Abstract
Background
Spinal intradural tumors are rare and heterogeneous in histological type, aggressiveness, and symptomatology, and there is a lack of data about them. This study investigated the epidemiological features of spinal intradural tumors.
Methods
This retrospective analysis included patients with spinal intradural tumors who underwent surgical treatment at the Myelopathy and Spondylosis Ward Beijing Jishuitan Hospital between January 2012 and December 2022.
Results
This study included 1321 patients [aged 47.19 ± 14.90 years, 603 (45.65%) males] with spinal intradural tumors. The most common histological subtype was schwannoma [n = 511 (38.68%)], followed by spinal meningioma [n = 184 (13.93%)] and ependymoma [n = 101 (7.65%)]. Fifteen (1.14%) patients were diagnosed with metastatic spinal intradural tumors as a presentation of another primary cancer type. The spinal intradural tumors were mostly found in the lumbar region [n = 436 (33.01%)], followed by the thoracic vertebrae [n = 390 (29.52%)], cervical vertebrae [n = 154 (11.66%)], and thoracolumbar region [n = 111 (8.40%)]. Schwannomas mostly affected the lumbar region [n = 256 (52.64%)], spinal meningiomas in the thoracic region [n = 153 (83.15)], and ependymomas in the lumbar region [56 (55.45%)]. The de novo metastases were mostly found in the lumbar region [n = 8 (53.33%)].
Conclusion
According to the results of our single-center study, the most common spinal intradural tumor in Northern China is schwannoma, followed by spinal meningioma and ependymoma.
Keywords: Spinal cord neoplasms, Spinal cord diseases, Epidemiology, Retrospective study
Background
Spinal tumors are heterogeneous in histological type, aggressiveness, and symptomatology [1, 2]. They may be benign and asymptomatic or have only mild symptoms, or high-grade and cause severe neurological disability [1]. Symptoms of spinal cord compression, myelopathy (neurological deficit due to diseases of the spinal cord), and degenerative spondylosis should be investigated to rule out a space occupying-lesion [3]. Some genetic disorders, such as neurofibromatosis and von Hippel-Lindau syndrome, may increase the risk of developing spinal tumors [2]. Exposure to radiation, especially in childhood, can increase the risk of spinal tumors [4].
Most spinal tumors are extradural metastases from other areas, reported in > 10% of patients with cancer [1, 5]. Primary spinal tumors are rare, comprising about 2-4% of all primary central nervous system tumors, with an incidence of 0.74 per 100,000 person-year [1, 2] and a 10-year survival of 64%. There are an estimated 7500 new cases of primary spinal tumors each year, compared with about 90,000 new metastatic cases [1, 2]. Primary spinal tumors are classified according to their location: (1) intramedullary (arising from cells within the spinal cord), including glioma (astrocytoma, oligodendroglioma, and ependymoma), hemangioblastoma, and ganglioglioma, representing about 10% of spinal cord tumors; (2) intradural extramedullary, including meningioma, schwannoma, neurofibroma, and others, representing about 70-80%; (3) extradural tumors, including neuroblastic tumors and spinal extradural angiolipoma (SEAL) [1, 2, 6]. In rare instances, spinal tumors can occur in uncommon locations. For instance, primary ependymomas have been documented to develop in the intradural extramedullary space [7].
The rarity and diversity of spinal tumors pose a major problem in understanding their epidemiological features [8]. Indeed, there is a lack of epidemiological data regarding primary tumors at this site [9]. This lack of data significantly impacts the patients regarding diagnosis, treatments, management, and healthcare planning. Most studies are single-site series and sometimes relatively old or focused on specific subtypes [10, 11]. Only one major study from the National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) databases on patients from the United States of America (USA) treated between 1999 and 2007 is available [12]. Only one study reported the epidemiology of spinal tumors in China [13], and many changes in socioeconomic status and lifestyle occurred in China since then. One study reported the epidemiology of primary spinal osseous tumors in Eastern China [14].
Therefore, this study aimed to examine the epidemiology of spinal intradural tumors at one specialized tertiary hospital in Beijing (China). The results could help the management of patients with such tumors.
Methods
Study design and patients
This retrospective analysis included patients with spinal intradural tumors who underwent surgical treatment and obtained a histopathologic diagnosis at the Myelopathy and Spondylosis Ward of our hospital between January 2012 and December 2022. The inclusion criteria were (1) underwent surgery, (2) confirmed histopathological diagnosis of intraspinal tumor, and (3) complete dataset. Patients with a known primary tumor at a non-spinal site before the diagnosis of intraspinal tumor were excluded. Patients who did not undergo surgery and those who were pathologically confirmed to have non tumors after surgery were excluded during patient screening. The patients were defined and grouped based on their primary diagnosis. This study was approved by the Ethics Committee of Beijing Jishuitan Hospital (JL [K2023] No. [144] − 00), and all participants provided written informed consent.
Data collection and definition
All data were collected from the patient charts. Age, sex, histological type, and tumor location of the patient were collected. The histological type included schwannoma, spinal meningioma, lipoma, hemangioma, ependymoma, astrocytoma, arachnoid cyst, dermoid cyst, lymphoma, metastatic tumor, hemangioblastoma, teratoma, melanoma, and others. Tumor location included cervical vertebra, cervicothoracic junction, thoracic vertebra, thoracolumbar, lumbar, lumbosacral, and sacrococcygeal region. The tumor location was also defined as cervical, thoracic, lumbar, and sacral based on the center of the tumor.
Statistical analysis
Only descriptive statistics were used. Continuous variables were presented as means ± standard deviations, and categorical variables were presented as n (%).
Results
This study included 1321 patients [aged 47.19 ± 14.90 years, 603 (45.65%) males] with spinal intradural tumors. The most common histological subtype was schwannoma, with [511 (38.68%)] patients, followed by spinal meningioma [184 (13.93%)] and ependymoma [101 (7.65%)]. Fifteen (1.14%) patients were diagnosed with metastatic spinal intradural tumors as a presentation of another primary cancer. The youngest age at presentation was observed in patients with dermoid cysts (34.39 ± 14.88 years), while the oldest age was observed for spinal meningioma (57.89 ± 13.28 years). The lowest male/female ratio (0.16) was observed for spinal meningioma, while the highest ratio (2.75) was for metastatic tumors (Table 1).
Table 1.
Distribution and proportion of pathological types, age, and sex
| Pathological type | n (%) | Mean age (years) | Male | Female | Male/female |
|---|---|---|---|---|---|
| Total | 1321 | 47.19 ± 14.90 | 603 (45.65) | 718 (54.35) | 0.84 |
| Schwannoma | 511 (38.68) | 47.94 ± 13.18 | 275 (53.82) | 236 (46.18) | 1.17 |
| Spinal meningioma | 184 (13.93) | 57.89 ± 13.28 | 25 (13.59) | 159 (86.41) | 0.16 |
| Lipoma | 46 (3.48) | 40.54 ± 17.00 | 18 (39.13) | 28 (60.87) | 0.64 |
| Hemangioma | 30 (2.27) | 47.67 ± 13.95 | 15 (50.00) | 15 (50.00) | 1 |
| Ependymoma | 101 (7.65) | 44.51 ± 13.75 | 46 (45.54) | 55 (54.46) | 0.84 |
| Astrocytoma | 10 (0.76) | 40.20 ± 14.05 | 3 (30.00) | 7 (70.00) | 0.43 |
| Arachnoid cyst | 16 (1.21) | 44.19 ± 13.38 | 7 (43.75) | 9 (56.25) | 0.78 |
| Dermoid cyst | 59 (4.47) | 34.39 ± 14.88 | 32 (54.24) | 27 (45.76) | 1.19 |
| Lymphoma | 5 (0.38) | 49.20 ± 20.61 | 3 (60.00) | 2 (40.00) | 1.50 |
| Metastatic tumor | 15 (1.14) | 59.13 ± 11.80 | 11 (73.33) | 4 (26.67) | 2.75 |
| Hemangioblastoma | 16 (1.21) | 49.13 ± 11.91 | 11 (68.75) | 5 (31.25) | 2.20 |
| Teratoma | 31 (2.35) | 35.29 ± 13.65 | 17 (54.84) | 14 (45.16) | 1.21 |
| Melanoma | 4 (0.30) | 50.50 ± 12.07 | 4 (100.00) | 0 | - |
| Other | 299 (22.63) | 44.52 ± 14.74 | 139 (46.49) | 160 (53.51) | 0.87 |
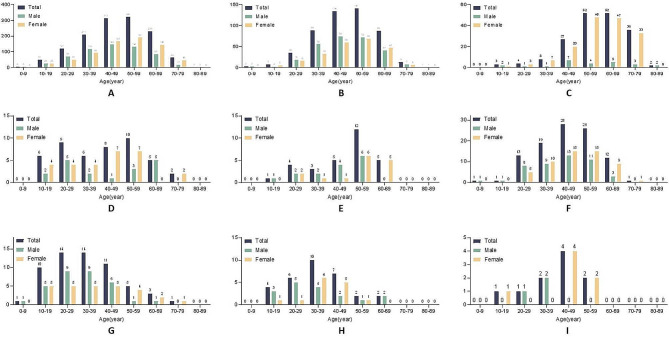
The distributions of the different histopathological subtypes according to age and sex showed that for intradural tumors (Fig. 1A), the peak occurrence was at 50–59 years in all patients or at 40–49 years in males and 50–59 years in females. Similar age and sex patterns were observed for nerve myelin sheath lesions (Fig. 1B). For meningiomas (Fig. 1C), the peak occurrence was at 50–69 years old in all patients. The occurrence was higher in females than males, peaking at 50–59 years in females and 40–49 years in males. Lipomas appeared to have a bimodal occurrence, peaking at 20–29 and 50–59 years. In males, the occurrence peaked at 20–29 and 60–69 years, while in females, it peaked at 40–59 years (Fig. 1D). The occurrence of hemangiomas was the highest at 50–59 years (Fig. 1E). The occurrence of ependymomas peaked at 40–49 years, or at 40–49 years in males and at 40–59 years in females (Fig. 1F). Dermoid cysts were mostly found at 20–39 years, or 20–39 years in males and 10–49 years in females (Fig. 1G). The occurrence of teratomas was the highest at 30–39 years, or 20–29 years in males and 30–39 years in females (Fig. 1H). Finally, astrocytomas were mainly observed at 40–49 years, or 30–39 years in males and 40–49 years in females (Fig. 1I).
Fig. 1.
Age and sex distribution of patients with A) intradural tumors, B) nerve myelin sheaths, C) spinal meningiomas, D) lipomas, E) hemangiomas, F) ependymomas, G) dermoid cysts, H) teratomas, and I) astrocytomas
The spinal intradural tumors were mostly found in the lumbar region [436 (33.01%)], followed by the thoracic vertebrae [390 (29.52%)], cervical vertebrae [154 (11.66%)], and thoracolumbar region [111 (8.40%)]. The most common tumor types, according to the location, were nerve myelin sheath in the cervical vertebra, cervicothoracic junction, thoracolumbar, and lumbar regions, while spinal meningioma was the most common in the thoracic vertebrae (Table 2).
Table 2.
Tumor types according to different locations
| Location of the tumor | N* | % | Most common | Secondary | Tertiary |
|---|---|---|---|---|---|
| Cervical vertebra | 154 | 11.66 | Nerve myelin sheath | Spinal meningioma | Other |
| Cervicothoracic junction | 41 | 3.10 | Nerve myelin sheath | Ependymoma | Spinal meningioma |
| Thoracic vertebra | 390 | 29.52 | Spinal meningioma | Nerve myelin sheath | Other |
| Thoracolumbar | 111 | 8.40 | Nerve myelin sheath | Other | Dermoid cyst |
| Lumbar | 436 | 33.01 | Nerve myelin sheath | Other | Ependymoma |
| Lumbosacral | 51 | 3.86 | Other | Nerve myelin sheath | Dermoid cyst |
| Sacrococcygeal region | 85 | 6.43 | Other | Nerve myelin sheath | Metastatic tumor |
Schwannomas mostly affected the lumbar region [256 (52.64%)], spinal meningiomas in the thoracic region [153 (83.15%)], and ependymomas in the lumbar region [56 (55.45%)]. The de novo metastases were mostly found in the lumbar region [8 (53.33%)]. Notably, most arachnoid cysts encompassed more than one vertebral region (Table 3).
Table 3.
Tumor types according to spinal level
| Pathological types | Cervical, n (%) | Thoracic, n (%) | Lumbar, n (%) | Sacral, n (%) |
|---|---|---|---|---|
| Schwannoma | 103 (20.16) | 184 (36.01) | 256 (52.64) | 18 (3.52) |
| Spinal meningioma | 27 (14.67) | 153 (83.15) | 19 (10.33) | 0 |
| Lipoma | 3 (6.52) | 21 (45.65) | 22 (47.83) | 9 (19.57) |
| Hemangioma | 1 (3.33) | 19 (63.33) | 13 (43.33) | 2 (6.67) |
| Ependymoma | 26 (25.74) | 33 (32.67) | 56 (55.45) | 4 (4.01) |
| Astrocytoma | 2 (20.00) | 8 (80.00) | 5 (50.00) | 0 |
| Arachnoid cyst | 0 | 13 (81.25) | 12 (75.00) | 0 |
| Dermoid cyst | 1 (1.69) | 14 (23.73) | 55 (93.22) | 11 (18.64) |
| Lymphoma | 0 | 5 (100.00) | 2 (40.00) | 0 |
| Metastatic tumor | 1 (6.67) | 6 (40.00) | 8 (53.33) | 3 (20.00) |
| Hemangioblastoma | 6 (37.50) | 7 (43.75) | 5 (31.25) | 0 |
| Teratoma | 1 (3.23) | 7 (22.58) | 25 (80.65) | 0 |
| Melanoma | 1 (25.00) | 1 (25.00) | 2 (50.00) | 0 |
| Other | 25 (8.36) | 74 (54.75) | 108 (36.12) | 89 (29.77) |
Discussion
This study could provide data for epidemiological features of spinal intradural tumors. In the present study, the most common spinal intradural tumor was schwannoma (38.68%), followed by spinal meningioma (13.93%) and ependymoma (7.65%). This epidemiology is not universal. An epidemiology study in the United States reporting on the data between 2006 and 2014 showed spinal schwannoma to be the third most common intradural spinal tumor following spinal meningioma and ependymoma [15]. A large epidemiological study based on the NPCR and SEER databases in the USA (1999–2007) showed that spinal meningioma was the most common lesion (32.6%), followed by tumors of the spinal nerves (26.7%) and ependymoma (21.2%) [12]. Schellinger et al. [16] also reported that the most common spinal intradural tumors were meningiomas (29%), nerve sheath tumors (24%), and ependymomas (23%). The results are also supported by a Croatian and an American study [17]. Engelhard et al. [18] from Chicago (USA) reported that the most common spinal intradural tumor types were meningioma (24.4%), ependymoma (23.7%), and schwannoma (21.2%). An early study in China (published in 1982) reported that the most common spinal cord tumor was neurinoma (46.8%), followed by meningioma (12.4%), dysembrioplastic tumors (11.0%), glioma (10.0%), and vascular tumors (6.0%) [13]. Our study represents the epidemiology in a specific population (Northern China), and had different results comparing to studies around the world. According to previous literature reports, the incidence rate of nervous system tumors in Asian people is 15.04/100,000 [19], while intraspinal tumors only account for about 1/10 of central nervous system tumors. Considering the prevalence and this being a single-center study, with most of the patients came from Northern China, we found the sample size to be adequate. Differences in epidemiology among studies can be due to several reasons, including the socioeconomic status of the study sites, medical technologies available, genetics among geographical areas, different tumor classification systems, different anatomical locations being analyzed (e.g., spinal cord vs. spinal), and the imaging methods available during the study periods. The present study was not designed to determine the reasons for the differences in epidemiology among geographical areas, and the fundamental differences among studies prevent any direct comparisons. Nevertheless, genetics and the use of hormonal treatments (i.e., hormonal contraceptives and hormonal replacement therapy) in Chinese women could play a role [20, 21]. It will have to be examined in future studies.
In the present study, some histopathological subgroups were too small to perform reliable analyses. For example, tanycytic ependymoma, which is rare subtype of ependymoma [22], was not observed in this study. Nevertheless, age- and sex-based differences could be observed among the lesions. Indeed, the youngest age at presentation was observed in patients with dermoid cysts, while the oldest age was observed for spinal meningioma. The lowest male/female ratio was observed for spinal meningioma, while the highest ratio was for metastatic tumors. It is consistent with the epidemiology of meningioma, i.e., that the median age at diagnosis is in the 60s and that women are more affected than men [23]. On the other hand, dermoid cysts develop at a younger age [24], as observed here. Bone metastases are commonly found in patients with lung, esophageal, or colon cancer, which are more common in men [25, 26], possibly explaining the higher representation of males, but the small subgroup precludes any firm conclusions. Nevertheless, generally speaking, spinal intradural tumors [4, 10], nerve myelin sheath lesions [27], meningiomas [23], hemangiomas [28], ependymomas [29], and astrocytomas [30] occur in middle-aged patients, while lipomas [31], dermoid cysts [24], and teratomas [32] occur at a younger age. Of note, only meningiomas showed a significant occurrence in older adults, consistent with the known epidemiology of meningiomas [23].
Metastatic lesions to the spine are reported to be much more frequent than primary ones, with an estimated ratio of 12.0. The present study excluded patients with a known history of primary cancer diagnosed before the spinal lesions. Nevertheless, 1.14% of the patients with intraspinal tumors had metastases from occult or undiagnosed primary cancers. The most common spinal metastases are bone metastases [33], but the present study focused on spinal intradural tumors, which could contribute to a lower frequency of spinal metastases included in the present study. Nevertheless, the results highlight the importance of investigating space-occupying spinal intradural lesions. Discovering metastases from an undetected or occult primary will, of course, change patient management.
Primary spinal cord tumors are mostly benign tumors, with the main adverse outcomes being limb sensory and motor dysfunction, and the most severe cases being paralysis [34, 35]. For extradural and subdural tumors, most patients have a good prognosis and may have residual local sensory dysfunction. For spinal cord intramedullary tumors, due to the deep location of the tumor, and the surgical process of cutting open the spinal cord, the incidence of limb motor dysfunction and postoperative neurological complications is higher, and it is prone to complete paralysis and urinary and fecal dysfunction [36]. A recent study of patients surgically treated for intramedullary spinal cord tumors reported that neurological deficits occurred in half of the patients in the postoperative chronic phase [37]. However, the occurrence of the above situations depends more on the patient’s preoperative functional status. If surgical intervention is carried out in the early stage of the disease, the prognosis is usually better. Optimal preoperative condition and advanced intraoperative monitoring might lead to improved functional outcomes [38].
The overall incidence rate of intraspinal tumors is low, and the existing literature lacks long-term large number of case reports [39]. Epidemiology studies are lacking in intraspinal tumors, especially in Asian areas. A recent study reported the burden of brain and central nervous system cancers in Asia [40]. However, it was not conducted in a clinical setting, and focused only on malignant tumors. We believe our study is unique due to its relatively large sample size on a rare disease, primarily comprising patients from a specific population (Northern China), and being conducted in a clinical setting. As neurosurgeons often focus on brain tumors, there is a lack of understanding and epidemiological evidence regarding spinal cord tumors among many doctors. As the center with relatively concentrated cases of spinal cord diseases, our hospital retrospectively summarizes ten years of case reports and summarizes its epidemiological characteristics, in hope of better guiding clinical practice.
This study has limitations. It was a single-center study. Despite the study center being a specialized tertiary center covering a large geographical area, the sample size was relatively small. Especially, several subgroups contained small numbers of patients, limiting the analyses. In addition, since the study center was a referral center, it is possible that there was a bias toward more complicated cases being referred, with simpler cases being managed locally. Furthermore, the retrospective nature of the study limited the data to those available in the charts. The pathological slides and imaging were not reviewed. The study only spanned a limited period, and no follow-up was available for many patients, preventing survival analyses.
Conclusion
In conclusion, according to the results of our single-center study, the most common spinal intradural tumors in Northern China is schwannoma, followed by spinal meningioma and ependymoma. The spine can be the presenting location of de novo metastatic disease. Additional multicenter studies with a larger sample size are necessary to determine the epidemiological patterns of individual histopathological subtypes.
Acknowledgements
Not applicable.
Abbreviations
- SEAL
Spinal extradural angiolipoma
- NPCR
National Program of Cancer Registries
- SEER
Surveillance, Epidemiology, and End Results
- USA
United States of America
Author contributions
YBS and LS carried out the studies, participated in collecting data, and drafted the manuscript. KDW and HBW performed the statistical analysis and participated in its design. LS and KDW participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Beijing Municipal Administration of Hospitals Incubating Program (grant no. PX2020018) and the Beijing Jishuitan Hospital Elite Young Scholar Program (grant no. XKGG202115).
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval and consent to participate
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of Beijing Jishuitan Hospital (JL [K2023] No. [144] − 00), and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Central Nervous System cancers. Version 1.2023. Fort Washington: National Comprehensive Cancer Network; 2023. [Google Scholar]
- 2.Pruitt AA. Neoplastic myelopathies. Continuum (Minneap Minn). 2021;27:121–42. [DOI] [PubMed] [Google Scholar]
- 3.Singleton JM, Hefner M. Spinal Cord Compression. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Matthew Hefner declares no relevant financial relationships with ineligible companies.2023.
- 4.Koeller KK, Shih RY. Intradural Extramedullary spinal neoplasms: radiologic-pathologic correlation. Radiographics. 2019;39:468–90. 10.1148/rg.2019180200 [DOI] [PubMed] [Google Scholar]
- 5.Fridley JS, Syed S, Niu T, Leary OP, Gokaslan ZL. Presentation of spinal cord and column tumors. Neurooncol Pract. 2020;7:i18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledbetter LN, Leever JD. Imaging of Intraspinal Tumors. Radiol Clin North Am. 2019;57:341–57. 10.1016/j.rcl.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 7.Duffau H, Gazzaz M, Kujas M, Fohanno D. Primary intradural extramedullary ependymoma: case report and review of the literature. Spine (Phila Pa 1976). 2000;25:1993–5. 10.1097/00007632-200008010-00021 [DOI] [PubMed] [Google Scholar]
- 8.Shobeiri P, Seyedmirzaei H, Kalantari A, Mohammadi E, Rezaei N, Hanaei S. The epidemiology of brain and spinal cord tumors. Adv Exp Med Biol. 2023;1394:19–39. 10.1007/978-3-031-14732-6_2 [DOI] [PubMed] [Google Scholar]
- 9.Sohn S, Chung CK. Epidemiology of spinal cord tumors. In: Chung CK, editor. Surgery of spinal cord tumors based on anatomy: an Approach based on anatomic compartmentalization. Singapore: Springer Singapore; 2021. pp. 1–6. [Google Scholar]
- 10.Samartzis D, Gillis CC, Shih P, O’Toole JE, Fessler RG. Intramedullary spinal cord tumors: part I-Epidemiology, pathophysiology, and diagnosis. Global Spine J. 2015;5:425–35. 10.1055/s-0035-1549029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tish S, Habboub G, Lang M, Ostrom QT, Kruchko C, Barnholtz-Sloan JS et al. The epidemiology of spinal schwannoma in the United States between 2006 and 2014. J Neurosurg Spine. 2019;1–6. [DOI] [PubMed]
- 12.Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer. 2012;118:4220–7. 10.1002/cncr.27390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng MK. Spinal cord tumors in the people’s Republic of China: a statistical review. Neurosurgery. 1982;10:22–4. 10.1227/00006123-198201000-00004 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Wang X, Wu Z, Huang W, Xiao J. Epidemiological characteristics of primary spinal osseous tumors in Eastern China. World J Surg Oncol. 2017;15:73. 10.1186/s12957-017-1136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tish S, Habboub G, Lang M, Ostrom QT, Kruchko C, Barnholtz-Sloan JS, et al. The epidemiology of spinal schwannoma in the United States between 2006 and 2014. J Neurosurg Spine. 2019;32:661–66. 10.3171/2019.10.SPINE191025 [DOI] [PubMed] [Google Scholar]
- 16.Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–9. 10.1007/s11060-007-9507-z [DOI] [PubMed] [Google Scholar]
- 17.Preston-Martin S. Descriptive epidemiology of primary tumors of the spinal cord and spinal meninges in Los Angeles County, 1972–1985. Neuroepidemiology. 1990;9:106–11. 10.1159/000110757 [DOI] [PubMed] [Google Scholar]
- 18.Engelhard HH, Villano JL, Porter KR, Stewart AK, Barua M, Barker FG, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010;13:67–77. 10.3171/2010.3.SPINE09430 [DOI] [PubMed] [Google Scholar]
- 19.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22:iv1–96. 10.1093/neuonc/noaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Chen M, Tan S, Qu X, Wang H, Liang X, et al. The socioeconomic and lifestyle determinants of contraceptive use among Chinese college students: a cross-sectional study. Reprod Health. 2020;17:125. 10.1186/s12978-020-00978-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin F, Tao M, Teng Y, Shao H, Li C, Mills E. Knowledge and attitude towards menopause and hormone replacement therapy in Chinese women. Gynecol Obstet Invest. 2015;79:40–5. 10.1159/000365172 [DOI] [PubMed] [Google Scholar]
- 22.Montemurro N, Lorenzini D, Ortenzi V, Giorgetti J. Stretched intradural extramedullary tanycytic ependymoma of the thoracic spine. Surg Neurol Int. 2022;13:426. 10.25259/SNI_647_2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggio I, Franceschi E, Tosoni A, Nunno VD, Gatto L, Lodi R, et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021;10:CNS72. 10.2217/cns-2021-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurya VP, Singh Y, Srivastava AK, Das KK, Bhaisora KS, Sardhara J, et al. Spinal dermoid and epidermoid cyst: an institutional experience and clinical insight into the neural tube Closure models. J Neurosci Rural Pract. 2021;12:495–503. 10.1055/s-0041-1724229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 26.Obermannova R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992–1004. 10.1016/j.annonc.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 27.Yeole U, Rao K, Beniwal M, Sivakoti S, Santosh V, Somanna S. Cranial and spinal malignant peripheral nerve sheath tumor: a pathological Enigma. J Neurosci Rural Pract. 2021;12:770–79. 10.1055/s-0041-1735325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tafti D, Cecava ND. Spinal Hemangioma. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Nathan Cecava declares no relevant financial relationships with ineligible companies.2023.
- 29.Ruda R, Bruno F, Pellerino A, Soffietti R. Ependymoma: evaluation and management updates. Curr Oncol Rep. 2022;24:985–93. 10.1007/s11912-022-01260-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogunlade J, Wiginton JGt, Elia C, Odell T, Rao SC. Primary spinal astrocytomas: a Literature Review. Cureus. 2019;11:e5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn MA, Walker ML. Spinal lipomas: clinical spectrum, embryology, and treatment. Neurosurg Focus. 2007;23:E10. 10.3171/FOC-07/08/E10 [DOI] [PubMed] [Google Scholar]
- 32.Senapati D, Mishra S, Shukla NK, Behera T. Long-segment Intradural Extramedullary Teratoma of Dorsolumbar spinal cord in an adolescent: a rare tumor with review of literature. Neurol India. 2023;71:760–63. 10.4103/0028-3886.383872 [DOI] [PubMed] [Google Scholar]
- 33.Ziu E, Viswanathan VK, Mesfin FB. Spinal Metastasis. StatPearls. Treasure Island (FL)2023. [PubMed]
- 34.Kumar N, Tan WLB, Wei W, Vellayappan BA. An overview of the tumors affecting the spine-inside to out. Neurooncol Pract. 2020;7:i10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang L, Liu X, Dang G, Jiang L, Wei F, Yu M, et al. Primary tumors of the spine: a review of clinical features in 438 patients. J Neurooncol. 2015;121:513–20. 10.1007/s11060-014-1650-8 [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Ma C, Li D, Li H, Dong X, Liu B, et al. The role of intraoperative neurophysiological monitoring in intramedullary spinal cord tumor surgery. Chin Neurosurg J. 2023;9:33. 10.1186/s41016-023-00348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tufo T, Grande E, Bevacqua G, Di Muccio I, Cioni B, Meglio M, et al. Long-term quality of life and functional outcomes in adults surgically treated for intramedullary spinal cord tumor. Front Psychol. 2023;14:1136223. 10.3389/fpsyg.2023.1136223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalil AM, Aboul-enein HA, Foad WA, Fayed AAA. Factors affecting Surgical Outcome of Intramedullary spinal cord tumors: a prospective one-year Follow-Up study. Egypt Spine J. 2021;37:35–46. 10.21608/esj.2021.60404.1167 [DOI] [Google Scholar]
- 39.Zhou D, Zhang Y, Liu H, Luo S, Luo L, Dai K. Epidemiology of nervous system tumors in children: a survey of 1,485 cases in Beijing Tiantan Hospital from 2001 to 2005. Pediatr Neurosurg. 2008;44:97–103. 10.1159/000113110 [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Cheng LC, Gao TY, Luo J, Zhang C. The burden of brain and central nervous system cancers in Asia from 1990 to 2019 and its predicted level in the next twenty-five years: Burden and prediction model of CNS cancers in Asia. BMC Public Health. 2023;23:2522. 10.1186/s12889-023-17467-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.