Abstract
This scientific commentary refers to ‘NMDA receptor autoantibodies primarily impair the extrasynaptic compartment’ by Jamet et al. (https://doi.org/10.1093/brain/awae163).
This scientific commentary refers to ‘NMDA receptor autoantibodies primarily impair the extrasynaptic compartment’ by Jamet et al. (https://doi.org/10.1093/brain/awae163).
The field of autoantibody-mediated neurological illnesses has expanded rapidly over the past 15 years, with many new forms of encephalitis identified. Today, the two most commonly diagnosed autoimmune encephalitides are those associated with autoantibodies against leucine-rich glioma-inactivated protein 1 (LGI1)1 or the N-methyl-D-aspartate receptor (NMDAR).2 In both conditions, autoantibodies are exclusively directed against known brain antigens, and passive transfer of these autoantibodies to experimental rodents mimics key aspects of the disease phenotype, fulfilling Witebsky’s criteria for pathogenicity.3 But despite these advances, the molecular mechanisms underlying pathogenicity remain poorly understood, limiting the development of new therapies for patients.
The presence of NMDAR antibodies has been associated with diverse psychiatric features, as well as with multifocal seizures, memory deficits, decreased consciousness and autonomic instability.2,4 Studies have concluded that these bivalent antibodies trigger internalization of surface neuronal NMDARs within 2 h (without affecting neighbouring synaptic proteins), leading to synaptic NMDAR hypofunction.2,5,6 This NMDAR internalization is not observed with monovalent Fab fragments. Autoimmune disease associated with NMDAR antibodies is therefore considered a pure NMDAR-IgG-opathy, with disruption of glutamatergic transmission leading to the induction of symptoms. Removal of autoantibodies from hippocampal neuronal cultures reverses this effect, restoring NMDARs to the cell surface2: this is considered the molecular mechanism for patient recovery. Current dogma thus upholds a simple pathophysiological model whereby bivalent autoantibodies cross-link synaptic NMDARs, leading to their internalization. In this issue of Brain, Jamet and colleagues7 challenge the details of this mechanism by reframing the primacy of synaptic dysfunction, the purity of the dysfunction and the relevance of antibody bivalency. Their findings call for a change in the classification and molecular nosology of NMDAR-antibody encephalitis, and potentially for other syndromes associated with autoantibodies that target key neuronal autoantigens.
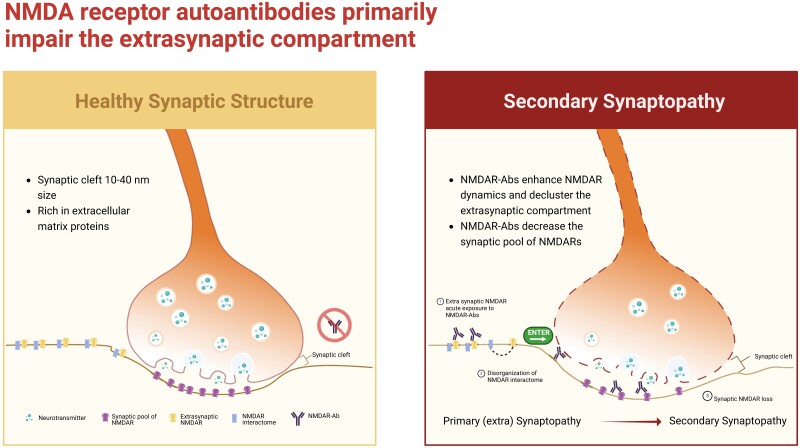
For several years, it has been recognized that the synaptic cleft, which is 10–40-nm wide and further constrained by the presence of abundant extracellular matrix proteins,8 is an inaccessible and inhospitable space for ∼10–15-nm high IgG molecules. The question then arises, are these disorders truly ‘synapt’-opathies and, if so, how do autoantibodies infiltrate this narrow space to trigger dysfunction of synaptic autoantigens (Fig. 1)?
Figure 1.
Dysregulation of the extrasynaptic compartment is proposed to result in secondary synaptopathy. Left: The synaptic cleft is a narrow space rich in extracellular matrix proteins; its small size means that molecules crossing it must also be small. Right: Overview of the findings of Jamet and colleagues.7 Figure created with BioRender.com.
To address these questions, Jamet and colleagues7 employed advanced single molecule-based imaging to observe the action of NMDAR antibodies on live dissociated hippocampal neurons. Their findings yield new insights which may reframe thinking in the field. First, they observed that within 30 min of incubation with neurons, patient CSF or monoclonal NMDAR antibodies markedly enhanced the dynamics of extrasynaptic NMDARs, without affecting synaptic (Homer-co-localized) NMDARs. These changes were accompanied by an increase in the area of the extrasynaptic surface protein interactome, with marked disorganization of membrane proteins in the extrasynaptic compartment.
Importantly, synaptic proteins were not altered over this time course. However, after 24 h of antibody incubation, synaptic dynamics and the synaptic interactome had also been disrupted. This suggests that the immediate effect of NMDAR antibodies was extrasynaptic, occurring in a subcellular region not constrained by the ∼20 nm synaptic cleft. Further, these rapid effects included modulation of proteins neighbouring the NMDAR, indicating a broader impact than previously understood. To complement co-localizations with canonical synaptic markers, the authors then conjugated NMDAR antibodies to 1-μm wide beads to definitively deny them access to the synaptic cleft. This purely extrasynaptic model effectively mimicked the effects of the patients’ antibodies over the 30-min and 24-h time courses. Finally, this study challenged the long-standing dogma of crosslinking-mediated internalization of NMDARs by showing that the number of GluN1-expressing clathrin-coated pits did not increase after antibody exposure; instead NMDARs were redistributed at the neuronal surface, as was also observed for Fab fragments.
The findings of Jamet and colleagues7 (summarized in Fig. 1) represent molecular, clinical and nosological advances in our understanding of NMDAR-antibody encephalitis. They suggest that primary autoantibody-induced dysfunction is extrasynaptic, and that the synaptic effects are secondary, occurring over a delayed time course. This is consistent with early reports of an extrasynaptic mechanism based on autoantibody specificity.9 According to this proposed mechanism, existing pathways of crosstalk between synaptic and extrasynaptic receptors serve as mediators of disease. The new findings also imply that clinical manifestations may be secondary to the disruption of other NMDAR-co-localizing proteins. This is intriguing given that some features seen in patients with NMDAR-antibody encephalitis are not easily explained by pure NMDAR dysfunction. For example, a neuroleptic malignant-like syndrome is classically considered secondary to blockade of dopaminergic pathways, while seizures are not easily squared with NMDAR hypofunction.
Overall, the current findings challenge the prevailing dogma in three ways, by suggesting that: (i) autoimmune channelopathies should potentially be reclassified as secondary synaptopathies occurring after a primary extra-synaptopathy; (ii) focusing exclusively on NMDAR dysfunction may be insufficient to fully explain the observed disease manifestations; and (iii) we should rethink the mechanisms by which molecules that target the NMDAR, such as allosteric inhibitors,10 could have therapeutic effects in patients with NMDAR-antibody encephalitis.
Finally, if these findings are validated, the concept of secondary synaptopathies could also have implications for the nosology of other autoantibody-mediated diseases thought to manifest with primary synaptic dysfunction. It is possible that LGI1-, CASPR2- and other autoantibody-mediated diseases may also turn out to be secondary synaptopathies.
Contributor Information
Meng Zhao, Department of Neurology, Mayo Clinic, Jacksonville, FL 32224, USA; Department of Neuroscience, Mayo Clinic, Jacksonville, FL 32224, USA.
David R Lynch, Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Sarosh R Irani, Department of Neurology, Mayo Clinic, Jacksonville, FL 32224, USA; Department of Neuroscience, Mayo Clinic, Jacksonville, FL 32224, USA; Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford OX3 9DU, UK; Department of Neurology, John Radcliffe Hospital, Oxford University Hospitals, Oxford OX3 9DU, UK.
Funding
This work was funded in whole or in part by fellowships from UKRI, Medical Research Council [MR/V007173/1], Wellcome Trust [104079/Z/14/Z] and by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests
D.R.L. receives royalties from testing for anti-NMDA receptor encephalitis. S.R.I. receives licensed royalties on patent application WO/2010/046716 entitled ‘Neurological Autoimmune Disorders’, and has filed two other patents entitled ‘Diagnostic method and therapy’ (WO2019211633 and US app 17/051,930; PCT application WO202189788A1) and ‘Biomarkers’ (WO202189788A1, US App 18/279,624; PCT/GB2022/050614). S.R.I. has received honoraria/research support from UCB, Immunovant, MedImmun, Roche, Janssen, Cerebral therapeutics, ADC therapeutics, Brain, CSL Behring, and ONO Pharma.
References
- 1. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today. 1993;14:426–430. [DOI] [PubMed] [Google Scholar]
- 4. Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladepeche L, Planaguma J, Thakur S, et al. NMDA receptor autoantibodies in autoimmune encephalitis cause a subunit-specific nanoscale redistribution of NMDA receptors. Cell Rep. 2018;23:3759–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamet Z, Mergaux C, Meras M, et al. NMDA receptor autoantibodies primarily impair the extrasynaptic compartment. Brain. 2024;147:2745-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuber B, Nikonenko I, Klauser P, Muller D, Dubochet J. The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc Natl Acad Sci U S A. 2005;102:19192–19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma R, Al-Saleem FH, Panzer J, et al. Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Ann Clin Transl Neurol. 2018;5:935–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mannara F, Radosevic M, Planaguma J, et al. Allosteric modulation of NMDA receptors prevents the antibody effects of patients with anti-NMDAR encephalitis. Brain. 2020;143:2709–2720. [DOI] [PubMed] [Google Scholar]