Abstract
Objective:
To assess the association between the onset of the COVID-19 pandemic and change in low-value cancer services.
Study Design:
In this retrospective cohort study, we used administrative claims from the HealthCore Integrated Research Environment, a repository of medical and pharmacy data from US health plans representing over 80 million members, between January 1, 2016, and March 31, 2021.
Methods:
We used linear probability models to investigate the relation between the onset of COVID-19 pandemic and 4 guideline-based metrics of low-value cancer care: 1) conventional fractionation radiotherapy instead of hypofractionated radiotherapy for early-stage breast cancer; 2) non-guideline-based antiemetic use for minimal-, low-, or moderate-to-high-risk chemotherapies; 3) off-pathway systemic therapy; and 4) aggressive end-of-life care. We identified patients newly diagnosed with breast, colorectal, and/or lung cancer. We excluded members who did not have at least 6 months of continuous insurance coverage and members with prevalent cancers.
Results:
Among 204,581 patients, the mean [SD] age was 63.1 [13.2], 68.1% were female, 83,593 (40.8%) had breast cancer, 56,373 (27.5%) had colorectal cancer, and 64,615 (31.5%) had lung cancer. The payer mix was 11.8% Medicare Advantage, 14.0% Medicare Supplemental, and 74.1% Commercial non-Medicare. Rates of low-value cancer services exhibited minimal changes during the pandemic: conventional radiotherapy, adjusted percentage point difference 3.93 (95% CI 1.50 to 6.36); off-pathway systemic therapy, adjusted percentage point difference 0.82 (95% CI -0.62 to 2.25); non-guideline-based antiemetics, adjusted percentage point difference -3.62 (95% CI -4.97 to -2.27); aggressive end-of-life care, adjusted percentage point difference 2.71 (95% CI -0.59 to 6.02).
Conclusions:
Low-value cancer care remained prevalent through the pandemic. Policymakers should consider changes to payment and incentive design to turn the tide against low-value cancer care.
Keywords: Low-value care, Quality of care, Health policy, End-of-life care, Value-based care, SARS-CoV-2, Cancer, COVID-19
Precis:
Among adults with newly diagnosed cancer, rates of low-value cancer services persisted throughout the pandemic in areas ranging from peri-diagnosis imaging to end-of-life care.
Introduction
Low-value health care services confer costs and risks to patients that exceed their benefits.(1) The importance of low-value services in US health care has been demonstrated by several lines of research outside of cancer care, and low-value health care has been a frequent target of performance-based measures from alternative payment models.(2–4) Studies have demonstrated how specific tests or treatments fail to produce health gains warranting their costs or adverse effects; many such services have been highlighted by medical specialty societies through the Choosing Wisely initiative.(5)
Low-value services are prevalent in cancer care, with rates of certain metrics such as bone scans in low-risk prostate cancer and tumor markers in non-metastatic breast cancer reaching over 50%.(6) In 2020, the onset of the COVID-19 pandemic caused a dramatic shift in cancer care delivery in an effort to reduce the risks of exposing patients with cancer to health care settings.(7) During this period of disruption, it is possible that health systems and clinicians seized the opportunity to reduce the use of lower-value services because of the potential for increased visits and hospitalizations to spread the virus among vulnerable populations.(8) Alternatively, clinicians may have preferred to use greater low-value therapeutics – including off-pathway supportive care medications and systemic therapies – to avoid patients receiving clinic-based infusional therapy. However, prior evidence suggests that considerable avoidance of cancer therapy during the pandemic with relatively minimal transition to oral chemotherapies, arguing against this hypothesis.(9,10)
We investigated the relation between the onset of COVID-19 pandemic and changes in several metrics of low-value cancer care. We hypothesized that the COVID-19 pandemic resulted in decreased provision of low-value cancer care. The rationale for this hypothesis was an assumption that health systems and patients would have sought to minimize potential COVID-19 exposure for vulnerable patients with cancer and thus decreased utilization of services that may be unnecessary during the pandemic. Furthermore, we would expect that any practice changes induced by the pandemic would be magnified among low-value practices, which may be marginal and thus more likely to change. We define low-value care using population-based metrics based on clinical guidelines, as per other studies outside of cancer care.(11,12)
Methods
Design
This retrospective cohort study examined trends in low-value care metrics for cancer patients using a large database of commercial insurance claims. The study was exempt from the primary institution’s institutional review board approval because it involved a limited study database with masked identifiers.
Data sources
We used administrative claims and health plan enrollment data from the HealthCore Integrated Research Environment for information on diagnoses, use of cancer treatment, costs, comorbidities, and rendering clinician identifiers. The HealthCore Integrated Research Environment is a repository of medical and pharmacy claims data for approximately 80 million geographically diverse members enrolled in individual, employer-sponsored, and Medicare Advantage plans starting in 2006. In 2016, the HealthCore Integrated Research Environment covered 6.6% of adults (≥20 years) in the United States.
Study sample
We identified enrollees in the health plans aged 18 years or older in fully insured or self-insured plans who had a new diagnosis of breast, colorectal, or lung cancer between January 1, 2016, and March 31, 2021. We identified new cancers using International Classification of Diseases, Ninth Revision or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes for breast, colorectal, or lung cancer (see Supplementary Table 1 in the Appendix for all diagnostic and procedure codes). We required members to have at least 6 months of continuous insurance coverage prior to initial cancer diagnosis date and excluded those who did not have at least 6 months of continuous coverage. We excluded cancers that had been previously diagnosed because it is difficult to ascertain prevalence of low-value cancer care practices across a heterogeneous cohort that may have received previous lines of therapy and be at different points in their cancer course. To exclude prevalent cancers and isolate incident cases, we excluded individuals who had any diagnosis code for an eligible cancer in the 6 months prior to incident index cancer diagnosis between 2016 and 2021. We further identified eligible populations for each low-value care outcome analysis as per published guidelines (see Supplementary Tables 1-2), such that each analysis for each low-value care outcome contained a different denominator of eligible patients. Baseline patient characteristics were measured during the 6-month period prior to diagnosis.
Low value care outcomes
The primary outcome for all analyses was receipt of low-value cancer care, defined as the percent of eligible patients per period who received a low-value service. Although there are no consensus definitions of low-value care in oncology care, we included published metrics from guideline bodies or peer-reviewed literature. These measures spanned across the cancer care continuum, from diagnosis to treatment to survivorship and end-of-life. We intentionally did not include metrics of cancer screening since the pandemic’s impact on declining cancer screening has been well-described.(13,14) Low-value care measures were identified from the American Society of Clinical Oncology and American Society for Radiation Oncology Choosing Wisely campaigns (15,16), the Hutchinson Institute for Cancer Outcomes Research (17), Anthem’s Cancer Care Quality Program treatment pathways (18), NCCN guidelines (19), and peer-reviewed literature on low-value antiemetic use (20). We defined four measures of low-value cancer care spanning the cancer care continuum (Supplementary Table 2): 1) conventional fractionation radiotherapy instead of hypofractionated radiotherapy for early-stage breast cancer only (15); 2) non-guideline-based antiemetic use for minimal-, low-, or moderate-to-high-risk chemotherapies across breast, colorectal, and lung cancers (16,19,20); 3) off-pathway systemic therapy across breast, colorectal, and lung cancers (18); and 4) aggressive end-of-life care (chemotherapy in the last 14 days of life, multiple emergency department visits in the last 30 days of life, intensive care unit utilization in the last 30 days of life, hospice initiation ≤3 days before death, and/or no hospice receipt before death) across breast, colorectal, and lung cancers (17). We intentionally chose measures of low-value care that involved both additional healthcare encounters (e.g. conventional radiotherapy, aggressive end-of-life care) and selection of lower-value diagnostics or treatment without increased encounters (e.g. low-value antiemetics, off-pathway systemic therapy). We hypothesized that metrics reflecting increased encounters would disproportionately decrease, compared to other low-value metrics, during the pandemic.
Covariates
We collected covariates for statistical adjustment including gender (male vs. female), age in years, Deyo-Charlson Comorbidity Index score, insurance type (Medicare Advantage, Medicare Supplemental, Commercial), geographic region (Northeast, Midwest, South, West), urban vs. rural domicile, and area-level socioeconomic status. Area-level socioeconomic status was specified as 1st quartile [lowest] to 4th quartile [highest], based on validated socioeconomic indicators such as median home value, median family income, the ratio of population below poverty, ratios of the population with less than high school education and at least 4 years of college, and unemployment rates, developed by the Agency for Healthcare Research and Quality applied to American Community Survey data.(21) For the conventional radiotherapy outcome, additional covariates (based on a prior study(22)) included service-site facility (office vs. outpatient facility), county-level radiation oncologist density, and a post-2018 indicator because ASTRO released guidance recommending hypofractionated radiotherapy for all patients with early-stage breast cancer at this time.
Statistical analysis
To verify the disruption in cancer care induced by the pandemic in this sample, we first described trends in cancer diagnosis rates. Linear probability models applied to patient-month level data were then used to evaluate the association of the COVID-19 period with each of the 4 low-value outcomes. The COVID-19 period was defined as March – December 2020, owing to the initiation of many state stay-at-home orders in March 2020. All analyses included a month fixed effect to account for seasonality, a linear time trend to account for secular changes in the outcome over time, and were adjusted for the above covariates. Linear time trends were defined as the month entering the regressions (e.g. month 1, 2, 3, etc.) and we controlled for differential trends in the pre- and post-periods. The key regression terms of interest were an indicator for the COVID-19 period and an interaction between that indicator and a linear time trend. After conducting each linear regression model, we used the Stata margins command to calculate the marginal effect of the COVID-19 period; these calculated marginal effects provide adjusted estimates that incorporate changes in both the outcome level and changes in the linear temporal trend in the outcome during the COVID-19 period. All analyses were conducted using Stata v16.1 (Stata Corp, College Station, TX). Statistical significance was set at 0.05 and all tests were two-sided.
Results
Among 204,581 members (mean [SD] age 63.1 [13.2], 68.1% female), 83,593 (40.8%) had breast cancer, 56,373 (27.5%) had colorectal cancer, and 64,615 (31.5%) had lung cancer (Table 1). The payer mix was 11.8% Medicare Advantage, 14.0% Medicare Supplemental, and 74.1% Commercial non-Medicare. We observed an initial steep decline in overall cancer diagnosis rates at the start of the COVID pandemic for all cancers that returned to baseline (Supplementary Figure 1). In unadjusted analyses, rates of low-value cancer care in the pre-COVID vs. COVID periods were: conventional fractionation radiotherapy (n=12,213): 22.1% vs. 9.4%; non-guideline-based antiemetics (n=81,315): 61.2% vs. 58.1%; off-pathway systemic therapy (n=41,487): 36.7% vs. 43.2%; aggressive end-of-life care (n=21,662): 75.7% vs. 73.3% (Figure 1). In adjusted analyses, the COVID period, relative to the pre-COVID period, was not associated with significant changes in off-pathway systemic therapy (adjusted percentage point difference 0.82, 95% CI, -0.62 to 2.25 pp, p=0.262) and aggressive end-of-life care (adjusted percentage point difference 2.71, 95% CI, -0.59 to 6.02, p=0.108) (Table 2). The COVID period was associated with an increase in conventional radiotherapy (adjusted percentage point difference 3.93, 95% CI, 1.50 to 6.36 pp, p=0.002), and a decrease in non-guideline-based antiemetics (adjusted percentage point difference -3.62, 95% CI, -4.97 to -2.27, p<0.001).
Table 1.
Demographic characteristics of the cohort
| Pre-COVID period (Jan 2016 – Feb 2020) | COVID period (Mar 2020 – Dec 2020) | |
|---|---|---|
| Number of members | 103,765 | 13,351 |
| Age, median (interquartile range) | 60 (53–69) | 59 (51–67) |
| Female (%) | 75,167 (72%) | 9,632 (72%) |
| Cancer type | ||
| Breast (%) | 52,920 (51%) | 6,809 (51%) |
| Colorectal (%) | 22,721 (22%) | 3,030 (23%) |
| Lung (%) | 28,383 (27%) | 3,479 (26%) |
| Urban domicile (%) | 81,397 (78%) | 10,363 (78%) |
| Region | ||
| Northeast (%) | 17,847 (17%) | 2,070 (16%) |
| Midwest (%) | 25,761 (25%) | 3,828 (29%) |
| South (%) | 34,419 (33%) | 4,638 (35%) |
| West (%) | 24,867 (24%) | 2,791 (21%) |
| Insurance type | ||
| Medicare (%) | 18,980 (18%) | 2,947 (22%) |
| Commercial (%) | 84,785 (82%) | 10,404 (78%) |
| Eligible populations in each low value metric | ||
| Conventional radiotherapy | 10,123 | 2,090 |
| Non-guideline based antiemetics | 69,511 | 11,804 |
| Off-pathway systemic therapy | 35,924 | 5,563 |
| Aggressive end-of-life care | 19,566 | 2,096 |
Source: HealthCore Integrated Research Environment.
Notes: Number of members refers to the number of unique members identified across measures.
Figure 1. Unadjusted patient-level trends in receipt low-value cancer care metrics, January 2016 to December 2020.
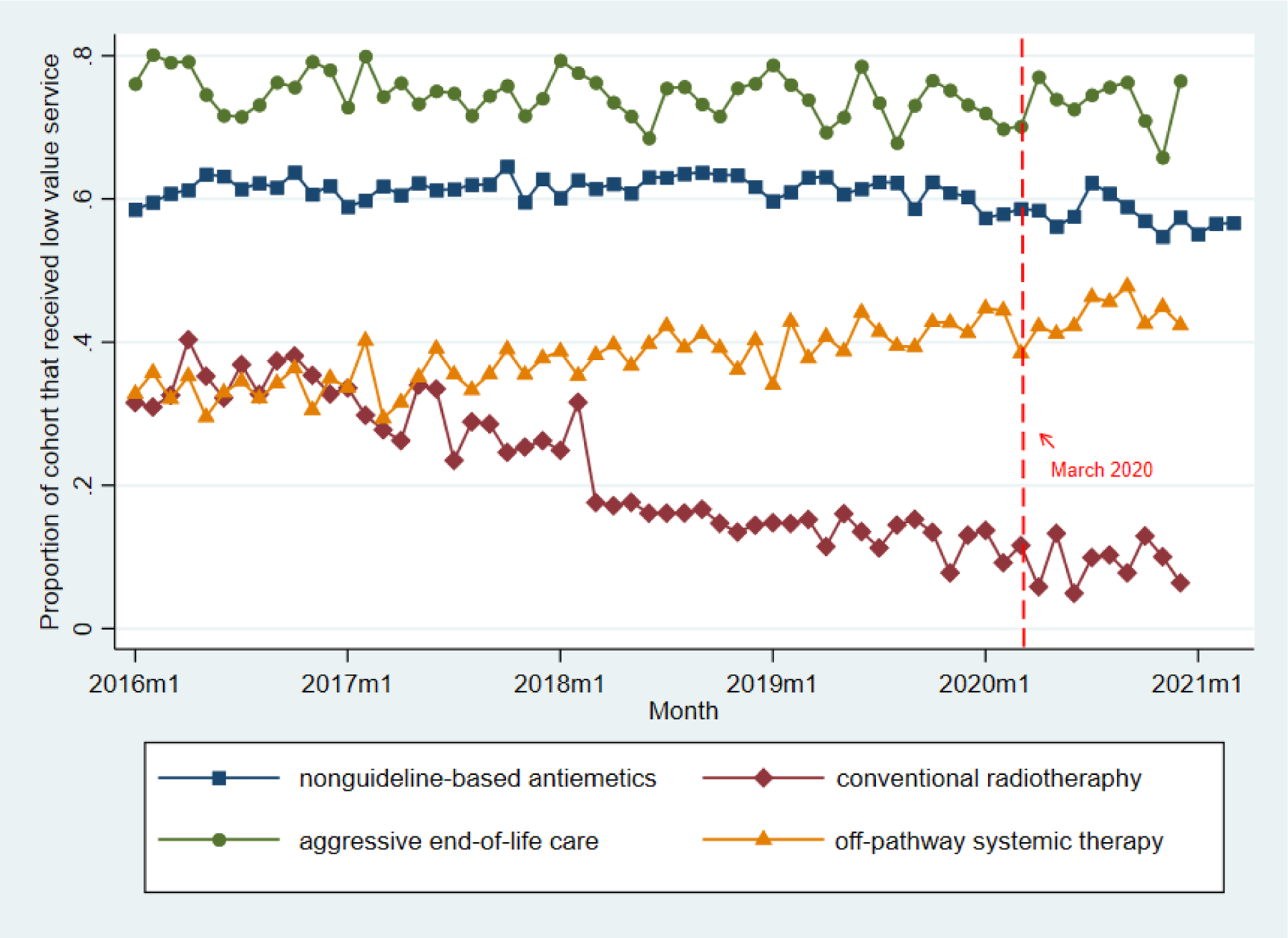
Source: HealthCore Integrated Research Environment.
Points represent monthly proportions of eligible patients who received low-value care measure in question. in the pre-COVID (January 2016 to February 2020) and COVID (March to December 2020) periods. Dotted lines represent March 1, 2020, which we defined as the beginning of the COVID pandemic period. Cohort refers to the eligible cohort for each measure.
Table 2.
Association between COVID pandemic and selected low-value cancer care metrics.
| Low-value care metric | Pre-COVID cohort size (n) | Post-COVID cohort size (n) | Unadjusted Pre-COVID rate (95% CI) | Unadjusted Post-COVID rate (95% CI) | Adjusted percentage point difference (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Conventional radiotherapy | 10,123 | 2,090 | 22.1% (21.3% to 23.0%) | 9.4% (8.2% to 10.7%) | 3.93 (1.50 to 6.36) | 0.002 |
| Non-guideline-based antiemetics | 69,511 | 11,804 | 61.2% (60.8% to 61.5%) | 58.1% (57.3% to 59.0%) | -3.62 (-4.97 to -2.27) | <0.001 |
| Off-pathway systemic therapy | 35,924 | 5,563 | 36.7% (36.2% to 37.2%) | 43.2% (41.9% to 44.5%) | 0.82 (-0.62 to 2.25) | 0.262 |
| Aggressive end-of-life care | 19,566 | 2,090 | 75.7% (75.2% to 76.4%) | 73.3% (71.4% to 75.2%) | 2.71 (-0.59 to 6.02) | 0.108 |
Source: HealthCore Integrated Research Environment.
Notes: Models used robust standard errors, clustered at year-month level (e.g. January 2018)
Adjusted percentage-point differences are estimated from linear probability models adjusted for age, Deyo-Charlson score, insurance type (Medicare Advantage, Medicare Supplemental, Commercial), urban/rural status, region (NE, MW, S, W), and area-level socioeconomic status. The conventional radiotherapy outcome was additionally adjusted for county-level radiation oncologist density.
Discussion
Among adults with breast, colorectal, or lung cancer, the onset of the COVID-19 pandemic was not associated with consistent changes in low-value cancer care. Rates of low-value cancer services persisted throughout the pandemic. Importantly, utilization-related metrics (conventional radiation, aggressive end-of-life care) did not show declines compared to non-utilization metrics that reflected discretionary care decisions (e.g. non-guideline-based antiemetics). Indeed, conventional radiation had a strong declining secular trend prior to the pandemic that appeared to plateau during the pandemic.
Pre-pandemic evidence suggests substantial physician variation in low-value practice patterns among both primary care and oncology clinicians.(11,23,24) Our study suggests that pandemic-related guidance to avoid unnecessary healthcare visits did not change low-value practice patterns of oncology clinicians. This is in line with prior evidence from smaller, limited-institution studies from outside of cancer, which suggest that pandemic-related disruptions did not meaningfully change metrics of hospice utilization near the end-of-life.(25) Of note, a national study among Medicare beneficiaries in 2020 showed some decreases in low-value cancer screening, but increases in low-value opioid use – thus representing an inconsistent effect.(26) Another national study of several low-value care practices outside of oncology care showed a sharp decline in April 2020 that normalized by the end of 2020, in line with this study.(27) Our study runs counter to prevailing notions that the utilization shock induced by the pandemic would disproportionately decrease low-value services (7,8). Indeed, both low-value and non-low-value cancer services may have decreased proportionately during the pandemic. Additionally, we have previously demonstrated that, despite clinical guidelines suggesting avoidance of discretionary infusion-based therapies during the pandemic, the prevalence of transitions from infusional or injection therapies to oral therapies was minimal.(10) It is possible that utilization declines were countered by practices intentionally increasing low-value services to mitigate revenue losses during the pandemic; future studies should investigate this troubling possibility. Of note, while the pandemic was associated with statistically significant changes in two low-value metrics (conventional radiotherapy, non-guideline-based antiemetics), the changes were <5 percentage-points in absolute value and were relatively low compared to the underlying prevalence of low-value care for each of these metrics; furthermore, these do not represent consistent effects, and thus we interpret these findings as not clinically impactful.
There are several limitations to our analysis. Claims-based analyses are unable to account for unmeasured patient-, provider-, practice-, or system-related factors that, if associated with the COVID pandemic, may have influenced our results. Thus, other factors may have impacted rates of low-value cancer care during the study period, though our analytic approach accounted for observed confounders, temporal trends, and unobserved confounders that were stable over time. Additionally, there is no consensus definition of low-value cancer care metrics after the point of cancer screening. While we chose several metrics that spanned the cancer care continuum, our selected metrics were not exhaustive. Greater efforts to define low-value cancer care practices are necessary. Finally, it is possible that certain low-value metrics such as low-value antiemetic use may be prone to measurement error because claims lack information on chemotherapy dosing. However, we assume that any measurement error be constant across time and not effected by COVID, such that comparing rates in post-covid to pre-covid would still be valid.
Oncology is a particular target of insurer efforts to curb expensive and potentially unnecessary health care services.(28) By definition, low-value cancer care poses additional costs and harms – including high out-of-pocket spending, medication side-effects, and care inconsistent with patient goals – to patients and the health care system, without meaningful benefits in patient outcomes. Thus, curbing low-value care represents an important policy priority given efforts to limit the costs of cancer care. Our study has implications for future strategies to curb low-value cancer care in oncology and beyond. Educational efforts, such as the American Board of Internal Medicine Choosing Wisely® campaign, and broad-based payment reform, including the Centers for Medicare and Medication Innovation Oncology Care Model, have had limited success in substantially curbing low-value cancer care.(29–31) Given that rates of low-value cancer care were persistently high through a massive health care disruption like the pandemic, it is clear that low-value care is a persistent and difficult problem. Policymakers should consider more targeted changes to payment and incentive design to turn the tide against low-value cancer care.
Conclusions
Low-value cancer care remained prevalent through the pandemic. Policymakers should consider changes to payment and incentive design to turn the tide against low-value cancer care.
Supplementary Material
Supplementary Figure 1
Caption: Trends in cancer diagnoses, January 2016 to March 2021.
Source: HealthCore Integrated Research Environment.
Legend: Points represent monthly number of newly diagnosed cancer patients in the pre-COVID (January 2016 to February 2020) and COVID (March to December 2020) periods. Dx = Diagnoses
Take Home Points:
Among adults with newly diagnosed cancer, rates of low-value cancer services persisted throughout the pandemic in areas ranging from peri-diagnosis imaging to end-of-life care.
Educational campaigns and payment reforms including the Centers for Medicare and Medication Innovation Oncology Care Model have had limited success in curbing low-value cancer care.
The COVID-19 pandemic caused a dramatic decrease in health care utilization, leading many to suspect that low-value cancer services may decrease.
We found that low-value care in areas ranging from cancer treatment, supportive care, and end-of-life care remained stubbornly high even during the height of stay-at-home orders in the US.
Policymakers should consider more targeted changes to payment and incentive design to turn the tide against low-value cancer care.
Acknowledgements:
The authors acknowledge Jay Fein, BA, for assistance in preparing this manuscript for publication.
Funding:
This work is funded by a National Cancer Institute Grant K08CA263541 (to RBP).
Role of the Funder:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med 2012. Jan 23;172(2):171–8. [DOI] [PubMed] [Google Scholar]
- 2.Song Z, Ji Y, Safran DG, Chernew ME. Health Care Spending, Utilization, and Quality 8 Years into Global Payment. N Engl J Med 2019. Jul 18;381(3):252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newhouse JP, Insurance Experiment Group. Free for All?: Lessons from the RAND Health Insurance Experiment [Internet]. Harvard University Press; 1993. Jan [cited 2022 Dec 22]. Available from: https://www.rand.org/pubs/commercial_books/CB199.html [Google Scholar]
- 4.Skinner J. Causes and Consequences of Regional Variations in Health Care. In: Pauly MV, Mcguire TG, Barros PP, editors. Handbook of Health Economics [Internet]. Elsevier; 2011. [cited 2022 Dec 22]. p. 45–93. (Handbook of Health Economics; vol. 2). Available from: https://www.sciencedirect.com/science/article/pii/B9780444535924000025 [Google Scholar]
- 5.Choosing Wisely Five Things Physicians and Patients Should Question [Internet]. 2012. [cited 2022 Dec 22]. Available from: https://www.choosingwisely.org/choosing-wisely-five-things-physicians-and-patients-should-question-press-release-april-4-2012/
- 6.Baxi SS, Kale M, Keyhani S, Roman BR, Yang A, Derosa AP, et al. Overuse of Health Care Services in the Management of Cancer: A Systematic Review. Med Care 2017. Jul;55(7):723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyawali B, Poudyal BS, Eisenhauer EA. Covid-19 Pandemic-An Opportunity to Reduce and Eliminate Low-Value Practices in Oncology? JAMA Oncol 2020. Nov 1;6(11):1693–4. [DOI] [PubMed] [Google Scholar]
- 8.Oakes AH, Segal J. The COVID-19 Pandemic Can Help Us Understand Low-Value Health Care. Health Affairs Forefront [Internet]. 2020. Oct 27 [cited 2022 Mar 1]; Available from: https://www.healthaffairs.org/do/10.1377/forefront.20201023.522078/full/ [Google Scholar]
- 9.Lang JJ, Narendrula A, Iyer S, Zanotti K, Sindhwani P, Mossialos E, et al. Patient-reported disruptions to cancer care during the COVID-19 pandemic: A national cross-sectional study. Cancer Med 2023. Feb;12(4):4773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh RB, Takvorian SU, Vader D, Paul Wileyto E, Clark AS, Lee DJ, et al. Impact of the COVID-19 Pandemic on Treatment Patterns for Patients With Metastatic Solid Cancer in the United States. J Natl Cancer Inst 2022. Apr 11;114(4):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of Physician Variation in Provision of Low-Value Services. JAMA Internal Medicine. 2019. Jan 1;179(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in Low-Value Services in Year 1 of the Medicare Pioneer Accountable Care Organization Program. JAMA Intern Med 2015. Nov;175(11):1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croswell JM, Corley DA, Lafata JE, Haas JS, Inadomi JM, Kamineni A, et al. Cancer screening in the U.S. through the COVID-19 pandemic, recovery, and beyond. Prev Med 2021. Oct;151:106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clinical Cancer Informatics. 2020. Nov;(4):1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn C, Kavanagh B, Bhatnagar A, Jacobson G, Lutz S, Patton C, et al. Choosing wisely: the American Society for Radiation Oncology’s top 5 list. Pract Radiat Oncol 2014. Dec;4(6):349–55. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Clinical Oncology. Ten things physicians and patients should question [Internet]. 2021. [cited 2022 Jul 15]. Available from: https://www.choosingwisely.org/societies/american-society-of-clinical-oncology/
- 17.Hutchinson Institute for Cancer Outcomes Research. Community Cancer Care in Washington State: Quality and Cost Report 2021. [Internet]. Seattle, WA: Fred Hutchinson Cancer Research Center; 2021.; [cited 2022 Jan 17]. Available from: https://www.fredhutch.org/content/dam/www/research/institute-networks-ircs/hicor/HICOR-Community-Cancer-Care-Report-2021.pdf [Google Scholar]
- 18.Bekelman JE, Gupta A, Fishman E, Debono D, Fisch MJ, Liu Y, et al. Association Between a National Insurer’s Pay-for-Performance Program for Oncology and Changes in Prescribing of Evidence-Based Cancer Drugs and Spending. J Clin Oncol 2020. Dec 1;38(34):4055–63. [DOI] [PubMed] [Google Scholar]
- 19.Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw 2017. Jul;15(7):883–93. [DOI] [PubMed] [Google Scholar]
- 20.Encinosa W, Davidoff AJ. Changes in Antiemetic Overuse in Response to Choosing Wisely Recommendations. JAMA Oncol 2017. Mar 1;3(3):320–6. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality, Centers for Medicare & Medicaid Services. Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries: Final Report. Penny Hill Press, editor. CreateSpace Independent Publishing Platform; 2017. 78 p. [Google Scholar]
- 22.Parikh RB, Fishman E, Chi W, Zimmerman RP, Gupta A, Barron JJ, et al. Association of Utilization Management Policy With Uptake of Hypofractionated Radiotherapy Among Patients With Early-Stage Breast Cancer. JAMA Oncol 2020. Apr 16; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermeyer Z, Powers BW, Makar M, Keating NL, Cutler DM. Physician Characteristics Strongly Predict Patient Enrollment In Hospice. Health Aff (Millwood). 2015. Jun;34(6):993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipitz-Snyderman A, Sima CS, Atoria CL, Elkin EB, Anderson C, Blinder V, et al. Physician-Driven Variation in Nonrecommended Services Among Older Adults Diagnosed With Cancer. JAMA Intern Med 2016. Oct 1;176(10):1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar A, Parulekar M, Sanders A. Impact of COVID-19 Pandemic on Utilization of the Inpatient Hospice Services (General Inpatient Hospice). Am J Hosp Palliat Care. 2022. Aug;39(8):996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine DM, Samal L, Neville BA, Burdick E, Wien M, Rodriguez JA, et al. The Association of the First Surge of the COVID-19 Pandemic with the High- and Low-Value Outpatient Care Delivered to Adults in the USA. J Gen Intern Med 2022. Nov;37(15):3979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahzad M, Zirui Song MD, Chernew M, Fendrick A. Changes in Use of Low-Value Services During the COVID-19 Pandemic. 2022. Apr 22 [cited 2023 Sep 1];28. Available from: https://www.ajmc.com/view/changes-in-use-of-low-value-services-during-the-covid-19-pandemic [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AL, Brennan TA, Verbrugge DJ, Newhouse JP. Measuring the Scope of Prior Authorization Policies: Applying Private Insurer Rules to Medicare Part B. JAMA Health Forum 2021. May 28;2(5):e210859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating NL, Jhatakia S, Brooks GA, Tripp AS, Cintina I, Landrum MB, et al. Association of Participation in the Oncology Care Model With Medicare Payments, Utilization, Care Delivery, and Quality Outcomes. JAMA 2021. Nov 9;326(18):1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapadia NS, Brooks GA, Landrum MB, Riedel L, Liu PH, Hassol A, et al. Association of the Oncology Care Model With Value-Based Changes in Use of Radiation Therapy. Int J Radiat Oncol Biol Phys 2022. Feb 9;S0360–3016(22)00090–6. [DOI] [PubMed] [Google Scholar]
- 31.Brooks GA, Landrum MB, Kapadia NS, Liu PH, Wolf R, Riedel LE, et al. Impact of the Oncology Care Model on Use of Supportive Care Medications During Cancer Treatment. JCO 2022. Jun;40(16):1763–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Caption: Trends in cancer diagnoses, January 2016 to March 2021.
Source: HealthCore Integrated Research Environment.
Legend: Points represent monthly number of newly diagnosed cancer patients in the pre-COVID (January 2016 to February 2020) and COVID (March to December 2020) periods. Dx = Diagnoses


