaling and function. Another explanation is that remnant cholesterol and LDL cholesterol are potentially related to the infarction size of the initial stroke, which could increase the risk of hemorrhagic transformation. 58
Limitations
The Chinese Stroke Center Alliance aimed to monitor and improve care quality and outcomes for patients with acute stroke and TIA. Although there have been substantial improvements in evidence‐based practice and common risk factor control, novel predictors of the adverse outcomes of stroke are still needed. 59 Our study was the first to evaluate the association of remnant cholesterol at admission with the in‐hospital outcomes of ischemic stroke or TIA during the acute stage. The findings indicated that a low remnant cholesterol level may predict bleeding events and support the joint assessment beyond LDL cholesterol. Several limitations should be acknowledged. First, this is a hospital‐based study, and we were unable to demonstrate the relationship of remnant cholesterol and long‐term prognosis outcomes, such as recurrent stroke, other cardiovascular events, or death within 3 or 12 months after discharge. Further follow‐up studies are needed to clarify the effect of remnant cholesterol on the long‐term outcomes of ischemic stroke. Second, the lipid profiles were measured at admission when patients were experiencing an acute phase of stroke. Lipoprotein can dynamically change during the early acute phase and impact the recovery and outcomes of stroke. 60 The concentrations of remnant cholesterol at admission and other phases (such as at discharge) could have different patterns with the outcomes of stroke, which needs further data and evidence. Third, we cannot rule out the potential confounding bias as some covariates were not collected in this study (eg, drinking status). Fourth, the analysis was conducted exclusively in Chinese patients. The proportion and sample size of bleeding events were small in this current analysis. The fact is that we are unable to differentiate the effects of remnant cholesterol on the subtype of bleeding events. This finding needs further validation in studies with larger sample sizes and non‐Asian populations.
Conclusions
In summary, low remnant cholesterol at admission was associated with a higher risk of bleeding events during the acute phase of ischemic stroke or TIA. Joint assessment of remnant cholesterol and LDL cholesterol at admission could further inform the risk of bleeding events in advance. The conclusion needs further validation and cautious interpretation due to the small proportion of bleeding events in this study.
Sources of Funding
Our work was funded by the Edith Cowan University Early‐Mid Career Researcher Grant Scheme (No. G1006465).
Disclosures
None.
Supporting information
Tables S1–S8
Figure S1
Acknowledgments
Study conception: Drs Wu, Han, Q. Zhang, Tao, and Guo; study design: Drs Wu, Xu, and H. Zhang; data analysis: Drs Wu, X. Li, and X. Li; manuscript drafting: Drs Wu, H. Zhang, and Balmer. All authors have approved the submitted version and agreed to publication.
This manuscript was sent to Monik C. Jiménez, SM, ScD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.034307
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Xiang Han, Email: hansletter@fudan.edu.cn.
Lixin Tao, Email: taolixin@ccmu.edu.cn.
References
- 1. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population‐based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, Zhou Y, Zhao X, Wang C, Liu L, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355–361. doi: 10.1111/j.1747-4949.2011.00584.x [DOI] [PubMed] [Google Scholar]
- 3. Pan Y, Li Z, Li J, Jin A, Lin J, Jing J, Li H, Meng X, Wang Y, Wang Y. Residual risk and its risk factors for ischemic stroke with adherence to guideline‐based secondary stroke prevention. J Stroke. 2021;23:51–60. doi: 10.5853/jos.2020.03391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population‐base cohort study. Stroke. 2020;51:2435–2444. doi: 10.1161/STROKEAHA.120.028992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paciaroni M, Caso V, Agnelli G, Mosconi MG, Giustozzi M, Seiffge DJ, Engelter ST, Lyrer P, Polymeris AA, Kriemler L, et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO‐EXTEND study. Stroke. 2022;53:2620–2627. doi: 10.1161/STROKEAHA.121.038239 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Wright N, Guo Y, Turnbull I, Kartsonaki C, Yang L, Bian Z, Pei P, Pan D, Zhang Y, et al. Mortality and recurrent vascular events after first incident stroke: a 9‐year community‐based study of 0·5 million Chinese adults. Lancet Glob Health. 2020;8:e580–e590. doi: 10.1016/S2214-109X(20)30069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qin H, Chen Y, Liu G, Turnbull I, Zhang R, Li Z, Wang Y, Liu L, Zhao X, Chen Z, et al. Management characteristics and prognosis after stroke in China: findings from a large nationwide stroke registry. Stroke Vasc Neurol. 2021;6:1–9. doi: 10.1136/svn-2020-000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoshino T, Ishizuka K, Toi S, Mizuno T, Nishimura A, Takahashi S, Wako S, Kitagawa K. Atherogenic dyslipidemia and residual vascular risk after stroke or transient ischemic attack. Stroke. 2022;53:79–86. doi: 10.1161/STROKEAHA.121.034593 [DOI] [PubMed] [Google Scholar]
- 9. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–1114. doi: 10.1016/S1474-4422(13)70195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 11. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha JK, Ducrocq G, Giroud M, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382:9–19. doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 12. Pan Y, Wangqin R, Li H, Jin A, Li J, Lin J, Meng X, Xian Y, Laskowitz DT, Wang Y. LDL‐C levels, lipid‐lowering treatment and recurrent stroke in minor ischaemic stroke or TIA. Stroke Vasc Neurol. 2022;7:276–284. doi: 10.1136/svn-2021-001317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee M, Cheng CY, Wu YL, Lee JD, Hsu CY, Ovbiagele B. Association between intensity of low‐density lipoprotein cholesterol reduction with statin‐based therapies and secondary stroke prevention: a meta‐analysis of randomized clinical trials. JAMA Neurol. 2022;79:349–358. doi: 10.1001/jamaneurol.2021.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Zhang X, Jin A, Pan Y, Li Z, Meng X, Wang Y. Trends and risk factors associated with stroke recurrence in China, 2007–2018. JAMA Netw Open. 2022;5:e2216341. doi: 10.1001/jamanetworkopen.2022.16341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, Hennerici M, MacLeod MJ, Sillesen H, Zweifler R, et al. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2009;40:3526–3531. doi: 10.1161/STROKEAHA.109.557330 [DOI] [PubMed] [Google Scholar]
- 16. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8 [DOI] [PubMed] [Google Scholar]
- 17. Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar‐Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, et al. Triglyceride‐rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies‐a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–557. doi: 10.1210/er.2018-00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85:550–559. doi: 10.1002/ana.25432 [DOI] [PubMed] [Google Scholar]
- 20. Wadström BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort‐based study. Eur Heart J. 2022;43:3258–3269. doi: 10.1093/eurheartj/ehab705 [DOI] [PubMed] [Google Scholar]
- 21. Li W, Huang Z, Fang W, Wang X, Cai Z, Chen G, Wu W, Chen Z, Wu S, Chen Y. Remnant cholesterol variability and incident ischemic stroke in the general population. Stroke. 2022;53:1934–1941. doi: 10.1161/STROKEAHA.121.037756 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu A, Wong KSL, Wang X, Wang Y. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol. 2018;3:117–130. doi: 10.1136/svn-2017-000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cloud GC, Williamson JD, Thao LTP, Tran C, Eaton CB, Wolfe R, Nelson MR, Reid CM, Newman AB, Lockery J, et al. Low‐dose aspirin and the risk of stroke and intracerebral bleeding in healthy older people: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6:e2325803. doi: 10.1001/jamanetworkopen.2023.25803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. doi: 10.1161/CIR.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 26. Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á, Martínez‐González M, Corella D, Salas‐Salvadó J, Estruch R, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76:2712–2724. doi: 10.1016/j.jacc.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Chen Z, Wang M, Mo J, Jing J, Yalkun G, Dai L, Meng X, Li H, Li Z, et al. Low LDL‐C level and intracranial haemorrhage risk after ischaemic stroke: a prospective cohort study. Stroke Vasc Neurol. 2023;8:127–133. doi: 10.1136/svn-2022-001612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Li Y, Zhu P, Xu J, Tang X, Qiao S, Yang W, Yang Y, Gao R, Yuan J, et al. Remnant cholesterol but not LDL cholesterol is associated with 5‐year bleeding following percutaneous coronary intervention. iScience. 2023;26:107666. doi: 10.1016/j.isci.2023.107666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, Lima J, Puri R, Nomura S, Tsai M, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42:4324–4332. doi: 10.1093/eurheartj/ehab432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, Guo Y, Xu X, Bian Z, Hu R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun L, Clarke R, Bennett D, Guo Y, Walters RG, Hill M, Parish S, Millwood IY, Bian Z, Chen Y, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25:569–574. doi: 10.1038/s41591-019-0366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alloubani A, Nimer R, Samara R. Relationship between hyperlipidemia, cardiovascular disease and stroke: a systematic review. Curr Cardiol Rev. 2021;17:e051121189015. doi: 10.2174/1573403X16999201210200342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Byrne P, Demasi M, Jones M, Smith SM, O'Brien KK, DuBroff R. Evaluating the association between low‐density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta‐analysis. JAMA Intern Med. 2022;182:474–481. doi: 10.1001/jamainternmed.2022.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. ApoB and non‐HDL cholesterol versus LDL cholesterol for ischemic stroke risk. Ann Neurol. 2022;92:379–389. doi: 10.1002/ana.26425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang G, Jing J, Wang A, Zhang X, Zhao X, Li Z, Wang C, Li H, Liu L, Wang Y, et al. Non‐high‐density lipoprotein cholesterol predicts adverse outcomes in acute ischemic stroke. Stroke. 2021;52:2035–2042. doi: 10.1161/STROKEAHA.120.030783 [DOI] [PubMed] [Google Scholar]
- 36. Park JH, Lee J, Ovbiagele B. Nontraditional serum lipid variables and recurrent stroke risk. Stroke. 2014;45:3269–3274. doi: 10.1161/STROKEAHA.114.006827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varbo A, Benn M, Tybjærg‐Hansen A, Jørgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026 [DOI] [PubMed] [Google Scholar]
- 38. Nordestgaard BG. Triglyceride‐rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 39. Langsted A, Madsen CM, Nordestgaard BG. Contribution of remnant cholesterol to cardiovascular risk. J Intern Med. 2020;288:116–127. doi: 10.1111/joim.13059 [DOI] [PubMed] [Google Scholar]
- 40. Vallejo‐Vaz AJ, Fayyad R, Boekholdt SM, Hovingh GK, Kastelein JJ, Melamed S, Barter P, Waters DD, Ray KK. Triglyceride‐rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation. 2018;138:770–781. doi: 10.1161/CIRCULATIONAHA.117.032318 [DOI] [PubMed] [Google Scholar]
- 41. Gao S, Xu H, Ma W, Yuan J, Yu M. Remnant cholesterol predicts risk of cardiovascular events in patients with myocardial infarction with nonobstructive coronary arteries. J Am Heart Assoc. 2022;11:e024366. doi: 10.1161/JAHA.121.024366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu HH, Guo YL, Zhu CG, Wu NQ, Gao Y, Xu RX, Dong Q, Qian J, Dou KF, Li JJ. Synergistic effect of the commonest residual risk factors, remnant cholesterol, lipoprotein(a), and inflammation, on prognosis of statin‐treated patients with chronic coronary syndrome. J Transl Med. 2022;20:243. doi: 10.1186/s12967-022-03448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura T, Obata JE, Hirano M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Watanabe K, Watanabe Y, Mishina H, et al. Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL‐cholesterol goals. Atherosclerosis. 2011;218:163–167. doi: 10.1016/j.atherosclerosis.2011.04.040 [DOI] [PubMed] [Google Scholar]
- 44. Heidemann BE, Koopal C, Bots ML, Asselbergs FW, Westerink J, Visseren FLJ. The relation between VLDL‐cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. Int J Cardiol. 2021;322:251–257. doi: 10.1016/j.ijcard.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 45. Endres M, Heuschmann PU, Laufs U, Hakim AM. Primary prevention of stroke: blood pressure, lipids, and heart failure. Eur Heart J. 2011;32:545–552. doi: 10.1093/eurheartj/ehq472 [DOI] [PubMed] [Google Scholar]
- 46. Chen ZM, Gu HQ, Mo JL, Yang KX, Jiang YY, Yang X, Wang CJ, Xu J, Meng X, Jiang Y, et al. U‐shaped association between low‐density lipoprotein cholesterol levels and risk of all‐cause mortality mediated by post‐stroke infection in acute ischemic stroke. Sci Bull (Beijing). 2023;68:1327–1335. doi: 10.1016/j.scib.2023.05.028 [DOI] [PubMed] [Google Scholar]
- 47. Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, Dalby AJ, Montalescot G, Braunwald E. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON‐TIMI 38). Circulation. 2011;123:2681–2689. doi: 10.1161/CIRCULATIONAHA.110.002683 [DOI] [PubMed] [Google Scholar]
- 48. Yang Q, Sun D, Pei C, Zeng Y, Wang Z, Li Z, Hao Y, Song X, Li Y, Liu G, et al. LDL cholesterol levels and in‐hospital bleeding in patients on high‐intensity antithrombotic therapy: findings from the CCC‐ACS project. Eur Heart J. 2021;42:3175–3186. doi: 10.1093/eurheartj/ehab418 [DOI] [PubMed] [Google Scholar]
- 49. Iijima R, Ndrepepa G, Mehilli J, Byrne RA, Schulz S, Neumann FJ, Richardt G, Berger PB, Schömig A, Kastrati A. Profile of bleeding and ischaemic complications with bivalirudin and unfractionated heparin after percutaneous coronary intervention. Eur Heart J. 2009;30:290–296. doi: 10.1093/eurheartj/ehn586 [DOI] [PubMed] [Google Scholar]
- 50. Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–e81. doi: 10.1161/ATV.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 51. Bang OY, Saver JL, Liebeskind DS, Starkman S, Villablanca P, Salamon N, Buck B, Ali L, Restrepo L, Vinuela F, et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology. 2007;68:737–742. doi: 10.1212/01.wnl.0000252799.64165.d5 [DOI] [PubMed] [Google Scholar]
- 52. Lin SF, Chao AC, Hu HH, Lin RT, Chen CH, Chan L, Lin HJ, Sun Y, Lin YY, Chen PL, et al. Low cholesterol levels increase symptomatic intracranial hemorrhage rates after intravenous thrombolysis: a multicenter cohort validation study. J Atheroscler Thromb. 2019;26:513–527. doi: 10.5551/jat.46151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tall AR, Yvan‐Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ho‐Tin‐Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. 2018;131:277–288. doi: 10.1182/blood-2017-06-742676 [DOI] [PubMed] [Google Scholar]
- 55. Zille M, Farr TD, Keep RF, Römer C, Xi G, Boltze J. Novel targets, treatments, and advanced models for intracerebral haemorrhage. EBioMedicine. 2022;76:103880. doi: 10.1016/j.ebiom.2022.103880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Song Q, Wang C, Wu S, Deng L, Li Y, Zheng L, Liu M. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. 2019;406:116445. doi: 10.1016/j.jns.2019.116445 [DOI] [PubMed] [Google Scholar]
- 57. Qi Z, Hu L, Zhang J, Yang W, Liu X, Jia D, Yao Z, Chang L, Pan G, Zhong H, et al. PCSK9 (proprotein convertase subtilisin/Kexin 9) enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation. 2021;143:45–61. doi: 10.1161/CIRCULATIONAHA.120.046290 [DOI] [PubMed] [Google Scholar]
- 58. Bodde MC, Hermans MPJ, Wolterbeek R, Cobbaert CM, van der Laarse A, Schalij MJ, Jukema JW. Plasma LDL‐cholesterol level at admission is independently associated with infarct size in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Cardiol Ther. 2019;8:55–67. doi: 10.1007/s40119-019-0126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gu HQ, Yang X, Wang CJ, Zhao XQ, Wang YL, Liu LP, Meng X, Jiang Y, Li H, Liu C, et al. Clinical characteristics, management, and in‐hospital outcomes in patients with stroke or transient ischemic attack in China. JAMA Netw Open. 2021;4:e2120745. doi: 10.1001/jamanetworkopen.2021.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plubell DL, Fenton AM, Rosario S, Bergstrom P, Wilmarth PA, Clark WM, Zakai NA, Quinn JF, Minnier J, Alkayed NJ, et al. High‐density lipoprotein carries markers that track with recovery from stroke. Circ Res. 2020;127:1274–1287. doi: 10.1161/CIRCRESAHA.120.316526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figure S1


