Abstract
Background
Endometriosis is a gynecological disease characterized by the presence of endometrial tissue in abnormal locations, leading to severe symptoms, inflammation, pain, organ dysfunction, and infertility. Surgical removal of endometriosis lesions is crucial for improving pain and fertility outcomes, with the goal of complete lesion removal. This study aimed to analyze the location and expression patterns of poly (ADP-ribose) polymerase 1 (PARP-1), epithelial cell adhesion molecule (EpCAM), and folate receptor alpha (FRα) in endometriosis lesions and evaluate their potential for targeted imaging.
Methods
Gene expression analysis was performed using the Turku endometriosis database (EndometDB). By immunohistochemistry, we investigated the presence and distribution of PARP-1, EpCAM, and FRα in endometriosis foci and adjacent tissue. We also applied an ad hoc platform for the analysis of images to perform a quantitative immunolocalization analysis. Double immunofluorescence analysis was carried out for PARP-1 and EpCAM, as well as for PARP-1 and FRα, to explore the expression of these combined markers within endometriosis foci and their potential simultaneous utilization in surgical treatment.
Results
Gene expression analysis revealed that PARP-1, EpCAM, and FOLR1 (FRα gene) are more highly expressed in endometriotic lesions than in the peritoneum, which served as the control tissue. The results of the immunohistochemical study revealed a significant increase in the expression levels of all three biomarkers inside the endometriosis foci compared to the adjacent tissues. Additionally, the double immunofluorescence analysis consistently demonstrated the presence of PARP-1 in the nucleus and the expression of EpCAM and FRα in the cell membrane and cytoplasm.
Conclusion
Overall, these three markers demonstrate significant potential for effective imaging of endometriosis. In particular, the results emphasize the importance of PARP-1 expression as a possible indicator for distinguishing endometriotic lesions from adjacent tissue. PARP-1, as a potential biomarker for endometriosis, offers promising avenues for further investigation in terms of both pathophysiology and diagnostic-therapeutic approaches.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12958-024-01264-0.
Keywords: PARP-1, EpCAM, FRα, Endometriosis, Intraoperative imaging, Tissue expression, Quantitative analysis
Introduction
Endometriosis is a debilitating gynecological condition defined by the implantation of endometrial tissue in ectopic places and usually associated with persistent inflammation resulting in pain, organ dysfunction, and infertility [1, 2]. Endometriosis most commonly occurs in the lower abdomen or pelvis, but it can appear anywhere in the body [2, 3]. Pelvic endometriosis can manifest as ovarian cysts (ovarian endometrioma), superficial peritoneal endometriosis (SPE), and deep infiltrating endometriosis (DIE), depending on the site and depth of implantation [1, 4]. DIE involves nodular lesions which invade the surrounding organs beneath the peritoneum. They are more aggressive and commonly found on the uterosacral ligaments, bladder, vagina, and intestine [5]. The complete surgical removal of endometriosis lesions can improve both pain symptoms and fertility outcomes, with the primary therapy goal being the removal of all visible lesions [6]. On the other hand, incomplete surgery is frequently associated with the recurrence of pain symptoms and the need for repeat surgery, with significant associated morbidity. Therefore, improvements in the resection technique are highly sought to improve outcomes, decrease functional loss and recurrence, and increase the patient’s quality of life. Minimally invasive surgery is the preferred surgical approach because it is usually associated with less pain, shorter hospital stays, and faster recovery [7]. Although endoscopy allows for magnification of the operative field, the identification of endometriosis implants is not always possible using white light because some implants may be very small or hidden, especially in case of DIE covered by healthy peritoneum or small superficial implant extending deeply retroperitoneum. Studies report that enhanced imaging allows for detecting of additional endometriotic lesions missed by conventional white-light laparoscopy [7, 8].
Fluorescent dyes such as fluorescein and indocyanine green (ICG) have been used for many years in clinical practice [9]. They can outline the vascular system and help identify areas of high perfusion or permeability. However, fluorescent dyes present drawbacks, such as speedy body clearance and the lack of precise targeting properties [10]. During the last years, molecular imaging probes have been developed to get around the limits of free dyes and visualize and measure biological activity in living systems [10, 11]. A molecular imaging probe includes a signal agent and a targeting moiety [12, 13]. The signal agent generates signals for imaging, whereas the targeting moiety interacts with a biomarker.
Intraoperative fluorescent molecular imaging is mainly used in cancer treatment, but it also has the potential to treat benign disorders like endometriosis [11]. Endometriosis is linked to gynecologic oncology, particularly ovarian cancer [14, 15]. The tumor niche in ovarian cancer and the pro-endometriotic niche in endometriosis, in particular, exhibit considerable chronic inflammatory and immunosuppressive characteristics [14].
Epithelial cell adhesion molecule (EpCAM) was one of the first cancer-related biomarkers to be identified. The first monoclonal antibody, edrecolomab, being tested on patients over thirty years ago [16]. Recent findings suggest that overexpression of EpCAM, accompanied by an epithelial-mesenchymal transition, might be involved in endometriosis [17]. In a previous study, both EpCAM and folate receptor alpha (FRα) seemed to be promising for targeted intraoperative imaging of endometriosis [18]. FRα is found on the apical surface of epithelial cell membranes in endometriotic lesions but it is not found in the surrounding normal tissue [18]. Nowadays, clinical interest has focused on the role of poly (ADP-ribose) polymerase 1 (PARP-1) which could represent a potential target for molecular imaging probes and therapy. Increased PARP-1 activity has been linked to several cancers and inflammation-related pathologies, such as asthma, sepsis, arthritis, atherosclerosis, and neurodegenerative disorders [19]. It has been suggested that PARPis, designed for cancer therapy, might be potentially used for inflammatory disorders treatment [19].
The main goal of this study was to investigate the presence and distribution of PARP-1, EpCAM, and FRα to identify a potential target for molecular imaging in endometriosis. As these three markers appear to have promising potential for targeted intraoperative imaging of endometriosis, we investigated their expression as both intensity signal and topographic distribution within endometriosis foci, comparing them with surrounding tissues [18].
Methods
Patients’ cohort
Tissue samples were selected from a retrospective database created by the IRCCS Burlo Garofolo, comprising specimens from patients who had undergone surgery for endometriosis. Following approval by the Institutional Review Board (IRB-BURLO 01/2022, 09.02.2022), patients were asked to sign an informed consent form. Tissue samples from 11 consecutive patients were used in the present study.
The inclusion criteria for patients’ selection were the histological confirmation of superficial peritoneal endometriosis (SPE) and deep infiltrating endometriosis (DIE). Exclusion criteria were: interdicted patients who were unable to provide informed consent; patients with other forms of endometriosis not reported in the inclusion criteria; patients with peritoneal inflammatory diseases (i.e., pelvic inflammatory disease, diverticulosis, etc.); and oncological patients with peritoneal involvement.
Gene expression analysis
We explored differentially expressed genes in peritoneum and endometriosis lesions using the Turku endometriosis database (EndometDB), freely accessible at https://endometdb.utu.fi/. The EndometDB, a public database, includes the expression data from 115 patients and 53 controls, with over 24,000 genes and clinical characteristics, such as age, disease stages, hormonal medication, menstrual cycle phase, and endometriosis lesion types [20].
Immunohistochemical analyses
For immunohistochemical (IHC) analysis, four-micrometer–thick formalin-fixed and paraffin-embedded (FFPE) tissue sections were deparaffinized, rehydrated, and unmasked using Epitope Retrieval Solutions (Novocastra) at pH6 and pH9 in thermostatic bath at 98 °C for 30 min. Subsequently, slides were washed in PBS at room temperature. After endogenous peroxidase neutralization with 3% H2O2 and Fc blocking with 0.4% casein in PBS (Novocastra), sections were incubated with antibodies.
We used the following primary antibodies: rabbit anti-human PARP-1 (clone EPR18461, 1:100 pH6, Abcam), rabbit anti-human EpCAM (1:400 pH9, Abcam), rabbit anti-human FRα (1:1000 pH9, Thermofisher). IHC staining was revealed using Novolink Polymer Detection System (Novocastra) and DAB (3,3’-Diaminobenzidine, Novocastra) as substrate chromogen.
Double fluorescent immunostainings for PARP-1/EpCAM and PARP-1/FRα were performed using Opal Multiplex IHC kit (Akoya Biosciences). After deparaffinization, antigen unmasking was carried out with Epitope Retrieval Solution (pH9, Novocastra) boiled at 100% power, followed by 20% power for 15 min using microwave technology (MWT). Sections were incubated with Blocking Buffer for 10 min at room temperature and then with primary antibody for 90 min at room temperature. Slides were then incubated with Polymeric Horseradish Peroxidase-conjugated (HRP) secondary antibody for 10 min, and signal was developed using Opal 520 fluorophore‐conjugated tyramide signal amplification (TSA, 1:100 dilution). To allow the next antigen detection, slides were again processed with microwave treatment for primary-secondary antibody complexes stripping; then they were incubated with second primary antibody, followed by Polymeric Horseradish Peroxidase‐conjugated (HRP) secondary antibody and Opal 620 fluorophore‐conjugated tyramide signal amplification (TSA, 1:100 dilution). Finally, sections were microwaved in Antigen Retrieval Buffer and nuclei were subsequently visualized with DAPI (4′,6‐diamidino‐2‐fenilindole).
Slides were analyzed with Axioscope A1 microscope (Zeiss) equipped with four fluorescence channels widefield IF. Microphotographs were collected using Axiocam 503 color digital camera with Zen 2.0 Software (Zeiss).
Quantitative immunolocalization analysis
Quantitative analyses of IHC staining were performed using two distinct algorithms capable of detecting nuclear labeling (PARP-1) and cytoplasmic labeling (EpCAM and FRα). In details quantitative analyses of IHC staining were carried out by calculating the average percentage of positive signals in endometriosis foci within all samples at low-power magnification (×100) using the Nuclear Hub (weak positivity: signal intensity threshold 210; moderate positivity: signal intensity threshold 188; strong positivity: signal intensity threshold 162) or Positive Pixel Count v9 (1 + weak positivity: signal intensity range 220–175; 2 + moderate positivity: signal intensity range 175–100; 3 + strong positivity: signal intensity range 100–0) Aperio ImageScope software version 12, distributed by Leica Biosystems. The percentage of positive signals is calculated based on the number of cells with nuclear or cytoplasmic labeling, normalized to the total cell count.
Statistical analysis
The paired two sample Bootstrap t-test [21] was applied to compare the average percentages between two groups (lesions vs. surrounding tissues). The boot.t.test function from the R package MKinfer was used to calculate the p-values. The p-values have been adjusted using the Benjamini-Hochberg correction. The differences with adjusted p-value < 0.05 were significant.
Results
Patients’ selection and clinical data
Tissue samples from 11 patients were used in the present study. The patient’s characteristics and medication are reported in Table 1. Collected surgical samples, their anatomical location, and pathological description are reported in Table 2.
Table 1.
Patient’s characteristics
| Patient | Age | Weight (BMI) | Endometriosis stages∗ |
Comorbidities | Ongoing hormonal therapy and menstrual cycle | Menstrual cycle phase | Other medications |
|---|---|---|---|---|---|---|---|
| 1 | 36 | 56 Kg (18.7) | III | / | Estroprogestinic therapy | / | / |
| 2 | 46 | 78 Kg (25.5) | IV | / | Spontaneous cycle | Not declared in preoperative evaluation | / |
| 3 | 32 | 65 Kg (28) | IV | Left hydronephrosis (grade II) | Progestinic therapy | / | / |
| 4 | 41 | 67 Kg (23.2) | IV | / | Progestinic therapy | / | / |
| 5 | 43 | 97 Kg (30) | IV | Systemic hypertension, Lichen sclerosus | Spontaneous cycle | Unknown, previous hysterectomy | ACE inhibitors |
| 6 | 33 | 79 Kg (26.7) | IV | / | Spontaneous cycle | Ovulatory phase | / |
| 7 | 39 | 63 Kg (20.8) | III | Anxious-depressive disorders | Estroprogestinic therapy | / | / |
| 8 | 28 | 79 Kg (25.5) | IV | / | Spontaneous cycle | Luteal Phase | / |
| 9 | 46 | 66 Kg (24.2) | II | Systemic hypertension, hypothyroidism (Hashimoto disease) | Progestinic therapy | / | ACE inhibitors, Levothyroxine |
| 10 | 40 | 74 Kg (28.9) | III | Systemic hypertension | Gonadotrophin-releasing hormone analogue | / | ACE inhibitors |
| 11 | 41 | 63 Kg (19.4) | II | / | Spontaneous cycle | Follicular Phase | / |
Note *The revised American Society for Reproductive Medicine score is currently the best-known classification of endometriosis and is the one most widely used throughout the world
Abbreviations BMI, body mass index; ACE, angiotensin-converting enzyme
Table 2.
Surgical samples characteristics
| Patient | Anatomical location of intra-operative samples | Material collected |
|---|---|---|
| 1 |
1a. Nodule of the left uterosacral ligament 1b. Nodule of the prevesical peritoneum |
1a. Fibroadipose tissue with blood extravasation site of endometriotic localization 1b. Fibrous tissue site of endometriotic lesion |
| 2 | Right lateral parameter | Soft tissue comprising multiple centers of endometriosis |
| 4 |
4a. Nodules of the rectus-sigma 4b. Nodules of the rectus-sigma |
4a. Soft tissue comprising multiple centers of endometriosis 4b. Soft tissue comprising multiple centers of endometriosis |
| 5 | Perirectal nodules, high rectal nodule, medium rectal nodule | Soft tissue comprising multiple centers of endometriosis |
| 6 |
6a. Sigma-rectum nodule 6b. Bladder-uterine plica nodule |
6a. Soft tissue comprising multiple centers of endometriosis 6b. Soft tissue comprising multiple centers of endometriosis |
| 7 |
7a. Biopsy of right uterine-sacral nodule 7b. Biopsy of the left pelvic peritoneum |
7a. Fibromuscular tissue with focus of endometriosis 7b. Fragment of fibro-adipose tissue with foci of endometriosis |
| 8 | Posterior leaflet of right broad ligament | Soft tissue comprising multiple centers of endometriosis |
| 9 | Nodule of the uterosacral ligament | Soft tissue comprising multiple centers of endometriosis |
| 10 | Left side parametrium | Left parametrial fibro-adipose tissue with foci of endometriosis associated with fibrosis and chronic inflammation |
| 11 | Biopsy of the left pelvic peritoneum | Fragment of fibro-adipose tissue with foci of endometriosis |
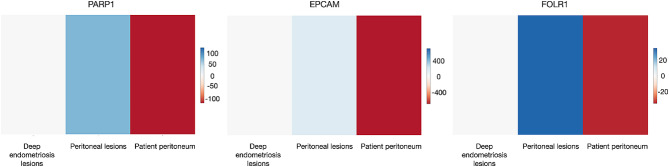
Gene expression analysis with EndometDB shows that PARP-1, EpCAM, and FOLR1 are overexpressed in the endometriotic lesion compared to the peritoneum
EndometDB is a user-friendly online interface that provides convenient access to a database of gene expression data from gathered samples. The database integrates clinical data and tissue types with transcriptome data, including over 48,000 measures. The EndometDB now has organized mRNA expression data from 115 patients and 53 controls. The database includes information on 190 lesions of various types. Users can perform targeted gene searches and navigate various tissue types to analyze and contrast gene expression patterns. The interactive functionalities enable comprehensive examination and display of the data. We examined the expression levels of PARP-1, EpCAM, and FOLR1 (FRα gene) in the peritoneum and both peritoneal and deep lesions using data from the EndometDB database (Fig. 1). All three markers demonstrate a significant increase in expression in lesions compared to peritoneal tissue. Deep lesions have a lower expression than peritoneal lesions.
Fig. 1.
Heatmap depicting the expression of selected genes in control and endometriosis patient samples. Endometriotic lesions have a considerable increase in gene expression compared to the peritoneum. EndometDB is used to collect data
Immunohistochemistry analysis shows that PARP-1, EpCAM, and FRa are overexpressed in the endometriotic lesion compared to the surrounding tissue
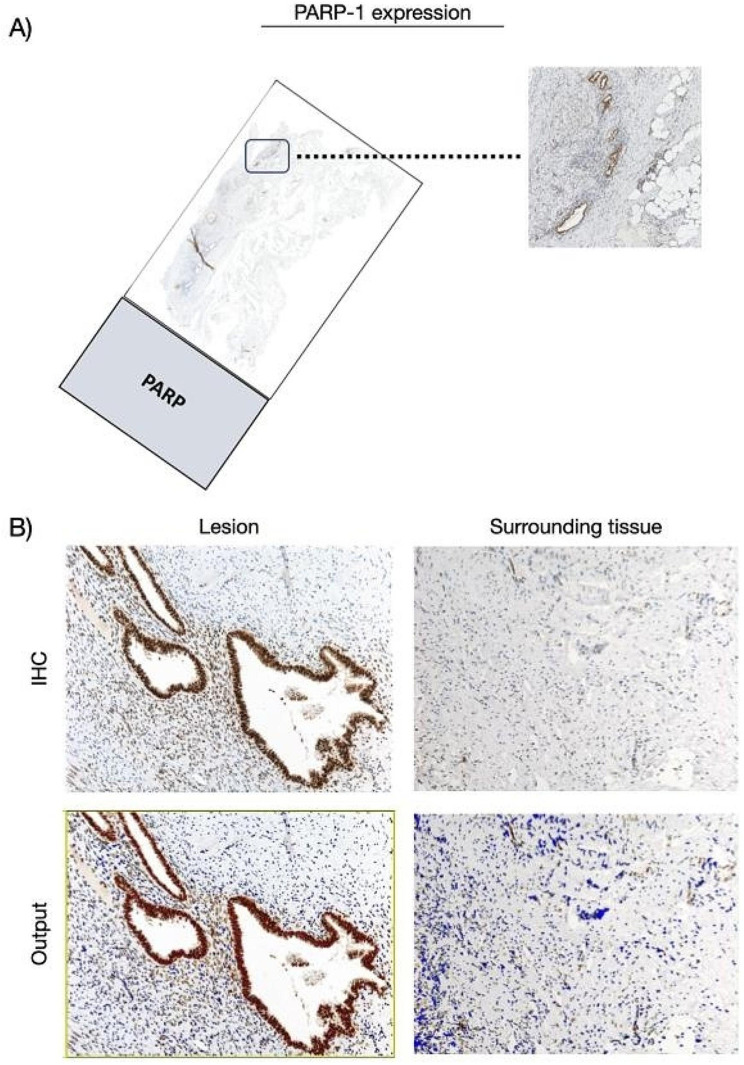
Immunostaining for PARP-1 showed overexpression in foci of endometriosis relative to the surrounding tissue. Indeed, PARP-1 is mainly expressed by glandular epithelial cells and a few cytogenic stromal cells, with nuclear labeling ranging from moderate to strong degree of intensity. In the surrounding tissue, we observed a mild nuclear expression by stromal cells, including fibroblasts and endothelial cells, and immune elements with lymphocyte and macrophage morphology (Fig. 2).
Fig. 2.
PARP-1 expression in the cohort of patients. (A) Whole slide image with a zoomed-in cross sectional area showing the endometriosis lesion. (B) Representative microphotographs showing expression of PARP-1 by IHC in endometriosis lesions and surrounding tissues. DAB (brown) chromogen was used to visualize the binding of the anti-human PARP-1 antibody. Magnification 100x; scale bars, 50 μm. In representative images of the output from the quantification analyses, the red signal corresponds to the 3 + signal (labeled as strong positive), the orange signal corresponds to the 2 + signal (labeled as positive), the yellow signal corresponds to the 1 + signal (labeled as weak positive), and finally, the blue signal corresponds to the negative expression (labeled as negative signal). For the average percentage calculation, we considered only strong positive signals and normal positive signals, as shown and described in Additional file 1
The endometrial tissue comprises the endometrial glandular epithelium and a specialized stroma known as the cytogenic stroma, which plays a pivotal role in the menstrual cycle and reproductive processes. The term “cytogenic” signifies their involvement in cell generation or development, while “stromal” indicates their location within the supportive connective tissue framework of the endometrium. These cells contribute to the cyclic changes that occur in the endometrium during the menstrual cycle, including proliferation, differentiation, and shedding of the endometrial lining during menstruation. Research on cytogenic stromal cells of the endometrium has often focused on their role in fertility, menstrual disorders, and conditions such as endometriosis and uterine leiomyoma. Immunostaining for PARP-1 revealed expression in both the endometrial tissue and immune cells. Specifically, we observed nuclear labeling ranging from moderate to strong intensity in the glandular epithelial cells. Additionally, a limited number of endometrial cytogenic stromal cells exhibited mild nuclear expression. Furthermore, beyond the endometrium, PARP-1 was also expressed by immune infiltrates, including lymphocytes and macrophages, displaying mild nuclear labeling.
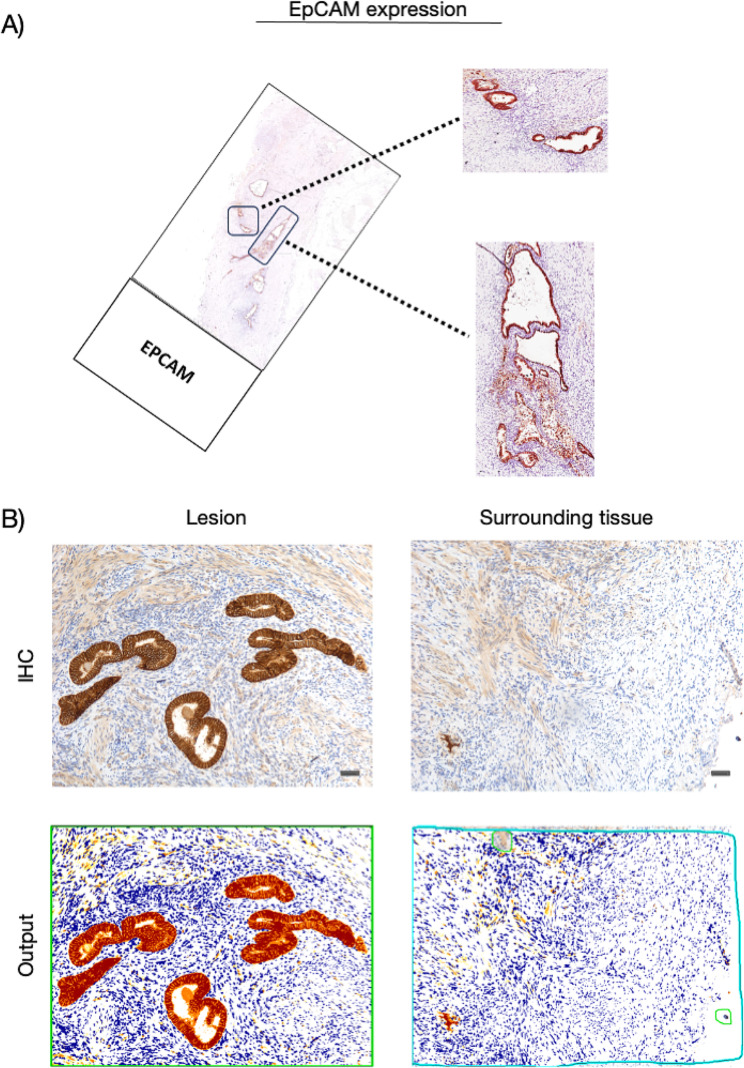
In endometriotic foci, with membrane and cytoplasmic labeling, immunohistochemical analysis revealed that glandular epithelial cells express EpCAM, exhibiting strong signal intensity (Fig. 3). Conversely, we observed few fibroblasts expressing EpCAM with membrane labeling and mild signal intensity in peri-endometriotic tissue.
Fig. 3.
EpCAM expression in the cohort of patients. (A) Whole slide image with a zoomed-in cross sectional area showing the endometriosis lesion. (B) Representative microphotographs showing expression of EpCAM by IHC in endometriosis lesions and surrounding tissues. DAB (brown) chromogen was used to visualize the binding of the anti-human EpCAM antibody. Magnification: 100x; scale bars, 50 μm. In representative images of the output from the quantification analyses, the red signal corresponds to the 3 + signal (labeled as strong positive), the orange signal corresponds to the 2 + signal (labeled as positive), the yellow signal corresponds to the 1 + signal (labeled as weak positive), and finally, the blue signal corresponds to the negative expression (labeled as negative signal). For the average percentage calculation, we considered only strong positive signals and normal positive signals, as shown and described in Additional file 2
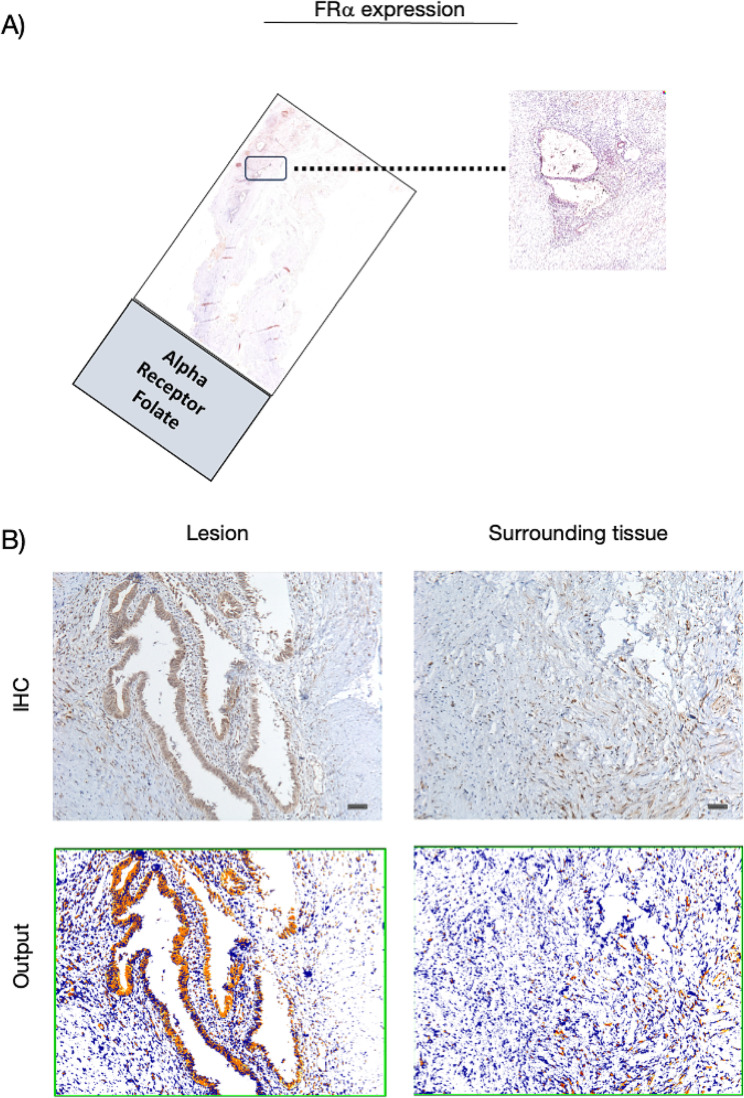
Regarding the FRα, we highlighted a higher expression in the endometrioid foci than the surrounding tissue (Fig. 4). Although with mild signal intensity compared to the other two markers, FRα is overexpressed in endometrioid glandular epithelial cells showing both membrane and cytoplasmic labeling. In contrast, surrounding tissue does not express it.
Fig. 4.
FRα expression in the cohort of patients. (A) Whole slide image with a zoomed-in cross sectional area showing the endometriosis lesion. (B) Representative microphotographs showing expression of FRα by IHC in endometriosis lesions and surrounding tissues. DAB (brown) chromogen was used to visualize the binding of the anti-human FRα antibody. Magnification 100x; scale bars, 50 μm. In representative images of the output from the quantification analyses, the red signal corresponds to the 3 + signal (labeled as strong positive), the orange signal corresponds to the 2 + signal (labeled as positive), the yellow signal corresponds to the 1 + signal (labeled as weak positive), and finally, the blue signal corresponds to the negative expression (labeled as negative signal). For the average percentage calculation, we considered only strong positive signals and normal positive signals, as shown and described in Additional file 3
Subsequently, this variable expression between endometriotic lesions and the surrounding area was confirmed by quantitative immunoassay analyses (Fig. 5, Additional file 1, 2 and 3).
Fig. 5.
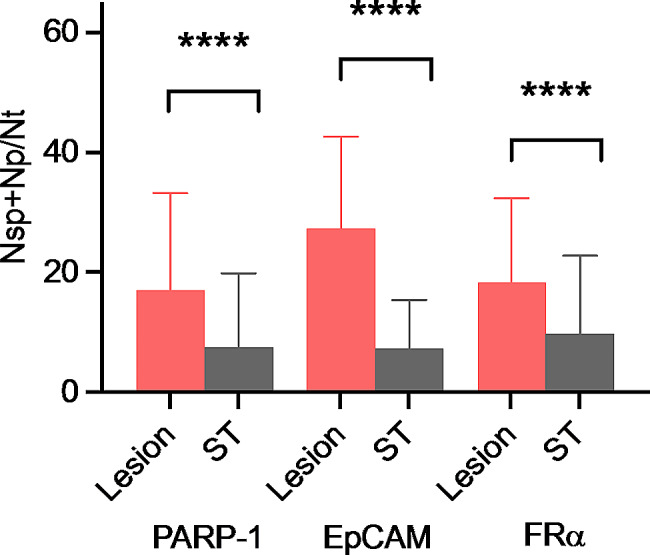
Comparison of the percentages of PARP-1, EpCAM, and FRα positivity between lesion samples and surrounding tissues (paired bootstrap t-test adjusted p-values < 10− 5). ST: Surrounding tissue, Nsp: number of strong positive cells, Np: number of positive cells, Nt: number of total cells
Opal dual immunostaining shows a clear colocalization of PARP-1 with EpCAM and FRα within the endometriotic lesion
While investigating expression of PARP-1, EPCAM and FRα protein level in endometriosis foci by immunohistochemistry, we noticed that their expression increased almost the whole in endometriosis foci compared to surround tissue, independently by localization, as confirmed by quantification analyses. Furthermore, we observed a considerable variability in terms of intensity signal among the considered markers and also heterogenous expression within the same marker.
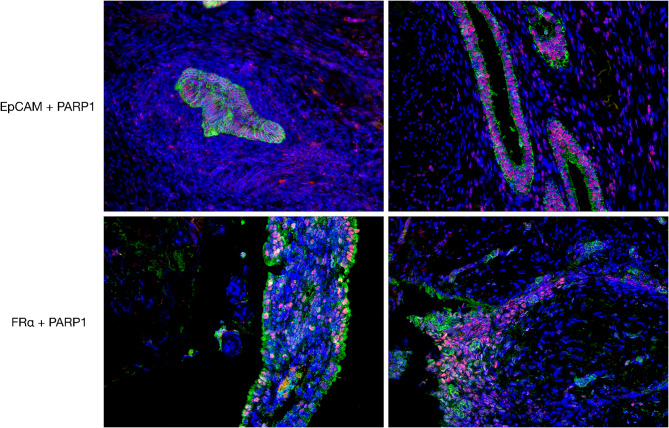
Subsequently we carried out double immunofluorescence opal assays for PARP-1 and EPCAM and for PARP-1 and FRα. We consistently observed their expression in endometriosis foci, highlighting an intense PARP-1 nuclear expression, a strong EPCAM expression with membrane labeling and mild intensity of signal was detected for FRα. These data suggest that for intraoperative detection of endometriosis foci it might be useful the simultaneous employment of the two fluorescent probes in order to improve the quality and the radicality of surgery cure (Fig. 6).
Fig. 6.
Representative microphotographs of double immunofluorescence staining for EpCAM, PARP-1, and FRα in endometriosis foci. The upper panels show EpCAM (green signal) and PARP-1 (red signal) expression. In the lower panels, FRα (green signal) and PARP-1 (red signal) are shown. Nuclei were stained with DAPI. Magnification 200x
Discussion
This study aimed to evaluate the expression of three proteins that could potentially serve as targets for intraoperative endometriosis imaging. The proteins examined were EpCAM and FRα, both membrane proteins, and the nuclear marker PARP-1. The focus of the study was on endometriotic lesions that would benefit the most from a targeted probe imaging technique, excluding ovarian lesions that are easily visible. Instead, superficial and deep infiltrating endometriosis lesions were analyzed.
The significance of PARP-1 lies in its role as a biomarker for targeted therapy, as it is known to be overexpressed in various cancer types. In the field of molecular imaging, PARP inhibitors (PARPi) are utilized as a model to develop specialized contrast agents [22]. Particularly, positron emission tomography (PET) imaging of PARP-1 has demonstrated successful results in noninvasively measuring physiologic levels of PARP-1 in patients and monitoring the therapeutic response to PARPi treatment [23]. Radiotracers are administered at low concentrations (subnanomolar) to minimize their pharmacological impact on the normal function of the PARP-1 enzyme. Moreover, the use of PARPi-FL has shown potential in assisting surgeons in detecting oral and tongue cancer, as well as determining the precise location and extent of the malignancy [24].
The significance of PARP-1 extends beyond its role in cancer, as it influences signaling pathways involved in immune response and inflammation. This suggests a potential rationale for developing radioligands to enable nuclear imaging of PARP-1 expression and activity in non-cancerous diseases such as cardiovascular disease, diabetes, and neurologic diseases [22].
This approach also aligns with the pathogenesis hypothesis of endometriosis, offering a new avenue for studying both PARP-1 and endometriosis [25, 26]. However, there is currently a lack of data on PARP-1 expression in endometriotic lesions. A study by Barreta et al. examined PARP-1 immunohistochemistry expression in both carcinomas and endometriosis and revealed that benign ovarian lesions associated with endometriosis exhibit similar levels of PARP-1 expression as ovarian carcinomas linked to endometriosis [27]. These findings prompted our investigation into the expression of this marker in endometriosis, recognizing the importance of filling this knowledge gap.
Previous studies have provided evidence of PARP-1 expression in the uterus during crucial reproductive processes such as embryo implantation and decidualization, with its regulation being influenced by ovarian hormones [28]. These findings strongly indicate the involvement of PARP-1 in the intricate process of embryo implantation [28]. Imamura et al. further contributed to this knowledge by reporting on the role of PARPs in the pre-implantation development and epigenetic modification of mouse zygotes [29]. Additionally, the research conducted by Ménissier de Murcia et al. confirmed the significance of PARP-1 and PARP-2 in embryogenesis. Collectively, these studies emphasize the importance of PARP-1 in embryogenesis and highlight its potential as a target for further exploration in the field of reproductive medicine [30].
In our study, first we performed immunohistochemical analysis of the endometrium FFPE samples in both the proliferative and secretory phases, revealing the expression of PARP-1 in various components including endometrial glandular elements, cytogenic stroma elements, and uncommon immune elements. Interestingly, the secretory phase exhibited a more prominent expression profile, particularly in the stromal layer (Additional file 4). Further examination of endometriotic lesions confirmed the staining pattern observed in the endometrium, consistently allowing for the identification of lesion boundaries based on PARP-1 expression. Qualitative analysis of the lesions within their tissue environment revealed a notable contrast highlighted by PARP-1 expression, with lower levels of PARP-1 expression in the surrounding tissues and higher levels observed in areas with a stronger immune response. Using advanced image analysis algorithms, we confirmed that the number of PARP-1-positive nuclei was higher within the lesions compared to the surrounding tissues in the patients studied. These findings emphasize the significance of PARP-1 expression as a potential marker for distinguishing endometriotic lesions from the surrounding tissue, providing valuable insights into the pathophysiology and detection of endometriosis.
EpCAM expression was observed to be low in the surrounding tissues, highlighting a potential significant contrast between the background and endometriotic lesions, where its expression was high. However, relying solely on detecting and imaging the epithelial component of the lesions may be limited due to the histological variations and diverse proportions of stromal and epithelial cells within endometriotic lesions. The conventional histological assessment of a “typical” endometriotic lesion typically emphasizes the presence of endometrioid glands and/or stroma exclusively in ectopic sites [1, 31, 32]. Nonetheless, there are a few exceptions observed, such as stromal-only endometriosis or cases where the stroma is absent or replaced by adipocytes, histocytes, or inflammatory or fibrotic infiltrates, resulting in only identifiable glands being present [31, 33, 34]. These variations in histological composition underscore the complexity of endometriotic lesions, emphasizing the importance of comprehensive characterization methods beyond solely targeting the epithelial component. Despite extensive scientific papers and global research priority reports, it is evident that endometriotic lesions exhibit considerable variability in their appearance [35–37].
Recent studies have shed light on the histological heterogeneity of these lesions, which can vary not only across different individuals but also within the same individual and even within a single biopsy [38]. Recognizing this diversity, multiplexed imaging has emerged as an intriguing approach for gathering comprehensive information from patient tissue samples by simultaneously examining multiple biomarkers [39]. This technique involves combining targeted or untargeted dyes to address the tissue and anatomical structural heterogeneity observed in endometriosis. Multi-wavelength fluorescence imaging methods using a wide range of dye-functionalized targeting agents have been explored in preclinical animal models, revealing the potential of this approach [39]. Therefore, it is plausible to consider the utilization of a combination of epithelium- and stroma-specific probes to enhance the imaging and characterization of endometriotic lesions, enabling a more comprehensive understanding of their complex nature.
It is essential to acknowledge a limitation of this study. It fails to consider other important factors that impact the ability of imaging probes to detect endometriosis lesions. These factors include the biodistribution of the imaging probe, its capability to reach the site of the endometriosis lesion, and its ability to bind to the specific molecular target. Future research is required to clarify these issues and improve our understanding of this field of study.
Conclusions
Overall, these three markers exhibit considerable potential for the successful visualization of endometriosis. The findings of this study underscore the significance of PARP-1 expression as a potential biomarker for differentiating endometriotic lesions from surrounding tissue. With the anticipation of our results serving as a catalyst, we hope to inspire further investigations and the implementation of molecular imaging techniques in the pursuit of achieving precision surgery. The potential of PARP-1 as a biomarker for endometriosis presents promising opportunities for additional research in understanding the underlying mechanisms of the disease and developing improved diagnostic and therapeutic strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1. Percentage of PARP-1 positive cells in both the lesion and the surrounding tissue, classified as either strong or normal. The quantitative analysis was performed by Nuclear v9 algorithm. Normal positivity: signal intensity threshold 188; strong positivity: signal intensity threshold 162.
Supplementary Material 2: Additional file 2.Number of total cells (Ntot), number of EpCAM positive cells (Np), and number of EpCAM strong positive cells (Nsp) quantified by the Positive Pixel Count v9 algorithm in the lesion and surrounding tissues over focus. Normal positivity: signal intensity range 175–100; Strong positivity: signal intensity range 100–0.
Supplementary Material 3: Additional file 3. Number of total cells (Ntot), number of FRα positive cells (Np), and number of FRα strong positive cells (Nsp) quantified by the Positive Pixel Count v9 algorithm in the lesion and surrounding tissues over focus. Normal positivity: signal intensity range 175–100; Strong positivity: signal intensity range 100–0.
Supplementary Material 4: Additional file 4. Immunohistochemical analysis of the FFPE samples of endometrium in proliferative and secretory phases.
Acknowledgements
Not applicable.
Author contributions
Study concept and design – S.B., A.Mang., G. Di L.; Methodology – S.S., B.Bo., S.M., C.B.; Analysis and interpretation of data – S.B., B.Be., G.L., G.B., A.Mart., G.Z., F.R., F.Z., A.R., A.G.; Drafting of manuscript – S.B., A.Mang.; Critical revision of the manuscript for important intellectual content – S.B., A.Mang., G. Di L., B.Be; Statistical analysis – G.B. and S.B.; Study supervision – S.B. and G.R. All authors reviewed the manuscript.
Funding
This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The study was conducted in accordance with the Helsinki Declaration and was approved by the Institutional Review Board (IRB-BURLO 01/2022, 09.02.2022), patients were asked to sign an informed consent form.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alessandro Mangogna, Email: alessandro.mangogna@burlo.trieste.it.
Stefania Biffi, Email: stefania.biffi@burlo.trieste.it.
References
- 1.Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E, ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod Oxf Engl. 2005;20:2698–704. 10.1093/humrep/dei135. 10.1093/humrep/dei135 [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC, Endometriosis. Lancet Lond Engl. 2004;364:1789–99. 10.1016/S0140-6736(04)17403-5. 10.1016/S0140-6736(04)17403-5 [DOI] [PubMed] [Google Scholar]
- 3.Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrão MS, Kho RM. Extrapelvic endometriosis: a systematic review. J Minim Invasive Gynecol. 2020;27:373–89. 10.1016/j.jmig.2019.10.004. 10.1016/j.jmig.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Tomassetti C, Johnson NP, Petrozza J, Abrao MS, Einarsson JI, Horne AW, Lee TTM, Missmer S, Vermeulen N, ZonderVan KT, Grimbizis G, de Wilde RL. An International Terminology for Endometriosis, 2021 †,‡, Facts Views Vis. ObGyn. 13 (n.d.) 295–304. 10.52054/FVVO.13.4.036 [DOI] [PMC free article] [PubMed]
- 5.Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, Vacher-Lavenu MC, Dubuisson JB. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod Oxf Engl. 2003;18:157–61. 10.1093/humrep/deg009. 10.1093/humrep/deg009 [DOI] [PubMed] [Google Scholar]
- 6.Working group of ESGE, ESHRE, and, Keckstein WESJ, Becker CM, Canis M, Feki A, Grimbizis GF, Hummelshoj L, Nisolle M, Roman H, Saridogan E, Tanos V, Tomassetti C, Ulrich UA, Vermeulen N, De Wilde RL. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis, Hum. Reprod. Open. 2020 (2020) hoaa002. 10.1093/hropen/hoaa002 [DOI] [PMC free article] [PubMed]
- 7.Aleksandrov A, Meshulam M, Smith AV, Chauvet P, Canis M, Bourdel N. Fluorescence-guided management of deep endometriosis. Fertil Steril. 2020;114:1116–8. 10.1016/j.fertnstert.2020.07.026. 10.1016/j.fertnstert.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 8.Maheux-Lacroix S, Belanger M, Pinard L, Lemyre M, Laberge P, Boutin A. Diagnostic accuracy of Intraoperative Tools for detecting endometriosis: a systematic review and Meta-analysis. J Minim Invasive Gynecol. 2020;27:433–e4401. 10.1016/j.jmig.2019.11.010. 10.1016/j.jmig.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Dai Z-Y, Shen C, Mi X-Q, Pu Q. The primary application of indocyanine green fluorescence imaging in surgical oncology. Front Surg. 2023;10:1077492. 10.3389/fsurg.2023.1077492. 10.3389/fsurg.2023.1077492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biffi S, Voltan R, Rampazzo E, Prodi L, Zauli G, Secchiero P. Applications of nanoparticles in cancer medicine and beyond: optical and multimodal in vivo imaging, tissue targeting and drug delivery, Expert Opin. Drug Deliv. 2015;12:1837–49. 10.1517/17425247.2015.1071791. 10.1517/17425247.2015.1071791 [DOI] [PubMed] [Google Scholar]
- 11.Bortot B, Mangogna A, Di Lorenzo G, Stabile G, Ricci G, Biffi S. Image-guided cancer surgery: a narrative review on imaging modalities and emerging nanotechnology strategies. J Nanobiotechnol. 2023;21:155. 10.1186/s12951-023-01926-y. 10.1186/s12951-023-01926-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Chen X. Design and development of Molecular Imaging Probes. Curr Top Med Chem. 2010;10:1227–36. 10.2174/156802610791384225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolting DD, Nickels ML, Guo N, Pham W. Molecular imaging probe development: a chemistry perspective. Am J Nucl Med Mol Imaging. 2012;2:273–306. [PMC free article] [PubMed] [Google Scholar]
- 14.Kajiyama H, Suzuki S, Yoshihara M, Tamauchi S, Yoshikawa N, Niimi K, Shibata K, Kikkawa F. Endometriosis and cancer, Free Radic. Biol Med. 2019;133:186–92. 10.1016/j.freeradbiomed.2018.12.015. 10.1016/j.freeradbiomed.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 15.Soroczynska K, Zareba L, Dlugolecka M, Czystowska-Kuzmicz M. Immunosuppressive extracellular vesicles as a linking factor in the development of Tumor and Endometriotic Lesions in the gynecologic tract. Cells. 2022;11:1483. 10.3390/cells11091483. 10.3390/cells11091483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald J, Henri J, Roy K, Hays E, Bauer M, Veedu RN, Pouliot N, Shigdar S. EpCAM Immunotherapy versus Specif Target Delivery Drugs Cancers. 2018;10:19. 10.3390/cancers10010019. 10.3390/cancers10010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Yang N, Liang Y, Chen M, Yang F, Liu L, Yao S. Increased expression of epithelial cell adhesion molecule and its possible role in epithelial-mesenchymal transition in endometriosis. J Obstet Gynaecol Res. 2020;46:2066–75. 10.1111/jog.14401. 10.1111/jog.14401 [DOI] [PubMed] [Google Scholar]
- 18.van den Berg LL, Crane LMA, van Oosten M, van Dam GM, Simons AHM, Hofker HS, Bart J. Analysis of biomarker expression in severe endometriosis and determination of possibilities for targeted intraoperative imaging. Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2013;121:35–40. 10.1016/j.ijgo.2012.10.025. 10.1016/j.ijgo.2012.10.025 [DOI] [PubMed] [Google Scholar]
- 19.Pazzaglia S, Pioli C. Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in Cancer and Non-cancer diseases. Cells. 2019;9:41. 10.3390/cells9010041. 10.3390/cells9010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel M, Fey V, Heinosalo T, Adhikari P, Rytkönen K, Komulainen T, Huhtinen K, Laajala TD, Siitari H, Virkki A, Suvitie P, Kujari H, Aittokallio T, Perheentupa A, Poutanen M. A relational database to identify differentially expressed genes in the endometrium and endometriosis lesions. Sci Data. 2020;7:284. 10.1038/s41597-020-00623-x. 10.1038/s41597-020-00623-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibshirani BE. R.J., An Introduction to the Bootstrap, Chapman and Hall/CRC, New York, 1994. 10.1201/9780429246593
- 22.Puentes LN, Makvandi M, Mach RH, Imaging M. PARP-1 and Beyond. J Nucl Med off Publ Soc Nucl Med. 2021;62:765–70. 10.2967/jnumed.120.243287. 10.2967/jnumed.120.243287 [DOI] [PubMed] [Google Scholar]
- 23.Ambur Sankaranarayanan R, Kossatz S, Weber W, Beheshti M, Morgenroth A, Mottaghy FM. Advancements in PARP1 targeted Nuclear Imaging and Theranostic Probes. J Clin Med. 2020;9:2130. 10.3390/jcm9072130. 10.3390/jcm9072130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Demétrio P, Kossatz C, Brand D, Karassawa Zanoni S, Roberts N, Guru D, Adilbay A, Mauguen C, Valero Mayor WA, Weber H, Schöder RA, Ghossein I, Ganly SG, Patel T, Reiner. A phase I study of a PARP1-targeted topical fluorophore for the detection of oral cancer. Eur J Nucl Med Mol Imaging. 2021;48:3618–30. 10.1007/s00259-021-05372-6. 10.1007/s00259-021-05372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611–26. 10.1586/eci.11.53. 10.1586/eci.11.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25:564–91. 10.1093/humupd/dmz018. 10.1093/humupd/dmz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barreta A, Sarian LO, Ferracini AC, Costa LBE, Mazzola PG, de Angelo Andrade L, Derchain S. Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometrioid carcinomas (type I) and endometriosis. Hum Pathol. 2019;85:72–81. 10.1016/j.humpath.2018.10.028. 10.1016/j.humpath.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 28.Vk JAMSM, V K, Cs B, G K, K H, Rk J. PARP1 during embryo implantation and its upregulation by oestradiol in mice. Reprod Camb Engl. 2014;147. 10.1530/REP-13-0588. [DOI] [PubMed]
- 29.Imamura T, Neildez TMA, Thenevin C, Paldi A. Essential role for poly (ADP-ribosyl)ation in mouse preimplantation development. BMC Mol Biol. 2004;5:4. 10.1186/1471-2199-5-4. 10.1186/1471-2199-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ménissier de Murcia J, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Amé J-C, Dierich A, LeMeur M, Sabatier L, Chambon P, de Murcia G. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–63. 10.1093/emboj/cdg206. 10.1093/emboj/cdg206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14:241–60. 10.1097/PAP.0b013e3180ca7d7b. 10.1097/PAP.0b013e3180ca7d7b [DOI] [PubMed] [Google Scholar]
- 32.Colgrave EM, Keast JR, Bittinger S, Healey M, Rogers PAW, Holdsworth-Carson SJ, Girling JE. Comparing endometriotic lesions with eutopic endometrium: time to shift focus? Hum Reprod. 2021;36:2814–23. 10.1093/humrep/deab208. 10.1093/humrep/deab208 [DOI] [PubMed] [Google Scholar]
- 33.Irving JA, Clement PB. Diseases of the Peritoneum, in: Kurman RJ, Ellenson LH, Ronnett BM, editors, Blaustein’s pathol. Female genit. Tract, Springer US, Boston, MA, 2011: pp. 625–78. 10.1007/978-1-4419-0489-8_13
- 34.Clement PB, Young RH. Two previously unemphasized features of endometriosis: Micronodular Stromal endometriosis and endometriosis with Stromal Elastosis. Int J Surg Pathol. 2000;8:223–7. 10.1177/106689690000800310. 10.1177/106689690000800310 [DOI] [PubMed] [Google Scholar]
- 35.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts Views Vis ObGyn. 2019;11:57. [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers PAW, Adamson GD, Al-Jefout M, Becker CM, D’Hooghe TM, Dunselman GAJ, Fazleabas A, Giudice LC, Horne AW, Hull ML, Hummelshoj L, Missmer SA, Montgomery GW, Stratton P, Taylor RN, Rombauts L, Saunders PT, Vincent K, Zondervan KT. Priorities in endometriosis, Research Priorities for Endometriosis, Reprod. Sci Thousand Oaks Calif. 2017;24:202–26. 10.1177/1933719116654991. WES/WERF Consortium for Research. 10.1177/1933719116654991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strehl JD, Hackl J, Wachter DL, Klingsiek P, Burghaus S, Renner SP, Fasching PA, Hartmann A, Beckmann MW. Correlation of histological and macroscopic findings in peritoneal endometriosis. Int J Clin Exp Pathol. 2013;7:152–62. [PMC free article] [PubMed] [Google Scholar]
- 38.Colgrave EM, Bittinger S, Healey M, Dior UP, Rogers PaW, Keast JR, Girling JE, Holdsworth-Carson SJ. Superficial peritoneal endometriotic lesions are histologically diverse and rarely demonstrate menstrual cycle synchronicity with matched eutopic endometrium. Hum Reprod Oxf Engl. 2020;35:2701–14. 10.1093/humrep/deaa249. 10.1093/humrep/deaa249 [DOI] [PubMed] [Google Scholar]
- 39.van Beurden F, van Willigen DM, Vojnovic B, van Oosterom MN, Brouwer OR, van der Poel HG, Kobayashi H, van Leeuwen FWB, Buckle T. Multi-wavelength fluorescence in image-guided surgery, clinical feasibility and future perspectives. Mol Imaging. 2020;19:1536012120962333. 10.1177/1536012120962333. 10.1177/1536012120962333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Additional file 1. Percentage of PARP-1 positive cells in both the lesion and the surrounding tissue, classified as either strong or normal. The quantitative analysis was performed by Nuclear v9 algorithm. Normal positivity: signal intensity threshold 188; strong positivity: signal intensity threshold 162.
Supplementary Material 2: Additional file 2.Number of total cells (Ntot), number of EpCAM positive cells (Np), and number of EpCAM strong positive cells (Nsp) quantified by the Positive Pixel Count v9 algorithm in the lesion and surrounding tissues over focus. Normal positivity: signal intensity range 175–100; Strong positivity: signal intensity range 100–0.
Supplementary Material 3: Additional file 3. Number of total cells (Ntot), number of FRα positive cells (Np), and number of FRα strong positive cells (Nsp) quantified by the Positive Pixel Count v9 algorithm in the lesion and surrounding tissues over focus. Normal positivity: signal intensity range 175–100; Strong positivity: signal intensity range 100–0.
Supplementary Material 4: Additional file 4. Immunohistochemical analysis of the FFPE samples of endometrium in proliferative and secretory phases.
Data Availability Statement
No datasets were generated or analysed during the current study.