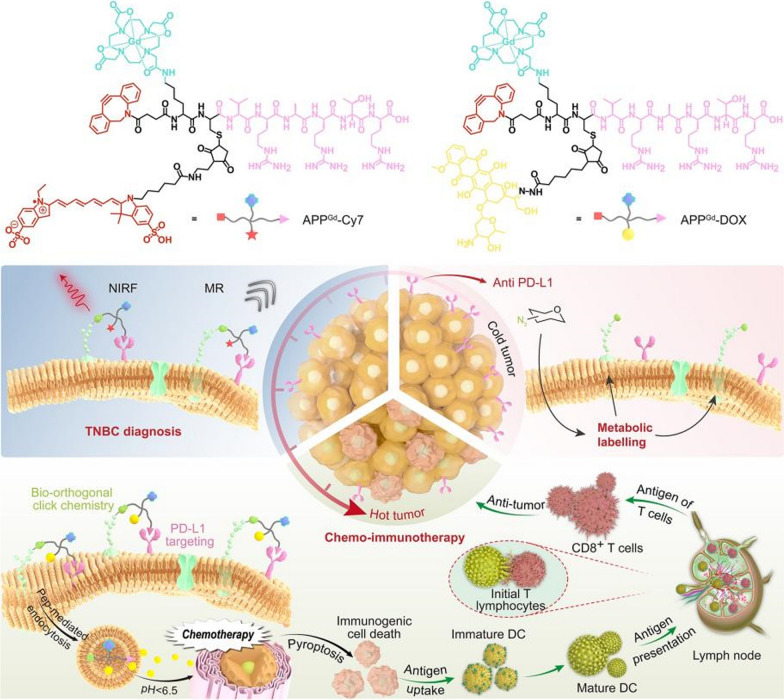
Abstract
Background
The combination of programmed cell death ligand-1 (PD-L1) immune checkpoint blockade (ICB) and immunogenic cell death (ICD)-inducing chemotherapy has shown promise in cancer immunotherapy. However, triple-negative breast cancer (TNBC) patients undergoing this treatment often face obstacles such as systemic toxicity and low response rates, primarily attributed to the immunosuppressive tumor microenvironment (TME).
Methods and results
In this study, PD-L1-targeted theranostic systems were developed utilizing anti-PD-L1 peptide (APP) conjugated with a bio-orthogonal click chemistry group. Initially, TNBC was treated with azide-modified sugar to introduce azide groups onto tumor cell surfaces through metabolic glycoengineering. A PD-L1-targeted probe was developed to evaluate the PD-L1 status of TNBC using magnetic resonance/near-infrared fluorescence imaging. Subsequently, an acidic pH-responsive prodrug was employed to enhance tumor accumulation via bio-orthogonal click chemistry, which enhances PD-L1-targeted ICB, the pH-responsive DOX release and induction of pyroptosis-mediated ICD of TNBC. Combined PD-L1-targeted chemo-immunotherapy effectively reversed the immune-tolerant TME and elicited robust tumor-specific immune responses, resulting in significant inhibition of tumor progression.
Conclusions
Our study has successfully engineered a bio-orthogonal multifunctional theranostic system, which employs bio-orthogonal click chemistry in conjunction with a PD-L1 targeting strategy. This innovative approach has been demonstrated to exhibit significant promise for both the targeted imaging and therapeutic intervention of TNBC.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02727-7.
Keywords: Click chemistry, Metabolic glycoengineering, Cancer immunotherapy, Prodrug, Triple negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) demonstrates the most aggressive subtype with poorest outcomes and highest propensity for metastasis among all breast cancers [1]. Over the past few years, immune checkpoint blockade (ICB) directed towards programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) have exhibited prolonged survival outcomes in solid tumors [2–6]. A greater prevalence of PD-L1 expression has been observed in TNBC in comparison to other subtypes of breast cancer, indicating that PD-L1 may represent a promising therapeutic target [7, 8]. Nevertheless, the efficacy of ICB monotherapy in TNBC is constrained by a poorly immunogenic tumor microenvironment (TME) and limited tumor penetration [1, 9].
Hence, reversal of immunosuppressive tumor microenvironment (ITM) is crucial to maximizing ICB efficacy in TNBC. Numerous studies have demonstrated that specific chemotherapeutic agents, including doxorubicin (DOX) [10], paclitaxel [11], and oxaliplatin [12], not only elicit antitumor cytotoxic effects but also facilitate immunogenic cell death (ICD). This process involves the release of immunostimulatory danger-associated molecular patterns (DAMPs) such as calreticulin (CRT), high mobility group box 1 (HMGB1), and adenosine triphosphate (ATP) [13]. DAMPs are essential in promoting dendritic cell (DC) maturation and facilitating antigen presentation to T cells within lymph nodes, ultimately leading to the activation of adaptive immunity [14]. Accumulating evidence indicate that the combination of ICB targeting the PD-1/PD-L1 signaling axis and ICD-inducing chemotherapy synergistically enhances the antitumor effects for TNBC [15–19]. The underlying mechanism may involve chemo-immunotherapy leading to the transformation of immunologically ‘cold’ tumors into ‘hot’ lesions, which are characterized by the infiltration of mature DCs and the presence of cytotoxic T lymphocytes (CTLs).
In recent years, PD-1/PD-L1 peptide-based inhibitors are gaining attention in cancer immunotherapy and tumor-targeted delivery [20–26]. Compared to monoclonal antibodies, which are most used in cancer immunotherapy, peptides offer several advantages in ligand targeting. For instance, peptides can penetrate tissues more effectively, have a lower likelihood of inducing immunogenicity, are more cost-effective to manufacture, and are easier to quality control during synthesis [9, 20, 23]. Nonetheless, peptides exhibit limitations, including low target affinity, rapid renal clearance, and susceptibility to degradation [27–29]. Thus, it is imperative to enhance the efficacy of PD-L1 antagonist peptides in blocking PD-1/PD-L1 interactions.
Azide/cyclooctyne-based bio-orthogonal chemistry occurs in vivo, while maintaining the structural integrity and biological activity [30–33]. Azides (–N3), a bio-orthogonal functional group, can be metabolically incorporated into cell-surface glycoproteins [33, 34]. In contrast to the transient binding typically observed in physical interactions between ligands and endogenous receptors, azides, acting as ‘chemical receptors,’ demonstrate high specificity and effectiveness in binding with cyclooctyne-labeled biomolecules with long-term retention [33, 35]. Herein, making use of the recognition by anti-PD-L1 peptides and cyclooctyne/azide-based bio-orthogonal click chemistry, we propose anti-PD-L1 peptide-based biomolecules to achieve efficient and sustained PD-L1 targeting to enhance the efficacy and sustainability of ICB therapy.
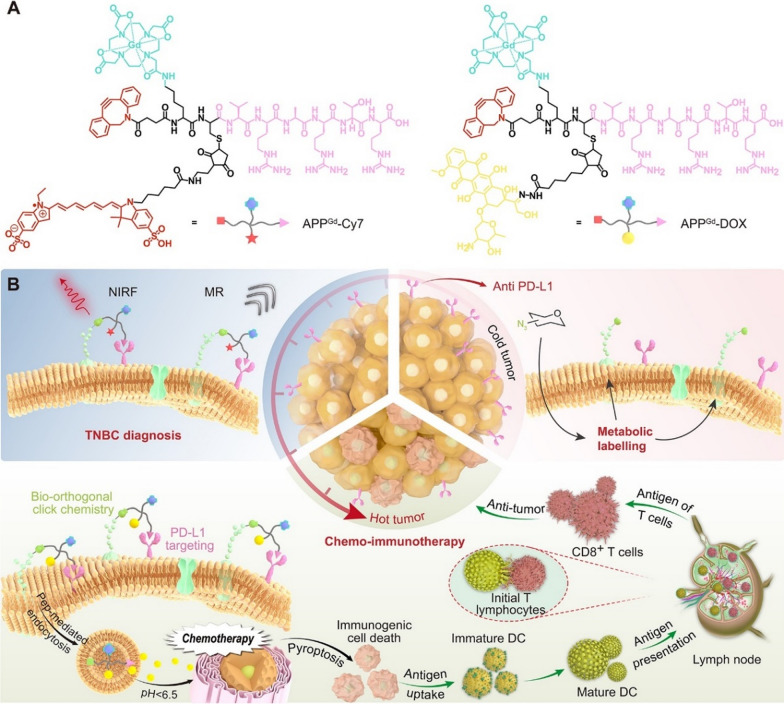
In this study, we present PD-L1-targeted theranostic systems modified with PD-L1 antagonist peptide (CVRARTR; Cys-Val-Arg-Ala-Arg-Thr-Arg), DOTA, and dibenzocyclooctyne (DBCO). We first designed a gadolinium (Gd)-based probe APPGd-Cy7, which is decorated with fluorescent sulfo-Cyanine7 (Cy7), aiming for achieving targeted PD-L1 magnetic resonance/near-infrared fluorescence (MR/NIRF) imaging in vivo for TNBC. Then, we replaced the fluorescence imaging module of Cy7 with the drug-loading module of DOX to generate prodrug APPGd-DOX, with the goal of synergistic chemo-immunotherapy for TNBC. As illustrated in Scheme 1, TNBC cells (4T1) overexpressing PD-L1 as natural receptors were pretreated with tetraacetyl-N-azidoacetylmannosamine (Ac4ManNAz) to produce azides as ‘chemical receptors’ on cell surfaces. APPGd-Cy7 can recognize PD-L1 expressed on 4T1 cells, followed by conjugating with azide-labeled glycoproteins on 4T1 cells via bio-orthogonal click chemistry, remarkably improving and extending MR/NIRF imaging. Following this, the prodrug APPGd-DOX accumulates in the tumor via PD-L1 targeting and covalently bind to the azide-rich tumor cell surfaces, significantly facilitating uptake and internalization of this prodrug in TNBC cells. The utilization of PD-L1 antagonist peptide conjugated prodrug facilitates the inhibition of PD-L1, thereby impeding tumor immune evasion and augmenting antitumor immunity. Additionally, the APPGd-DOX prodrug enhances drug delivery efficiency by acidic pH-responsive DOX release, evoking the ICD effect and DAMPs release, further promoting DC maturation and activation of CTLs. Consequently, the enhanced targeted delivery of APPGd-DOX prodrug demonstrated significant efficacy in inhibiting tumor growth by inducing a potent immune response against the tumor, ultimately resulting in the restoration of immune tolerance in TNBC.
Scheme 1.
Illustration of the bio-orthogonal click chemistry strategy for PD-L1-targeted imaging and pyroptosis-mediated chemo-immunotherapy of TNBC. A Chemical structure of PD-L1-targeted probe APPGd-Cy7 and prodrug APPGd-DOX. B In vivo MR/NIRF imaging process involves metabolism glycoengineering, and the PD-L1 targeting accumulation of APPGd-Cy7 in tumor tissue; In vivo synergistic chemo-immunotherapy strategy includes metabolism glycoengineering, PD-L1 targeting, and pH-responsive drug release and pyroptosis-mediated regulation of antitumor immunity
Materials and methods
Agents
Lyso-Tracker, Mito-Tracker, and DiO green, DAPI solution, Hoechst 33,342 working solution (1 ×), and CCK-8 assay kit were sourced from Beyotime Ltd. The Millipore filtration system was utilized to purify water. Flow cytometry antibodies, including anti-CD3-APC, anti-CD4-FITC, anti-CD8-PercpCy5.5, anti-IFN-γ-PE/Cy7, anti-CD25-PE/Cy7, anti-Foxp3-V450, anti-MHC II-V450, anti-CCR7-PE, anti-CD80-FITC, and anti-CD40-APC antibodies were provided by BioLegend, Inc. Anti-PD-L1-antibody was sourced from Proteintech (Chicago, USA, 66248-1-Ig). Anti-Calreticulin, anti-HMGB1, anti-Cleaved-Caspase-3, anti-Cleaved-GSDME, and anti-GSDME antibodies were obtained from Abcam (Cambridge, MA, ab92516, ab79823, ab52101, ab222470, and ab215191). Doxorubicin hydrochloride (DOX) was obtained from Bide Pharmatech Ltd. (Shanghai, China), while Ac4ManNAz was procured from Sigma-Aldrich (Saint Louis, USA, 900917).
Cell culture
The murine 4T1 and HC11 (mouse mammary epithelial cells) cell lines were procured from the Cell Bank of the Chinese Academy of Sciences in Shanghai, China. These cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution at 37 °C in a 5% CO2 humidified environment.
Synthesis of APPGd-Cy7, APPGd-Cy5, and APPGd-DOX
The peptide was produced on a rink amide resin (loading: 0.81 mmol/g) with the assistance of a CEM Liberty one microwave-assisted peptide synthesizer. Following the attachment of all amino acids and DOTA, the resultant products were transferred to a glass peptide synthesis vessel. Following deprotection of the Fmoc group, the compounds were conjugated with N-succinimidyl 4-[(5-aza-3,4:7,8-dibenzocyclooct-1-yne)-5-yl]-4-oxobutyrate (DBCO-NHS). After conjugation of DBCO, the resultant product was dissociated from its solid support through treatment using a cleavage cocktail composed of trifluoroacetic acid-triisopropylsilane-H2O (95:2.5:2.5). Subsequently, the solution was eliminated, and the resulting white solids were precipitated by adding dry diethyl ether, followed by centrifugation at a speed of ~ 6500 rpm for ~ 15 min. The peptide was subsequently purified and isolated using preparative high-performance liquid chromatography (HPLC) with a C-18 column. The compounds APPGd-Cy7 and APPGd-Cy5 were synthesized by coordinating gadolinium (Gd) ions with the DOTA chelator on APP and then conjugating them with fluorescent Cy7 or sulfo-cyanine 5 (Cy5) through a thiol-maleimide reaction. Similarly, the prodrug APPGd-DOX was prepared by labeling with Gd3+ ions and conjugating with the 6-maleimidocaproylhydrazone derivative of doxorubicin (Mal-DOX) using a thiol-maleimide reaction.
Physical characterization and drug release profile
The 1H nuclear magnetic resonance (1H-NMR) and attenuated total reflectance Fourier transform infrared (ATR FT-IR) spectrum of APP, APPGd-Cy5, APPGd-Cy7 and APPGd-DOX were obtained at room temperature with a Bruker ADVANCE NEO 600 MHz NMR spectrometer and a Nicolet 6700 FTIR spectrometer, respectively. The absorption and fluorescence spectra of APPGd-Cy7, APPGd-Cy5 and APPGd-DOX were assessed at varying concentrations (2.5–10 μM). The cumulative release of DOX was evaluated through dialysis of the APPGd-DOX prodrug in phosphate-buffered saline (PBS) at varying pH levels (7.4, 6.5, 6.0, and 5.0). Dialysis was conducted using a dialysis tube with a molecular weight cutoff (MWCO) of 1000 for a duration of 10 h. The release of DOX was monitored by measuring absorbance values at 490 nm using a UV–visible spectrometer (Varian Cary 100 Conc).
Cellular uptake and colocalization analysis
4T1 cells were treated with APPGd-Cy5 (10 μM) following a 3-day incubation with 50 μM Ac4ManNAz, and subsequently stained with DAPI to visualize the cell nucleus. To examine intracellular distribution, 4T1 cells were exposed to APPGd-Cy5 (10 μM) for 4 h, followed by staining with 5 μM DiO green, 50 nM Lyso-Tracker green or 100 nM Mito-Tracker green for 30 min, and Hoechst33342 working solution (1 ×) for an additional 15 min as per standard protocol. Following washes with PBS twice, fluorescence imaging was conducted utilizing a confocal laser scanning microscope (CLSM, Leica TCS SP5, Germany). The cellular uptake of DOX was assessed in 4T1 cells following treatment with DOX, APPGd-DOX, and Click-APPGd-DOX at a concentration of 2 μM.
In vitro cytotoxicity assay
To assess the cytotoxicity of Ac4ManNAz, 4T1 and HC11 cells were cultured in 96-well plates and incubated with varying concentrations of Ac4ManNAz (0–50 μM) for a duration of three days. Subsequently, cell viability was subsequently assessed using a CCK-8 assay. Following this, the same cells were cultured with either 0 or 50 μM Ac4ManNAz for three days in 96-well plates, and then treated with DOX and APPGd-DOX at different concentrations (0–10 μM) for a period of 24 h. Cell viability was evaluated again using the CCK-8 assay.
Western blot analysis of cells
4T1 cells were exposed to either 0 or 50 μM Ac4ManNAz for a duration of 3 days, subsequently treated with different agents (DOX, APP, and APPGd-DOX) at a uniform concentration of 2 μM for a period of 24 h. A protease inhibitor-supplemented RIPA buffer was used to lyse the cells following treatments. The expression levels of PD-L1, CRT, HMGB1, GSDME-FL, GSDME-N, and Cleaved Caspase-3 were then evaluated through western blot analysis.
RNA sequencing
4T1 cells were collected following treatment with or without APPGd-DOX, and total RNA was extracted for RNA sequencing. The resulting transcriptomic sequencing data was analyzed and visualized utilizing an online platform available at https://www.bioinformatics.com.cn. All genes with an adjusted p-value < 0.05 and fold change > 1.5 were considered significant.
Animal experiment
Female BALB/c mice, 5–6 weeks old, were sourced from the Shanghai Experimental Animal Center and kept in a pathogen-free facility. All animal experiments followed approved guidelines from the Ethics Committee of Xinhua Hospital, affiliated with Shanghai Jiao Tong University School of Medicine (XHEC-F-2023-014). Subcutaneous injections of 1.0 × 106 4T1 cells suspended in 100 μL PBS were administered to each mouse on the flanks.
In vivo and ex vivo fluorescence (FL) imaging
Mice were divided into three groups (n = 3 per group): PEG-Cy7, APPGd-Cy7, and Click-APPGd-Cy7. Prior to this, mice with 4T1 tumors received intraperitoneal injections of either 40 mg/kg Ac4ManNAz once daily for 3 days or were injected with PBS. Subsequently, the cohorts were intravenously injected with 1 mg/kg PEG-Cy7 or 1 mg/kg APPGd-Cy7. Under isoflurane anesthesia, in vivo fluorescence imaging of the 4T1 tumor-bearing mice was carried out using the IVIS spectrum system (PerkinElmer), with excitation at 710 nm and emission collected at 760 ± 20 nm. Imaging and analysis of the mice were conducted at specific time points.
In vivo MR imaging
Following the formation of the subcutaneous tumor, Ac4ManNAz (40 mg/kg) was administered intraperitoneally once daily for three days to the Click-APPGd-Cy7 group (n = 3). The Gd-DOTA and APPGd-Cy7 groups (n = 3 per group) received PBS injections as the control. Subsequently, mice with 4T1 tumors were intravenously injected with Gd-DOTA (150 μL, 1 mg/kg) or APPGd-Cy7 (150 μL, 1 mg/kg). Magnetic resonance images were obtained prior to the injection of Gd-DOTA or APPGd-Cy7, as well as at 1-, 2-, 3-, and 4-h(s) post-injection.
Antitumor activity
After reaching a subcutaneous tumor volume of approximately 150 mm3, 4T1 tumor-bearing mice were randomly assigned to five groups (n = 5 per group): (1) PBS, (2) DOX, (3) APPGd-DOX, (4) Click-APP, and (5) Click-APPGd-DOX. In the click groups, Ac4ManNAz (40 mg/kg) was administered intraperitoneally once daily for 3 days, while other groups received PBS injections. Subsequently, all groups were treated every seven days with PBS, DOX, APP, or APPGd-DOX (5 mg/kg). Body weights and tumor volumes were measured every two days for 14 days. Tumor volume (V) was calculated according to the formula: V (mm3) = 0.5 × L (mm) × W2 (mm2).
Histological examination
Fresh tumors and organs were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for various analyses, including H&E staining, immunohistochemical staining, and immunofluorescence staining. For immunofluorescence staining, primary antibodies against PD-L1, CRT, HMGB1, Caspase-3, CD4, and CD8 were utilized. The process involved deparaffinization, heat-induced antigen retrieval using a citrate solution, and blocking with 5% BSA. The samples were subjected to overnight incubation with primary antibodies at 4 ℃, followed by a 1-h incubation with secondary antibodies at room temperature. DAPI was utilized as a nuclear counterstain for immunofluorescence staining.
Flow cytometry analysis, HMGB1 release, and cytokine assay
Following various treatments, spleens, tumors, and tumor-draining lymph nodes (TDLNs) from all experimental groups (n = 5 per group) were excised and subsequently subjected to enzymatic digestion using collagenase IV at 37 °C for a duration of 1 h. The digested tissues were subsequently filtered through a 70 μm cell strainer to obtain single-cell suspensions. These isolated single cells were then suspended in a staining buffer and labeled with fluorescent-conjugated antibodies to identify specific cell populations, including CTLs (CD3+CD4−CD8+), CD4+T cells (CD3+CD4+CD8−), mature DCs (CD40+CD80+MHC II+CCR 7+), and Tregs (CD4+CD25+Foxp3+). Enzyme-linked immunosorbent assay (ELISA) kits were employed to quantify the levels of HMGB1, IFN-γ, TNF-α, and IL-12 in serum specimens obtained from mice following different treatments.
Statistics
Quantitative values were presented as mean ± standard deviation (SD), and statistical analyses were conducted using a two-tailed Student's t-test and one-way ANOVA. A significance level of P < 0.05 was employed to determine statistical significance, denoting a distinction between the groups under comparison.
Results and discussion
Preparation and characterization of APPGd-Cy5, APPGd-Cy7 and APPGd-DOX
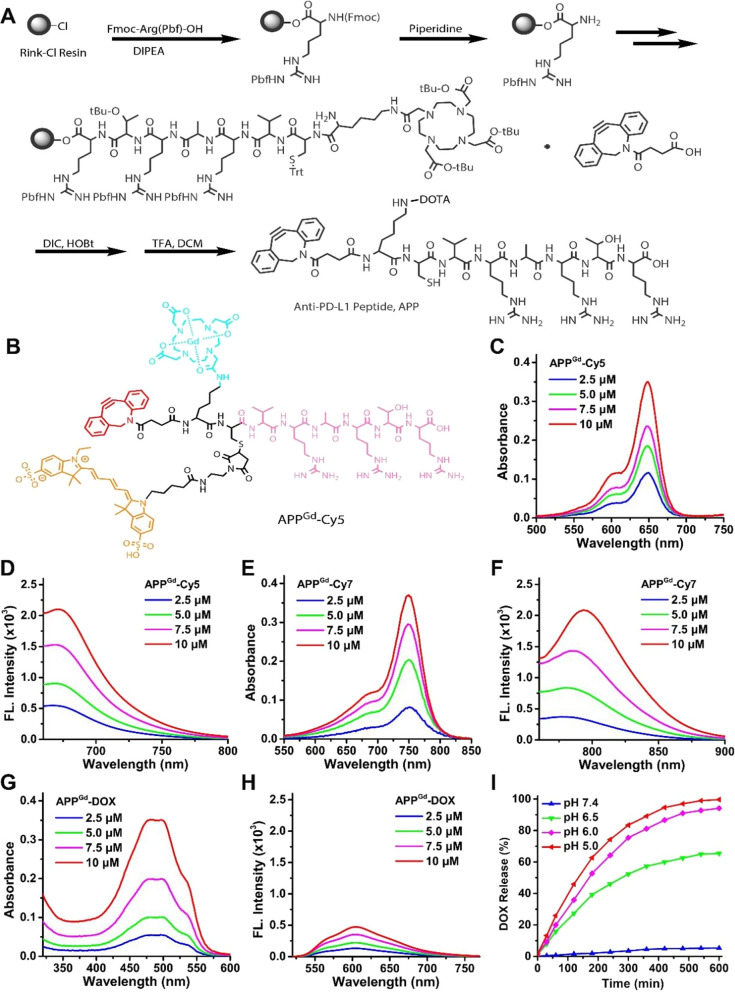
The APP modified with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was synthesized via conventional Fmoc-based solid-phase peptide (SPPS) methodology, as illustrated in Fig. 1A. Characterization of the isolated APP was conducted utilizing high-performance liquid chromatography (HPLC) and mass spectrometry (Figures S1 and S2). For MR/fluorescence imaging purposes, the compounds APPGd-Cy5 and APPGd-Cy7 were prepared by labeling the peptide with gadolinium (Gd) ions through coordination with the DOTA chelator, followed by conjugation with fluorescent sulfo-cyanine 5 (Cy5) or sulfo-cyanine7 (Cy7) using a thiol-maleimide reaction (Scheme 1A and Fig. 1B). Similarly, the compound APPGd-DOX prodrug was labeled with Gd3+ ions and the acid-labile hydrazone bond-linked Mal-DOX using a thiol-maleimide reaction (Scheme 1A). The chemical structures of APP, APPGd-Cy5, APPGd-Cy7 and APPGd-DOX were further characterized by 1H-NMR and ATR FT-IR spectrum (Figures S3–S10). Absorbance and fluorescence spectra of the labeled compounds, including APPGd-Cy5, APPGd-Cy7, and APPGd-DOX, were analyzed in PBS (pH 7.4). The absorption and fluorescence peaks of APPGd-Cy5 were observed at ~ 650 nm and 670 nm, respectively (Fig. 1C, D). APPGd-Cy7 exhibited absorbance at 750 nm and strong fluorescence intensity at 780 nm, facilitating enhanced photon penetration through tissues while minimizing the impact of tissue autofluorescence (Fig. 1E, F). Moreover, the absorbance of APPGd-DOX was noted at 480 nm, with a low fluorescence intensity detected at 610 nm (Fig. 1G, H).
Fig. 1.
Preparation and characterization of APPGd-Cy5, APPGd-Cy7, and APPGd-DOX prodrug. A Schematic synthesis route of APP. B Chemical structure of APPGd-Cy5. C–H The absorption and fluorescence spectra of APPGd-Cy5, APPGd-Cy7, and APPGd-DOX prodrug at various concentrations (2.5–10 μM). I The cumulative release of DOX from the APPGd-DOX prodrug was observed following incubation at pH levels of 7.4, 6.5, 6.0, and 5.0, respectively
Given that DOX was linked to APPGd-DOX through an acid-labile hydrazone bond, it is expected to exhibit pH-sensitive release within endo/lysosomal environments (pH ~ 5.0). To assess this, the pH-responsive degradation kinetics of APPGd-DOX were quantitatively analyzed using dialysis tubes with a molecular weight cutoff of 1000 Da in PBS at varying pH levels (7.4, 6.5, 6.0, and 5.0). Analysis depicted in Fig. 1I revealed minimal DOX release (~ 5%) from APPGd-DOX over 600 min in PBS at pH 7.4, suggesting the robust stability of the prodrug in physiological condition. In contrast, the activation of APPGd-DOX demonstrated rapid kinetics, leading to the release of over 60% and 90% of DOX after 10 h of incubation in PBS at pH levels of 6.5 and 6.0, respectively. Conversely, near 100% of DOX was released from APPGd-DOX at pH 5.0 within the same time, highlighting the sensitivity of APPGd-DOX to endo-/lysosomal pH. This characteristic suggests that APPGd-DOX has the potential to minimize premature drug release and reduce adverse effects in healthy tissues, while facilitating targeted intracellular drug delivery, thereby offering significant advantages in cancer treatment.
In vitro cellular uptake and cytotoxicity evaluation
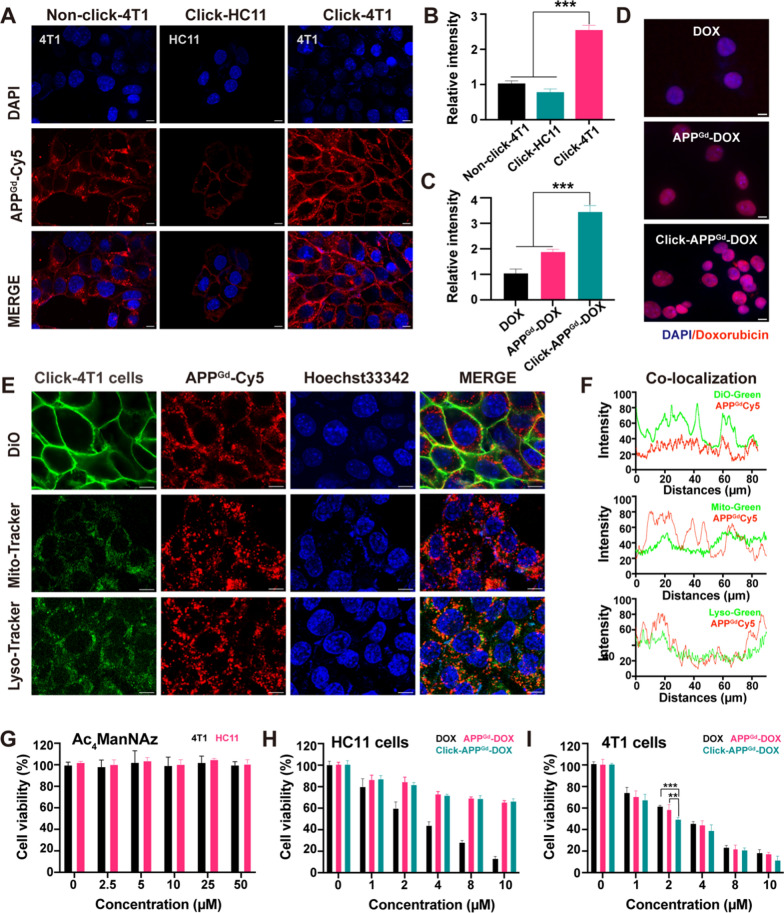
We first labeled 4T1 cells with azide groups by treating them with 50 μM Ac4ManNAz. Subsequently, Ac4ManNAz-pretreated 4T1 cells were incubated with 10 μM APPGd-Cy5. The uptake efficiency of APPGd-Cy5 reflected variations in the formation of azides. After incubation with APPGd-Cy5, Ac4ManNAz-pretreated 4T1 cells exhibited a 2.52 ± 0.16-fold higher fluorescence intensity compared to non-pretreated 4T1 cells (Fig. 2A, B). We also conducted azide group labeling on HC11 cells using Ac4ManNAz pretreatment. Following incubation with APPGd-Cy5, Ac4ManNAz-pretreated HC11 cells exhibited a weaker fluorescence intensity compared to non-pretreated 4T1 cells (Fig. 2A, B), indicating PD-L1 targeting for 4T1 cells. Overall, in Ac4ManNAz-pretreated 4T1 cells, the click efficiency of APPGd-Cy5 with azide groups significantly increased. This enhanced click efficiency suggests promising potential for tumor-targeted drug delivery. We further investigated the subcellular organelle localization behavior of APPGd-Cy5 in Ac4ManNAz-pretreated 4T1 cells, and the results were quantified utilizing ImageJ. As illustrated in Fig. 2E, F a substantial amount of APPGd-Cy5 was internalized into lysosomes. A notable degree of co-localization between APPGd-Cy5 (red) and lysosomes (green) was observed in Ac4ManNAz-pretreated 4T1 cells following a 4-h incubation period. Additionally, the fluorescence intensity of DOX showed a 3.42 ± 0.28-fold increase in Click-APPGd-DOX, and a 1.85 ± 0.13-fold increase in APPGd-DOX than in DOX (Fig. 2C, D). Substantial anti-PD-L1 peptide was internalized into lysosomes. Recent studies have highlighted that the controlled targeting of PD-L1 for lysosomal degradation is pivotal in preventing the undesirable recycling of PD-L1 [36, 37]. Consequently, the intracellular disposition of APPGd-DOX has been shown to enhance the lysosomal degradation of PD-L1, thereby potentially increasing its therapeutic efficacy. Furthermore, APPGd-DOX specifically releases DOX and accumulated in 4T1 cells, amplifying the enhanced cytotoxicity of DOX.
Fig. 2.
A CLSM images of APPGd-Cy5 (10 μM) in 4T1 cells treated with either 0 or 50 μM Ac4ManNAz (Non-click-4T1 or Click-4T1), and HC11 cells treated with 50 μM Ac4ManNAz (Click-HC11). B Relative FL intensities of APPGd-Cy5 (10 μM) in 4T1 cells treated with either 0 or 50 μM Ac4ManNAz, and HC11 cells treated with 50 μM Ac4ManNAz. (C) Relative FL intensities and (D) fluorescence images of DOX in 4T1 cells incubated with DOX, APPGd-DOX, and Click-APPGd-DOX (2 μM). DOX. E CLSM images of 4T1 cells treated with 10 μM APPGd-Cy5, co-stained with 5 μM DiO, or 100 nM Mito-Tracker or 50 nM Lyso-Tracker green. F Co-localization curves. Scale bars: 10 µm. Cell viabilities of (G) HC11 and 4T1 cells treated with Ac4ManNAz, (H) HC11 cells or (I) 4T1 cells treated with DOX, APPGd-DOX, and Click-APPGd-DOX
We further examined the cytotoxicity of Ac4ManNAz and observed no significant harm to normal HC11 cells and 4T1 cells at concentrations up to 50 μM (Fig. 2G). Subsequently, we evaluated the cytotoxic effects of DOX, APPGd-DOX, and Click-APPGd-DOX in HC11 and 4T1 cells. As shown in Fig. 2H, DOX displayed non-selective cytotoxicity, with comparable levels of toxicity observed in both cell types. As illustrated in Fig. 2I, APPGd-DOX demonstrated an enhanced cytotoxic response against 4T1 cells at a concentration of 2 μM, compared to DOX alone. This enhanced response is attributed to the PD-L1 targeting effect of APPGd-DOX, which allows for killing 4T1 cells at relatively low drug doses, thereby minimizing non-specific cytotoxicity. Furthermore, Click-APPGd-DOX exhibited more potent tumor cell-specific cytotoxicity than APPGd-DOX at the same concentration of 2 μM, suggesting that the bio-orthogonal click chemistry strategy enhances PD-L1-targeted delivery efficacy.
In vitro PD-L1 binding
Western blotting was used to assess the expression level of PD-L1 in vitro. 4T1 cells exhibited a higher PD-L1 level compared to murine mammary epithelial HC11 cells (Figure S3). Several pre-clinical and clinical studies demonstrated PD-L1 as a potently promising target for TNBC [8, 16, 38–40]. DOX treatment also induced an elevation in PD-L1 expression of 4T1 cells [41, 42], resulting in exhaustion of T cells and immune tolerance, key components of tumor immune escape [43–47]. In addition, several studies have revealed a significant upregulation of PD-L1 protein levels in tumor specimens derived from TNBC patients following DOX chemotherapy, which is beneficial for enhancing the efficacy of PD-1/PD-L1 blockade immunotherapy [38, 48]. The mechanism of PD-L1 upregulation after DOX chemotherapy may include inflammatory signaling [49], the DNA damage response pathway [50], activation of NF-κB pathway [51], and oxidative stress [52]. Nevertheless, the administration of APPGd-DOX in Ac4ManNAz-pretreated 4T1 cells showed reduced PD-L1 expression levels compared to the control group (Fig. 3E). CVRARTR, a peptide antagonist of PD-L1, serves as a promising alternative to monoclonal antibodies for ICB, which has been widely studied in recent years [20, 37, 53, 54]. The internalization-induced downregulation of PD-L1 by binding peptides not only directly impedes the binding of PD-L1 but also indirectly augments their efficacy in inhibiting the PD-1/PD-L1 interaction by reducing the cell surface availability of PD-L1 [20, 37]. Therefore, we speculate that the binding of anti-PD-L1-Peptide (CVRARTR) to PD-L1-overexpressing cells is specifically mediated through PD-L1, and the downregulation of PD-L1 to the inhibition of PD-L1 by PD-L1-binding peptides.
Fig. 3.
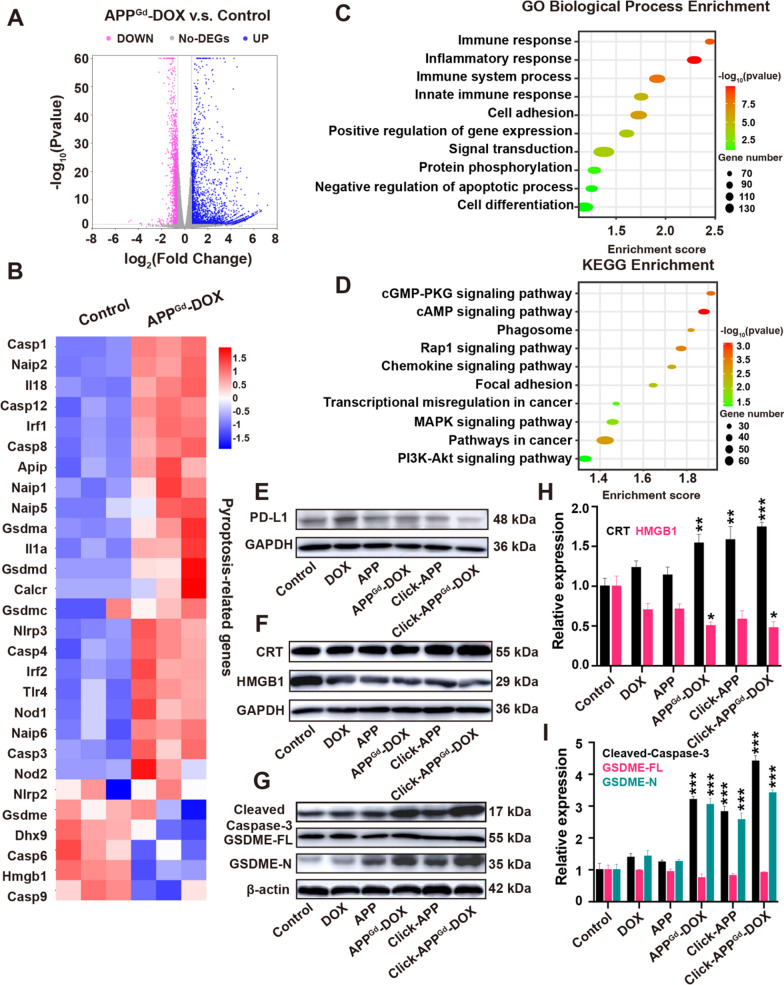
A Volcano plot of DEGs, (B) heatmap to visualize the expression of pyroptosis-related genes, (C) GO biological process analyses of DEGs, and (D) KEGG analyses of DEGs in the control vs APPGd-DOX. E–I Western blot analysis of PD-L1, CRT, HMGB1, Cleaved-caspase-3, GSDME-FL, and GSDME-N in 4T1 cells after treatments with Control, DOX, APP, APPGd-DOX, Click-APP, and Click-APPGd-DOX, respectively. *P < 0.05, **P < 0.01, and ***P < 0.001 vs Control group
Evaluation of ICD and pyroptosis in vitro
RNA sequencing of 4T1 cells was conducted following APPGd-DOX treatment. Comparative analysis with the control group revealed 3781 differentially expressed transcripts in the APPGd-DOX group, with 1439 downregulated and 2342 upregulated. These differentially expressed genes (DEGs) are displayed on a standard volcano plot (Fig. 3A). The Gene Ontology (GO) function analysis histogram, presented in Figure S13, delineates biological process, cellular ingredients, and molecular functions. GO analysis within the biological processes category (Fig. 3C) revealed enrichment in genes related to immune response, immune system processes, and innate immune responses. KEGG pathway analysis (Fig. 3D) indicated enrichment in proteins associated with pathways in cancer, cAMP signaling, MAPK signaling, and PI3K-Akt signaling. The enrichment of genes in these pathways indicates that pyroptosis plays a complex role across a range of biological processes. Pyroptosis aids in the removal of harmful pathogens and cells that have been compromised, and it also has the capacity to modulate inflammatory pathways, which could have implications for the behavior of tumors.
The immunogenicity of ICD is predominantly facilitated by DAMPs, including exposed CRT on cell surfaces, secreted ATP, and released HMGB1 [55]. These DAMPs, generated during ICD, can interact with pattern recognition receptors on the surface of DCs, initiating a cascade of cellular responses. These responses ultimately lead to the activation of immune responses, thereby augmenting the effectiveness of immunotherapy. Several studies have provided evidence that immune response against tumors can be augmented by ICD in conjunction with ICB therapy [14, 56, 57]. Moreover, the presence of CRT on cell surfaces and the release of HMGB1 from the nucleus are considered hallmarks signifying the induction of ICD. To substantiate our hypothesis and probe the mechanisms involved, we conducted western blot analysis in vitro to assess the expression of CRT and HMGB1, as indicators of ICD. Figure 3F, H suggests that upon treatment with Click-APPGd-DOX, there is a marked increase in CRT exposure in 4T1 cells, along with a noticeable decrease in intracellular HMGB1 secretion, potentially indicating substantial release from the nucleus. This implies that the combination of chemo-immunotherapy and biorthogonal click chemistry strategies could achieve a potent synergistic therapeutic effect, resulting in significant induction of ICD and enhancing TNBC's immunogenicity.
Furthermore, Pyroptosis, a newly identified form of programmed cell death with unique morphological traits distinct from those of apoptosis and necrosis, is capable of inducing the release of antigens and subsequently triggering potent antigen-specific immune responses [58]. A heatmap displaying DEGs related to pyroptosis in vitro after APPGd-DOX was generated by transcriptomic analysis (Fig. 3B). Western blot analysis was further conducted to validate the occurrence of pyroptosis in vitro. Given that Caspase-3 precisely cleaves the middle linker of gasdermin E (GSDME) to generate the GSDME-N domain, subsequently triggering pyroptosis through the formation of membrane pores, Cleaved-Caspase-3 level and GSDME expression levels were assessed in response to various in vitro treatments [59]. As displayed in Fig. 3G, I, the Cleaved Caspase-3 showed a notable elevation in the Click-APPGd-DOX group. Similarly, GSDME-N was observed in Click-APPGd-DOX treated cells and exhibited the highest expression level. These findings affirm the ability of Click-APPGd-DOX to trigger Caspase-3-mediated GSDME-dependent pyroptosis in tumor cells.
In vivo and ex vivo for FL imaging
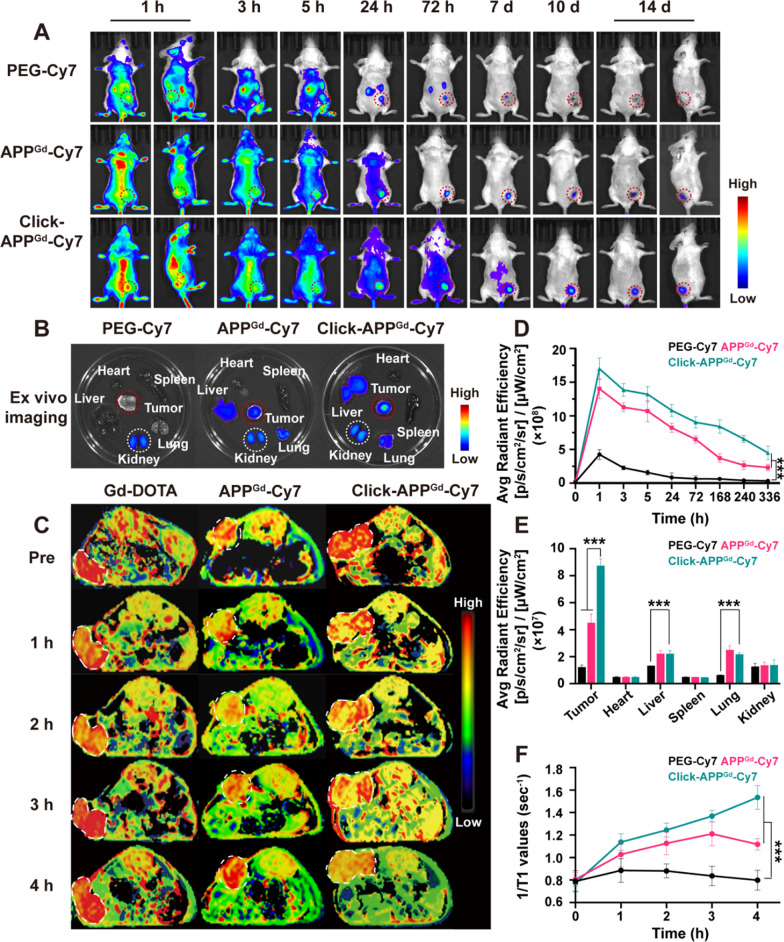
To assess enhanced tumor retention and biodistribution, in vivo FL imaging of mice with 4T1 tumors was conducted in three groups (n = 3): (1) PEG-Cy7, (2) APPGd-Cy7, and (3) Click-APPGd-Cy7. Upon reaching a tumor volume approximately 150 mm3, the group receiving click-containing treatment were intraperitoneally injected with Ac4ManNAz (40 mg/kg) daily for 3 days. Subsequently, mice were intravenously administered 150 μL (1 mg/kg) of PEG-Cy7 and APPGd-Cy7 via the tail vein. Using the IVIS spectrum, we recorded selected time points from 1 h to 14 days post-injection, as depicted in Fig. 4A. Compared to PEG-Cy7, mice injected with APPGd-Cy7 and Click-APPGd-Cy7 displayed significantly higher fluorescence signals during the imaging period, demonstrating prolonged tumor retention and efficient accumulation of PD-L1 targeting. Additionally, the fluorescence signals in Click-APPGd-Cy7 were the highest among the three groups, showing 4.00–15.81 times and 1.21–2.48 times higher signals compared to PEG-Cy7 and APPGd-Cy7 after injection, respectively (Fig. 4D). These results confirm the effective bio-orthogonal click reaction and PD-L1 targeting function to gain favorable retention of the APPGd-Cy7 in the tumor.
Fig. 4.
A In vivo FL imaging after injection of PEG-Cy7, APPGd-Cy7, or Click-APPGd−Cy7. B Ex vivo FL imaging. C In vivo MR-T1 mapping images of mice after injection of Gd-DOTA, APPGd-Cy7, or Click-APPGd−Cy7. D FL intensities in tumor regions and (E) ex vivo FL intensities of organs and tumors. F Time-dependent 1/T1 changes in MR-T1 mapping images after injection of Gd-DOTA, APPGd-Cy7, and Click-APPGd−Cy7. ***P < 0.001
To compare organ distribution in 4T1 tumor-bearing mice, tumors and major organs were excised on day 14, and the fluorescence intensity from excised organs and tumor tissues were measured using the IVIS spectrum. As illustrated in Fig. 4B, E, tumor retention of Click-APPGd-Cy7 group was the highest than the other two groups. Furthermore, the fluorescence intensities of tumors in Click-APPGd-Cy7 group were greatly higher compared to those in other tissues, such as the liver and kidneys, suggesting excellent retention and distribution efficiency within the tumor. Besides, the fluorescence signals of Click-APPGd-Cy7 and APPGd-Cy7 in liver and lungs were only surpassed by those in the tumor. We hypothesize that PD-L1 is highly expressed on cancer cells and macrophages, while macrophages are predominantly found in organs such as the liver and lungs [46].
In vivo MR-T1 mapping imaging
Subsequently, in vivo MR-T1 mapping imaging was performed on 4T1-tumor-bearing mice, randomly divided into 3 groups (n = 3 per group): (1) Gd-DOTA, (2) APPGd-Cy7, (3) Click-APPGd-Cy7, as depicted in Fig. 4C. At 1- and 2-h post-injection, all three groups exhibited enhanced tumor signals. By 3 h post-injection, signals in the Gd-DOTA group had returned to baseline levels, while signal enhancement continued in the Click-APPGd-Cy7 and APPGd-Cy7 groups. Furthermore, the signal enhancement in the APPGd-Cy7 reduced at 4-h post-injection, whereas Click-APPGd-Cy7 continued to exhibit sustained enhancement. To quantitatively evaluate the signal intensities of tumor retention, 1/T1 values were measured and illustrated in Fig. 4F. The 1/T1 values in both the Click- and non-click-APPGd-Cy7 groups consistently exceeded those in the Gd-DOTA group post-injection, indicating the enhanced active PD-L1 targeting. Furthermore, the highest 1/T1 values in Click-APPGd-Cy7 at 4-h post-injection were 1.98-times higher compared to the baseline scale, while the highest 1/T1 values in APPGd-Cy7 at 3 h post-injection were 1.39-times higher compared to the baseline scale. These results from in vivo MR-T1 mapping imaging and the change in 1/T1 values demonstrate the reliable and prolonged tumor retention in the Click-APPGd-Cy7 group, highlighting efficient click reactions and enhanced PD-L1 targeting.
Antitumor efficacy in vivo
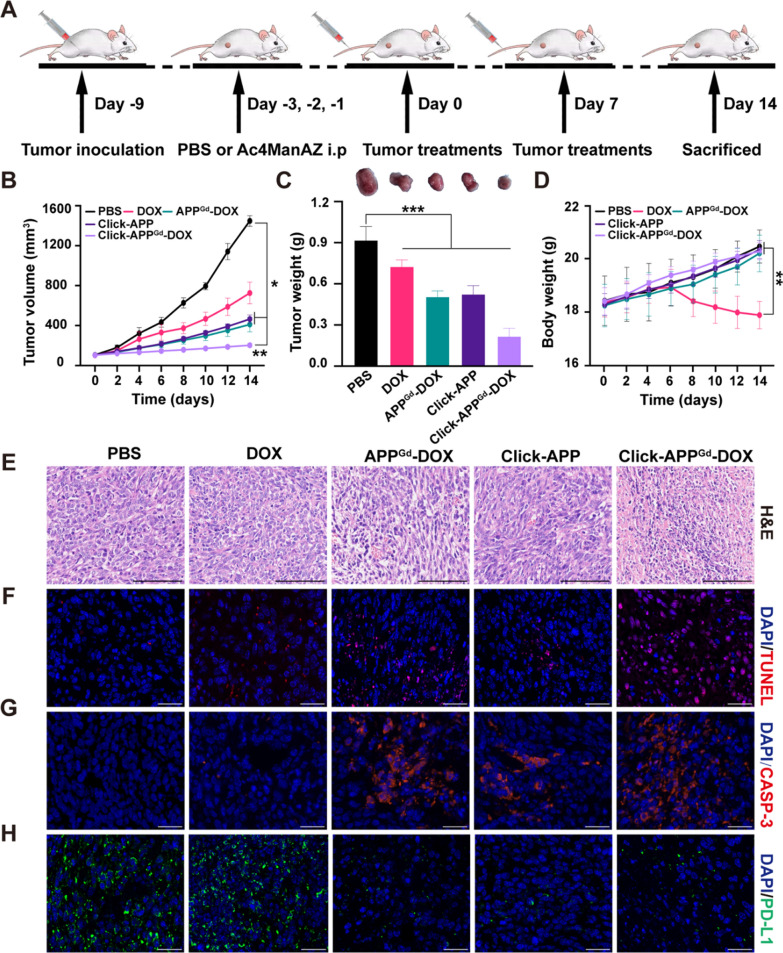
Encouraged by the favorable in vitro antitumor activity and improved in vivo tumor retention, we subsequently evaluated the therapeutic efficacy of different treatments in vivo. As illustrated in Fig. 5A, 4T1 tumor-bearing mice were randomly assigned into five groups (n = 5 per group): (1) i.v. PBS, (2) i.v. DOX, (3) i.v. APPGd-DOX, (4) i.p. Ac4ManNAz-treated (40 mg/kg) + i.v. APP, and (5) Ac4ManNAz-treated (40 mg/kg) + i.v. APPGd-DOX, at a dose of 5 mg/kg. Initially, all groups exhibited gradual increases in body weights, except for the DOX group, which experienced severe weight loss due to in vivo systemic toxicity (Fig. 5D). Compared to the PBS group, DOX exhibited a modest 50.00% decrease in tumor growth, suggesting restricted therapeutic effectiveness with a single administration of chemotherapy (Fig. 5B). In contrast, both APPGd-DOX and Click-APP exhibited significant delays in tumor growth, achieving reductions of 67.49% and 71.79%, respectively, after treatment. Notably, Click-APPGd-DOX demonstrated the most profound antitumor effect, achieving an approximately 86.12% inhibition of tumor growth. This enhanced antitumor efficacy of Click-APPGd-DOX can be attributed to improved tumor accumulation facilitated by efficient bio-orthogonal click chemistry and enhanced PD-L1 targeting, as well as synergistic chemo-immunotherapy.
Fig. 5.
A Illustration of 4T1 tumor inoculation and therapeutic interventions in BALB/c mice. B Body weights, (C) tumor volume, (D) tumor weights and tumor photos after various treatments, including (1) PBS, (2) DOX, (3) APPGd-DOX, (4) Click-APP, and (5) Click-APPGd-DOX. **P < 0.01 and ***P < 0.001. E–H Representative H&E staining, TUNEL, Caspase-3, and PD-L1 immunofluorescence staining of tumor slides. Scale bar: 100 μm
Tumor weights and photos at the end of treatments are displayed in Fig. 5C. The Click-APPGd-DOX group decreased to 23.58% compared to the PBS control group and 29.83% compared to the DOX-treated group, indicating a substantial tumor inhibition rate of 76.42%. H&E analysis revealed significant therapeutic effects, including extensive cellular atrophy and nuclear condensation after Click-APPGd-DOX therapy (Fig. 5E). The TUNEL assay and Caspase-3 immunofluorescence staining confirmed effective tumor eradication within the Click-APPGd-DOX group (Fig. 5F, G, Figure S14A, B). Additionally, the Click-APPGd-DOX markedly downregulated the PD-L1 expression in tumor cells (Fig. 5H and Figure S14C), indicating effective PD-L1 blockade. Moreover, evaluation of treatment-related toxicity through H&E analyses on major organs showed substantial damage in the DOX group compared to PBS-treated mice, while only negligible structural abnormalities were observed in APPGd-DOX, Click-APPGd, and Click-APPGd-DOX groups (Figure S15). In summary, the enhanced tumor-targeted delivery of APPGd-DOX using bio-orthogonal click chemistry and PD-L1 targeting demonstrated synergistic chemo-immunotherapy, resulting in the inhibition of tumor progression. Moreover, Click-APPGd-DOX mitigated off-target toxicity associated with chemotherapeutic drugs.
In vivo ICD and immune response induced by Click-APPGd−DOX
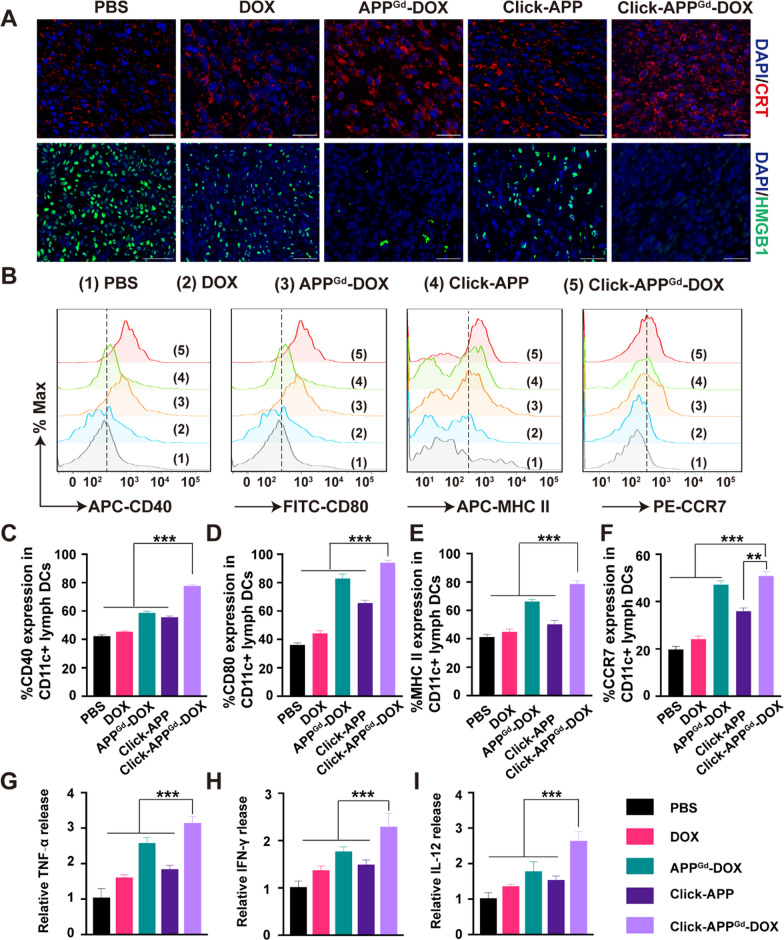
The in vivo ICD effect induced by Click-APPGd-DOX was assessed through immunofluorescence staining and the ELISA kit, as shown in Fig. 6A, Figure S14D, E and Figure S16. Consistent with the observed antitumor effect, the Click-APPGd-DOX group exhibited the highest levels of CRT expression and the most substantial release of HMGB1 among all the treated groups. This result could be explained by the heightened accumulation of DOX at the tumor site, leading to enhanced DAMPs release and presentation for DC recruitment, which is crucial for initiating immune responses [60, 61].
Fig. 6.
Click-APPGd-DOX induced ICD, DC maturation, and proinflammatory cytokine secretion in vivo. A Representative tumor sections of CRT, and HMGB1 immunofluorescence staining. Scale bar is 100 μm. B Markers including CD40, CD80, MHC II, and CCR7 on the DC membrane from TDLNs in groups treated with (1) PBS, (2) DOX, (3) APPGd-DOX, (4) Click-APP, and (5) Click-APPGd-DOX groups were evaluated by flow cytometry. C–F Quantification of CD40, CD80, MHC II and CCR7. Relative concentration of the (G) TNF-α, (H) IFN-γ, and (I) IL-12. **P < 0.01 and ***P < 0.001
Mature DCs, functioning as a major subset of antigen-presenting cells (APCs), aid in presenting tumor associated antigens to T cells, thereby initiating T cell activation [62–65]. To evaluate the maturation of DCs in vivo, cells obtained TDLNs were stained with fluorescently labeled antibodies targeting CD80, CD40, MHC II, and CCR7, followed by analyses using flow cytometry. In comparison to the PBS group, the levels of co-stimulatory molecules CD40 and CD80 were obviously elevated in the APPGd-DOX and Click-APPGd-DOX groups (Fig. 6B). CD40 and CD80 expression levels in the Click-APPGd-DOX groups were 1.85 and 2.59 times higher, respectively, than in the PBS group (Fig. 6C, D). Major histocompatibility complex class II (MHC II) molecules are essential for capturing peptides in the endocytic pathway to produce T cell receptor ligands, facilitating the maturation of DCs [66, 67]. Click-APPGd-DOX groups induced 78.70 ± 2.25% of MHC-II expression in TDLNs, significantly higher than that of APPGd-DOX (66.38 ± 1.41%), Click-APP (50.34 ± 2.63%), or DOX (45.04 ± 1.85%) (Fig. 6E). C–C chemokine receptor 7 (CCR7) guides mature DCs from the peripheral tissues to lymph nodes, regulating T cell activation and adaptative immunity [68, 69]. As shown in Fig. 6F, Click-APPGd-DOX facilitated the highest level of CCR7 expression, demonstrating a 1.08-fold increase compared to APPGd-DOX, a 1.41-fold increase compared to Click-APP, and a 2.10-fold increase compared to DOX. These findings underscore the potent capability of matured DC migration. In summary, the above results collectively demonstrate that the combination of the bio-orthogonal click chemistry strategy and synergistic chemo-immunotherapy promotes the maturation and migration of DCs.
The levels of circulating cytokines TNF-α, IFN-γ, and IL-12 were quantified using ELISA, as shown in Fig. 6I. Both the APPGd-DOX and Click-APPGd-DOX formulations demonstrated significant elevations in TNF-α, IFN-γ, and IL-12 concentrations within TME, indicative of heightened activity of TILs producing these cytokines. Specifically, the relative levels of TNF-α, IFN-γ, and IL-12 in the Click-APPGd-DOX group were approximately 1.22-fold, 1.30-fold, and 1.49-fold higher than those in the APPGd-DOX group, respectively, suggesting a robust antitumor immune response.
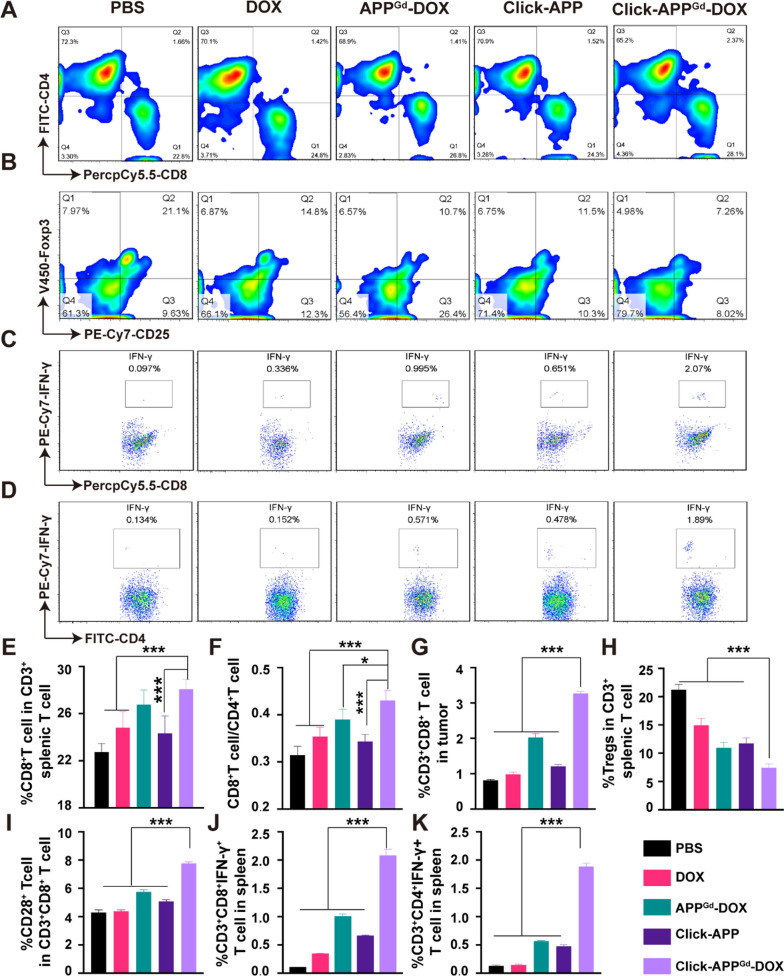
Subsequently, infiltration of T cells is investigated by immunofluorescence staining, as depicted in Figure S17. Fluorescence signals detected in the Click-APPGd-DOX group highlight a substantial infiltration of CD8+ T cells and a comparatively lower presence of regulatory T cells (Tregs) in the tumor, underscoring the potential for activating antitumor immunity. To further explore the in vivo immunoregulatory effects, we conducted flow cytometry analyses CTLs, CD4+ T cells, Tregs, and tumor-infiltrating T cells (TILs) (Figure S18). Mature DCs, assisting in antigen presentation to naïve T lymphocytes, effectively initiate the activation of CTLs, ultimately leading to tumor rejection [70]. Figure 7A, E demonstrate a statistically notable increase in the proportion of CTLs in the Click-APPGd-DOX-treated group (28.08 ± 0.81%) compared to the PBS control group (22.76 ± 0.71%), in accordance with the results obtained through immunofluorescence staining. Moreover, the CD8+/CD4+ T cell ratio within the CD3+ lymphocyte subset serves as a crucial indicator of adaptive T cell immunity, with a decrease in this ratio frequently associated with unfavorable outcomes in immunotherapy. Compared to the PBS group (0.32 ± 0.02%), the Click-APPGd-DOX-treated group (0.43 ± 0.02%) exhibited a noteworthy increase in the CD8+/CD4+ T cell ratio (Fig. 7F), indicating a significant improvement. This enhancement is primarily attributed to the infiltration of CTLs into TME. The percentage of intratumoral CTL infiltration notably increased from 0.819 ± 0.02% in the PBS group to 3.274 ± 0.05% in the Click-APPGd-DOX group, indicating a substantial improvement facilitated by Click-APPGd-DOX in intratumoral CTL infiltration (Figure S19 and Fig. 7G). Consequently, the combination of bio-orthogonal click chemistry and chemo-immunotherapy effectively increases CTL infiltration and demonstrates robust systemic immune activation.
Fig. 7.
T cell immune responses were assessed in vivo following treatment with (1) PBS, (2) DOX, (3) APPGd-DOX, (4) Click-APP, and (5) Click-APPGd-DOX. Representative flow cytometry analyses of (A) CD4+ and CD8+ splenic T cells, B splenic Tregs, (C) splenic IFN-γ+CD8+ T cells, and (D) splenic IFN-γ+ CD4+ T cells. E Percentage of CTLs in splenic CD3+ T cells. F The ratio of CD8+/CD4+ T cells. G Percentages of intratumoral CD3+CD8+ T cells, H Tregs in splenic CD3+ T cells, (I) CD28+ T cells in CD3+CD8+ splenic T cells, J splenic IFN-γ+ CD8+ T cells, and (K) splenic CD4+IFN-γ+ T cells. *P < 0.05, **P < 0.01, and ***P < 0.001
Tregs constitute a critical component of the TME, exerting immunosuppressive effects on antitumor immunity [71, 72]. As displayed in Fig. 7B, H, the proportion of splenic Tregs in the group treated with APPGd-DOX (11.00 ± 0.94%) was markedly reduced compared to both the PBS group (21.28 ± 0.88%) and the DOX group (14.98 ± 1.14%). Moreover, the Tregs percentage in the group treated with Click-APPGd-DOX (7.47 ± 0.66%) was significantly lower compared to both the groups treated with APPGd-DOX (11.00 ± 0.94%) and Click-APP (11.78 ± 0.93%). Furthermore, the presence of CD3+CD8+CD25+Foxp3+ Treg (T8regs) cells was also examined. As depicted in Figure S20, Click-APPGd-DOX demonstrated a 3.41% reduction in CD8+ Tregs in comparison to the control group treated with PBS. This observation suggest that Click-APPGd-DOX depletes CD8+ Treg cells effectively. In addition to enhancing the proliferation of CTLs, the treatment with Click-APPGd-DOX exhibited the potential to suppress immunosuppressive Tregs.
CD28, a crucial costimulatory molecule expressed on CD8+ T cells, exerts a significant influence on the activity of effector T cells [73]. As depicted in Figure S21 and Fig. 7I, the Click-APPGd-DOX group (7.78 ± 0.09%) exhibited a notable increase in the proportion of CD28+ cells among the CD3+CD8+ T cell subset compared to the PBS group (4.31 ± 0.16%). This enhancement contributes to the improved function of effector T cells and bolster immune responses. IFN-γ+ CTLs facilitate the differentiation of CD8+ T cells into antigen-specific CTLs, while IFN-γ+ CD4 + T cells support the differentiation of CD4+ T cells into Th1 cells [74]. Flow cytometry analyses were conducted to assess these phenomena, as shown in Fig. 7C, D. In comparison to the PBS group (0.10 ± 0.01%), the percentage of IFN-γ+CD3+CD8+ T cells in the Click-APPGd-DOX (2.07 ± 0.12%) demonstrated a statistically significant increase (Fig. 7J). Furthermore, Click-APPGd-DOX displayed the highest proportion of IFN-γ+CD3+CD4+ T cells, exhibiting a 3.95-, 3.31-, and 12.45-fold increase when compared to APPGd-DOX, Click-APP, or DOX, respectively (Fig. 7K).
Conclusion
In summary, we have developed the bio-orthogonal muti-functional theranostic system using bio-orthogonal click chemistry and PD-L1 targeting strategy. Following the administration of Ac4ManNAz, tumor cells efficiently introduced azide groups on their cell surfaces, subsequently engaging bio-orthogonal click reactions with DBCO-modified theranostic system. APPGd-Cy7 achieve enhanced and prolonged MR/NIRF imaging in vivo for PD-L1 targeting of TNBC. Furthermore, the APPGd-DOX prodrug with PD-L1 targeting performs pH-responsive DOX release, inducing ICD and DAMPs release in tumor cells, thereby initiating robust antitumor immune responses, and attracting various immune cells to the TME. The combination of ICB with the ICD-inducing chemotherapy could mitigate the suppressive influence of tumor cells on the antitumor immune response, leading to a significant reduction in tumor growth. Overall, our study presents a promising approach utilizing bio-orthogonal click chemistry, PD-L1 targeting, and pH-responsive drug release for combined MR/NIRF imaging and synergistic chemo-immunotherapy in TNBC.
Supplementary Information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82071870, 92159203, 81901870), the Sponsored Program of Shanghai Science and Technology Committee (No. 21S31905000), the Shanghai Rising-Star Program (22QA1406000) and Natural Science Foundation of Shanghai (21ZR1441600).
Author contributions
Author contributions Yan Wang: Validation, Investigation, Visualization, Writing-original draft. Yanhong Chen, Yuelin Huang, Weixi Huang, and Xue Dong: Validation, Visualization. Ding-Kun Ji: Methodology, Investigation. Defan Yao: Conceptualization, Methodology, Investigation, Formal analysis, Writing—review and editing, Funding acquisition. Dengbin Wang: Supervision, Writing—review and editing, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Laboratory animal ethical and welfare committee of XinHua Hospital Affiliated to Shanghai JiaoTong University School of Medicine, approved the animal experiment protocol (XHEC-F-2024-014).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Defan Yao, Email: yaodefan@xinhuamed.com.cn.
Dengbin Wang, Email: wangdengbin@xinhuamed.com.cn.
References
- 1.Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18(4):479–89. 10.6004/jnccn.2020.7554 [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 4.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–52. 10.1016/j.immuni.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45. 10.1186/s13045-021-01056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett D, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69(1):25–34. 10.1111/his.12904 [DOI] [PubMed] [Google Scholar]
- 8.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70. 10.1158/2326-6066.CIR-13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci USA. 2015;112(47):E6506–14. 10.1073/pnas.1519623112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701. 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, Cheung TH, McNeish IA, Kwong J. Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-dependent exocytosis. Cancer Immunol Res. 2020;8(8):1099–111. 10.1158/2326-6066.CIR-19-0616 [DOI] [PubMed] [Google Scholar]
- 12.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91. 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41. 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 14.Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–80. 10.1002/anie.201804882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–26. 10.1056/NEJMoa2202809 [DOI] [PubMed] [Google Scholar]
- 16.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 17.Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100. 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 19.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–88. 10.1093/annonc/mdz158 [DOI] [PubMed] [Google Scholar]
- 20.Gurung S, Khan F, Gunassekaran GR, Yoo JD, Poongkavithai Vadevoo SM, Permpoon U, Kim SH, Kim HJ, Kim IS, Han H, et al. Phage display-identified PD-L1-binding peptides reinvigorate T-cell activity and inhibit tumor progression. Biomaterials. 2020;247: 119984. 10.1016/j.biomaterials.2020.119984 [DOI] [PubMed] [Google Scholar]
- 21.Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM, Li WW, Zhou XM, Ma WW, Fu CY, et al. Blocking of the PD-1/PD-L1 interaction by a d-peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54(40):11760–4. 10.1002/anie.201506225 [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Song Y, Zhang Y, He S, Liu X, Yang X, Gong T, Huang Y, Gao H. Sequential delivery of PD-1/PD-L1 blockade peptide and IDO inhibitor for immunosuppressive microenvironment remodeling via an MMP-2 responsive dual-targeting liposome. Acta Pharm Sin B. 2023;13(5):2176–87. 10.1016/j.apsb.2023.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Zhao Z, Zhang L, Li Y, Jain A, Barve A, Jin W, Liu Y, Fetse J, Cheng K. Discovery of low-molecular weight anti-PD-L1 peptides for cancer immunotherapy. J Immunother Cancer. 2019;7(1):270. 10.1186/s40425-019-0705-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Zhang N, Zhou J, Ding C, Jin Y, Cui X, Pu K, Zhu Y. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6(2):178–88. 10.1158/2326-6066.CIR-17-0035 [DOI] [PubMed] [Google Scholar]
- 25.Magiera-Mularz K, Skalniak L, Zak KM, Musielak B, Rudzinska-Szostak E, Berlicki Ł, Kocik J, Grudnik P, Sala D, Zarganes-Tzitzikas T, et al. Bioactive macrocyclic inhibitors of the PD-1/PD-L1 immune checkpoint. Angew Chem Int Ed Engl. 2017;56(44):13732–5. 10.1002/anie.201707707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song S, Shim MK, Yang S, Lee J, Yun WS, Cho H, Moon Y, Min JY, Han EH, Yoon HY, Kim K. All-in-one glycol chitosan nanoparticles for co-delivery of doxorubicin and anti-PD-L1 peptide in cancer immunotherapy. Bioact Mater. 2023;28:358–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaman R, Islam RA, Ibnat N, Othman I, Zaini A, Lee CY, Chowdhury EH. Current strategies in extending half-lives of therapeutic proteins. J Control Release. 2019;301:176–89. 10.1016/j.jconrel.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 28.Cicero AFG, Fogacci F, Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol. 2017;174(11):1378–94. 10.1111/bph.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco-Miguez A, Gutierrez-Jacome A, Perez-Perez M, Perez-Rodriguez G, Catalan-Garcia S, Fdez-Riverola F, Lourenco A, Sanchez B. From amino acid sequence to bioactivity: the biomedical potential of antitumor peptides. Protein Sci. 2016;25(6):1084–95. 10.1002/pro.2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo H, Lee S, Na JH, Kim SH, Hahn SK, Choi K, Kwon IC, Jeong SY, Kim K. Bioorthogonal copper-free click chemistry in vivo for tumor-targeted delivery of nanoparticles. Angew Chem Int Ed Engl. 2012;51(47):11836–40. 10.1002/anie.201206703 [DOI] [PubMed] [Google Scholar]
- 31.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36(8):1249–62. 10.1039/B613014N [DOI] [PubMed] [Google Scholar]
- 32.Yi W, Xiao P, Liu X, Zhao Z, Sun X, Wang J, Zhou L, Wang G, Cao H, Wang D, Li Y. Recent advances in developing active targeting and multi-functional drug delivery systems via bioorthogonal chemistry. Signal Transduct Target Ther. 2022;7(1):386. 10.1038/s41392-022-01250-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Xu J, Sun Y, Mo L, Liu B, Pan X, Liu Z, Tan W. Aptamer-based logic computing reaction on living cells to enable non-antibody immune checkpoint blockade therapy. J Am Chem Soc. 2021;143(22):8391–401. 10.1021/jacs.1c02016 [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Sobral MC, Zhang DKY, Cartwright AN, Li AW, Dellacherie MO, Tringides CM, Koshy ST, Wucherpfennig KW, Mooney DJ. Metabolic labeling and targeted modulation of dendritic cells. Nat Mater. 2020;19(11):1244–52. 10.1038/s41563-020-0680-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willems LI, van der Linden WA, Li N, Li KY, Liu N, Hoogendoorn S, van der Marel GA, Florea BI, Overkleeft HS. Bioorthogonal chemistry: applications in activity-based protein profiling. Acc Chem Res. 2011;44(9):718–29. 10.1021/ar200125k [DOI] [PubMed] [Google Scholar]
- 36.Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y, Ji T, Wu H, Wang B, Anderson GJ, et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18(5):3250–8. 10.1021/acs.nanolett.8b01071 [DOI] [PubMed] [Google Scholar]
- 37.Moon Y, Shim MK, Choi J, Yang S, Kim J, Yun WS, Cho H, Park JY, Kim Y, Seong JK, Kim K. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics. 2022;12(5):1999–2014. 10.7150/thno.69119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Shi W, Wu Y, He Z, Sun J, Cai S, Luo Q. Immunogenic nanovesicle-tandem-augmented chemoimmunotherapy via efficient cancer-homing delivery and optimized ordinal-interval regime. Adv Sci (Weinh). 2022;10(1): e2205247. 10.1002/advs.202205247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilad Y, Eliaz Y, Yu Y, Han SJ, O’Malley BW, Lonard DM. Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci Rep. 2019;9(1):15099. 10.1038/s41598-019-51537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. 10.1016/S1470-2045(19)30689-8 [DOI] [PubMed] [Google Scholar]
- 41.Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci USA. 2018;115(6):E1239–48. 10.1073/pnas.1718197115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Zhao Z, Qiu N, Zhou Q, Wang G, Jiang H, Piao Y, Zhou Z, Tang J, Shen Y. Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy. Nat Commun. 2021;12(1):2425. 10.1038/s41467-021-22407-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19(5):287–305. 10.1038/s41571-022-00601-9 [DOI] [PubMed] [Google Scholar]
- 44.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–90. 10.1038/s41571-022-00689-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 47.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–8. 10.1038/s41591-019-0432-4 [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–201. 10.1016/j.celrep.2017.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T, Zheng S, Liu Y, Li X, Wu J, Sun Y, Liu G. DNA damage response and PD-1/PD-L1 pathway in ovarian cancer. DNA Repair (Amst). 2021;102: 103112. 10.1016/j.dnarep.2021.103112 [DOI] [PubMed] [Google Scholar]
- 51.Xu HZ, Li TF, Wang C, Ma Y, Liu Y, Zheng MY, Liu ZJ, Chen JB, Li K, Sun SK, et al. Synergy of nanodiamond-doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J Nanobiotechnol. 2021;19(1):268. 10.1186/s12951-021-01017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailly C. Regulation of PD-L1 expression on cancer cells with ROS-modulating drugs. Life Sci. 2020;246: 117403. 10.1016/j.lfs.2020.117403 [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Lyu B, Yang C, He B, Zhang H, Wang X, Zhang Q, Dai W. An enzyme-responsive and transformable PD-L1 blocking peptide-photosensitizer conjugate enables efficient photothermal immunotherapy for breast cancer. Bioact Mater. 2023;22:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu Z, Lu Z, Huang J, Zhong Y, Yan N, Kong R, Cheng H. Self-reinforced photodynamic immunostimulator to downregulate and block PD-L1 for metastatic breast cancer treatment. Biomaterials. 2023;303: 122392. 10.1016/j.biomaterials.2023.122392 [DOI] [PubMed] [Google Scholar]
- 55.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75. 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 56.Jiang M, Zeng J, Zhao L, Zhang M, Ma J, Guan X, Zhang W. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo-immunotherapy. Nanoscale. 2021;13(41):17218–35. 10.1039/D1NR05512G [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Yang S, Zhang T, Wang S, Yang Q, Xiao Y, Shi X, Xue P, Kang Y, Liu G, et al. Bioresponsive immune-booster-based prodrug nanogel for cancer immunotherapy. Acta Pharm Sin B. 2022;12(1):451–66. 10.1016/j.apsb.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. 10.1186/s13045-020-00946-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 60.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. 10.1146/annurev.iy.09.040191.001415 [DOI] [PubMed] [Google Scholar]
- 61.Reise Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–83. 10.1038/nri1845 [DOI] [PubMed] [Google Scholar]
- 62.Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2(3):229–34. 10.1038/85286 [DOI] [PubMed] [Google Scholar]
- 63.Feng Q, Ma X, Cheng K, Liu G, Li Y, Yue Y, Liang J, Zhang L, Zhang T, Wang X, et al. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater. 2022;34(40): e2206200. 10.1002/adma.202206200 [DOI] [PubMed] [Google Scholar]
- 64.Chen Q, Liu L, Lu Y, Chen X, Zhang Y, Zhou W, Guo Q, Li C, Zhang Y, Zhang Y, et al. Tumor microenvironment-triggered aggregated magnetic nanoparticles for reinforced image-guided immunogenic chemotherapy. Adv Sci (Weinh). 2019;6(6):1802134. 10.1002/advs.201802134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- 66.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–7. 10.1126/science.288.5465.522 [DOI] [PubMed] [Google Scholar]
- 67.Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reise Sousa C, Germain RN, Mellman I, Steinman RM. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191(6):927–36. 10.1084/jem.191.6.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21(2):279–88. 10.1016/j.immuni.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 69.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. 10.1016/S0092-8674(00)80059-8 [DOI] [PubMed] [Google Scholar]
- 70.Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1(3):145–9. 10.1158/2326-6066.CIR-13-0102 [DOI] [PubMed] [Google Scholar]
- 71.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18. 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71. 10.1038/s41571-019-0175-7 [DOI] [PubMed] [Google Scholar]
- 73.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–33. 10.1126/science.aaf1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–47. 10.1038/s41577-018-0044-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.