Abstract
Objectives:
Psychotropic medications are frequently used in the treatment of dementia. Little is known, however, about the patterns of psychotropic medication use in community-dwelling minority persons with dementia (PWD). The purpose of this study was to investigate racial/ethnic differences in psychotropic medication use across a diverse population of community-dwelling PWD and to examine the extent to which caregiver characteristics influence this use.
Method:
Data were drawn from the baseline assessment of the Resources for Enhancing Alzheimer’s Caregiver Health II trial. Generalized linear models were used to identify racial/ethnic differences in psychotropic medication use. Akaike Information Criterion (AIC) model selection was used to evaluate possible explanations for observed differences across racial/ethnic group including caregiver characteristics, such as confidence managing problematic behaviors, and PWD characteristics including pain, problem behaviors, cognitive impairment, and functional impairment.
Results:
Differences in anxiolytic and antipsychotic medication use were observed across racial/ethnic groups; however, race/ethnicity alone was not sufficient to explain those differences. Perceptions of caregiving and caregiver socioeconomic status were important predictors of anxiolytic use while PWD characteristics, including cognitive impairment, functional impairment, problem behavior frequency, pain, relationship to the caregiver, sex, and age were important for antipsychotic use.
Conclusion:
Racial/ethnic differences in psychotropic medication use among community-dwelling PWD cannot be explained by race/ethnicity alone. The importance of caregiver characteristics in predicting anxiolytic medication use suggest that interventions aimed at caregivers may hold promise as an effective alternative to pharmacotherapy and may help maintain PWD in the community.
Keywords: dementia, race/ethnicity, psychotropic medications, community-dwelling
Introduction
There are currently two classes of medications approved by the FDA for the treatment of Alzheimer’s disease and related disorders: cholinesterase inhibitors for the early to moderate stages of dementia, and NMDA receptor antagonists for moderate to severe disease stages. Previous investigations have uncovered racial/ethnic differences in utilization of these drugs such that Non-Hispanic White patients receive more prescriptions relative to minority patients (Hernandez, McClendon, Zhou, Sachs, & Lerner, 2010; Poon, Lal, Ford, & Braun, 2009; Zuckerman et al., 2008). This difference is particularly concerning considering that older African Americans and Hispanic/Latinos are part of the fastest growing sector of the US older adult population, estimated to make up more than 53% of the U.S. population by 2050 (Passel & Cohn, 2008), and are more likely than older Non-Hispanic Whites to have Alzheimer’s disease and other dementias (Valle & Lee, 2002).
In addition to cholinesterase inhibitors and NMDA receptor antagonists, psychotropic medications including anxiolytics, antipsychotics, and antidepressants are used in the treatment of dementia, with nearly one-third of those diagnosed taking antidepressants or antipsychotics (Gruber-Baldini et al., 2007). While antidepressant treatment may slow disease progression (Lauterbach et al., 2010), improve domains of neuropsychiatric symptoms (Drye et al., 2011), increase hippocampal neurogenesis, and improve cognition (Malberg, 2004), antipsychotic medication is associated with increased mortality (Simoni-Wastila et al., 2009), risk of falls (Woolcott et al., 2009), and rapid decline in cognitive and functional ability (Rosenberg et al., 2012). Although not approved by the FDA, these medications are often prescribed off-label to manage neuropsychiatric symptoms despite concerns about their safety and effectiveness (Wang, Brookhart, Setoguchi, Patrick, & Schneeweiss, 2006).
Relatively little is known about racial/ethnic differences in the prescription and use of psychotropic medication for the behavior complications of dementia. A majority of the existing work in this area focuses on relatively homogeneous nursing home populations or inpatients (Kamble, Sherer, Chen, Aparasu, & Pharm, 2010; Weston, Weinstein, Barton, & Yaffe, 2009). Few studies of psychotropic medication use among older adults living in the community exist (Aparasu, Mort, & Brandt, 2003, Cook, Reeves, Teufel, & Postolache, 2015; Jano, Johnson, Chen, & Aparasu, 2008), and only a few focus exclusively on persons with dementia (PWD). Typically, a comprehensive evaluation of potential racial/ethnic differences is not possible due to either the absence of race/ethnicity data in the analysis (Kunik et al., 2010) or the dichotomization of race into “White and non-White categories” (Chan, Kasper, Black, & Rabins, 2007); however, a post-hoc analyses of US Veteran’s Affairs patients diagnosed with dementia found that African Americans were more likely to be prescribed haloperidol versus olanzapine or quetiapine than Non-Hispanic Whites (Kim, Chiang, Kales, 2011). This difference in prescribing is alarming as typical antipsychotics such as haloperidol have been shown to increase the risk of death in older adult patients relative to atypical antipsychotics (Aparasu, Chatterjee, Mehta, & Chen, 2012; Huybrechts et al., 2012).
The interpretation of racial/ethnic differences in psychotropic medication use among dementia patients is not straightforward. The relative lack of psychotropic medication use in a particular group may represent an advantage given the minimal benefit and increased risk of death associated with these medications. However, lack of antidepressant use may represent a disadvantage as these medications may slow disease progression (Lauterbach et. al., 2010), improve several domains of neuropsychiatric symptoms (Drye, et. al., 2011), increase hippocampal neurogenesis, and improve cognition (Malberg, 2004). Given the documented racial/ethnic differences in approved anti-dementia treatments, and the array of potential benefits and harms that accompany off-label use of psychotropic medication in dementia, examining psychotropic medication use in a culturally diverse dementia population is a priority.
Multiple conceptual models are available to help understand the determinants of psychotropic medication use in dementia caregiving and also to understand how racial/ethnic differences in medication use arise (Andersen, & Newman, 2005; Pearlin, Mullan, Semple, Skaff, 1990). These models highlight the multifactorial nature in which race/ethnicity can influence caregiving outcomes including differential exposure to hazards or stressors that influence health and exacerbate disease; unequal access to financial and educational resources that buffer the effects of stressors; and variability in cultural norms that influence perceptions of caregiving, coping strategies, and social support availability. A majority of the existing work on racial/ethnic differences in anti-dementia medication among community-dwelling older adults relies on billing data (Perryman, Lewis, & Rivers, 2009) or cohorts that focus solely on the PWD, thereby lacking information on informal caregivers (Hernandez et al., 2010; Mehta, Yin, Resendez, & Yaffe, 2005; Zuckerman et al., 2008). Informal caregivers are key agents for the plan of care for PWD, and caregivers from different racial/ethnic groups may vary in the perceived intensity of stressors and coping strategies relevant to health outcomes (Pinquart & Sörensen, 2005). The purpose of this study was to investigate racial/ethnic differences in psychotropic medication use and to examine the extent to which caregiver characteristics influence PWD psychotropic medication across a diverse population of community-dwelling dementia patients.
Using data from the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II randomized trial, we first focused on documenting racial/ethnic differences in the use of three psychotropic medications (anxiolytics, antipsychotics, and antidepressants). We then identified variables that could explain racial/ethnic differences in psychotropic medication as potential targets for future intervention. We hypothesized that the prevalence of psychotropic medication would be higher in Non-Hispanic Whites compared to Hispanics/Latinos or African Americans, and that observed differences between racial/ethnic groups would be explained by caregiver socioeconomic factors, PWD characteristics, caregiver health, perceptions of caregiving, or non-financial resources.
Methods
Sample
The data for this study were drawn from the baseline assessment of REACH II (ClinicalTrials.gov Identifier NCT00177489). Recruitment procedures, eligibility criteria, and psychometric properties of measures and intervention outcomes are described elsewhere (Belle et al., 2006). The primary goal of the REACH II trial was to evaluate a multi-component, psychosocial intervention aimed at improving the quality of life of Alzheimer’s caregivers. In total, 642 community-dwelling PWDs and their caregivers were recruited throughout 2001–2004 from five sites across the country (Birmingham, AL; Memphis, TN; Miami, FL; Palo Alto, CA; and Philadelphia, PA). This analysis included only caregivers who were the same race/ethnicity as the PWDs. All participants needed to have full information on study predictors and outcomes (N=543).
Outcome Measures
This study focused on PWD use of anxiolytic, antipsychotic, and antidepressant medications using the “brown bag” method of data collection (Psaty et al., 1992). Caregivers were asked to display all currently administered medications to the in-home interviewer. Medication names were recorded by study personnel and were later assigned a therapeutic classification code (Aloisi, 2002). Although more detailed information on drug dosages and duration of use is desirable, these were not collected as part of the REACH II trial.
Predictors
Several caregiver and PWD characteristics were examined as predictors of PWD psychotropic medication use. Race/ethnicity, the focal variable of this study, was obtained through caregiver report and recorded as Non-Hispanic White, Hispanic/Latino, or African American. Sampling was clustered by site and considered in the investigation. Other variables of interest reported by the caregiver included socioeconomic status as measured by current employment status, years of education, yearly household income before taxes, and income adequacy.
Several PWD characteristics included baseline cognitive status as measured by the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975); functional impairment as measured by the ability to independently perform basic and instrumental activities of daily living (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963); and the number of behavior complications exhibited in the past week as measured by the Revised Memory and Behavior Problem Checklist (Teri et al., 1992).
No direct measure of pain was collected in REACH II; however, information on PWD analgesic medication use was available. Previous research supports the use of analgesic medication as a proxy for pain (Norton et al., 2010); therefore, PWD use of a narcotic or COX-2 inhibitor was utilized as a dichotomous surrogate for pain. Non-steroidal anti-inflammatory agents (NSAIDs) were not considered as they have historically been used to manage low levels of chronic pain that cannot be eliminated (Ferrell et al., 2009). Additionally, NSAIDs such as aspirin are often used to decrease platelet aggregation and prevent blood clots (Alhusban & Fagan, 2011). An overwhelming majority of the NSAID use in this study was aspirin (84.2%). Therefore, we focused on the presence of a narcotic or COX-2 inhibitor as a surrogate for pain. PWD sex, age at baseline, and relationship to the caregiver (spouse/non-spouse) were also considered.
Several variables representing caregiver perceptions of caregiving were used in the analyses and included overall caregiving burden as measured by an abbreviated, 12-item version of the Zarit Caregiver Burden Inventory (Bédard et al., 2001; Zarit, Orr, & Zarit, 1985); the extent to which a caregiver was bothered by assisting with PWD functional limitations (daily care bother; Gitlin et al., 2005); the extent to which caregivers were bothered by PWD problem behaviors (Teri et al., 1992); the amount of confidence caregivers had in handling the problem behaviors (Teri et al., 1992); caregiving mastery, assessed by eight items developed by REACH investigators (Hilgeman et al., 2009); vigilance, measured by the hours per day a caregiver reported needing to be “on duty” to care for the PWD (Hilgeman et al., 2009); and the nine-item Positive Aspects of Caregiving Scale (Tarlow et al., 2004).
Caregiver health was measured by self-report (Schulz et al., 1997) and depression, as measured by the 10-item version of the Center for Epidemiological Studies-Depression Scale (CES-D), (Radloff, 1977). Non-financial resources were captured by spiritual/religious coping and social network. Spiritual/religious coping was assessed by nine questions asking caregivers to rate the extent to which religious and spiritual beliefs affect their caregiving (Pargament et al., 1990); while multiple dimensions of social support including network size, support satisfaction, and negative social interactions were captured from several previous measures of social interaction and support (Krause & Markides, 1990; Krause, 1995; Lubben, 1988). Social network size was assessed with two questions regarding the number of people who can be counted on to provide help. Caregiver satisfaction with the help received from social contacts was assessed with three questions. Finally, the presence of negative social interactions was assessed with four questions asking caregivers to rate the frequency of negative interactions on a four-point scale. The final resource considered was dementia knowledge measured by the caregiver’s general knowledge of memory loss, dementia, and end of life legal issues (Hilgeman et al., 2009).
Statistical Analysis
Descriptive statistics were computed for demographic variables. To determine whether there were racial/ethnic differences in the use of psychotropic medication, generalized linear models with a logit link function were fit using each medication as an outcome and race/ethnicity as a predictor. Two common methods used in the epidemiologic literature were considered for evaluating explanations for racial/ethnic differences in psychotropic medication use: including successive addition of variables that may attenuate the effect of race/ethnicity, and the addition of interaction terms to determine whether the risk of medication associated with a variable of interest differs across race/ethnicity. For several reasons, these methods were considered insufficient for the current study. Consequently, Akaike Information Criterion (AIC) model selection, an information-theoretic approach presented by Burnham and Anderson (2002) was chosen to address study hypotheses concerning differing patterns of medication use between racial/ethnic groups. This approach allowed us to determine whether observed racial/ethnic differences in psychotropic medication use could be explained by caregiver socioeconomic status, PWD characteristics, caregiver perceptions of caregiving, caregiver health, or non-financial caregiving resources.
The objective of the AIC-based model selection is to find the smallest number of parameters for adequate representation of the data, resulting in a model that achieves the optimal balance in the trade-off between bias and variance. The AIC model selection approach has been used extensively in the ecology literature and has been recognized in the social sciences as a theoretically rigorous method for selecting an optimal model from various pre-specified models (Burnham & Anderson, 2004). Briefly, this method uses AIC to quantify the amount of information in a given set of pre-specified models relative to the amount of noise. The model with the lowest AIC (AIC minimal model) is the most optimal. Remaining models are then ranked based on the AIC (lower is better). Differences in AIC (ΔAIC) are used to compare the optimal model to each remaining model, with the larger values of ΔAIC (typically greater than 2) indicating poorer fit (Burnham & Anderson, 2002; Burnham & Anderson, 2004).
Differences in model AIC can also be used to calculate the likelihood of a model given the data. These likelihoods represent the strength of evidence for each model and can be used to produce evidence ratios. Evidence ratios represent the relative strength of evidence for one model versus the other, and quantify the amount of variation in the selected best model from sample to sample if we could draw repeated, independent samples from the population. Evidence ratios close to one indicate that there is little evidence in favor of either model (Burnham & Anderson, 2002; Burnham & Anderson, 2004). Given our a priori interests, we employed this approach to determine whether models containing some combinations of these variable sets without race/ethnicity were more parsimonious than the equivalent model containing race/ethnicity, thus implying that racial/ethnic differences in psychotropic medication can be explained by these other factors. All combinations of variable sets were investigated in main effects, logistic regression models. Trimmed models were not presented because it is inappropriate to use AIC-selection criteria and then revise models based on p-values, as this mixes statistical paradigms (Anderson, Link, Johnson, & Burnham, 2001).
Results
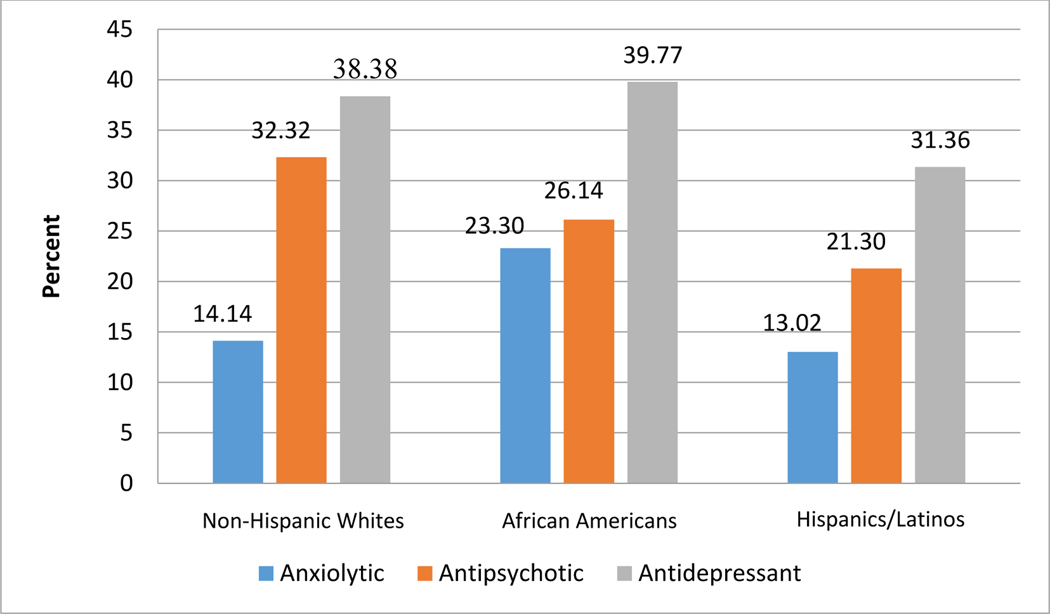
Demographic characteristics are shown in Table 1. As shown in Table 2, PWDs across racial/ethnic groups exhibited, on average, approximately eleven behavioral complications, causing caregivers “a little” to “a moderate” amount of bother. On average, Non-Hispanic White and African American caregivers reported “very much” confidence managing behavior complications whereas Hispanic/Latino caregivers reported only “moderate” levels of confidence. Figures 1 and 2 display the distribution of PWD psychotropic medication use for each racial/ethnic group. As shown in Figure 1, antidepressants were the most prevalent psychotropic medication across all racial groups, followed by antipsychotics, and anxiolytics. Within Non-Hispanic White PWDs the percentage of people taking an antipsychotic is slightly over two times the percentage taking an anxiolytic; however, that relation does not hold within African American PWDs where the prevalence of anxiolytics is almost equal to that of antipsychotics. Within Hispanic/Latino PWDs, the prevalence of antipsychotic use is approximately 1.5 times greater than the use of anxiolytics.
Table 1.
Demographics of Study Participants
| Non-Hispanic Whites | African Americans | Hispanics/Latinos | ||||
|---|---|---|---|---|---|---|
| Demographics | Caregiver | Care recipient | Caregiver | Care recipient | Caregiver | Care recipient |
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age† | 59.98 (12.68) | 77.84 (10.26) | 62.28 (12.82) | 79.78 (8.41) | 58.79 (14.12) | 79.80 (8.98) |
| Sex n (%)† | ||||||
| Female | 161 (81.31) | 101 (51.01) | 149 (84.66) | 110 (62.50) | 136 (80.47) | 113 (66.86) |
| Male | 37 (18.69) | 97 (48.99) | 27 (15.34) | 66 (37.50) | 33 (19.53) | 56 (33.14) |
| Employment n (%)§ | ||||||
| Unemployed | 19 (9.60) | - | 20 (11.36) | - | 20 (11.83) | - |
| Retired | 92 (46.46) | - | 64 (36.36) | - | 51 (30.18) | - |
| Homemaker | 32 (16.16) | - | 30 (17.05) | - | 41 (24.26) | - |
| Employed | 55 (27.78) | - | 62 (35.43) | - | 57 (33.73) | - |
| Education§ | 13.78 (1.96) | - | 13.05 (2.14) | - | 11.04 (3.95) | - |
| Household Income§ | 46,161.15 (25,026.24) | - | 31,718 (22,382.91) | - | 25,783.54 (21,750.45) | - |
| Income Adequacy | 1.72 (1.02) | - | 1.66 (1.06) | - | 1.47 (1.00) | - |
| Relationship n(%)§ | ||||||
| Spouse | 111 (56.06) | - | 52 (29.55) | - | 62 (36.69) | - |
| Non-spouse | 87 (43.94) | - | 124 (70.45) | - | 107 (63.31) | - |
| Years of care | 3.98 (5.54) | - | 3.99 (3.96) | - | 6.22 (9.34) | - |
p≤0.05 for chi-square test of homogeneity for PWD variable
p≤0.05 for chi-square test of homogeneity (discrete variable) or ANOVA (continuous variable) for caregiver variable
Table 2.
Descriptive Statistics for Study Predictors and Outcomes
| Non-Hispanic Whites | African Americans | Hispanics/Latinos | ||
|---|---|---|---|---|
| Range | Mean (SD) | Mean (SD) | Mean (SD) | |
| PWD | ||||
| Cognitive status | 0–30 | 11.61 (7.38) | 12.62 (7.68) | 12.78 (6.92) |
| Functional impairment | 0–14 | 10.43 (2.80) | 10.39 (2.84) | 9.63 (3.39) |
| Number of problem behaviors | 0–24 | 10.56 (4.11) | 10.70 (4.04) | 10.67 (3.83) |
| Pain n (%) | - | 22 (11.11) | 30 (17.05) | 17 (10.06) |
| Caregiver | ||||
| Self-reported health | ||||
| Overall current | 0–4 | 2.10 (1.01) | 2.06 (1.05) | 2.24 (1.08) |
| Current versus 6 months previous | 0–4 | 2.06 (0.81) | 2.10 (0.91) | 2.27 (0.84) |
| Depression | 0–60 | 9.58 (6.35) | 9.66 (6.41) | 10.75 (6.58) |
| Burden | 0–48 | 16.88 (8.67) | 17.03 (8.73) | 17.81 (9.11) |
| Daily care bother | 0–4 | 0.73 (0.76) | 0.81 (0.83) | 0.76 (0.77) |
| Problem behavior bother | 0–4 | 1.42 (0.89) | 1.56 (0.93) | 1.44 (0.88) |
| Problem behavior confidence | 0–4 | 2.19 (0.90) | 2.04 (0.93) | 1.91 (0.93) |
| Mastery | 0–6 | 5.93 (2.70) | 6.32 (2.96) | 5.65 (2.94) |
| Vigilance | 0–24 | 18.86 (6.70) | 19.82 (6.24) | 19.33 (6.95) |
| Positive aspects of caregiving | 0–36 | 24.74 (8.93) | 26.09 (8.82) | 26.08 (8.70) |
| Spiritual/religious coping | 0–18 | 15.22 (3.20) | 15.13 (3.39) | 13.95 (3.81) |
| Social Network | ||||
| Size | 0–10 | 6.70 (2.31) | 6.63 (2.28) | 5.90 (2.29) |
| Social support satisfaction | 0–9 | 5.31 (2.58) | 5.51 (2.86) | 4.17 (2.82) |
| Negative social interaction | 0–12 | 2.71 (2.57) | 2.93 (3.03) | 3.07 (2.78) |
| Dementia knowledge | 0–4 | 2.93 (1.30) | 2.24 (1.26) | 1.90 (1.35) |
| Outcomes, n (%) | ||||
| Anxiolytics† | - | 28 (13.93) | 41 (23.30) | 22 (12.94) |
| Antipsychotics† | - | 65 (32.34) | 46 (26.14) | 36 (21.18) |
| Antidepressants | - | 77 (38.31) | 73 (39.77) | 54 (31.76) |
p≤0.05 for chi-square test of homogeneity
p≤0.05 for ANOVA
Figure 1. Psychotropic Medication Prevalence by Race/Ethnicity among Persons with Dementia.
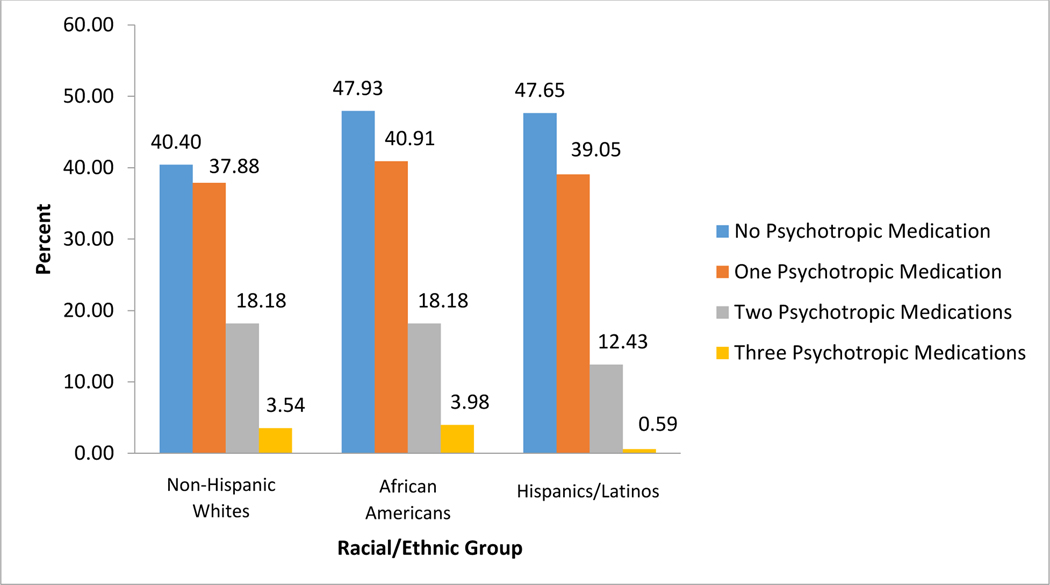
Figure 2. Distribution of Psychotropic Medications by Race/Ethnicity.
The distribution of the number of psychotropic medications taken by PWDs is displayed in Figure 2. African Americans and Hispanics/Latinos demonstrate the lowest prevalence of psychotropic medication use with approximately 48% of PWDs receiving no psychotropic medication (47.93% and 47.65%, respectively). Approximately 40% of Non-Hispanic White PWDs received no psychotropic medication.
Significant racial/ethnic differences were observed for the use of anxiolytics (Wald Χ2=9.86, df=2, p≤ 0.01), with African American PWDs having significantly higher odds of anxiolytic use relative to Non-Hispanic White PWDs (OR=1.83; 95% confidence interval (CI): (1.07, 3.13)). Significant racial/ethnic differences were also observed for antipsychotics (Wald Χ2=6.68, df=2, p=0.04) with Hispanics/Latinos having significantly lower odds of antipsychotic use versus Non-Hispanic Whites (OR=0.49; 95% CI: (0.28, 0.86)). No significant racial/ethnic differences in antidepressant use were observed; thus, no further investigation of between-race/ethnicity differences in antidepressant medication was performed.
The results of the AIC model selection process for anxiolytics and antipsychotics are presented in Table 3. Recall that models are numbered by rank, with 1 being the most parsimonious. If a model without race/ethnicity is more parsimonious than the equivalent model containing it, racial/ethnic differences in psychotropic medication can be explained by other variables in the model. Table 3 displays the AIC information for the top three models predicting anxiolytics and antipsychotics in direct comparison to the equivalent model with or without race/ethnicity, as a majority of the weight was contained in the top model for both medications. The model containing race/ethnicity alone and the model containing race/ethnicity with all sets of predictors are also shown for reference.
Table 3.
AIC Model Fit for Models Predicting Anxiolytic and Antipsychotic Use
| Variable Set Included in the Model* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Rank | PWD Race/Ethnicity | Site | A | B | C | D | E | AIC | ΔAIC | Weight | Rank of Equivalent Model Without Race | Evidence Ratio† |
| Anxiolytics | ||||||||||||
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 477.85 | 0.00 | 0.58 | 4 | 14.38 |
| 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 481.83 | 3.98 | 0.08 | 10 | 6.00 |
| 3 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 483.16 | 5.31 | 0.04 | 21 | 16.97 |
| 21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 489.32 | 11.32 | <0.01 | NA | NA |
| 110 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 499.74 | 21.89 | <0.01 | 125 | 11.95 |
| Antipsychotics | ||||||||||||
| 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 616.26 | 0.00 | 0.51 | 2 | 3.03 |
| 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 618.48 | 2.22 | 0.17 | - | - |
| 3 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 619.29 | 3.03 | 0.11 | 4 | 3.88 |
| 50 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 632.21 | 16.21 | <0.01 | NA | NA |
| 82 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 636.41 | 20.16 | <0.01 | 67 | 0.33 |
Inclusion in the model is indicated by 1, exclusion is indicated by 0
Set A: PWD variables (cognitive impairment, functional impairment, problem behavior frequency, pain, relationship to the caregiver, sex, and age
Set B: caregiver health variables (self-reported health both current and current compared to six months previous, and depression)
Set C: perceptions of caregiving (caregiving burden, bother assisting with functional impairments, bother handling problem behaviors, confidence handling problem behaviors, caregiving mastery, vigilance, and positive aspects of caregiving)
Set D: non-financial caregiving resources (spiritual and religious coping, social network size, social network satisfaction, negative social interaction, and dementia knowledge
Set E: caregiver socioeconomic status (education, employment, income, and income adequacy)
Evidence ratio comparing model with race to an equivalent model without race
For anxiolytics, Model 1 accounts for over half of the model weight and contains PWD race/ethnicity, in addition to the sets of variables representing perceptions of caregiving, and caregiver socioeconomic status. The evidence ratio comparing Model 1 to the same model without race/ethnicity (Model 4 not shown) is 14.38, indicating that the relative likelihood of Model 1 is 14.38 times greater than the equivalent model without race/ethnicity. We can examine the importance of other variable sets in the same way that the importance of race/ethnicity in anxiolytic medication use was evaluated. For example, the difference between the top two models predicting anxiolytic use is the presence of socioeconomic status in Model 1. The evidence ratio comparing Model 1 to Model 2 is 7.25, indicating that there is considerably more support for the model containing socioeconomic status in addition to race/ethnicity and perceptions of caregiving, rather than race/ethnicity and perceptions of caregiving alone. Together, the AIC model selection results suggest that race/ethnicity is necessary for explaining anxiolytic use, even when accounting for relevant caregiving variables.
For antipsychotic medication, Model 1, the AIC optimal model, accounts for over half of the total model weight and includes race/ethnicity, study site, and PWD characteristics. The equivalent model without race/ethnicity is ranked second with an evidence ratio of 3.01, indicating that there is approximately three times more evidence for the model containing race/ethnicity. This is much weaker evidence for the role of race/ethnicity than was observed for anxiolytics, and suggests that caregiver attributes may better explain racial/ethnic differences in PWD’s use of anxiolytics versus antipsychotics. Another notable difference between anxiolytic and antipsychotic medication is that study site appears in each of the top ten models for antipsychotic use (not shown), indicating substantial geographic variation in use of antipsychotic medication.
Tables 4 and 5 present effect estimates and confidence intervals from the AIC optimal models predicting anxiolytics and antipsychotics, as well as the models with race/ethnicity alone. As shown in Table 4, the effect of race/ethnicity on anxiolytic medication increases when variation in perceptions of caregiving and caregiver socioeconomic status is accounted for. Additionally, the odds of PWD anxiolytic use were significantly higher for each additional hour the caregiver needed to be “on duty” (vigilance) and for caregivers with higher levels of income. Unlike anxiolytics, the association between race/ethnicity and antipsychotic medication use does not change between the AIC optimal model and the model with race/ethnicity alone. This is congruent with the AIC model results showing weak evidence for the role of race/ethnicity in the use of antipsychotic medications. The use of analgesics (pain proxy) increased the odds of psychotropic medication while higher cognitive status decreased the odds.
Table 4.
Logistic Regression Models Predicting Anxiolytic Medication (The AIC optimal model and the reduced model with only race)
| Variable | Race Only* | AIC Optimal Model* | ||
|---|---|---|---|---|
|
| ||||
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Race | ||||
| Non-Hispanic Whites | REF | - | REF | - |
| African Americans | 1.83 | (1.07, 3.13) | 2.17 | (1.20, 3.93) |
| Hispanics/Latinos | 0.69 | (0.35, 1.35) | 0.85 | (0.39, 1.84) |
| Perceptions of caregiving (Variable Set C) | ||||
| Overall caregiving burden | - | - | 1.01 | (0.97, 1.05) |
| Bother handling problem behaviors | - | - | 1.30 | (0.91, 1.87) |
| Confidence handling problem behaviors | - | - | 0.81 | (0.61, 1.07) |
| Bother handling functional impairment | - | - | 0.69 | (0.46, 1.05) |
| Mastery handling caregiving responsibilities | - | - | 1.00 | (0.89, 1.12) |
| Vigilance | - | - | 1.06 | (1.01, 1.11) |
| Positive aspects of caregiving | 0.98 | (0.95,1.01) | ||
| Caregiver socio-economic status (Variable Set E) | ||||
| Education | - | - | 0.98 | (0.89, 1.19) |
| Employment | - | - | 1.19 | (0.98, 1.43) |
| Income | - | - | 1.13 | (1.01, 1.26) |
| Income adequacy | - | - | 0.74 | (0.58, 0.95) |
Set C: perceptions of caregiving (caregiving burden, bother assisting with functional impairments, bother handling problem behaviors, confidence handling problem behaviors, caregiving mastery, vigilance, and positive aspects of caregiving)
Set E: caregiver socioeconomic status (education, employment, income, and income adequacy)
Site was also included in the model as a nuisance variable to account for clustering by site.
Table 5.
Logistic Regression Models Predicting Antipsychotic Medication (The AIC optimal model and the reduced model with only race)
| Variable | Race Only* | AIC Optimal Model* | ||
|---|---|---|---|---|
|
| ||||
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Race | ||||
| Non-Hispanic Whites | REF | - | REF | - |
| African Americans | 0.67 | (0.34, 1.39) | 0.68 | (0.41, 1.10) |
| Hispanics/Latinos | 0.49 | (0.28, 0.86) | 0.49 | (0.28, 0.88) |
| Care Recipient variables (Variable Set A) | ||||
| Age | - | - | 0.99 | (0.96, 1.01) |
| Sex | - | - | 1.54 | (0.92, 2.59) |
| Relationship to the caregiver | - | - | 0.94 | (0.56, 1.59) |
| Cognition | - | - | 0.97 | (0.94, 1.00) |
| Functional impairment | ||||
| Number of problem behaviors | 1.01 | (0.96, 1.06) | ||
| Pain | - | - | 1.82 | (1.04, 3.21) |
Set A: CR variables (cognitive impairment, functional impairment, problem behavior frequency, pain, relationship to the caregiver, sex, and age)
Site was also included in the model as a nuisance variable to account for clustering by site. Estimates are not provided here.
Discussion
This study utilized a diverse sample of community-dwelling PWDs and their caregivers to examine racial/ethnic patterns of psychotropic medication use among demented adults. Comparing the prevalence of medication among PWDs from three different racial/ethnic groups, we observed significant differences in the use of anxiolytic and antipsychotic medication. To examine reasons for these differences, we used AIC model selection techniques to determine whether models containing some combinations of variable sets representing PWD characteristics, caregiver socioeconomic status, caregiver perceptions of caregiving, caregiver health, and non-financial caregiving resources were more parsimonious than the equivalent model containing race/ethnicity, thus implying that racial/ethnic differences in psychotropic medication must be considered within the context of these other factors.
African American PWDs were almost twice as likely to use anxiolytic medication compared to Non-Hispanic White PWDs. These results are in contrast to cross-sectional and longitudinal studies of the Established Populations for Epidemiologic Studies of the Elderly (EPESE) cohort. Those studies have consistently found higher rates of psychotropic medication use among community-dwelling, elderly Non-Hispanic Whites versus African Americans (Blazer et al., 2000).
Given that Non-Hispanic White and African American PWDs demonstrated similar levels of impairment and behavior complications in our study, one potential explanation for these disparate findings may be the time period in which the studies were conducted. Data used in the EPESE studies were collected prior to the approval of rivastigmine, galantamine, and memantine (Jones, 2011). Limited choice of FDA approved medications to manage dementia would likely increase the off label use of psychotropic medication for dementia symptoms during the time period of the EPESE studies. Additionally, minority dementia patients tend to receive a diagnosis later in the disease process compared to Non-Hispanic Whites, and once diagnosed, are less likely to access available treatment, which may have resulted in a higher prevalence of anxiolytic use among Non-Hispanic Whites (Cooper, Tandy, Balamurali, & Livingston, 2010). Data for REACH II were collected during the release of three cholinesterase inhibitors and memantine, an NMDA receptor antagonist. Research has demonstrated racial/ethnic differences in the use of new prescription drugs, with Non-Hispanic Whites receiving more novel medications than African Americans (Wang et al., 2007). Therefore, it is possible that the higher prevalence of anxiolytic use by African American PWDs in this study is a result of Non-Hispanic White PWDs transitioning to newer, FDA approved medications.
We also found that Hispanic/Latino PWDs were approximately 40% less likely to use an antipsychotic medication than Non-Hispanic White PWDs. Previous studies of psychotropic medication use among community dwelling elderly did not detect a difference in antipsychotic medication use (Aparasu et al., 2003; Jano et al., 2008); however, our results are consistent with findings from studies of FDA approved anti-dementia medication (Hernandez et al., 2010; Mehta et al., 2005; Zuckerman et al., 2008) that found a higher prevalence of cholinesterase inhibitor use among Non-Hispanic White dementia patients versus Hispanics/Latinos. The discrepancy between our study and the null results from previous work may be due to differences in the study samples; if the difference was strongest among older adults with dementia, the prior studies would not detect it.
Results from the AIC model selection analyses revealed that caregiver and PWD characteristics did not adequately explain racial/ethnic differences in anxiolytic and antipsychotic medication use. This finding is commensurate with a study of approved dementia treatment among Medicare beneficiaries that showed racial/ethnic differences in medication use that could not be fully explained by demographic, economic, health status, access to health care, or health care utilization (Zuckerman et al., 2008). Similarly, the racial/ethnic differences observed by Hernandez et al. could not be accounted for by gender, age, education, marital status, clinical referral, severity, and racial composition of the community (Hernandez et al., 2010). The current study adds to this literature by considering care recipient variables that were not included in previous work.
The finding of persistent racial/ethnic inequalities in medication used to treat dementia appears to be robust across FDA approved and non-approved medications, suggesting that there are still important explanations that have not been considered such as medication adherence. A study of U.S. veterans with hypertension and dementia found that African American and Hispanic/Latino patients demonstrated lower adherence to anti-hypertensive and anti-dementia medications relative to Non-Hispanic White patients (Poon et al., 2009). Another study of Medicaid patients found that after adjustment for income, Hispanics/Latinos were more likely to avoid filling prescriptions due to cost, resulting in higher rates of cost-related non-adherence in Hispanic/Latino enrollees compared to Non-Hispanic enrollees (Frankenfield et al., 2010). It is possible that the racial/ethnic differences in medication use observed in our study result from differing rates of adherence secondary to income inequalities between the racial/ethnic groups. Participants in the REACH trials were asked to supply all currently used medications, making it difficult to know whether absence of a medication represents non-adherence. Future studies investigating racial/ethnic differences in psychotropic drug use among community-dwelling dementia patients should collect detailed information on prescribed medications, filled prescriptions, and medication routines in order to address issues of adherence.
As in any research, this study has limitations. First, the variable sets representing caregiver socioeconomic factors, PWD characteristics, caregiver health, perceptions of caregiving, and non-financial resources were constructed using secondary data and subsequently, are neither exhaustive nor targeted for the current research questions. No formal examination of the extent to which variables within a set cluster together was made; however, all variables were chosen based on face validity and are reasonably expected to represent an important component of the variable set.
Another limitation is that the AIC model selection method used to assess racial/ethnic differences in psychotropic medication use depends on the models specified by the user. We based our choice of models on stress process models supported in the literature that outline determinants of psychotropic medication use in dementia caregiving and also how racial/ethnic differences in medication use may arise. We chose to include only main effects models in our analysis (Cranwell-Bruce, 2010) because evaluating interactions between multiple variable sets would necessitate a prohibitively large number of models.
Another limitation concerns the construction of the racial/ethnic groups. In order to obtain sufficient sample size for an analysis of Hispanic/Latino PWDs, REACH combined Hispanic/Latino caregivers from different cultural subgroups, largely Cuban and Mexican Americans. Despite speaking the same language, these people represent distinct cultural groups that may differ with respect to perceptions of caregiving and PWD health outcomes (Yeo & Gallager-Thompson, 2013). Additionally, REACH did not account for acculturation of the caregiver or PWD. Previous research has shown differences in neuropsychological measures of cognition and caregiver perceptions of caregiving by levels of acculturation (Cohen, Bulatao, & Anderson, 2004). Future studies should attempt to differentiate between cultural groups and include acculturation measures.
REACH II data were collected before the release of the first FDA black box warning on the increased risk of death associated with antipsychotics in the elderly. Therefore, current dementia treatment patterns may differ from those observed here. Although we cannot specifically address this issue, a study by Singh and Nayak (2015) found that warnings and labelling changes regarding the use of atypicals has had minimal impact on their use in noninstitutionalized individuals with dementia, suggesting that understanding the predictors of antipsychotic drug use in community-dwelling dementia patients is still timely and important. Finally, it is important to note that REACH II was a randomized clinical trial including individuals who were willing to participate in an intervention study. These people may not be representative of all community-dwelling persons with dementia and their caregivers.
Within the context of these limitations, this study establishes a point of reference for evaluating racial and ethnic differences in psychotropic medication use among dementia patients living in the community. Moreover, it suggests that there are racial/ethnic differences in the use of psychotropic medication, particularly anxiolytics, by community-dwelling PWDs and that race/ethnicity alone is not sufficient for accounting for these differences. To our knowledge, this is the first study to examine predictors of psychotropic medication among racial and ethnic minority individuals with dementia living in the community. Perhaps our most significant findings suggest that caregiver characteristics are important to consider in the examination of racial/ethnic differences in use of anxiolytics whereas PWD characteristics seem more important in the use of antipsychotics. Different intervention targets may be needed to decrease racial/ethnic differences in the use of these medications and to improve quality of care for all persons with dementia. Specifically, caregiver interventions may hold promise as an effective alternative to anxiolytic use and may help maintain dementia patients in the community.
Contributor Information
Elsie L. Grace, Center for Social Epidemiology and Population Health, School of Public Health, University of Michigan, Ann Arbor, MI, USA
Rebecca S. Allen, Alabama Research Institute on Aging and Department of Psychology, University of Alabama, Tuscaloosa, AL USA.
Keisha D. C. Ivey, Alabama Research Institute on Aging and Department of Psychology, University of Alabama, Tuscaloosa, AL USA
Shannon M. Knapp, University of Arizona, Statistics Consulting Lab, Bio5 Institute, 1657 East Helen Street, PO Box 210240, Tucson, AZ, USA
Louis D. Burgio, Burgio Geriatric Consulting, 26 The Downs, Tuscaloosa, AL 35401, USA
References
- Alhusban A, & Fagan SC (2011). Secondary prevention of stroke in the elderly: a review of the evidence. The American journal of geriatric pharmacotherapy, 9(3), 143–152. doi: 10.1016/j.amjopharm.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Aloisi P. (2002). IDI instant drug index. Blackwell Publishing. [Google Scholar]
- Anderson DR, Link WA, Johnson DH, & Burnham KP (2001). Suggestions for presenting the results of data analyses. The journal of wildlife management, 373–378. doi: 10.2307/3803088 [DOI] [Google Scholar]
- Andersen R, & Newman JF (2005). Societal and individual determinants of medical care utilization in the United States. Milbank quarterly, 83(4), Online-only. doi: 10.1111/j.1468-0009.2005.00428.x [DOI] [PubMed] [Google Scholar]
- Aparasu RR, Chatterjee S, Mehta S, & Chen H. (2012). Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents. Medical care, 50(11), 961–969. doi: 10.1097/MLR.0b013e31826ec185 [DOI] [PubMed] [Google Scholar]
- Aparasu RR, Mort JR, & Brandt H. (2003). Psychotropic Prescription Use by Community-Dwelling Elderly in the United States. Journal of the American Geriatrics Society, 51(5), 671–677. doi: 10.1034/j.1600-0579.2003.00212.x [DOI] [PubMed] [Google Scholar]
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, & O’Donnell M. (2001). The Zarit Burden interview a new short version and screening version. The gerontologist, 41(5), 652–657. doi: 10.1093/geront/41.5.652 [DOI] [PubMed] [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, Gitlin L, Klinger J, Koepe KM, Lee CC, Martindale-Adams J, Nichols L, Schulz R, Stahl S, Stevens A, Winter L, & Zhang S. (2006). Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Annals of internal medicine, 145(10), 727–738. doi: 10.7326/0003-4819-145-10-200611210-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D, Hybels C, Simonsick E, & Hanlon JT (2000). Sedative, hypnotic, and antianxiety medication use in an aging cohort over ten years: a racial comparison. Journal of the American Geriatrics Society, 48(9), 1073–1079. doi: 10.1111/j.1532-5415.2000.tb04782.x [DOI] [PubMed] [Google Scholar]
- Burnham KP, & Anderson DR (2002). Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media. [Google Scholar]
- Burnham KP, & Anderson DR (2004). Multimodel inference understanding AIC and BIC in model selection. Sociological methods & research, 33(2), 261–304. doi: 10.1177/0049124104268644 [DOI] [Google Scholar]
- Chan DC, Kasper JD, Black BS, & Rabins PV (2007). Clinical diagnosis of dementia, not presence of behavioral and psychological symptoms, is associated with psychotropic use in community-dwelling elders classified as having dementia. Journal of geriatric psychiatry and neurology, 20(1), 50–57. doi: 10.1177/0891988706297088 [DOI] [PubMed] [Google Scholar]
- Cohen B, Bulatao RA, & Anderson NB (Eds.). (2004). Critical perspectives on racial and ethnic differences in health in late life. National Academies Press. [PubMed] [Google Scholar]
- Cook TB, Reeves GM, Teufel J, & Postolache TT (2015). Persistence of racial disparities in prescription of first-generation antipsychotics in the USA. Pharmacoepidemiology and drug safety, 24(11), 1197–1206. [DOI] [PubMed] [Google Scholar]
- Cooper C, Tandy AR, Balamurali TB, & Livingston G. (2010). A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. The American Journal of Geriatric Psychiatry, 18(3), 193–203. doi: 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- Cranwell-Bruce LA (2010). Drugs for Alzheimer’s disease. Medsurg Nursing, 19(1), 51. [PubMed] [Google Scholar]
- Drye LT, Martin BK, Frangakis CE, Meinert CL, Mintzer JE, Munro CA, Porsteinsson AP, Rabins PV, Rosenberg PB, Schneider LS, Weintraub D, & Lyketsos CG (2011). Do treatment effects vary among differing baseline depression criteria in depression in Alzheimer’s disease study±2 (DIADS-2)?. International journal of geriatric psychiatry, 26(6), 573–583. doi: 10.1002/gps.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell B, Argoff CE, Epplin J, Fine P, Gloth FM, Herr K, Katz JD, Mehr DR, Reid MC, Reisner L, Radcliff S, Addleman K, Fierstein C, Ickowics E, & Lundebjerg N. (2009). Pharmacological management of persistent pain in older persons. Journal of the American Geriatrics Society, 57(8), 1331. doi: 10.1111/j.1532-5415.2009.02376.x [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Frankenfield DL, Wei II, Anderson KK, Howell BL, Waldo D, & Sekscenski E. (2010). Prescription medication cost-related non-adherence among Medicare CAHPS respondents: disparity by Hispanic ethnicity. Journal of health care for the poor and underserved, 21(2), 518–543. doi: 10.1353/hpu.0.0314 [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Roth DL, Burgio LD, Loewenstein DA, Winter L, Nichols L, Argüelles S, Corcoran M, Burns R, & Martindale J. (2005). Caregiver appraisals of functional dependence in individuals with dementia and associated caregiver upset psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. Journal of aging and health, 17(2), 148–171. doi: 10.1177/0898264304274184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Stuart B, Zuckerman IH, Simoni-Wastila L, & Miller R. (2007). Treatment of Dementia in Community-Dwelling and Institutionalized Medicare Beneficiaries. Journal of the American Geriatrics Society, 55(10), 1508–1516. doi: 10.1111/j.1532-5415.2007.01387.x [DOI] [PubMed] [Google Scholar]
- Hernandez S, McClendon MJ, Zhou XHA, Sachs M, & Lerner AJ (2010). Pharmacological treatment of Alzheimer’s disease: effect of race and demographic variables. Journal of Alzheimer’s Disease, 19(2), 665–672. doi: 10.3233/JAD-2010-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgeman MM, Durkin DW, Sun F, DeCoster J, Allen RS, Gallagher-Thompson D, & Burgio LD (2009). Testing a theoretical model of the stress process in Alzheimer’s caregivers with race as a moderator. The Gerontologist, 49(2), 248–261. doi: 10.1093/geront/gnp015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, Lucas JA, & Schneeweiss S. (2012). Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ, 344, e977. 10.1136/bmj.e977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jano E, Johnson M, Chen H, & Aparasu RR (2008). Determinants of atypical antipsychotic use among antipsychotic users in community-dwelling elderly, 1996–2004. Current medical research and opinion, 24(3), 709–716. 10.1185/030079908X260989 [DOI] [PubMed] [Google Scholar]
- Jones RW (2011). Drug treatment for people with dementia. Clinical medicine, 11(1), 67–71. doi: 10.7861/clinmedicine.11-1-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble P, Sherer J, Chen H, Aparasu R, & Pharm M. (2010). Off-label use of second-generation antipsychotic agents among elderly nursing home residents. Psychiatric Services. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, & Jaffe MW (1963). Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. Jama, 185(12), 914–919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- Kim HM, Chiang C, & Kales HC (2011). After the black box warning: predictors of psychotropic treatment choices for older patients with dementia. Psychiatric Services. [DOI] [PubMed] [Google Scholar]
- Krause N. (1995). Negative interaction and satisfaction with social support among older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 50(2), P59–P73. doi: 10.1093/geronb/50B.2.P59 [DOI] [PubMed] [Google Scholar]
- Krause N, & Markides K. (1990). Measuring social support among older adults. The International Journal of Aging and Human Development, 30(1), 37–53. doi: 10.2190/CY26-XCKW-WY1V-VGK3 [DOI] [PubMed] [Google Scholar]
- Kunik ME, Snow AL, Davila JA, McNeese T, Steele AB, Balasubramanyam V, Doody R, Schulz PE, Kalavar JS, Walder A, & Morgan RO (2010). Consequences of aggressive behavior in patients with dementia. The Journal of neuropsychiatry and clinical neurosciences. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, & Mendez MF (2010). Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. The Journal of neuropsychiatry and clinical neurosciences. [DOI] [PubMed] [Google Scholar]
- Lubben JE (1988). Assessing social networks among elderly populations. Family & Community Health, 11(3), 42–52. [Google Scholar]
- Malberg JE (2004). Implications of adult hippocampal neurogenesis in antidepressant action. Journal of Psychiatry and Neuroscience, 29(3), 196. [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Yin M, Resendez C, & Yaffe K. (2005). Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology, 65(1), 159–162. doi: 10.1212/01.wnl.0000167545.38161.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MJ, Allen RS, Snow LA, Hardin MJ, & Burgio LD (2010). Predictors of need-driven behaviors in nursing home residents with dementia and associated certified nursing assistant burden. Aging & mental health, 14(3), 303–309. doi: 10.1080/13607860903167879 [DOI] [PubMed] [Google Scholar]
- Pargament KI, Ensing DS, Falgout K, Olsen H, Reilly B, Van Haitsma K, & Warren R. (1990). God help me:(I): Religious coping efforts as predictors of the outcomes to significant negative life events. American journal of community psychology, 18(6), 793–824. doi: 10.1007/BF00938065 [DOI] [Google Scholar]
- Passel JS, & Cohn DVUS (2008). US population projections: 2005–2050. [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, & Skaff MM (1990). Caregiving and the stress process: An overview of concepts and their measures. The gerontologist, 30(5), 583–594. doi: 10.1093/geront/30.5.583 [DOI] [PubMed] [Google Scholar]
- Perryman M, Lewis M, & Rivers PA (2009). Treatment Disparities in Medication Prescribing for Alzheimer’s: Disease among Ethnic Groups. Journal of health care finance, 35(4), 64. [PubMed] [Google Scholar]
- Pinquart M, & Sörensen S. (2005). Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: A meta-analysis. The Gerontologist, 45(1), 90–106. doi: 10.1093/geront/45.1.90 [DOI] [PubMed] [Google Scholar]
- Poon I, Lal LS, Ford ME, & Braun UK (2009). Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Annals of Pharmacotherapy, 43(2), 185–193. doi: 10.1345/aph.1L368 [DOI] [PubMed] [Google Scholar]
- Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M, & Cardiovascular Health Study Collaborative Research Group. (1992). Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. Journal of clinical epidemiology, 45(6), 683–692. doi: 10.1016/0895-4356(92)90143-B [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rosenberg PB, Mielke MM, Han D, Leoutsakos JS, Lyketsos CG, Rabins PV, ... & Zuckerman IH (2012). The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer’s disease. International journal of geriatric psychiatry, 27(12), 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Newsom J, Mittelmark M, Burton L, Hirsch C, & Jackson S. (1997). Health effects of caregiving: the caregiver health effects study: an ancillary study of the Cardiovascular Health Study. Annals of Behavioral Medicine, 19(2), 110–116. doi: 10.1007/BF02883327 [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Ryder PT, Qian J, Zuckerman IH, Shaffer T, & Zhao L. (2009). Association of antipsychotic use with hospital events and mortality among Medicare beneficiaries residing in long-term care facilities. The American Journal of Geriatric Psychiatry, 17(5), 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, & Nayak R. (2015). Impact of FDA Black Box Warning on Psychotropic Drug Use in Noninstitutionalized Elderly Patients Diagnosed With Dementia A Retrospective Study. Journal of pharmacy practice, 0897190015579451. doi: 10.1177/0897190015579451 [DOI] [PubMed] [Google Scholar]
- Tarlow BJ, Wisniewski SR, Belle SH, Rubert M, Ory MG, & Gallagher-Thompson D. (2004). Positive Aspects of Caregiving Contributions of the REACH Project to the development of new measures for Alzheimer’s caregiving. Research on aging, 26(4), 429–453. doi: 10.1177/0164027504264493 [DOI] [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, & Vitaliano PP (1992). Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychology and aging, 7(4), 622. 10.1037/0882-7974.7.4.622 [DOI] [PubMed] [Google Scholar]
- Valle R, & Lee B. (2002). Research priorities in the evolving demographic landscape of Alzheimer disease and associated dementias. Alzheimer Disease & Associated Disorders, 16, S64–S76. [DOI] [PubMed] [Google Scholar]
- Wang PS, Brookhart MA, Setoguchi S, Patrick AR, & Schneeweiss S. (2006). Psychotropic medication use for behavioral symptoms of dementia. Current neurology and neuroscience reports, 6(6), 490–495. doi: 10.1007/s11910-006-0051-6 [DOI] [PubMed] [Google Scholar]
- Wang J, Zuckerman IH, Miller NA, Shaya FT, Noel JM, & Mullins CD (2007). Utilizing New Prescription Drugs: Disparities among Non-Hispanic Whites, Non-Hispanic Blacks, and Hispanic Whites. Health services research, 42(4), 1499–1519. doi: 10.1111/j.1475-6773.2006.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AL, Weinstein AM, Barton C, & Yaffe K. (2009). Potentially inappropriate medication use in older adults with mild cognitive impairment. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, glp158. doi: 10.1093/gerona/glp158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, & Marra CA (2009). Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Archives of internal medicine, 169(21), 1952–1960. [DOI] [PubMed] [Google Scholar]
- Yeo G, & Gallager-Thompson D. (Eds.). (2013). Ethnicity and the Dementias Second Edition. Routledge. [Google Scholar]
- Zarit SH, Orr NK, & Zarit JM (1985). Families under stress: Caring for the patient with Alzheimer’s disease and related disorders. New York: UniversityPress. [Google Scholar]
- Zuckerman IH, Ryder PT, Simoni-Wastila L, Shaffer T, Sato M, Zhao L, & Stuart B. (2008). Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 63(5), S328–S333. doi: 10.1093/geronb/63.5.S328. [DOI] [PMC free article] [PubMed] [Google Scholar]