Abstract
Background
The purpose of this systematic review and meta-analysis was to synthesize the current literature to determine the safety and efficacy of using subcutaneous insulin compared to an intravenous (IV) insulin infusion in managing diabetic ketoacidosis (DKA).
Methods
We searched Ovid-Medline, EMBASE, SCOPUS, BIOSIS and CENTRAL from inception to April 26, 2024. Randomized controlled trials (RCTs) and observational studies that assessed the use of subcutaneous compared to intravenous insulin for the treatment of mild to moderate DKA were included. Data extraction and quality assessment were performed by two independent reviewers and disagreements were resolved through further discussion or by a third reviewer. The Cochrane Risk of Bias tool version 2.0 was used to evaluate the RCTs and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS)-I tool was used to evaluate the observational studies. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria. Meta-analyses were conducted using random-effects models. We followed the PRISMA guidelines for reporting our findings.
Results
Six RCTs (245 participants) and four observational studies (8444 patients) met our inclusion criteria. Some studies showed a decreased length of stay (Mean Difference [MD] in days: -0.39; 95% CI: -2.83 to 2.08; I2: 0%) among individuals treated with subcutaneous insulin compared to intravenous insulin. There was no difference in the risk of all-cause mortality, time to resolution of DKA (MD in hours: 0.17; 95% confidence interval [CI]: -3.45 to 3.79; I2: 0%) and hypoglycemia (Risk Ratio [RR]: 1.02; 95% CI: 0.88 to 1.19; I2: 0%) between the two groups.
Conclusion
Treatment of DKA with subcutaneous insulin may be a safe and effective alternative to IV insulin in selected patients. The limited available evidence underscores the need for further studies to explore optimal dosing, patient selection criteria and long-term outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-024-01666-6.
Keywords: Diabetes, Diabetic ketoacidosis, Subcutaneous insulin, Systematic review
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes. DKA most commonly occurs in patients with type 1 diabetes; however it can also present among people with type 2 diabetes with concomitant insulin deficiency [1] and among people with ketosis-prone type 2 diabetes. Given the increasing prevalence of diabetes [2], DKA is a common presentation in the emergency room and an indication for intensive care unit (ICU) admission. Ramphul et al. [2] recently showed that the incidence of hospitalizations due to DKA in the United States increased from 2003 to 2017, with the incidence per 10,000 admissions rising from 32.04 in 2003 to 61.60 in 2017 according to the 2017 National Inpatient Sample.
The diagnostic criteria for DKA includes the triad of uncontrolled hyperglycemia, increased total serum ketone concentration and metabolic acidosis. DKA commonly presents with a precipitating cause, such as an acute illness or increased physiological stress [1]. DKA management includes treating the precipitating cause, correcting dehydration with intravenous (IV) fluids, managing electrolyte abnormalities, and administering insulin to correct the ketoacidosis and hyperglycemia. The standard of care for managing DKA is through the administration of a continuous IV insulin infusion [3]. DKA is usually treated in a monitored setting, such as the resuscitation area in the emergency department or the ICU. As per Diabetes Canada, IV insulin is the standard of care for DKA management [4]. On the other hand, the American Diabetes Association (ADA) recommends IV insulin administration for moderate-to-severe DKA and suggests the possibility of using subcutaneous rapid-acting insulin for mild and uncomplicated DKA [3].
There are few observational studies and randomized controlled trials (RCTs) that have evaluated the safety and efficacy of using a subcutaneous insulin protocol in managing DKA outside of a monitored setting. To our knowledge, the last systematic review conducted on this topic was done by Andrade-Castellanos et al. in 2016 [5]. The studies presented in this previous review demonstrated that insulin therapy is effective in managing DKA regardless of the route of administration in selected patients. However, it only included RCTs, excluding observational studies. Given that additional studies have been conducted on this topic since 2016, we performed an updated systematic review to assess the safety and efficacy of using subcutaneous insulin in managing DKA compared to IV insulin. The main purpose of this study was to assess the safety and efficacy of treatment with subcutaneous insulin versus IV insulin among adult patients with mild to moderate DKA.
Methods
Our systematic review was conducted by following a prespecified protocol and is reported based on the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6]. Our protocol was registered with Prospero (CRD42022339577). Material deviations to the prespecified protocol are described below.
We systematically searched Ovid-Medline, EMBASE, SCOPUS, BIOSIS and CENTRAL from inception until April 26th, 2024 to identify studies that compared the use of subcutaneous insulin to continuous IV insulin for the treatment of mild to moderate DKA. Keywords and search strategies used are available in Appendix 1.
We included RCTs and observational studies that compared subcutaneous insulin and a continuous IV insulin infusion in managing adult patients with mild-to-moderate DKA. Subcutaneous insulin included either short-acting or rapid-acting insulin. Inclusion was restricted to studies that included adults age ≥ 18 years old, who were identified as having diabetes with DKA, defined as having arterial pH ≤ 7.3, serum bicarbonate ≤ 18 mmol/L and anion gap is > 12 mmol/L with positive serum and/or urine ketones. Our definition of DKA was based on the criteria outlined in the ADA guidelines [3]. Mild DKA is defined as pH 7.25 to 7.30 and serum bicarbonate 15 to 18 mmol/L, whereas moderate was defined as pH 7.00 to < 7.24 and serum bicarbonate 10 to < 15 mmol/L [3] Studies were required to report at least one of the following outcomes: length of stay, time to resolution of DKA, mortality, need for intensive care stay, hypoglycemia or hypokalemia. Studies that compared subcutaneous insulin versus IV insulin in severe DKA, or in patients with hypotension despite fluid resuscitation, comatose state, and other medical conditions that warranted ICU admission were excluded.
Literature reviews, systematic reviews, meta-analyses, letters to the editor and commentaries, and animal studies were excluded. However, prior systematic reviews and literature reviews were assessed for potential studies that were eligible for inclusion. Finally, we excluded conference abstracts as they did not contain sufficient information to properly assess the study quality. After removing duplicates, two independent reviewers (AA and TM) screened the titles and abstracts of identified publications, and any publication deemed potentially relevant by either reviewer was carried forward to a full-text review. Discrepancies during full-text review between reviewers were resolved by consensus or a third reviewer (OY).
Data extraction and quality assessment were performed for all included studies by two independent reviewers (AA and TM), and disagreements were resolved through further discussion or by a third reviewer (OY). First, we extracted information regarding the study design, population characteristics (population size, location of the study, study period, body mass index [BMI]), precipitants of DKA, inclusion and exclusion criteria, and outcomes of interest (length of hospital stay, time to resolution of DKA, cost of hospitalization, complications). The Cochrane Risk of Bias tool version 2.0 was used to evaluate the RCTs [7] and the Risk of Bias In Non-randomized Studies of Interventions (ROBINS)-I tool was used to evaluate observational studies [8].
Risk of Bias Assessment in Included Studies
We assessed the risk of bias for RCTs using the Risk of Bias 2 (RoB2), the tool recommended by the Cochrane Collaboration. We judged the “Risk of Bias” criteria as “low risk”, “some concerns” or “high risk” and evaluated different bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Version 6) [7]. We assessed the following domains: the risk of bias arising from the randomization process, the risk of bias due to deviations from the intended interventions (effect of assignment to intervention and adhering to intervention), missing outcome data, the risk of bias in measurement of the outcome, and the risk of bias in selection of the reported result. We synthesized an overall risk of bias judgement based on the outcomes within each domain.
For the observational studies, a quality assessment was conducted by using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool from the Cochrane Review [8]. We judged the “Risk of Bias” as “low”, “moderate”, “serious”, “critical” or “no information (NI)”. We assessed the following domains: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviation from intended intervention, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result. We synthesized the overall risk of bias based on the outcomes within each domain. The overall quality of the evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [9].
We conducted meta-analyses using the Dersimonian and Laird random-effects models with inverse variance weighting to pool data across studies for each outcome of interest at the suggestion of journal reviewers [10, 11]. A random-effects approach was used due to the clinical heterogeneity across the various studies. If the measures were continuous, we estimated mean differences (MDs) and for count data, we estimated pooled risk ratios (RRs). We also conducted a sensitivity analysis whereby we conducted meta-analyses stratified by the type of study (RCTs and observational studies). The meta-analyses were conducted using R software, version 4,2,3. The data used to conduct the meta-analysis is available in Supplementary Table 1.
Results
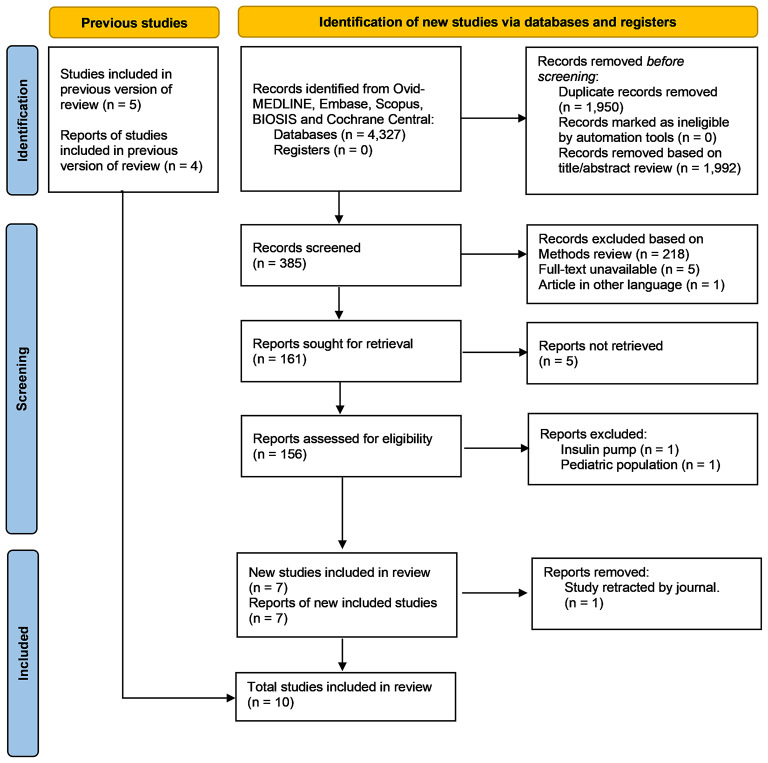
After conducting our updated systematic search, six RCTs and five retrospective cohort studies were included from a total of 4327 articles that were initially identified in our search. However, one retrospective study was retracted by the journal and thus, it was excluded from our systematic analysis [12] (Fig. 1). For all included studies, the intervention of interest was the use of intermittent doses of subcutaneous insulin compared to a continuous IV infusion for the treatment of DKA among adults. Most of the studies retrieved included individuals with uncomplicated DKA [13–20]. However, the RCT done by Fisher et al. did not characterize the severity of DKA in their study population [21]. Rao et al. was the only study that characterized the severity of DKA, which included 330 patients with mild DKA, 4,966 with moderate DKA and 2,693 patients with severe DKA [22].
Fig. 1.
PRISMA 2020 flow diagram for updated systematic reviews which included searches of databases and registers only
The included RCTs had a total of 245 participants, and the observational studies had a total of 8,444 patients. Of these 8,689 individuals, 712 received a subcutaneous rapid-acting insulin analogue, 7,962 received intravenous regular insulin and 15 individuals received intramuscular insulin. All included participants finished their assigned treatment. Baseline patient demographics and the interventions studied in the RCTs and observational studies are summarized in Table 1.
Table 1.
Summary of studies and patient characteristics comparing the use of subcutaneous insulin versus intravenous insulin in the management of mild to moderate diabetic ketoacidosis (DKA)
| Author (Year) |
Study design Size (N) | Causes of DKA | Mean Age (years) | BMI (kg/m2) |
Included participants | Intervention |
|---|---|---|---|---|---|---|
| Fischer et al. (1977) |
Randomized controlled trial (N = 45) |
N/A |
IM Group: 40.7 SC Group: 44.3 IV Insulin: 37.2 |
Inclusion criteria: Adult patients with plasma glucose > 300 mg/dl; blood acetone positive; pH < 7.3; HCO3 < 15 mEq/L; glycosuria with ketonuria. Exclusion criteria: None. |
SC Group: Initial dose of 0.33U/kg regardless of the plasma glucose then 7U/hr. IM group: Initial dose of 0.33U/kg regardless of the initial plasma glucose then 7U/hr. If the ketoacidosis was not under control (HCO3 < 15 mEq/L; pH < 7.3 and plasma acetone still positive), then 4 to 12U of regular insulin every 2 h in the same route is given. IV Insulin group: Initial dose of 0.33U/kg regardless of the initial plasma glucose, then 7 U/hr until plasma glucose < 250 mg/dl. |
|
| Umpierrez et al. (2004) |
Randomized controlled trial (N = 40) |
SC Insulin: Poor compliance: 60% IV Insulin: Poor compliance: 70%, 15% new onset of diabetes. |
SC Insulin: 37 (12) IV Insulin: 39 (14) |
SC Insulin: 26 ± 7 IV Insulin: 27 ± 9 |
Inclusion criteria: Adult patients with diabetic ketoacidosis, glucose < 250 mg/dL, bicarbonate 15 mEq/L, pH < 7.3, and positive serum ketones. Exclusion criteria: Individuals with persistent hypotension, comatose state, acute myocardial ischemia, heart failure, end stage renal disease, anasarca, dementia or pregnancy were excluded. |
SC Insulin Lispro Group: Initial injection of 0.3 units/kg of body weight, followed by 0.1 units/kg/h until blood glucose levels reached 250 mg/dL. The insulin dose was then reduced to 0.05 units/kg/h and fluids were changed to D5W in 0.45% NS until resolution of DKA. IV Regular Insulin Group: Received an initial bolus of 0.1 units/kg, followed by a continuous infusion of regular insulin calculated to deliver 0.1 units/kg/h until blood glucose levels decreased to approximately 250 mg/dL (13.9 mmol/). Fluids changed to D5W, and insulin infusion decreased to 0.05 unit/kg/h until resolution of DKA. |
| Umpierrez et al. (2004) |
Randomized controlled trial (N = 45) |
SC-1 h: Poor compliance 53%, New-onset diabetes 20% SC-2 h: Poor compliance 60%, Medical illnesses 27%. New-onset diabetes 20%; IV Regular Insulin: Poor compliance 60%, New-onset diabetes 13%. |
SC-1 h: 36 (8) SC-2 h: 38 (12) IV insulin: 40 (13) |
SC-1 h: 27 ± 6 SC-2 h: 29 ± 7 IV Insulin: 27 ± 7 |
Inclusion criteria: Adult patients with DKA, a plasma glucose level > 13.8 mmol/L (250 mg/dl), a serum bicarbonate level < 15 mmol/L, a venous pH < 7.30, and a positive serum ketone level, and/or a serum beta-hydroxybutyrate level > 3.0 mmol/L. Exclusion criteria: Individuals with persistent hypotension, comatose state, acute myocardial ischemia, heart failure, end stage renal disease, anasarca, dementia or pregnancy were excluded. |
SC-1 h Group: 15 patients received an initial insulin Aspart injection of 0.3 units/kg body weight, followed by 0.1 units/kg/hr until blood glucose reached 13.8 mmol/L (250 mg/dL). SC-2 h Group: 15 patients received an initial dose of 0.3 units/kg followed by 0.2 units/kg 1 h later and every 2 h until blood glucose reached 13.8 mmol/L (250 mg/dL). IV Insulin Group: received an initial IV regular insulin bolus of 0.1 units/kg, followed by a continuous infusion of regular insulin calculated to deliver 0.1 units/kg/hr until blood glucose levels reached ≤ 13.8 mmol/L (250 mg/dL). |
| Ersöz et al. (2006) |
Randomized controlled trial (N = 20) |
N/A |
SC Insulin: 39 (20) IV Insulin: 49 (18) |
Inclusion criteria: Adult patients with mild to moderate DKA. Exclusion criteria: Individuals with severe concomitant illness, with persistent hypotension, hypothermia, plasma glucose > 33.3mmol/L (> 600 mg/dL), arterial pH level < 7.0 or serum bicarbonate level < 10 mmol/L at admission were excluded. |
L Group (SC Insulin): Following a bolus injection of 0.15 U/kg IV regular insulin, the patients received half of this dose as hourly SC insulin lispro. R Group (IV Insulin): Received standard IV regular insulin infusion. |
|
| Karoli et al. (2011) |
Randomized controlled trial (N = 50) |
SC insulin: 34 (13) IV insulin: 35 (11) |
IV Insulin: 24 ± 2 SC Insulin: 25 ± 3 |
Inclusion criteria: Adult patients with mild to moderate DKA. Exclusion criteria: Individuals with severe DKA and required admission to the ICU, loss of consciousness, acute myocardial ischemia, congestive heart failure, end-stage renal disease, anasarca, pregnancy, serious co-morbidities, and persistent hypotension were excluded. |
Group 1 (Regular IV Insulin): an initial bolus of regular insulin 0.1 units/kg intravenously followed by continuous infusion of regular insulin calculated to deliver 0.1 units/kg/h until blood glucose levels decreased to approximately 250 mg/dL, then insulin infusion rate was decreased to 0.05 units/kg/h until resolution of DKA. Group 2 (SC Insulin Lispro): received subcutaneous insulin lispro as initial bolus of 0.3 units/kg followed by 0.2 units/kg 1 h later and then 0.2 units/kg every 2 h until blood glucose reached 250 mg/dL. |
|
| Prasad et al. (2015) |
Randomized controlled trial (N = 45) |
SC-1 h: Infection 53.3%, poor compliance 40%, new onset 6.7% SC-2 h Infection 60%, poor compliance 33.3%, new onset 6.7% IV Insulin: Infection 53.3%. Poor compliance 40%, new onset 6.7% |
SC-1 h: 35 (11) SC-2 h: 34 + 11 IV Insulin: 38 + 11 |
SC-1 h: 28 ± 9 SC-2 h: 29 ± 7 IV Insulin: 29 ± 7 |
Inclusion criteria: Patients with diabetes mellitus on the medicine ward with random blood sugar > 250mg/dL, serum bicarbonate < 18 mmol/L, arterial pH <7.30. Urinary ketones were present. Exclusion criteria: Participants with acute myocardial infarction, end stage renal disease, hepatic failure and pregnancy. |
SC-1 h SC-2 h IV Insulin regular |
| Balili et al. (2017) |
Retrospective cohort study (N = 21) |
SC Group: Infection 8, missed insulin 2, new onset diabetes 5 IV Insulin: Infection 8, missed insulin 2, new onset diabetes 2 |
SC Insulin: 50.33 ± 19.08 IV Insulin: 58.00 ± 12.31 |
Inclusion criteria: Patients with mild to moderate DKA who received either IV regular insulin or SC rapid insulin analog from January 2012 until December 2015. Exclusion criteria: Patients with severe DKA (persistent hypotension, acute and chronic decompensated liver disease with serum transaminase levels > 3x upper limit of normal range, chronic kidney disease (eGFR < 15 cc/min), decompensated heart failure, dementia, coma, steroid use, pregnant at time of admission, and initial treatment with IV and SC insulin in combination) were excluded. |
IV Insulin group: Received continuous intravenous regular insulin infusion. SC Insulin group: Received intermittent (one to two hourly) subcutaneous rapid-acting insulin analogue. |
|
| Rao et al. (2022) |
Retrospective cohort study (N = 7989) |
N/A |
Intervention Site: Pre -implementation: 37.5 (15.5) Post-implementation 39.6 (16.9) Standard care sites: Pre-implementation: 42.2 (17.6) Post-implementation: 43.0 (17.8) |
Inclusion criteria: Adult hospitalized patients with DKA at 21 hospitals between January 1, 2010, and December 31, 2019. Exclusion criteria: Patients age < 18 years old, pregnancy, any other medical condition that would require ICU admission (i.e. septic shock, cardiogenic shock, ST-elevation myocardial infarction and Glasgow Coma Scale score < 8) were excluded. |
SC Insulin Group: Glargine and Lispro (Intervention Site) IV Insulin Group |
|
| Stuhr et al. (2023) |
Retrospective cohort study (N = 257) |
SC Group: 33.3% non-compliance, 17.3% new onset of diabetes, 22.7% infection, 33.3%: Others/not reported. IV insulin (with bolus): 45.2% non-compliance, 9.7% new onset of diabetes, 17.7% infection, 43.5%: Others/not reported. IV insulin (without bolus): 32.5% non-compliance, 13.3% new onset of diabetes, 19.2% infection, 43.3%: Others/not reported |
SC Group: 44.43 IV Insulin (with bolus) 42.65 IV Insulin (without bolus): 45.01 |
SC Group: 29.27 IV Insulin (with bolus) 26.54 IV Insulin (without bolus): 26.76 |
Inclusion criteria: Adult patients 18 years or older and admitted with a primary diagnosis of mild to moderate DKA from the ED at one of the study institutions and within the institution-specific time frames. Exclusion criteria: Patients were excluded if the DKA was unresolved prior to discharge, they were admitted from other institutions, had transitioned to IV insulin infusion for failed SC treatment, or had severe DKA, end-stage renal disease, pregnancy, or currently incarceration. Patients who presented to the ED with DKA are excluded from consideration of SC insulin if they are persistently hypotensive; have renal failure, defined by serum creatinine > 3 mg/dL, pH < 7.1, lactate > 4 mmol/L, and not resolving after initial resuscitation, or are not able to communicate symptoms of hypoglycemia due to severe altered mental status. In addition, they are excluded from SC protocol initiation if there are no beds available on the designated acute care floor. Lastly, if they meet other criteria for ICU admission, including but not limited to pH < 7.0 or presence of shock. |
SC Group: 0.3 units/kg insulin lispro SC once, then 0.1–0.2 units/kg insulin lispro SC depending on the AG and BG according to the protocol. IV Insulin (with bolus): 0.1 units/kg/h infusion + 0.1 units/kg bolus, then insulin infusion drip titrated according to BG to target 90–140 mg/dL. IV Insulin (with no bolus): 0.1 units/kg/h infusion, then insulin infusion drip according to BG to target 90–140 mg/dL. |
| Griffey et al. (2023) |
Retrospective cohort study (N = 177) |
N/A | N/A | N/A |
Inclusion criteria: Adult patients who presented to the ED with mild to moderate DKA between August 1, 2021 until February 2022 were assigned to the SQuID protocol on a predesignated, hospitalist-run observation floor in the hospital. Exclusion criteria: Pregnant patients or patients with concurrent infections, active co-morbidities, concerns for myocardial infarction, altered level of consciousness or need for surgery or if deemed too sick for the predesignated floor by the ED team were excluded. |
SQuID versus traditional during the study period SQuID versus preintervention period SQuID versus pre-COVID control period |
Abbreviations SC: subcutaneous; IV: intravenous; IM: intramuscular; DKA: diabetic ketoacidosis ; HCO3: bicarbonate ; BMI: body mass index ; RBS: random blood sugar ; mg: milligram ; dl: deciliter; u/h: Unit/hour ; kg: kilogram
Primary Outcomes
Overall, the studies included in our systematic review demonstrated that subcutaneous insulin is equally as effective in the treatment of mild to moderate DKA and in certain studies, there was a decreased length of hospitalization, and shorter length of stay in the emergency department among individuals treated with subcutaneous insulin compared to IV insulin [13, 14, 19]. Furthermore, there was no significant difference in time to resolution of DKA, all-cause mortality, hypoglycemic and hypokalemic events between both groups. Our primary outcomes of interest are summarized in Table 2.
Table 2.
Summary of outcomes assessing the use of subcutaneous insulin compared to intravenous insulin for the management of mild to moderate diabetic ketoacidosis (DKA)
| Author (Year) |
Length of stay (days) | Time to resolution of DKA (hours) | Mortality | Hypoglycemia (< 4 mmol/L) or Hypokalemia (< 3.5 mmol/L) |
Findings |
|---|---|---|---|---|---|
| Fischer et al. (1977) | N/A | N/A | N/A |
No episodes of hypoglycemia. 4 cases of hypokalemia (uncertain which intervention group) |
Authors report no significant difference between the two groups in terms of hypoglycemia and hypokalemia risks. |
| Umpierrez et al. (2004) |
SC: 4 ± 2 IV Insulin: 4 ± 1 |
SC: 10 ± 3 IV Insulin: 11 ± 4 |
None |
Hypoglycemia: One in each group Hypokalemia: N/A |
No significant difference was found between the two groups in terms of major outcome variables. However, treatment of diabetic ketoacidosis in a non–intensive care setting (step-down unit or general medicine ward) was associated with a 39% lower hospitalization cost than was treatment with IV insulin in the ICU. |
| Umpierrez et al. (2004) |
SC-1 h: 3.4 ± 3; SC-2 h: 3.9 ± 5; IV Insulin: 4.5 ± 3 |
SC-1 h: 10 ± 3; SC-2 h: 10.7 ± 3; IV Insulin: 11 ± 3 |
None |
Hypoglycemia: One in each group |
Treatment of DKA with SC insulin analogs every 1–2 h represents a safe and effective alternative to treatment with IV regular insulin as no statistically significant differences were found among the 3 groups in terms of biochemical parameters, changes in plasma glucose, length of hospital stay, mortality, hypoglycemic events and recurrence of DKA |
| Ersöz et al. (2006) | N/A |
L Group (SC Group): Time to reach glucose < 200 mg/dL = 9.4 ± 8.9, pH > 7.3 = 8.2 ± 5.6, serum bicarbonate > 18mEq/L = 14.8 ± 7.0, β-hydroxybutyrate < 0.6 mmol/L = 11.2 ± 4.9 and urine ketones negative = 17.2 ± 7.0; R Group (IV Insulin): Time to reach glucose < 200 mg/dL = 12.7 ± 7.5, pH > 7.3 = 6.8 ± 5.7, serum bicarbonate > 18mEq/L = 13.2 ± 7.5, β-hydroxybutyrate < 0.6 mmol/L = 15.3 ± 8.7 and urine ketones negative: 22.3 ± 10.9 |
N/A | N/A |
No significant difference between the two groups in terms of rate of decline of plasma glucose, Beta-hydroxybutyrate, urinary ketone excretion, effective plasma osmolality and the mean duration of treatment until correction of the ketoacidosis. No serious side effects between the two groups. Total amount of insulin delivered was not different between the two groups. Treatment of mild and moderate DKA with hourly SC insulin lispro administration represents a safe and effective alternative to IV regular insulin administration. |
| Karoli et al. (2011) |
Group 1 (IV Insulin): 6.6 ± 1.5 Group 2 (SC Insulin): 6 ± 1.2 |
Group 1 (IV Insulin): 11 ± 1.6 Group 2 (SC Insulin): 12 ± 2.2 |
None |
Hypoglycemia: Group 1 (IV Insulin): 2 patients Group 2 (SC Insulin): 1 patient Hypokalemia: N/A |
No significant differences between the two groups were observed in terms of major primary outcomes. The patients with uncomplicated DKA under appropriate supervision and careful monitoring can be managed in medical wards or non-ICU setting. |
| Prasad et al. (2015) |
SC-1 h: 6.2 ± 4; SC-2 h: 5.9 ± 3; IV Insulin: 6.8 ± 3 |
SC-1 h: 10.3 ± 3; SC-2 h: 10.8 ± 3; IV Insulin: 10.5 ± 3 |
None | None |
The mean duration of treatment until glucose concentration was < 13.8 mmol/L (< 250 mg/dL) was not statistically different between patients treated with SC-1 h (6.8 ± 3 h) and SC-2 h (6.5 ± 3 h) or with IV regular insulin (6.9 ± 4 h). Similarly, the mean duration of treatment until resolution of ketoacidosis was not statistically different among treatment groups 10.3 ± 3 h, 10.8 ± 3 h and 10.5 ± 3 h respectively. |
| Balili et al. (2017) |
SC group: 5.22 (3–11) IV Insulin group: 11.42 (4–28) |
SC Insulin group: 24.11 ± 7.70 IV Insulin group: 25.67 ± 8.56 |
One died in SC insulin analogue group due to septic shock secondary to hospital-acquired infection. |
Hypoglycemia: SC group: 1 event IV insulin group: 2 events Hypokalemia: Occurred more frequently in the IV group. |
Length of hospital stay was significantly shorter by a mean of six days for those in the SC insulin group compared to those in the IV insulin group. Two patients (17%) in the IV insulin group and one patient (11%) in the SC insulin group developed hypoglycemia. One patient died in the SC insulin group due to septic shock secondary to a hospital-acquired infection.1 Hypokalemia occurred more frequently in the IV insulin group (50%) compared to the SC insulin group (11%). |
| Rao et al. (2022) |
Intervention Site: Overall mean hospital stay 64.6 h (Pre-implementation) and 56.2 h (Post-implementation); Standard Care Sites: Overall mean hospital stay 62.5 h (Pre-implementation) and 58.5 h (Post-implementation) |
Intervention Site: Time to glucose < 250 mg/dL = 9.5 h (Pre-implementation) and 11.3 h (Post-implementation); Standard Care Sites: Time to anion gap < 16 = 9.4 h (Pre-implementation) and 9.4 h (Post-implementation) |
Intervention Site: Mortality within 30 days 0 (Pre-implementation) and 1 (Post-implementation); Standard Care Sites: Mortality within 30 days 48 (Pre-implementation) and 35 (Post-implementation) |
Intervention Site: Direct admissions to ICU: (Pre-implementation: 202; Post-implementation: 34), Late admissions to ICU: (Pre-implementation: 6; Postimplementation:4); Standard Care Sites: Direct admissions to ICU: (Pre-implementation: 3357; Post-implementation: 2488), Late admissions to ICU: (Pre-implementation, 64; Post-implementation, 45). |
The SC insulin protocol was associated with a 57% relative decrease in ICU admissions at intervention sites and a 50% relative decrease in 30-day readmissions compared with standard care sites. |
| Stuhr et al. (2023) |
SC: 3.4 IV Insulin (with bolus): 4.5 IV Insulin (without bolus): 6.5 |
SC: 12.7 IV Insulin (with bolus): 13.1 IV Insulin (without bolus): 13.9 |
N/A |
Hypoglycemia: SC: 45 events IV Insulin (with bolus): 69 events. IV Insulin (without bolus): 100 events Hypokalemia: N/A |
Using a SC insulin protocol may be equally effective in treating mild to moderate DKA compared with an IV insulin infusion protocol, while also decreasing LOS and incidence of hypoglycemic events. With a reduction in LOS and the ability to successfully treat DKA outside of the ICU environment, utilization of a SC DKA treatment protocol in this population can potentially lead to reduced healthcare costs and increased ICU resources availability. |
| Griffey et al. (2023) |
SQuID versus traditional during the study period: -3.0 (95% CI -8.5 to -1.4) SQuID versus preintervention period: -1.4 (95% CI -3.1 to -0.1) SQuID versus pre-COVID control period: -3.6 (95% CI -7.5 to -1.8) |
N/A | N/A | N/A | Mild to moderate DKA can be managed on a non-ICU floor using subcutaneous insulin injections instead of a traditional insulin drip – known as the SQuID protocol, however more data are needed from other sites to address the safety and efficacy. ED LOS was statistically shorter in patients treated with SQUiD protocol compared to the traditional protocol. Decrease in ICU admissions across all mild to moderate DKA patients was seen over time with no statistical difference between intervention period to pre-intervention and pre-COVID periods. |
Abbreviations BUN: blood urea nitrogen; CRP: C-reactive protein; dl: deciliter; DKA: diabetic ketoacidosis; FBS: fasting blood sugar; ICU: intensive care unit; IV: intravenous; kg: kilogram; mg: milligram; LOS: length of stay; SC: subcutaneous; SCr: serum creatinine
Time to Resolution of DKA
Most RCTs and observational studies demonstrated that the time to resolution of DKA with IV and subcutaneous insulin were similar in both groups [13–18, 20–22]. In the meta-analysis, there was no difference in the mean difference of time to resolution of DKA in people treated with subcutaneous insulin versus intravenous insulin (mean difference in hours [MD]: 0.17; 95% confidence interval [CI]: -3.45 to 3.79; I2: 0%) (Fig. 2).
Fig. 2.
Pooled mean difference of the time to resolution of diabetic ketoacidosis (hours) in patients treated with subcutaneous insulin versus intravenous insulin for the management of diabetic ketoacidosis. Abbreviations CI, confidence interval; IV, intravenous; MD, mean difference; SC, subcutaneous; SD, standard deviation
Length of Stay
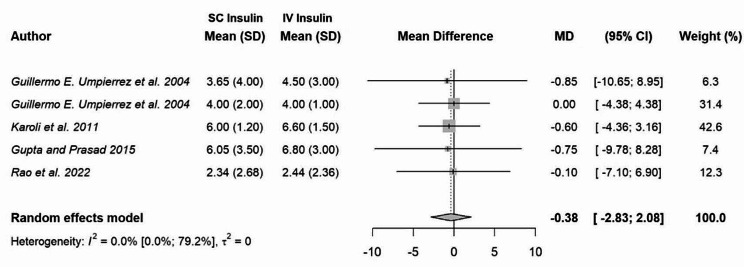
Results regarding the length of stay varied across studies. Four RCTs [16–18, 20] and one observational study [22] showed no statistical difference between both groups in terms of length of hospital stay. The observational study by Balili et al. observed that the length of hospital stay was significantly shorter by a mean of six days for those in the subcutaneous insulin group compared to the IV insulin group [14]. The observational study done by Stuhr et al. demonstrated that the length of stay was significantly longer in the IV insulin with no bolus group compared to the subcutaneous insulin group, notably by a mean of 3.4 days in the subcutaneous insulin group compared to 6.1 days in the IV insulin with no bolus group [13]. The prospective study by Griffey et al. demonstrated that the length of stay in the emergency department was shorter in patients treated with a subcutaneous insulin protocol compared to the traditional insulin infusion with a point estimate of -3.0, 95% CI -8.5 to -1.4. [19] Two RCTs [15, 21] did not evaluate this specific outcome. We conducted a meta-analysis assessing this outcome in five studies. Given that Griffey et al. did not report actual values for the length of stay, we were unable to include their results in the meta-analysis. In the meta-analysis, there was no difference in the length of hospital stay among people treated with subcutaneous insulin versus intravenous insulin for the management of DKA (MD in days: -0.39; 95% CI: -2.83 to 2.08; I2: 0%) (Fig. 3).
Fig. 3.
Pooled mean difference in days on length of hospital stay in patients treated with subcutaneous insulin versus intravenous insulin for management of diabetic ketoacidosis. Abbreviations CI, confidence interval; IV, intravenous; mean difference; RR, risk ratio; SC, subcutaneous; SD, standard deviation
All-Cause Mortality
There were no deaths reported in the RCTs [15–18, 20, 21]. However, there was one death reported in the retrospective cohort study by Balili et al. The patient died in the subcutaneous insulin group due to septic shock secondary to a hospital-acquired infection, which he acquired multiple days after the resolution of DKA. As such, his death was attributed to sepsis rather than the treatment of DKA itself [14]. There were no other mortalities reported in the observational studies. [13, 19]
Hypoglycemia
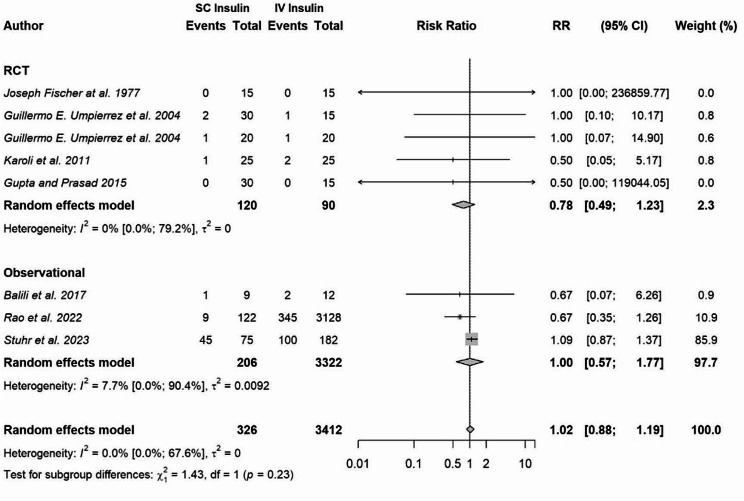
One RCT [16] and two observational studies [13, 14] reported increased episodes of hypoglycemia among individuals in the IV insulin group compared to those in the subcutaneous insulin group, whereas four RCTs [15, 17, 18, 20] and one observational study [22] showed no difference in the episodes of hypoglycemia among both groups. One RCT [21] and one observational study [19] did not report any hypoglycemic events. In our meta-analysis of eight studies, there was no increased risk of hypoglycemia among people treated with subcutaneous insulin versus IV insulin for DKA management (Risk Ratio [RR]: 1.02; 95% CI: 0.88 to 1.19; I2: 0%) (Fig. 4). To determine if the results differed based on study design, we repeated the meta-analysis by assessing hypoglycemic events in RCTs (RR: 0.78; 95% CI 0.49 to 1.23; I2: 0%) and observational studies separately (RR: 1.00; 95% CI: 0.57 to 1.77; I2: 7.7%) (Fig. 4).
Fig. 4.
Pooled risk ratio of hypoglycemia in patients treated with subcutaneous insulin versus intravenous insulin in the management of diabetic ketoacidosis. Abbreviations CI, confidence interval; IV, intravenous; mean difference; RR, risk ratio; SC, subcutaneous; SD, standard deviation
Hypokalemia
Only two studies [14, 21] assessed the risk of hypokalemia given that most studies followed an electrolyte replacement protocol. Balili et al. observed higher occurrences of hypokalemia in the IV insulin group compared to the subcutaneous insulin group (six versus one episode, respectively) [14]. Fisher et al. reported four episodes of hypokalemia, but they did not specify in which treatment group these episodes occurred [21]. Given the lack of data related to this specific outcome, we were not able to conduct meta-analysis for this outcome.
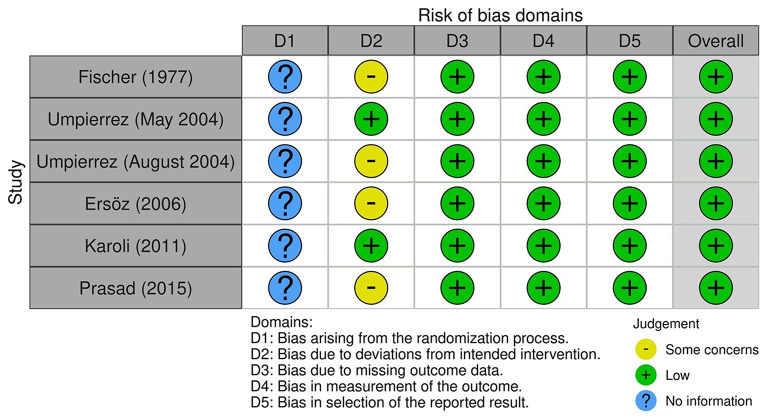
Based on our assessment using RoB2 from the Cochrane Review, the risk of bias for the RCTs was overall low [15–18, 20, 21] (Fig. 5). Using the ROBINS-I tool, we concluded that the risk of bias for the observational studies was low to moderate risk [13, 14, 19, 22] (Fig. 6).
Fig. 5.
Risk of bias assessment for randomized control trials using the Cochrane Risk of Bias tool
Fig. 6.
Risk of bias assessment for observational studies using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS)-I tool
The overall quality of evidence using the GRADE criteria was low for all-cause mortality given that there were no reports of deaths in the included studies. The quality of evidence for hypoglycemia, time to resolution of DKA and length of hospital stay were moderate due to the few studies conducted for these outcomes and potential risk of bias. Finally, the quality of evidence for hypokalemia was considered very low due to the paucity of studies that assessed this outcome and potential bias in the studies (Table 3).
Table 3.
Summary of assessment of quality of evidence using grading of recommendations Assessment, Development, and evaluation (GRADE) assessment criteria
| Outcome | Effect estimates (95% CI) |
Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| All-cause mortality | Not estimable. |
8,190 (4 randomized controlled trials, 2 observational studies) |
⊕⊕◯◯ Low |
No deaths reported. |
| Time to resolution of diabetic ketoacidosis (DKA) |
Mean difference in hours: 0.17 (-3.45 to 3.79) |
8,467 (4 randomized controlled trials, 1 observational study) |
⊕⊕⊕◯ Moderate |
Few studies. Potential bias from one study. |
| Length of hospital stay (LOS) |
Men difference in days: -0.38 (-2.83 to 2.08) |
8, 624 (4 randomized controlled trials, 1 observational study) |
⊕⊕⊕◯ Moderate |
Few studies. |
| Hypoglycemia |
Risk ratio: 1.02 (0.88 to 1.19) |
8,447 (5 randomized controlled trials, 3 observational studies) |
⊕⊕⊕◯ Moderate |
Few studies. Potential bias from one study. |
| Hypokalemia | Not estimable. |
66 (1 randomized controlled trial, 1 observational study) |
⊕◯◯◯ Very Low |
Most studies used a protocol for potassium replacement. |
Discussion
In this systematic review, we synthesized the available evidence to determine the safety and efficacy of using subcutaneous insulin in the management of patients with DKA compared to the current standard of care of using a continuous IV insulin infusion. Based on the included RCTs and observational studies, we found that subcutaneous insulin was associated with a significant improvement in clinical and patient-centered outcomes, specifically a decrease in the length of emergency department and hospital stay [13, 14, 19]. Most importantly, the studies reported no observed difference in adverse outcomes between the two treatment groups.
Based on our assessment using RoB2 and ROBINS-I tool, the overall risk of bias in the RCTs and observational studies were low. The observational study conducted by Balili et al. [14] had some concerns in terms of the quality given that they did not adjust for confounding factors, such as, BMI, the time of admission, co-morbidities, and initial laboratory values on presentation. Additionally, they did not have enough individuals in each group to achieve a power of 0.8 and alpha error of 0.05 due to incomplete data. The quality of evidence was overall very low to moderate for the outcomes assessed due to the limited number of studies, and potential bias from one study [14]. No deaths associated with DKA management were reported in the studies retrieved.
Overall, the studies included in this systematic review demonstrate that both the use of subcutaneous insulin and IV insulin are equally effective in treating mild-to-moderate DKA and no evidence of increased adverse outcomes among individuals treated with subcutaneous insulin. Some studies also showed that subcutaneous insulin is associated with an overall shorter duration of hospitalization [13, 14], which may lead to a reduction in hospitalization costs. The lower hospitalization costs are partly due to the fact that subcutaneous insulin does not need to be administered in a monitored setting given that hospitalization costs for people admitted to the ICU with DKA are higher when compared to a step-down unit. A comparative study conducted in 2018 compared the outcomes and costs between adult patients with DKA who were admitted to the ICU versus a step-down unit. There was no difference in the risk of hospital mortality between both groups; however, the total mean hospitalization cost was higher for a hospitalization in the ICU versus a step-down unit (i.e., $20,428 versus $6,484 Canadian dollars, respectively) [23].
To the best of our knowledge, no previous study has evaluated the safety and efficacy of subcutaneous insulin in people with euglycemic DKA. Only one study in this systematic review included patients with severe DKA [22]. The majority of studies conducted so far on this topic have mainly focused on the use of subcutaneous insulin in managing mild to moderate DKA.
Limitations
Our study has some potential limitations. First, the present data is only applicable to patients with mild-to-moderate DKA without severe illness given that most of the studies excluded patients who were critically ill with multiple co-morbidities. Second, there were no documented cases of euglycemic DKA in the included studies. However, in one retrospective study, the authors included a protocol to treat euglycemic DKA [22]. Thus, further studies are required to clarify the safety of using subcutaneous insulin for the management of DKA in this specific patient population. Third, the studies included individuals with DKA and did not perform sub-analyses for causes of DKA or underlying type of diabetes. It is unclear whether the efficacy and safety of using subcutaneous insulin compared to a continuous IV insulin varies among individuals with different precipitants of DKA and varying forms of diabetes. Fourth, even though Rao et al. [22] had a relatively large sample size with noteworthy reductions in direct ICU and hospital readmissions by using subcutaneous insulin, the number of DKA cases in the subcutaneous insulin group is significantly smaller than that of the IV insulin treatment group (subcutaneous insulin group: 420 DKA cases versus IV insulin group: 7569 DKA cases). There remains a need for larger-scale studies to better understand the effectiveness and feasibility of using subcutaneous insulin in managing DKA. Finally, this systematic review only included a total of ten studies, most of which had a relatively small sample size and no documented close follow-up. Hence, larger RCTs with established protocols and regular follow-up are needed to confirm the clinical significance of using subcutaneous insulin compared to IV insulin and to explore optimal dosing and administration strategies for subcutaneous insulin in the management of DKA.
New Insights
We conducted an updated systematic review based on the initial systematic review done by Andrade-Castellanos et al. in 2016, which has allowed us to gain further insight into the use of subcutaneous insulin in the management of DKA. There are also a few differences in terms of the study population, specifically the exclusion of the pediatric population. Our systematic review included four additional observational studies [13, 14, 19, 22] and two RCTs [20, 21], which were published after the systematic review conducted by Andrade-Castellanos et al. [5]. By gathering data from observational studies as well, we were able to conclude that all included studies support the use of subcutaneous insulin as an effective alternative to IV insulin in the management of mild to moderate DKA. Based on the updated data, we were able to conduct a meta-analysis for certain outcomes.
Conclusion
Our systematic review and meta-analysis did not identify important differences in overall mortality and adverse outcomes following treatment of mild-to-moderate DKA with subcutaneous insulin compared to a continuous IV insulin infusion. Studies conducted to date have demonstrated that subcutaneous insulin is associated with a decreased length of hospital stay and hospitalizations costs when compared to a continuous IV insulin infusion [13, 14, 18]. However, the sample sizes of the retrieved studies were relatively small with no follow-up data available to assess outcomes after discharge. Further studies are necessary to determine the effectiveness and safety of using subcutaneous insulin in the treatment of DKA in specific populations, including DKA during pregnancy, euglycemic DKA, severe DKA, DKA from various precipitants and among individuals with different forms of diabetes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Genevieve Gore and Andrea Quaiattini, for developing the search strategies and helping us retrieve the articles.
Author contributions
O.Y. and A.A. developed the protocol for the systematic review. A.A. and T.M. conducted the literature search and screened the abstracts. A.A. and T.M. reviewed the full text articles and conducted the data extraction. P.R. and K.F. provided expertise with conducting meta-analysis. P.R. conducted the meta-analysis. A.A. and T.M. wrote the main manuscript text and all authors reviewed the manuscript.
Funding
No funding was given to conduct this study. Dr. Filion is supported by a salary support award from the Fonds de recherche du Québec - santé and a William Dawson Scholar award from McGill University.
Data availability
All data generated or analysed during this study are included in this published article (i.e. data extracted to conduct the meta-analyses are available in Supplementary Table 1).
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. 10.1038/s41572-020-0165-1 [DOI] [PubMed] [Google Scholar]
- 2.Ramphul K, Joynauth J. An update on the incidence and Burden of Diabetic Ketoacidosis in the U.S. Diabetes Care. 2020;43(12):e196–7. 10.2337/dc20-1258 [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43. 10.2337/dc09-9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Canada Clinical Practice Guidelines, Expert C, Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Can J Diabetes. 2018;42(Suppl 1):S109–14. [DOI] [PubMed] [Google Scholar]
- 5.Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Gonzalez-Padilla DA. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst Rev. 2016;1:CD011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schünemann HBJ, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. 2013. Available from: guidelinedevelopment.org/handbook.
- 10.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 11.Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plann Inference. 2010;140:961–70. 10.1016/j.jspi.2009.09.017 [DOI] [Google Scholar]
- 12.Pan Y, Wang Q, Zhao F, Shen J, Zhong X. Effect of Continuous Subcutaneous Injection of Insulin Analogues in pregnant women with diabetes Mellitus complicated with ketoacidosis. J Healthc Eng. 2021;2021:8670474. 10.1155/2021/8670474 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Stuhr K, LeeMaster R, Hickman AW, Reachi B, Pace W, Meek C. Subcutaneous insulin Versus Traditional Intravenous insulin infusion in treatment of mild to Moderate Diabetic Ketoacidosis. J Emerg Med. 2023;65(3):e221–8. 10.1016/j.jemermed.2023.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Balili CAV, Gomez MHS. Efficacy and safety of subcutaneous insulin analogue versus intravenous insulin infusion among patients with mild to moderate diabetic ketoacidosis at the university of Santo Tomas hospital. Phillippine J Intern Med. 2017;55(1).
- 15.Ersöz HO, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60(4):429–33. 10.1111/j.1368-5031.2006.00786.x [DOI] [PubMed] [Google Scholar]
- 16.Karoli R, Fatima J, Salman T, Sandhu S, Shankar R. Managing diabetic ketoacidosis in non-intensive care unit setting: role of insulin analogs. Indian J Pharmacol. 2011;43(4):398–401. 10.4103/0253-7613.83109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27(8):1873–8. 10.2337/diacare.27.8.1873 [DOI] [PubMed] [Google Scholar]
- 18.Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117(5):291–6. 10.1016/j.amjmed.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Griffey RT, Schneider RM, Girardi M, Yeary J, McCammon C, Frawley L, et al. The SQuID protocol (subcutaneous insulin in diabetic ketoacidosis): impacts on ED operational metrics. Acad Emerg Med. 2023;30(8):800–8. 10.1111/acem.14685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad A, Gupta A. Role of insulin aspart in Management of Diabetic Ketoacidosis. Indian J Res. 2015;4(7):61–2. [Google Scholar]
- 21.Fisher JN, Shahshahani MN, Kitabchi AE. Diabetic Ketoacidosis: low-dose insulin therapy by various routes. N Engl J Med. 1977;297(5):238–41. 10.1056/NEJM197708042970502 [DOI] [PubMed] [Google Scholar]
- 22.Rao P, Jiang SF, Kipnis P, Patel DM, Katsnelson S, Madani S, et al. Evaluation of outcomes following hospital-wide implementation of a Subcutaneous insulin protocol for Diabetic Ketoacidosis. JAMA Netw Open. 2022;5(4):e226417. 10.1001/jamanetworkopen.2022.6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernando SM, Bagshaw SM, Rochwerg B, McIsaac DI, Thavorn K, Forster AJ, et al. Comparison of outcomes and costs between adult diabetic ketoacidosis patients admitted to the ICU and step-down unit. J Crit Care. 2019;50:257–61. 10.1016/j.jcrc.2018.12.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (i.e. data extracted to conduct the meta-analyses are available in Supplementary Table 1).