Summary
Chlamydia trachomatis (Ct) is the most common cause for bacterial sexually transmitted infections (STIs) worldwide with a tremendous impact on public health. With the aim to unravel novel targets of the chlamydia life cycle, we screen a compound library and identify 28 agents to significantly reduce Ct growth. The known anti-infective agent pentamidine—one of the top candidates of the screen—shows anti-chlamydia activity in low concentrations by changing the metabolism of host cells impairing chlamydia growth. Furthermore, it effectively decreases the Ct burden upon local or systemic application in mice. Pentamidine also inhibits the growth of Neisseria gonorrhea (Ng), which is a common co-infection of Ct. The conducted compound screen is powerful in exploring antimicrobial compounds against Ct in a medium-throughput format. Following thorough in vitro and in vivo assessments, pentamidine emerges as a promising agent for topical prophylaxis or treatment against Ct and possibly other bacterial STIs.
Keywords: Chlamydia trachomatis, sexually transmitted infections, compound screen, mouse model, Neisseria gonorrhea, prevention, pre-exposure prophylaxis, antibacterial
Graphical abstract
Highlights
-
•
A compound screen identifies 28 non-antibiotics inhibiting Chlamydia trachomatis
-
•
Pentamidine inhibits chlamydia replication indirectly via the host cells
-
•
Systemic and intrauterine pentamidine treatment decreases chlamydia burden in mice
-
•
Pentamidine is a promising candidate for prophylaxis against bacterial STIs
As the numbers of sexually transmitted infections are rising, innovative prophylactic measures are needed. Knapp et al. performed a medium-throughput compound screen to identify new drugs inhibiting Chlamydia trachomatis growth in cell lines. The top hits were tested in a Chlamydia trachomatis mouse model for their ability to prevent infection.
Introduction
The risk of death or serious health restraints from infectious diseases has decreased in developed countries based on the availability of efficient antimicrobial measures, including prophylaxis and treatment.1,2 However, many sexually transmitted infections (STIs) are currently increasing in North America and Europe,3,4 especially among individuals engaging in high-risk sex practices, including unprotected sex with multiple partners.5 This trend was even intensified by the COVID-19 pandemic due to reduced screening and access to health care facilities.6,7
The most common bacterial STIs are Chlamydia trachomatis (Ct) infections with an estimated prevalence of around 4% in the Americas and around 2% in Europe with highest infection rates in the population between 15 and 24 years.8,9 There are different Ct serovars characterized by their major outer membrane proteins: serovars A–C primarily cause ocular infection, while serovars D–K preferentially infect the urogenital tract, and serovars L1–L3 can lead to lymphogranuloma venereum.10 Ct is an intracellular pathogen utilizing the host cell machinery for replication.11 Briefly, Ct elementary bodies (EBs) can interact with various surface receptors on epithelial cells, which facilitate the uptake of the pathogen.11 Using cell cytoskeletal proteins, EBs form an inclusion within the endocytic vacuoles where they replicate in the form of reticulate bodies (RBs) utilizing nutrients provided by the host cell. After finishing their replication cycle, RBs transform back into EBs and leave the cell by cell lysis or extrusion.11,12 Since the Ct developmental cycle is strictly dependent on the host cell, treatment approaches do not need to solely rely on antibiotics inhibiting, e.g., bacterial protein synthesis but may also consider alternative mechanisms by interfering with cell entry or modulating host cell structures.13 Alternative strategies involve, among others, inhibiting type III secretion systems of Ct, blocking chlamydial attachment by destroying the bacterial membrane or binding certain membrane structures, and enhancing host cell defense mechanisms by cytokines or blocking of metabolic processes utilized by Chlamydia spp.13,14 Even though human chlamydial infections are well treatable with antibiotics, there are studies reporting tetracycline resistance by the presence of a Tet(C)-island in the genome in the species-specific strain Chlamydia suis infecting pigs.13,15
Another STI of concern with high rates of antibiotic resistance is Neisseria gonorrhea (Ng). A large portion of clinical Ng isolates are resistant to early developed antibiotics like penicillin, sulfonamides, and tetracyclines, and additional resistance to extended-spectrum cephalosporins and macrolides, which are currently recommended for treatment, is on the rise.16,17 Moreover, co-infections of Ng with various STIs are common,18 as there exists an increased susceptibility of already infected individuals to other STIs.19,20 A significant problem with both Ct and Ng infections is posed by the high rates of asymptomatic cases that can still cause severe long-term sequelae in females like pelvic inflammatory disease resulting in infertility or ectopic pregnancy.21
Preventive measurements include screening programs for individuals at high risk. Nevertheless, screening is expensive for the health care system, and a cost-effectiveness assessment is often not available.22,23 The ultimate goal would be to develop a vaccine as preventive measure, which was not successful so far despite 60 years of research with only one vaccine candidate currently being in a clinical trial.24,25,26,27 Other preventive strategies involve pre- and post-exposure prophylaxis (PrEP/PEP) with doxycycline (doxy) in patient groups with high-risk behavior for the acquisition of chlamydial infections and syphilis that are frequently using human immunodeficiency virus (HIV)-PrEP.28,29,30 However, the concern of antimicrobial resistance of Ng and also other sexually transmitted pathogens as well as commensals greatly limits the doxy-PEP. In a recent study from Luetkemeyer et al., the development of resistance in Ng and Staphylococcus aureus isolates was increased in the doxy-PEP group.30 Therefore, alternatives to doxy to prevent and treat STIs are of growing importance.
In this project, we report a systematic screen for molecules that reduce Ct growth in vitro. We identified pentamidine as a candidate compound that was also effective in reducing Ct burden (by topical or systemic treatment) in an in vivo mouse model of genital Ct infection. In addition, we demonstrated that already low concentrations modulate the host metabolism and inhibit the growth of Ct and Ng, making it an ideal agent for PrEP or PEP of STIs.
Results
Identification of reagents inhibiting chlamydia growth in a medium-throughput compound screen
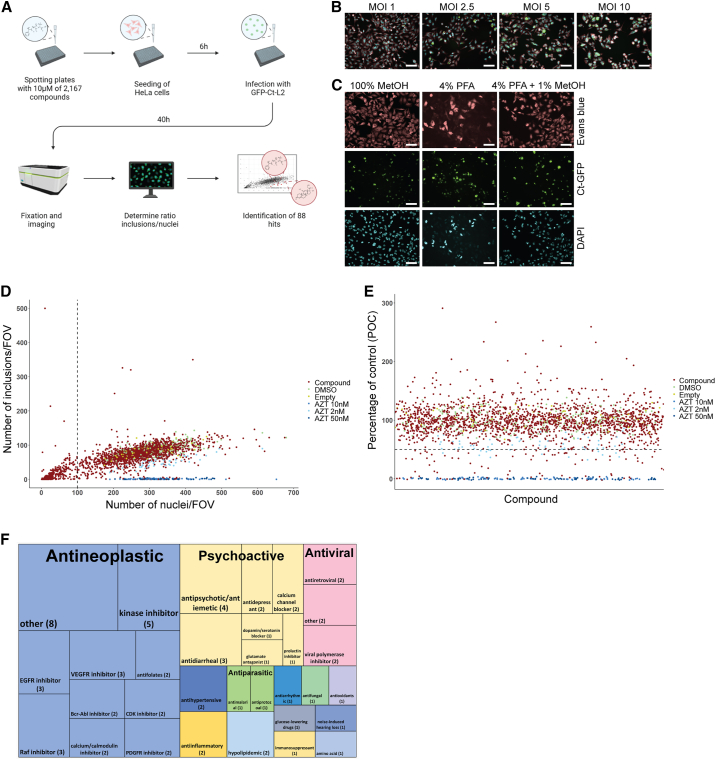
To identify novel classes of pharmacological agents inhibiting chlamydia growth, we chose a library of ∼2,200 compounds, which include approved drugs and well-described experimental molecules. As Ct-infected cells are not dividing, we defined the optimal ratio of 2,000 HeLa cells per 384-well infected with Ct-L2-GFP at a multiplicity of infection (MOI) of 2.5 (Figures 1A and 1B). To determine the number of nuclei and inclusions in each condition, we fixed the cells with 4% paraformaldehyde/1% methanol, which allowed for the detection of the endogenous GFP signal of Ct inclusions and nuclear staining with DAPI and cell staining with Evans blue for HeLa cells (Figure 1C). A decrease in the number of cell nuclei per field of view to less than 100 was considered as cytotoxic effect of a given compound and excluded from analysis (Figure 1D). To test the efficiency of the screening compounds to reduce Ct growth, we set the cutoff to 50% in relation to the negative control (DMSO) (percentage of control, POC < 50) and the positive control (azithromycin 50 nM) (Figure 1E). In the initial screening approach, we identified 88 compounds having the potential to control chlamydia infection. We only identified 26 compounds that are known antibiotics. These compounds were excluded from subsequent analysis as the aim was to identify novel inhibitors. The screen revealed the following main substance classes as effective: (1) antineoplastics, (2) antivirals, and (3) psychoactive drugs (Figure 1F; Table S1). In a validation experiment, dose responses of the remaining 62 reagents in 3-fold dilutions revealed 28 reagents that showed a reduction of chlamydia growth by more than 40% (POC < 60) in two or more concentrations (Figure 2A; Table S2). These 28 compounds were further evaluated in various conditions to select the most effective candidates for chlamydia inhibition.
Figure 1.
Medium-throughput screen reveals compounds inhibiting chlamydia growth
(A) Schematic of the experimental setup of the medium-throughput screen. HeLa cells were seeded in 384-well plates containing 10 μM of compounds. Ct-containing media were added after 6 h on top. Plates were incubated for 40 h.
(B and C) Overview of test conditions.
(B) Overlay of fluorescent images of DAPI (blue), Evans blue (red), and Ct inclusions (green) infected with different MOI (PerkinElmer Operetta high-content automated confocal microscope, 20× long field WD); representative images; scale bars, 100 μm.
(C) One representative field of view (FOV) per well is depicted after fixation with either 100% methanol (MetOH), 4% paraformaldehyde (PFA), or 4% PFA containing 1% methanol.
(D) Plot showing number of nuclei vs. number of inclusions of initial screen. Each dot represents one test compound (Cpd). DMSO-treated wells as negative controls (DMSO), empty wells (Empty), and azithromycin-treated wells as positive controls (AZT 10 nM, AZT 2 nM, AZT 50 nM). The cutoff for cytotoxic drugs was 100 nuclei per FOV.
(E) Percentage of control vs. compound ID for compounds being not cytotoxic. Hits are compounds that reduce chlamydia growth to less than 50% of control.
(F) Tree map plot of the drug classes and subclasses identified to inhibit Ct growth.
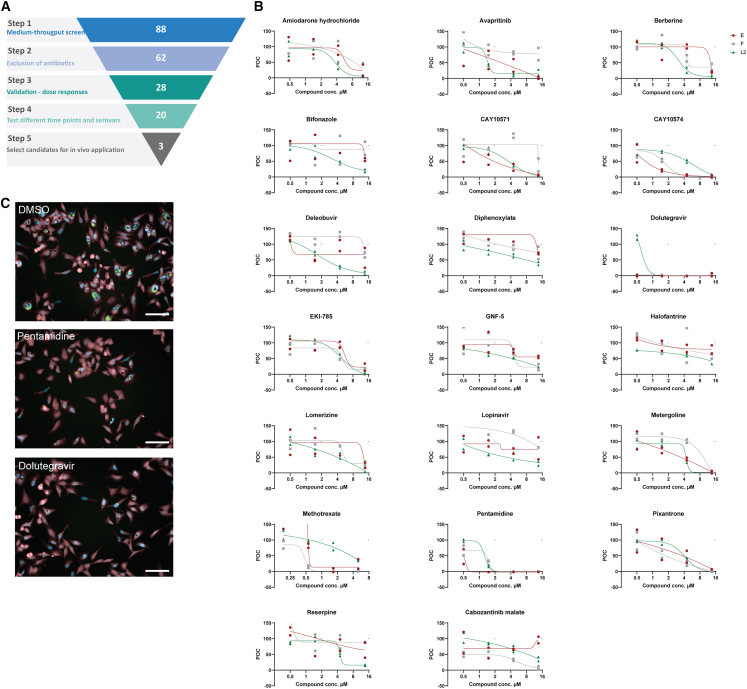
Figure 2.
Validation experiments reveal inhibition of Ct growth across various serovars
(A) Schematic of the compound sets used in this study and the sequential screening steps applied to obtain a selection of a final set of 3 drugs for in vivo experiments.
(B) Dose-response curves for the 20 compounds remaining after the validation conditions. 3-fold dilutions in technical duplicates starting from 13.5 μM for Ct serovars E, F, and L2 were assessed. POC, percentage of DMSO-treated control.
(C) Representative images of indicated compounds in 13.5 μM concentration (PerkinElmer Operetta high-content automated confocal microscope, 20× long field WD); scale bars, 100 μm.
Validation reveals candidate drugs being effective against different genital chlamydia serovars
Ct-L2 causing lymphogranuloma venereum is more invasive and grows faster in vitro than genital serovars Ct-E and Ct-F.31 Therefore, we investigated if the compounds validated from the primary screen are reactive against different genital Ct serovars in various concentrations (Figure 2A; Table S3). The most efficient compounds identified are avapritinib, CAY10571, CAY10574, dolutegravir, EKI-785, metergoline, methotrexate, pentamidine, and pixantrone. Most drug candidates showed a similar trend in all three Ct serovars (Figure 2B). By using the CellTiter-Glo, we demonstrated that compounds used in the validation screen are well tolerated by the cells as viability was above 90% for all compounds (Figure S1). In addition to the experimental layout with the compound treatment before Ct infection (Figure 1A), we assessed the effectivity of drugs if added 1 h after infection (Figure S2; Table S4). This time point of infection was chosen to identify drugs that have an effect during early chlamydia life cycle. We observed a slight decrease for dolutegravir and pentamidine if compounds are added after infection (Figure S2). This suggests that those compounds might play a role during early events in chlamydia replication or might take some time until they are fully active within the cells. We selected pentamidine, dolutegravir, and metergoline to test in a mouse model for genital chlamydia infection as (1) these compounds significantly block Ct growth in various conditions, (2) their targets in the chlamydia life cycle have not yet been described, and (3) they are approved drugs that allow for easier translation to patients (Figure 2C).
Pentamidine is effective in a mouse model for genital chlamydia infection
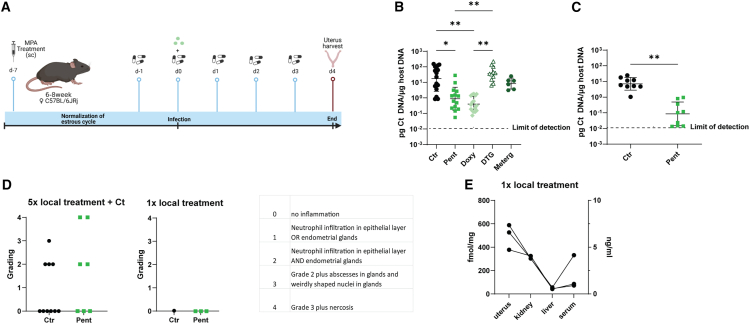
To investigate if the compounds inhibiting Ct growth in HeLa cells also work in vivo during female genital tract infection, we established a mouse model of Ct infection for prophylactic compound treatment. Mice received drug doses every 24 h starting 1 day before infection, resembling continuous schemata for pre-exposure prophylaxis.29 At the peak of chlamydia burden 4 days after genital Ct infection,32 uteri were harvested to quantify chlamydia burden (Figure 3A). Mice receiving a combination of systemic and local treatment with doxy or pentamidine had a significantly lower chlamydia burden compared with DMSO-treated mice (Figure 3B). Treatment with either dolutegravir or metergoline did not protect mice from chlamydia replication within their uteri (Figure 3B).
Figure 3.
Pentamidine prevents Ct infection in vivo
(A) Schematic of setup of animal experiments. Created with BioRender.
(B) Bacterial burden in mouse uteri at the end of experiment in different groups under systemic plus local treatment: mock-treated mice (Ctr), pentamidine (Pent), doxy, dolutegravir (DTG), or metergoline (Meterg)-treated mice. Bacterial burden is indicated as pg Ct DNA per μg host DNA. One-way ANOVA and Tukey’s multiple comparisons tests were performed to find differences between treatment groups (∗p < 0.05, ∗∗p < 0.01). Data are presented as the mean of biological replicates ± SD.
(C) Bacterial burden in mouse uteri after local Pent or mock (Ctr) treatment was analyzed by unpaired t test (∗∗p < 0.01). Data are presented as the mean of biological replicates ± SD.
(D) Histopathological grading of uteri. Uteri from mice receiving 5× local treatment and Ct infection (same mice as shown in C, n = 7–10) and mice treated once locally (n = 3) were stained with H&E and assessed by a pathologist 24 h after last treatment.
(E) Pentamidine absorption 24 h after 1× local treatment in different organs (fmol/mg) and serum (ng/μL).
Next, we explored the effect of local application of pentamidine in the mouse model for genital Ct infection starting 1 day prior infection. Local pentamidine treatment significantly reduces the chlamydia burden in mice (Figure 3C). In comparison to the systemic pentamidine treatment where all mice presented with bradykinesia, we did not observe these therapy-related adverse events in mice with the local treatment regime. Histopathological assessment of uteri of locally treated mice revealed similar grading of inflammation between pentamidine-treated and -untreated Ct-infected mice (Figure 3D). Additionally, three mice received only one local dose of pentamidine to assess pathology (Figure 3D) and absorption (Figure 3E) of pentamidine upon transcervical drug inoculation. Singular drug doses do not cause any pathological changes in the uterus, liver, or kidney of these mice, and the highest drug levels could be detected within the uterus (Figure 3E). Therefore, pentamidine represents an interesting compound for prophylaxis against Ct infection in a mouse model, having potential as a local microbicide preventing human Ct infection.
Lack of effectiveness of dolutegravir against Ct infection in people with HIV
For dolutegravir, we analyzed a cohort of people living with HIV receiving dolutegravir as part of their antiretroviral therapy. Individuals receiving dolutegravir are as likely to acquire a Ct infection as patients without dolutegravir intake (Figure S3). Therefore, our results suggest that in the mouse model for genital chlamydia infection and in humans, dolutegravir does not reach inhibitory levels in tissue to prevent Ct infection.
Pentamidine impairs Ct rapidly and permanently
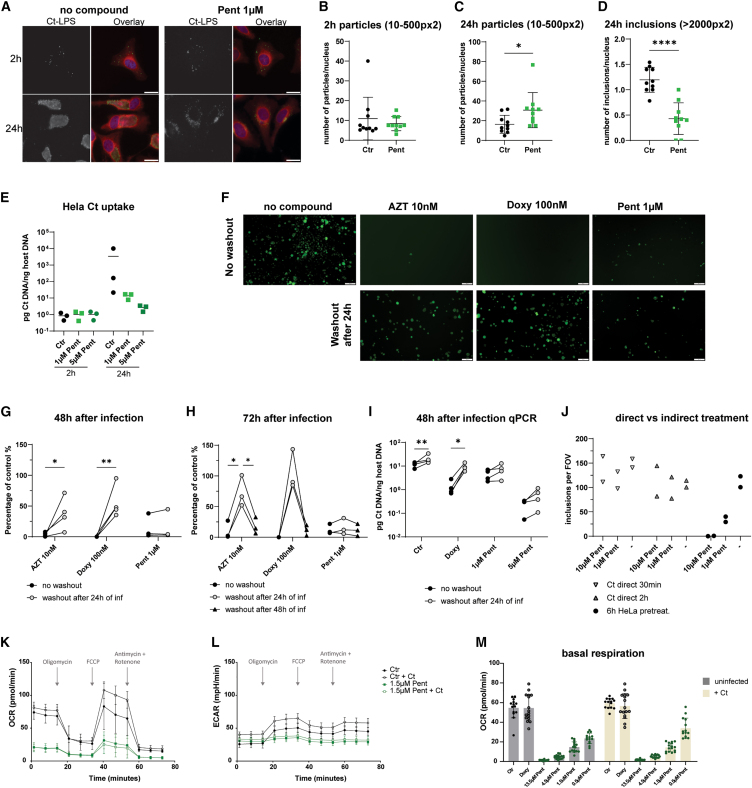
The mode of action for pentamidine in protozoan infection is not well described. Pentamidine is reported as an inhibitor of (1) DNA, RNA, or protein biosynthesis33 and topoisomerase,34,35 (2) polyamine synthesis,36 (3) folate metabolism,37,38 and (4) membrane integrity.39 To learn if pentamidine has a direct effect on Ct or an indirect effect by inhibiting host cell metabolic processes, we first tested if pentamidine acts directly on Ct before they enter host cells rather than acting indirectly via affecting the host cell metabolism. Upon treatment of Ct with different concentrations of pentamidine 30 min or 2 h before adding Ct to the untreated cells, we did not observe a Ct growth reduction in HeLa cells (Figure 4J). Next, we investigated if pentamidine interferes with Ct uptake or with later steps during replication. 2 h after infection, the number of bacterial particles taken up in both conditions was comparable (Figures 4A, 4B, and 4E). After 24 h, however, Ct inclusions only occur in control cells, whereas single Ct particles are present in pentamidine-treated cells (Figures 4A–4E). To determine if Ct particles taken up in pentamidine-treated cells are showing hallmarks of bacterial growth, we used N-[7-(4-Nitrobenzo-2-oxa-1,3-diazole)]-6-aminocaproyl-D-erythro-sphingosine (C6-NBD-ceramide) labeling. Upon uptake in the cells, C6-NBD-ceramide is modified to sphingomyelin at the Golgi apparatus, resulting in the transfer and retention of sphingomyelin in Ct inclusions upon bacterial growth.40,41 We performed confocal imaging of infected cells treated with C6-NBD-ceramide to assess the recruitment of C6-NBD-sphingomyelin to the inclusions. C6-NBD-sphingomyelin accumulates in the Golgi apparatus, and our findings demonstrate a clear colocalization of C6-NBD-sphingomyelin with Ct-lipopolysaccharide (LPS)-positive vesicles and inclusions in both controls and pentamidine-treated samples. Notably, in the pentamidine-treated cells, there was a halt in the development of the inclusions (Figure S4). This suggests that the uptake and early steps in the Ct life cycle are not impaired by pentamidine, but only later stages, including bacterial replication, are affected by pentamidine.
Figure 4.
Pentamidine acts more rapidly against chlamydia than antibiotics by an indirect effect via host cells
(A–D) HeLa cells pre-treated with 1 μM Pent for 6 h were infected with Ct-L2 (no GFP) at MOI 100. Ct uptake was quantified after washing 2× with PBS to remove unbound Ct, PFA fixation and antibody staining for Ct-LPS in 10 FOV per condition (Olympus IX53, LUCPlanFL N, 40×). Significant differences were tested by unpaired t tests (∗p < 0.05, ∗∗∗∗p < 0.0001). Data are presented as the mean of 10 FOV ± SD. (A) Representative images of chlamydia uptake and inclusion formation 2 and 24 h after infection. Scale bars, 20 μm; Ct-LPS (green), Evans blue (red), DAPI (blue). (B) Ratio of chlamydia particles (size = 10–500 px2) and nuclei per FOV 2 h after infection. (C) Ratio of chlamydia particles (size = 10–500 px2) and nuclei per FOV 24 h after infection. (D) Ratio of chlamydia inclusions (size > 2,000 px2) and nuclei per FOV 24 h after infection.
(E) Quantification of Ct uptake with qPCR. HeLa cells were treated with 1 or 5 μM Pent and infected with Ct-L2 (no GFP, MOI 100). HeLa cells were harvested 2 or 24 h after infection and ratios of Ct DNA/host DNA were determined (n = 3 independent experiments).
(F–I) Medium containing 10 nM azithromycin (AZT), 100 nM doxy, and 1 μM or 5 μM Pent was replaced every 24 h supplemented with freshly prepared compounds or just medium. Significant differences were tested by two-way ANOVA with to Šídák’s multiple comparisons test (∗p < 0.05, ∗∗p < 0.01). (F) Representative images of washout experiments 72 h after infection (Olympus IX53, CPlan N, 10×). Scale bars, 100 μm. (G) HeLa cells treated with indicated compounds, analysis 48 h after infection (n = 4 independent experiments, 10 FOV per experiment were analyzed, Olympus IX53, LCAch N, 20×). (H) HeLa cells treated with indicated compounds, analysis 72 h after infection (n = 3 independent experiments, 10 FOV per experiment were analyzed, Olympus IX53, LCAch N, 20×). (I) Quantification of Ct burden with qPCR. HeLa cells were harvested 48 h after infection and ratios of Ct DNA/host DNA were determined (n = 4 independent experiments). (J) Ct pretreated with pentamidine before infection. HeLa cells were infected with pre-treated Ct (for 30 min or 2 h; indicated pentamidine concentration during Ct-pretreatment) or HeLa cells were pre-treated with pentamidine 6 h before infection (final concentration of 10 or 1 μM concentration). Quantification of inclusions per FOV (Olympus IX53, LCAch N, 20×) normalized to inclusions in untreated control samples (n = 2 independent experiments, expressed as mean of 10 FOV per experiment).
(K–M) Seahorse analysis using the mitotic stress test kit, performed on 8,000 HeLa cells treated with compounds (Ctr = DMSO, 3-fold dilutions of Pent starting at 13.5 μM or 100 nM doxy) and/or Ct-L2-GFP (MOI 2.5) for 48 h. Data are pooled replicates from two independent runs (n = 14–16), mean ± SD. (normalized to cell number). (K) Oxygen consumption rate (OCR) in pmol/min. (L) Extracellular acidification rate (ECAR) in mPH/min. (M) Basal respiration of all treatment conditions (OCR).
To elaborate on the function of pentamidine in Ct infection, we investigated the kinetics of clearance of chlamydia upon in vitro treatment. Therefore, HeLa cells were pre-treated with compounds in a similar setup as in our initial screen. Next, compounds were washed out 24 or 48 h after infection, respectively (Figures 4F–4I). While Ct persists and still proliferates in azithromycin- and doxy-treated samples if washout occurs already 24 h after infection, Ct growth is significantly impaired after the 24 h washout in pentamidine-treated samples (Figures 4F, 4G, and 4I). A comparable effect to pentamidine is achieved for azithromycin and doxy if they are present for at least 48 h (Figure 4H). This suggests that pentamidine permanently inhibits Ct growth at an early time point and that it does not only suppress bacterial translation, as it occurs in the presence of macrolides and tetracyclines.
In summary, we show that Ct is taken up into pentamidine-treated cells and early steps of inclusion formation occur. However, replication of Ct is significantly impaired, and after washout of pentamidine, the inhibitory effect persists.
Impairment of Ct growth by modulation of host cell metabolism
As we did not observe direct disruption of Ct by pentamidine, we assessed other mechanisms of pentamidine inhibiting Ct growth indirectly via host cells. Solute carriers (SLCs) might be essential for Ct growth by providing necessary factors of the bacterial life cycle. The reduced folate carrier SLC19A1 was shown to be transporting antimetabolites like methotrexate and pentamidine.37 Treatment of SLC19A1−/− cells with methotrexate resulted in a loss of anti-chlamydia effects, whereas treatment with pentamidine led to a similar reduction of Ct growth as observed in Renilla−/− control cells (Figure S6). We therefore conclude that pentamidine and the antifolate methotrexate have different modes of action in their activity against Ct.
We tested the possibility that the host metabolism might be influenced by pentamidine in a way that prevents further Ct growth in infected cells. We performed Seahorse analysis, using the mitotic stress test kits on HeLa cells treated with pentamidine and infected with Ct. When assessing the oxygen consumption rate, we noticed that pentamidine-treated cells have reduced basal respiration and that their spare capacity for mitochondrial respiration is reduced (Figure 4K). Interestingly, infected control cells perform more oxidative phosphorylation at baseline. This difference is not observed in pentamidine-treated samples. Similarly, pentamidine-treated cells perform less glycolysis as shown by the extracellular acidification rate and increased glycolysis in infected control cells, indicating that Ct proliferation is reflected by high energy demand by the host cells (Figure 4L). This effect of reduced metabolic activity in pentamidine-treated cells is dose dependent, while doxy-treated cells show similar basal respiration as control cells (Figure 4M). These data illustrate that, by reducing the basal metabolism in host cells via pentamidine, growth of Ct within the host cells is inhibited. We next investigated if the metabolic effect of pentamidine results in reduced viability and proliferation of the host cells. By performing lactate dehydrogenase (LDH) assays and fluorescence-activated cell sorting-based viability assays (7-Aminoactinomycin (7-AAD) staining and CellTrace Violet staining), we observed that HeLa cell viability and proliferation is not affected by pentamidine (Figures S5A–S5C). As HeLa cells are robust cells, we additionally assessed the viability of primary cervical epithelial cells upon pentamidine treatment, which validated our observations from HeLa cells in this more physiologically relevant cell type (Figures S5D and S5E). These data show that the inhibitory effect on bacterial replication observed in pentamidine-treated cells is probably due to metabolic changes within the host cells. However, the drug doses used in our study do not impact the viability and proliferation of HeLa cells and primary cervical epithelial cells as host cells of Ct.
Pentamidine inhibits the growth of Ng, while commensals of the physiologic vaginal flora retain replication capacity
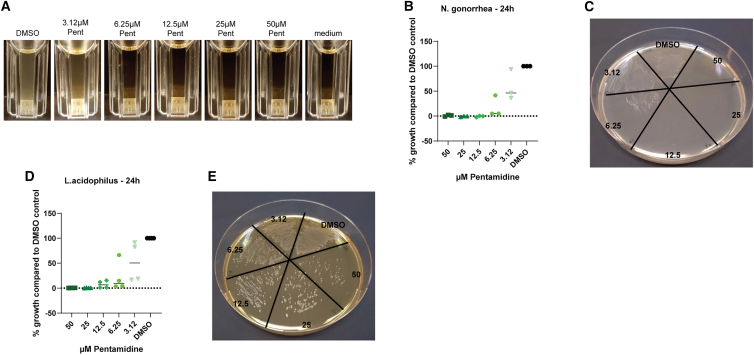
As Ct and Ng infections often coincide, PrEP or PEP strategies are usually designed to be effective against Ct and Ng infection.28,30 We therefore assessed the antimicrobial potential of pentamidine against Ng as it was reported to have some inhibitory effect on other gram-negative bacteria such as E. coli.42 By treating liquid cultures of Ng with pentamidine, we identified the minimal inhibitory concentration between 3.12 and 6.25 μM (Figures 5A and 5B). By spreading the liquid cultures after 24 h of pentamidine treatment on untreated plates, we determined that the minimal inhibitory concentration corresponded to the minimal bactericidal concentration, as regrowth of Ng was absent (Figure 5C).
Figure 5.
Pentamidine inhibits Ng growth in vitro but does not stop the growth of Lactobacilli
(A) Liquid 24 h cultures of Ng in the presence of 2-fold serial dilutions of Pent (starting at 50 μM).
(B) Quantification of growth of Ng in Pent-treated cultures in comparison to DMSO control cultures. Data are presented as the means of 3 independent experiments.
(C) Representative image of Ng bacterial smears derived from Pent-treated 24 h liquid cultures.
(D) Quantification of growth of L. acidophilus in Pent-treated cultures in comparison to control cultures. Data are presented as the means of 4 independent experiments.
(E) Representative image of L. acidophilus bacterial smears derived from Pent-treated 24 h liquid cultures.
Vaginal dysbiosis is a common side effect of antibiotic treatment.43 As prophylactic treatment of STIs should not permanently affect the urogenital microbiome, we next assessed the effect of pentamidine on Lactobacillus acidophilus as a representative species of the female vaginal flora. We observed reduced growth of the bacteria in the presence of pentamidine; however, the drug did not have a bactericidal effect on L. acidophilus in any concentration tested as bacteria grew back after withdrawal of the drug (Figures 5D and 5E). Our data indicate that the bactericidal pentamidine concentrations do not have a lasting detrimental effect on the human female genital tract microbiome.
In summary, we successfully applied a medium-throughput screen to Ct infection and validated the most promising compounds in a mouse model for genital Ct infection. Pentamidine emerged as the lead candidate demonstrating efficacy against Ct in vivo and inhibiting Ng growth in vitro, all while preserving L. acidophilus, a key component of the natural vaginal flora. Furthermore, when applied topically in the Ct mouse model, pentamidine successfully prevented Ct infection. However, its mode of action appears to be mediated through the host cells, because direct incubation with pentamidine does not affect Ct. Instead, pentamidine modifies the metabolic activity within the host cells. In comparison to azithromycin and doxy, the effect of pentamidine is rapid and long lasting, making it an ideal candidate as topical agent against bacterial STIs in a therapeutic or even prophylactic setting.
Discussion
In this study, we utilized a medium-throughput discovery screen to evaluate the potential of approximately 2,200 compounds in inhibiting the intracellular growth of Ct. As a result, we successfully identified and validated 28 non-antibiotic compounds that demonstrated the ability to reduce Ct inclusion formation. Notably, we found that pentamidine, when topically or systemically applied, effectively prevented Ct infection in vivo.
In their global health sector strategy on STIs, the World Health Organization aims to reduce the global cases of bacterial STIs from 374 million in 2020 to below 150 million until 2030. The main pillars will focus on prevention, screening programs for priority populations, and innovative approaches to treatment and vaccines.44 Considering the absence of available vaccines against any of the most common bacterial STIs, namely Ct, Ng, and Mycoplasma genitalium, coupled with the escalating antibiotic resistance observed among these pathogens, it is crucial to investigate novel strategies for STI prevention.
In a similar setup as described in this study, Mojica et al. tested 339 Australian natural products with an mCherry-expressing Ct strain.45 They identified mainly tetrahydroanthraquinone and thiaplakortone compounds as hits, which had been classified as antiparasitic agents against malaria or trypanosomes.45 We tested our top candidates—pentamidine, dolutegravir, and metergoline—for their efficacy in a mouse model of female genital tract Ct infection. Pentamidine was the only compound of the three drugs that significantly reduced Ct burden in the mice comparable to antibiotic treatment upon systemic and local treatment. In comparison to the antibacterial activity of metergoline against the intracellular bacterium Salmonella typhimurium in a murine Salmonella infection model,46 we did not see a Ct growth reduction upon metergoline application in vivo. For the intraperitoneal application, we used the same dosage, but the frequency of application was every 24 h, in contrast to the mouse model for Salmonella infections where application followed a 12 h interval. Explanations for the absence of effect in our mouse model could be that (1) the agent was not sufficiently concentrated in the female genital epithelium, (2) the dose is not high enough for Ct inhibition in vivo, or (3) more dosages would be required due to the half-life of metergoline. Dolutegravir was the most effective drug against Ct in vitro but did not show an effect in the mouse model for Ct infection. Even though the usual application route is oral, we chose systemic treatment by injection and determined the optimal/maximal dosage based on previous literature using either oral application47 or injection with dolutegravir equivalents.48 Dolutegravir was shown to modify the folate metabolism, which might be its potential mode of action during Ct infection,49,50 similarly to methotrexate.51 Dolutegravir leads to the downregulation of SLC19A1 in placental cells, thereby reducing the uptake of both methotrexate and folic acid.50 Only 5%–7% of plasma concentration of dolutegravir reaches the cervical tissue, which could be one reason for the lack of activity against Ct, as only very low concentrations are needed for its activity against HIV.52,53 Follow-up experiments could assess if long-acting dolutegravir derivatives can reach a dosage sufficient for chlamydia growth inhibition in vivo.48,54 Recently, the dolutegravir derivative 7-methoxy-4-methyl-6,8-dioxo-N-(3-(1-(2-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)phenyl)-3,4,6,8,12,12a-hexahydro-2H-pyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide (DTHP) was shown to inhibit cancer cell growth in a murine xenograft model after intraperitoneal application.55 Recognizing the importance of refining the delivery method as well as the dosage for in vivo application, our research specifically emphasized pentamidine as a promising prophylactic agent against Ct.
The concept of PrEP and PEP proved efficient against STI such as HIV.56 Recent findings suggest doxy prophylaxis as PEP against bacterial STIs in certain individuals at high risk for STI acquisition.28,29,30 A potential caveat with doxy prophylaxis is the development of antibiotic resistance of the pathogens and also commensals. In C. suis, which is related to the human-adapted pathogen Ct, tetracycline resistance is common. C. suis can also infect other hosts than pigs, as infection is documented in humans working in life stock facilities,57,58 where selective pressure occurs due to the enormous use of antibiotics.59 In co-infected individuals with C. suis and Ct, horizontal transfer of the tet(C) gene might occur, which was demonstrated in vitro between C. suis and other chlamydia strains.60,61 For Ng, antibiotic resistance is very common and even multidrug-resistant strains are frequently observed in some countries. Doxy-PEP reduced Ng cases depending on the resistance frequency in the respective study area.28,30
We propose pentamidine identified in this study as a promising alternative agent for STI prophylaxis. In a PrEP setting in mice in vivo, pentamidine reduced the chlamydial burden similarly to doxy. We observed inhibition of Ng growth in the presence of >6.25 μM pentamidine. However, minimum inhibitory concentration (MIC) breakpoints and pharmacokinetic or pharmacodynamic models for pentamidine and Ng are not established so far. Pentamidine is reported to have antibacterial properties by targeting the bacterial membrane.42,62 Gram-negative bacteria can become more susceptible to antibiotics by disrupting their membrane and making it permeable for other drugs.39,62 This synergistic effect of combining pentamidine with antibiotics was shown for Acinetobacter baumannii in a mouse model,39 a finding that could be translated to multidrug-resistant bacteria strains, thereby increasing the treatment options and delaying the development of resistance.62,63,64,65,66 With regard to potential side effects associated with pentamidine, ongoing advances are being made in the development of pentamidine analogs that exhibit improved performance in disrupting bacterial membranes while being less cytotoxic to the host organism, thereby increasing their potential as adjuvants for antimicrobial therapies.67,68,69 It remains to be investigated if these analogs are also effective against Ct as the mechanism of action is probably more complex than just disrupting the membrane of the pathogen.
In the clinics, pentamidine is already used as prophylaxis against pneumocystis pneumonia with either systemic or topical (inhalation) application once every 3–4 weeks. During systemic treatment, pentamidine can have side effects like ventricular arrhythmia, hypotension, nephrotoxicity, or hepatic dysfunction.70 During local application by inhalation with a nebulizer, it can cause cough, chest pain, difficulty breathing, or skin rash.70 Nevertheless, also doxy can cause symptoms like headache, nausea, vomiting, diarrhea, skin rash, or bacterial vaginitis. In the mouse model for genital Ct infection, we observed bradykinesia and dizziness after systemic treatment with pentamidine, while mice treated only locally did not present with obvious adverse events. In vitro, pentamidine has an immediate and long-lasting effect, as Ct inclusions do not grow back as observed for the bacteriostatic compounds azithromycin and doxy if treatment is stopped after 24 h. Thus, the bactericidal compound pentamidine might facilitate a long-lasting effect or even better clearance in vivo, which would make the application more convenient and less error-prone than daily intake as required for doxy-PrEP or intake after every risk contact as recommended for doxy-PEP. This enhanced effectiveness may be attributed to the ability of pentamidine to enhance the fitness of host cells. Through the utilization of various incubation strategies, encompassing both Ct alone and in combination with host cells, we observed that pentamidine may not directly affect Ct but rather bolsters the resilience of host cells in combatting this pathogen by reducing the metabolic activity in host cells to a level that does not kill the host but significantly impairs the pathogen. This mechanism holds promise in reducing both the mutation pressure exerted on Ct and the development of resistance.
Pentamidine was shown to be effective in a mouse model of Leishmania infection after topical application on the skin as a cream formulation.71 It remains to be tested if pentamidine is suitable for being applied locally to the cervix in form of an ointment, as we dissolved it in 0.9% saline solution and applied it directly into the murine cervix before Ct infection. Previous publications describe the development of vaginal microbicides like salicylidene acylhydrazides restricting iron in Ct and Ng infection or the LPS-binding molecule alkylpolyamine DS-96 blocking Ct attachment.72,73 Approved for other clinical applications, long-term clinical experience is an advantage for our approach to pentamidine treatment. Histopathological assessment revealed that single local treatments do not cause any irritation or organ toxicity in the female reproductive tract. The effect on the local microbiome was assessed by susceptibility testing of L. acidophilus, a commensal of the female genital tract, which revealed to survive Ct and Ng-bactericidal pentamidine dosages. In addition, there is evidence for synergistic effects of pentamidine in combination with antibiotics, which could contribute substantially to reducing total antibiotic load and increase the variability of different antibiotic classes that can be used to treat certain pathogens.
In conclusion, we identified 28 highly effective compounds from diverse substance classes that demonstrated significant efficacy against Ct in vitro. Some of these compounds belong to classes that were previously shown to be associated with pathways involved in chlamydia replication. Furthermore, our study provides the first evidence of pentamidine’s efficacy in a mouse model of Ct infection. Based on these findings, we propose that pentamidine holds potential as an alternative topically or systemically applied agent for prophylactic strategies (PrEP and PEP) not only against Ct but also against other bacterial STIs, such as Ng, without disrupting the genital flora. Given the substantial increase in Ng antibiotic resistance, pentamidine could serve as an adjuvant in combination with antibiotics, offering a backup plan for multidrug-resistant strains of Ng and other bacteria like Mycoplasma genitalium. Future endeavors should focus on developing a formulation of pentamidine or one of its analogs suitable for local application in humans or as a low-dosage systemic antibiotic adjuvant. One emphasis will be the development of a pentamidine derivative that exhibits bactericidal properties while minimizing severe side effects linked to the original compound. Additionally, it is crucial to investigate whether lower concentrations of the drug remain effective. It is imperative to investigate the inhibitory effect of pentamidine on other STIs besides Ct in vivo and explore the development of resistance to pentamidine.
Limitations of the study
We provide a proof of concept for the use of pentamidine as a locally active prophylactic agent against Ct infection. However, the precise mode of action remains unclear, even though extensive in vitro infection experiments revealed that pentamidine most likely acts via metabolic alterations in host cells, thereby limiting the proliferation capacity of bacteria. It remains to be elucidated if long-term treatment and related metabolic alterations lead to toxic side effects in epithelial tissue. Another challenge with pentamidine to prevent STIs is the effect on the local microbiome, which needs to be carefully assessed with other species than L. acidophilus and with in vivo studies. The next step will be the translation of topical drug application in humans. While pentamidine application is reasonably feasible with creams and suppositories at the penis and anal region, respectively, topical preventative considerations for the female lower genital tract and especially the cervix are more challenging. Possible options include vaginal application by cream, suppository, or sprays that might allow dissemination of pentamidine to the cervix. Developing pentamidine derivatives that are suitable for easy systemic distribution or innovative topical administration are options for future studies in the context of topical pentamidine prophylaxis. Pentamidine as a lead compound could help to identify pentamidine analogs or structurally related compounds using structure-activity relationship studies for similarly active compounds with fewer side effects on host cells and the microbiome.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Ct LPS FITC, clone B410F | Invitrogen | Cat # MA1-7339; RRID: AB_1016792 |
| Ct LPS, clone 512F | Invitrogen | Cat # MA5-16287; RRID: AB_2537804 |
| Goat anti-mouse IgG AF680 | Invitrogen | Cat # A-21058; RRID: AB_2535724 |
| Bacterial and virus strains | ||
| CTL2P-pGFP:pSW2 | Chlamydia Biobank | CT401 |
| Ct serovars L2 | DSMZ-German Collection of Microorganisms and Cell Culture GmBH | DSM 19102 |
| Ct serovars E | DSMZ-German Collection of Microorganisms and Cell Culture GmBH | DSM 19131 |
| Ct serovars F | DSMZ-German Collection of Microorganisms and Cell Culture GmBH | DSM 19410 |
| Neisseria gonorrhea | ATCC | ATCC 49226 |
| Lactobacillus acidophilus | ATCC | ATCC 4356 |
| Biological samples | ||
| Healthy adult cervical tissue | Medical University of Vienna | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Azithromycin | Synovo | N/A |
| C6-NBD Ceramide | Focus Biomolecules/Biotrend | Cat# 10-5496-1mg |
| Doxycycline Vibravenös | Pfizer | N/A |
| Dolutegravir | MedChemExpress | Cat# HY-13238 |
| Cycloheximide | Sigma | Cat# 01810 |
| Gastrografin | Bayer | Cat# 80375310 |
| Medroxyprogesterone acetate | TCA | Cat# M1964 |
| Metergoline | Sigma | Cat# M3668-500MG |
| Penicillin | Sigma | Cat# P3032-1MU |
| Pentamidine isethionate | Thermo Fisher Scientific | Cat# 461860010 |
| Critical commercial assays | ||
| CellTrace Violet | Thermo Fisher Scientific | Cat# C34557 |
| 7-AAD | BD | Cat# 559925 |
| LDH-Cytox™ Assay Kit | Biolegend | Cat# 426401 |
| QIAamp DNA mini kit | Qiagen | Cat# 51306 |
| Phalloidin AF594 | Invitrogen | Cat# A12381 |
| Luna® Universal Probe qPCR Master Mix | New England Biolabs | Cat# M3004X |
| Seahorse XF Cell Mito Stress Test | Agilent | Cat# 103015-100 |
| Experimental models: Cell lines | ||
| HeLa cells | ATCC | ATCC® CCL-2™; RRID:CVCL_0030 |
| McCoy cells | ATCC | ATCC® CRL-1696™; RRID:CVCL_3742 |
| HCT116-SLC19A1-KO-c4 | RESOLUTE | CE0540-U; RRID:CVCL_D4IH |
| HCT116-Renilla-KO-c1 | RESOLUTE | CE04CG-T |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6JRj | Janvier labs/Charles River | RRID: IMSR_RJ:C57BL-6JRJ |
| Oligonucleotides | ||
| Primer Ct 16S DNA | IDT | F: 5′-GGA GGC TGC AGT CGA GAA TCT-3′ R: 5′-TTA CAA CCC TAG AGC CTT CAT CAC A-3′ Probe: 5′-[6-FAM]-TCG TCA GAC TTC CGT CCA TTG CGA-[TAMRA]-3′ |
| Rodent GAPDH VIC | Applied Biosystems | Cat# 4308313 |
| Human GAPDH VIC | Thermo Fisher Scientific | Cat# 4448489 |
| Software and algorithms | ||
| FlowJo Version 10.8.1 | FlowJo | https://www.flowjo.com/ |
| Olympus FV31S-SW software | Olympus | https://www.olympus-lifescience.com/en/support/downloads/ |
| Prism Version 8 & 9 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| BioRender | BioRender | https://www.biorender.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Georg Stary (georg.stary@meduniwien.ac.at).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Bacterial strains and cell culture
Ct serovar CTL2P-pGFPpSW2 (referred Ct-L2-GFP) was purchased from the Chlamydia Biobank (University of Southampton).74 Ct serovars L2 (DSM 19102), E (DSM 19131) and F (DSM 19410) were purchased from the DSMZ-German Collection of Microorganisms and Cell Culture GmBH. Serovar Ct-L2-GFP was propagated in McCoy cell monolayers in DMEM supplemented with 10% FBS (Biowest, Nuaille, France), 1x non-essential amino acids (MEM NEAA 100X, Gibco, Thermo Fisher Scientific) in the presence of 10 U ml−1 penicillin (Sigma, St. Louis, USA) to select for GFP expressing bacteria. Strains L2, E and F were propagated in HeLa cells and 1 μg mL−1 cycloheximide (Sigma, Burlington, USA) was added to the medium as described previously. All strains were purified using gastrografin (Bayer, Germany) gradient centrifugation and titers were determined as described previously.75,76,77 Purified stocks were stored in sucrose-phosphate-glutamate buffer at −80°C and thawed immediately before use (220 mM sucrose, 8.6 mM Na2HPO4, 3.8 mM KH2PO4 and 5 mM L-glutamic acid).
HeLa cells (ATCC CCL-2) and McCoy cells (ATCC CRL-1696) were maintained in DMEM with 10% FBS. During infection, all cells were cultured in DMEM with 10% FBS (supplemented with 10 U ml−1 penicillin if Ct-L2-GFP was used). HeLa cells were authenticated using highly polymorphic short tandem repeat loci (STRs).
CRISPR/Cas9 knock out lines of HCT116 cells transduced with sgRNA targeting either SLC19A1 (CE0540-U) or Renilla luciferase (CE04CG-T) cDNA were obtained from RESOLUTE37 and cultured in RPMI containing 10% FBS. During infection, all cells were cultured in DMEM with 10% FBS (supplemented with 10 U ml−1 penicillin).
Primary cervical epithelial cells were derived from hysterectomy samples from premenopausal healthy donors which were recruited at the University Hospital, Medical University of Vienna, Austria after obtaining appropriate fully informed written consent. Cervical biopsies were digested overnight at 4°C with dispase II (2U ml−1). Epithelial layer is scraped off with tweezers and cultivated in Keratinocyte Growth Medium-2 (Lonza) and CnT-IsoBoost until the first split (CnT-ISO-50, CELLnTEC). The study was approved by the local ethics committee, Medical University of Vienna (ECS 1503/2020).
For experiments with Neisseria gonorrhea, the fully antibiotics susceptible strain ATCC 49226 was used. Ng were cultured on homemade agar plates with gonococcal base medium supplemented with Kellogg’s supplement I and II.78 For experiments with Lactobacillus acidophilus, the ATCC 4356 strain was cultured on MRS plates.
Mice
All mouse experiments were approved by the Institutional Review Board of the Austrian Ministry of Sciences (BMBWF 2020–0.380.439). Female 6–8-week-old C57BL/6JRj mice were purchased from Janvier labs/Charles River and maintained under specific pathogen free conditions in Biosafety Level 2 (BSL-2) facilities at the Medical University of Vienna. Mice were housed in standard cages in a temperature and humidity-controlled room with a 12 h light/dark cycle.
Method details
Medium-throughput compound screening
The compound screening was performed in collaboration with the CeMM Molecular Discovery Platform using a customized library of 2167 compounds. The compounds derived from the NIH clinical collection, CeMM library of unique drugs CLOUD, and collections of anti-cancer agents, natural products, epigenetic compounds, metabolites and kinase inhibitors were spotted on 384-well assay plates at concentrations of typically 10 μM (ranging from 10 to 50 μM) in 0.1% DMSO. As positive control, azithromycin at concentrations of 2, 10 and 50 nM was used. 2,000 HeLa cells were seeded per well in 25 μL and incubated for 6 h to ensure adherence of cells. Then, Ct-L2-GFP at MOI 2.5 were added on top in 25 μL suspension and incubated for 42 h. Cells were fixed with 3.7% paraformaldehyde with 1% methanol and stained with 1 μg mL−1 DAPI and 0.002% Evans blue. Number of nuclei and chlamydia inclusions were counted for each well. Z-factors were calculated using negative controls (DMSO) and positive controls (azithromycin 50 nM) from each plate individually.79 All plates passed the quality control with Z‘>0. By linear regression, the percentage of control (POC) was calculated, setting the mean of negative controls to 100% and the mean signal of positive controls to 0% separately for each plate. Hits were defined as compounds with POC <50 and number of nuclei >100. Compounds with less than 100 nuclei were considered toxic (306 in total).
For the dose response validation, all hits except known antibiotics were tested in an 8-point dose-response in duplicates in 3-fold dilutions typically starting at 13.5 μM. Azithromycin was used at an assay concentration of 67.5 nM. Top hits must fulfill the criteria of POC <60 and the number of nuclei must be >150. The 20 top candidates from the validation screen were tested also in serovar E and F. As for these serovars, a centrifugation step is crucial to obtain sufficient infectivity, the plates were centrifuged for 30 min at 600 g before incubation for 46 h. In experiments with serovars not expressing GFP, cells were permeabilized with a 0.1% saponin/PBS solution for 20 min before immunofluorescence staining with FITC-conjugated anti-Ct LPS monoclonal antibody (B410F, Invitrogen, 1:100) in 0.1% Saponin in PBS +2% BSA for 30 min.
Compounds for in vitro tests
For other assays than medium-throughput screening, the following compounds and suppliers were used: azithromycin (Synovo, Tübingen, Germany), doxycycline (Vibravenös, Pfizer, Pocé-sur-Cisse, France), dolutegravir (MedChemExpress, Monmouth Junction, USA), metergoline (Sigma, St. Louis, USA), pentamidine isethionate (Thermo Fisher Scientific, Germany). Compounds were dissolved in DMSO and diluted in 0.9% NaCl to final concentrations indicated in the respective experiments (final DMSO concentration in the cultures <1%).
In vitro experiments with cell lines
For in vitro uptake and washout experiments, HeLa cells were seeded in the presence of pentamidine and infected with Ct-L2 (MOI 100 for uptake, MOI 2.5 for washout) after 6 h. For microscopy, cells were fixed with 4% paraformaldehyde and stained as described for the Ct screen. Acquisition was performed with an Olympus IX53 microscope (LUCPlanFL N, 40x). For qPCR, DNA was isolated with Qiagen DNA mini kit. For viability assays with HeLa cells and primary epithelial cells either calorimetric assays (CellTiter-Glo and LDH-assays) or flow cytometry-based assays (staining with CellTrace Violet and 7-AAD) were used. Cells were cultured in the presence of pentamidine for 48 h before viability was assessed. For CellTrace Violet labeling, 1 million HeLa cells were incubated in 1 mL PBS containing 1 μM CellTrace Violet for 15 min. Cells are washed with 5 mL medium before plating. After harvest, cells were stained with 2.5 μg mL−1 7-AAD in PBS for 10 min and directly acquired with a Cytek Aurora flow cytometer. FACS data were analyzed using FlowJo (Version 10.8.1).
For C6-NBD-sphingomyelin staining and confocal microscopy, HeLa cells were treated with 1 μM pentamidine or DMSO in controls for 6 h before infection with Ct-L2 MOI 2.5 (no GFP-expressing). At indicated time points (2 h, 6 h or 18 h after infection), C6-NBD-Ceramide was added to the medium (final concentration 5 μM C6-NBD-Ceramide in 0.05% BSA) and incubated for 30 min at 37°C. Cells were washed 2x with PBS and cells were incubated with fresh DMEM +10% FCS for 60 min at 37°C to allow back-exchange. Cells are fixed with 4% PFA solution for 20 min at 37°C. Subsequently, cells are stained with anti-Ct-LPS antibody (Clone 512F, Invitrogen, 1.3 μg mL−1 in 0.1% Saponin in 2% BSA/PBS) for 30 min. Secondary antibody staining with anti-mouse-IgG (AF680, Invitrogen, 5 μg mL−1 in 0.1% Saponin in 2% BSA/PBS) was combined with Phalloidin staining (AF594, Invitrogen, 1:100) for 30 min at RT. After washing with PBS, counterstaining with DAPI for 5 min, coverslips were mounted onto slides. Samples were acquired at a confocal laser scanning microscope (Olympus, FLUOVIEW-FV 3000, equipped with OBIS lasers: 405, 488, 561, 640 nm and ×60 UPlanXApo objectives and Olympus FV31S-SW software).
For assessment of cellular metabolic activity, we performed a Seahorse XF Cell Mito Stress Test (Agilent). 8,000 HeLa cells were seeded per well in 80 μL DMEM supplemented with 10% FCS in the presence of pentamidine. Cells were incubated for 6 h before adding 80 μL medium containing Ct-L2-GFP (MOI 2.5). Cells are incubated for 40 h at 37°C, 5% CO2 before exchanging the culture medium with assay medium (Agilent Seahorse DMEM, 1 mM pyruvate, 2 mM glutamine and 10 mM glucose). Plates were incubated for 1 h at 37°C without CO2 before loading them into the Agilent XFe96 Extracellular Flux Analyzer. The assay was performed using 1 μM oligomycin, 1 μM FCCP and 0.5 μM rotenone/antimycin A.
Mouse model of Ct infection
Mice were treated with 2.5 mg medroxyprogesterone acetate (MPA; TCA, Tokyo, Japan) in 100 μL PBS subcutaneously 7 days before infection to normalize the estrous cycle. Prophylactic treatment with compounds was started one day before infection and was repeated every 24 h until the end of experiment. The doses chosen for in vivo treatment were according to the highest still tolerated systemic doses reported in the literature46,47,80 and suitable for dilution in small volume for transcervical application. Doses were calculated for an average mouse weight of 20 g in our study. For systemic treatment, doses were 0.8 mg pentamidine, 0.45 mg dolutegravir, 0.25 mg doxy or 0.1 mg metergoline in 100 μL 0.9% NaCl containing 10% DMSO administered intraperitoneally. For local treatment, mice received half of the systemic dosage in 15 μL 0.9% NaCl with 10% DMSO alone in control conditions or containing 0.4 mg pentamidine, 0.22 mg dolutegravir, 0.125 mg doxy or 0.05 mg metergoline by intrauterine application using an NSET device. For intrauterine infection, 1x 106 IFU of Ct-L2-GFP in sucrose-phosphate-glutamate buffer were added to the local treatment dose and administered to the uterus using an NSET device as previously described.32 On day 4 after infection, uteri were minced and snap frozen.
For absorption studies, mice were treated once transcervically with 15 μL 0.9% NaCl with 0.4 mg pentamidine and organs and serum were harvested 24 h after treatment. Organs were either snap frozen in liquid nitrogen for targeted MS/MS or paraffin embedded for histopathological assessment.
qPCR to assess Ct burden
To assess the chlamydia burden in the murine uteri or in HeLa cells, DNA was isolated using the QIAamp DNA mini kit (Qiagen) and host GAPDH DNA and chlamydia 16S DNA were quantified by qPCR using Luna Universal Probe qPCR Master Mix (New England Biolabs) on a StepOnePlus Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific) in a multiplexed manner as previously described.32,81 Using standard curves from known amounts of Ct and host DNA, the amount of chlamydia DNA (in pg) per unit weight of host DNA (in μg) allowed to calculate the ratio of pathogen DNA/host DNA.
Antimicrobial susceptibility testing
For susceptibility testing of Ng, liquid gonococcal base medium containing Kellogg’s supplement I and II and NaHCO3 was prepared as previously described.78 Pentamidine was added at a concentration of 3.12, 6.25, 12.5, 25 and 50 μM and samples were inoculated with a 0.5 McFarland bacterial suspension in NaCl 1:100.
For susceptibility testing of L. acidophilus, inoculum was prepared by dissolving single colonies in 0.9% NaCl solution at a McFarland standard of 0.5 and using bacterial solution 1:500 in MRS broth containing various pentamidine concentrations.
OD600 was measured after 24 h and growth rate was calculated comparing to optical density of medium only and untreated cultures. To assess bactericidal effect of pentamidine, 5 μL of 24 h liquid cultures treated with various drug concentrations were spread out on fresh agar plates and incubated for 48 h.
Chlamydia incidence in HIV cohort receiving DTG
For this analysis, all Ct-positive results from 04/2014-11/2020 from males visiting the HIV- and STI-clinic of the Medical University of Vienna were retrospectively evaluated, and patients’ characteristics (HIV status and DTG exposure) were retrieved from the medical records. GraphPad Prism 8 was used to perform the statistical analyses. Nominal variables were plotted as number and percentage of patients with a specific feature. To calculate the incidence rate of infections and the respective 95% confidence interval (95% CI), the person-time method was used. Reinfections during the observational period were analyzed using a Kaplan-Meier curve and a log rank test was used to compare the incidence of Ct-reinfections by HIV-status. The presented analysis complies with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The Local Ethics Committee of the Medical University of Vienna provided the ethical approval (2175/2020). Due to the retrospective design, the need for an informed consent had been waived.
Quantification and statistical analysis
Statistical analysis of medium-throughput screen was done in R calculating Z-factors and linear regression models. All other statistical analyses were done in GraphPad Prism version 9.5.0. If not stated differently, data are expressed as mean ± standard deviation (s.d.). For analysis of mouse experiments, outliers were excluded using Grubbs’ method with alpha = 0.05. One-way ANOVA and Tukey’s multiple comparisons were performed to find differences between treatment groups. For comparison between only two groups, unpaired t-tests were performed. For statistical analysis of in vitro experiments, two-way ANOVA with matching across row and multiple comparison testing according to Šídák’s (comparing 2 means) or Tukey’s (more than 2 means) was used.
Acknowledgments
We thank all members of the Stary laboratory for discussions and feedback and especially A.E. Aguilar González for preparation of reagents and A. Kopf for her assistance with the confocal microscope. We are grateful to G. Superti-Furga for scientific input and RESOLUTE for providing the HCT116 cell lines. We thank M. Haller and A. Stary from the Outpatients Centre for the Diagnosis of Venero-Dermatological Diseases, Pilzambulatorium Schlösselgasse, Vienna, for sharing the Ng strains with us. We are grateful to B. Willinger and B. Selitsch from the Department of Laboratory Medicine of the Medical University of Vienna for providing the L. acidophilus strain. This project was supported by the Austrian Science Fund (FWF, P31494) and the Medical Scientific Fund of the Mayor of the City of Vienna (21201). Graphical images were created with BioRender (G.S. owns a license for publishing).
Author contributions
K.K. planned and conducted the experiments, analyzed the data, and drafted the figures and manuscript. R.K. planned and conducted the experiments and analyzed the data. A.K. conducted screening experiments and analyzed the data. M.S. conducted and analyzed the Seahorse experiments. R.D.-H. conducted the experiments. M.D. performed the histopathological grading. J.S. performed the mass spectrometry experiments. D.C. collected and analyzed patient data of the dolutegravir cohort. M.K. and C.G. recruited patients to obtain primary material and planned experiments. A.B., S.K., H.S., and G.S. provided reagents and revised the manuscript. H.S. and G.S. supervised the project.
Declaration of interests
K.K., R.K., A.K., S.K., H.S., and G.S. are inventors on a patent application entitled “Pentamidine in the treatment of genital infections and/or STIs” (EU application number EP 24 177 796.0) filed by the Medical University of Vienna that relates to the use of pentamidine against genital infections.
Published: July 8, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101643.
Supplemental information
2,167 compounds were tested for their ability to inhibit Ct growth for more than 50% compared to controls in serovar L2. Azithromycin served as a positive control and DMSO as a negative control. Nuclei and Ct inclusions per FOV were determined.
The non-antibiotics inhibiting Ct growth identified in the primary drug screen were tested in serovar L2 in an 8-point dose response with duplicates in 3-fold dilutions, typically starting at 13.5 μM. Number of nuclei and inclusions were determined in one FOV per well.
The non-antibiotics which were validated to inhibit Ct growth in serovar L2 were applied to serovar E and serovar F in 4-point serial dilutions. Number of nuclei and inclusions were determined in one FOV per well.
Cells were infected with Ct, and compounds were added at indicated concentrations only 1 h after infection.
References
- 1.Roser M., Ritchie H., Spooner F. 2021. Burden of Disease.https://ourworldindata.org/burden-of-disease [Google Scholar]
- 2.el Bcheraoui C., Mokdad A.H., Dwyer-Lindgren L., Bertozzi-Villa A., Stubbs R.W., Morozoff C., Shirude S., Naghavi M., Murray C.J.L. Trends and Patterns of Differences in Infectious Disease Mortality Among US Counties, 1980-2014. JAMA. 2018;319:1248–1260. doi: 10.1001/jama.2018.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2021. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021.https://www.who.int/publications/i/item/9789240027077 [Google Scholar]
- 4.Centers for Disease Control and Prevention Sexually Transmitted Disease Surveillance 2022. 2024 https://www.cdc.gov/std/statistics/ [Google Scholar]
- 5.Bosetti D., Mugglin C., Calmy A., Cavassini M., Stöckle M., Braun D., Notter J., Haerry D., Hampel B., Kovari H., et al. Risk Factors and Incidence of Sexually Transmitted Infections in the Swiss HIV Cohort Study. Open Forum Infect. Dis. 2022;9 doi: 10.1093/ofid/ofac592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright S.S., Kreisel K.M., Hitt J.C., Pagaoa M.A., Weinstock H.S., Thorpe P.G. Impact of the COVID-19 Pandemic on Centers for Disease Control and Prevention-Funded Sexually Transmitted Disease Programs. Sex. Transm. Dis. 2022;49:E61–E63. doi: 10.1097/OLQ.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagaoa M., Grey J., Torrone E., Kreisel K., Stenger M., Weinstock H. Trends in Nationally Notifiable Sexually Transmitted Disease Case Reports during the US COVID-19 Pandemic, January to December 2020. Sex. Transm. Dis. 2021;48:798–804. doi: 10.1097/OLQ.0000000000001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huai P., Li F., Chu T., Liu D., Liu J., Zhang F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020;20:589. doi: 10.1186/s12879-020-05307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman L., Rowley J., Hoorn S.V., Wijesooriya N.S., Unemo M., Low N., Stevens G., Gottlieb S., Kiarie J., Temmerman M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens M.P., Twin J., Fairley C.K., Donovan B., Tan S.E., Yu J., Garland S.M., Tabrizi S.N. Development and evaluation of an ompA quantitative real-time PCR assay for Chlamydia trachomatis serovar determination. J. Clin. Microbiol. 2010;48:2060–2065. doi: 10.1128/JCM.02308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitsels A., Sanders N., Vanrompay D., Dandekar T., Ball S.G. Chlamydial Infection From Outside to Inside. Front. Microbiol. 2019;10:2329. doi: 10.3389/fmicb.2019.02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witkin S.S., Minis E., Athanasiou A., Leizer J., Linhares I.M. Chlamydia trachomatis: the Persistent Pathogen. Clin. Vaccine Immunol. 2017;24:e00203-17. doi: 10.1128/cvi.00203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bommana S., Polkinghorne A. Mini review: Antimicrobial control of chlamydial infections in animals: Current practices and issues. Front. Microbiol. 2019;10:113–119. doi: 10.3389/fmicb.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou C., Jin Y., Wu H., Li P., Liu L., Zheng K., Wang C. Alternative strategies for Chlamydia treatment: Promising non-antibiotic approaches. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.987662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dugan J., Rockey D.D., Jones L., Andersen A.A. Tetracycline resistance in Chlamydia suis mediated by genomic islands inserted into the chlamydial inv-like gene. Antimicrob. Agents Chemother. 2004;48:3989–3995. doi: 10.1128/AAC.48.10.3989-3995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unemo M., Shafer W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: Past, evolution, and future. Clin. Microbiol. Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unemo M., Lahra M.M., Escher M., Eremin S., Cole M.J., Galarza P., Ndowa F., Martin I., Dillon J.A.R., Galas M., et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: a retrospective observational study. Lancet. Microbe. 2021;2:e627–e636. doi: 10.1016/S2666-5247(21)00171-3. [DOI] [PubMed] [Google Scholar]
- 18.Althaus C.L., Turner K.M.E., Mercer C.H., Auguste P., Roberts T.E., Bell G., Herzog S.A., Cassell J.A., Edmunds W.J., White P.J., et al. Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: Observational study, systematic reviews and mathematical modelling. Health Technol. Assess. 2014;18:1–99. doi: 10.3310/hta18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard C.A., Schoborg R.V., Low N., Unemo M., Borel N. Pathogenic Interplay Between Chlamydia trachomatis and Neisseria gonorrhoeae that Influences Management and Control Efforts—More Questions than Answers? Curr. Clin. Microbiol. Rep. 2019;6:182–191. doi: 10.1007/s40588-019-00125-4. [DOI] [Google Scholar]
- 20.Su X., Le W., Zhu X., Li S., Wang B., Madico G., Yang Z., Chaisson C.E., McLaughlin R.E., Gandra S., et al. Neisseria gonorrhoeae Infection in Women Increases With Rising Gonococcal Burdens in Partners: Chlamydia Coinfection in Women Increases Gonococcal Burden. J. Infect. Dis. 2022;226:2192–2203. doi: 10.1093/infdis/jiac408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies B., Turner K.M.E., Frølund M., Ward H., May M.T., Rasmussen S., Benfield T., Westh H., Danish Chlamydia Study Group Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. Lancet Infect. Dis. 2016;16:1057–1064. doi: 10.1016/S1473-3099(16)30092-5. [DOI] [PubMed] [Google Scholar]
- 22.Low N., Hocking J.S., van Bergen J. The changing landscape of chlamydia control strategies. Lancet. 2021;398:1386–1388. doi: 10.1016/S0140-6736(21)02002-X. [DOI] [PubMed] [Google Scholar]
- 23.US Preventive Services Task Force Screening for Chlamydia and Gonorrhea: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:949–956. doi: 10.1001/jama.2021.14081. [DOI] [PubMed] [Google Scholar]
- 24.Phillips S., Quigley B.L., Timms P. Seventy years of Chlamydia vaccine research - Limitations of the past and directions for the future. Front. Microbiol. 2019;10:70. doi: 10.3389/fmicb.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham S., Juel H.B., Bang P., Cheeseman H.M., Dohn R.B., Cole T., Kristiansen M.P., Korsholm K.S., Lewis D., Olsen A.W., et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019;19:1091–1100. doi: 10.1016/S1473-3099(19)30279-8. [DOI] [PubMed] [Google Scholar]
- 26.Luis M. de la M., Zhong G., Brunham R.C. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017;24:1–25. doi: 10.1128/CVI.00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Maza L.M., Darville T.L., Pal S. Chlamydia trachomatis vaccines for genital infections: where are we and how far is there to go? Expert Rev. Vaccines. 2021;20:421–435. doi: 10.1080/14760584.2021.1899817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina J.M., Charreau I., Chidiac C., Pialoux G., Cua E., Delaugerre C., Capitant C., Rojas-Castro D., Fonsart J., Bercot B., et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect. Dis. 2018;18:308–317. doi: 10.1016/S1473-3099(17)30725-9. [DOI] [PubMed] [Google Scholar]
- 29.Bolan R.K., Beymer M.R., Weiss R.E., Flynn R.P., Leibowitz A.A., Klausner J.D. Doxycycline Prophylaxis to Reduce Incident Syphilis among HIV-Infected Men who have Sex with Men who Continue to Engage in High Risk Sex: A Randomized, Controlled Pilot Study. Sex. Transm. Dis. 2015;42:98–103. doi: 10.1097/OLQ.0000000000000216.Doxycycline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luetkemeyer A.F., Donnell D., Dombrowski J.C., Cohen S., Grabow C., Brown C.E., Malinski C., Perkins R., Nasser M., Lopez C., et al. Postexposure Doxycycline to Prevent Bacterial Sexually Transmitted Infections. N. Engl. J. Med. 2023;388:1296–1306. doi: 10.1056/NEJMoa2211934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faris R., Andersen S.E., McCullough A., Gourronc F., Klingelhutz A.J., Weber M.M. Chlamydia trachomatis Serovars Drive Differential Production of Proinflammatory Cytokines and Chemokines Depending on the Type of Cell Infected. Front. Cell. Infect. Microbiol. 2019;9:399. doi: 10.3389/fcimb.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondek D.C., Olive A.J., Georg Stary M.N.S. CD4+ T cells are necessary and sufficient to confer protection against C. trachomatis infection in the murine upper genital tract. J. Immunol. 2012;23:1–7. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun T., Zhang Y. Pentamidine binds to tRNA through non-specific hydrophobic interactions and inhibits aminoacylation and translation. Nucleic Acids Res. 2008;36:1654–1664. doi: 10.1093/nar/gkm1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh G., Dey C.S. Induction of apoptosis-like cell death by pentamidine and doxorubicin through differential inhibition of topoisomerase II in arsenite-resistant L. donovani. Acta Trop. 2007;103:172–185. doi: 10.1016/j.actatropica.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro T.A., Englund P.T. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. USA. 1990;87:950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basselin M., Badet-Denisot M.-A., Lawrence F., Robert-Gero M. Effects of Pentamidine on Polyamine Level and Biosynthesis in Wild-Type, Pentamidine-Treated, and Pentamidine-Resistant Leishmania. Exp. Parasitol. 1997;85:274–282. doi: 10.1006/expr.1996.4131. [DOI] [PubMed] [Google Scholar]
- 37.Girardi E., César-razquin A., Lindinger S., Papakostas K., Konecka J., Hemmerich J., Kickinger S., Kartnig F., Gürtl B., Klavins K., et al. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 2020;16:469–478. doi: 10.1038/s41589-020-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waalkes T.P., Makulu D.R. Pharmacologic aspects of pentamidine. Natl. Cancer Inst. Monogr. 1976;43:171–177. [PubMed] [Google Scholar]
- 39.Stokes J.M., Macnair C.R., Ilyas B., French S., Côté J.P., Bouwman C., Farha M.A., Sieron A.O., Whitfield C., Coombes B.K., Brown E.D. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017;2:17028. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mital J., Miller N.J., Dorward D.W., Dooley C.A., Hackstadt T. Role for Chlamydial Inclusion Membrane Proteins in Inclusion Membrane Structure and Biogenesis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackstadt T., Scidmore M.A., Rockey D.D. Lipid metabolism in Chlamydia trachomatis-infected cells: Directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bichowsky-Slomnitzki L. The effect of aromatic dismidines on bacterial growth; the mechanism of action. J. Bacteriol. 1948;55:27–31. doi: 10.1128/JB.55.1.27-31.1948. [DOI] [PubMed] [Google Scholar]
- 43.Coudray M.S., Madhivanan P. Bacterial vaginosis—A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization . 2022. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030.https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies [Google Scholar]
- 45.Mojica S.A., Eriksson A.U., Davis R.A., Bahnan W., Elofsson M., Gylfe Å. Red Fluorescent Chlamydia trachomatis Applied to Live Cell Imaging and Screening for Antibacterial Agents. Front. Microbiol. 2018;9:3151. doi: 10.3389/fmicb.2018.03151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis M.J., Tsai C.N., Johnson J.W., French S., Elhenawy W., Porwollik S., Andrews-Polymenis H., McClelland M., Magolan J., Coombes B.K., Brown E.D. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat. Commun. 2019;10 doi: 10.1038/s41467-018-08190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heredia A., Hassounah S., Medina-Moreno S., Zapata J.C., Le N.M., Han Y., Foulke J.S., Davis C., Bryant J., Redfield R.R., Wainberg M.A. Monotherapy with either dolutegravir or raltegravir fails to durably suppress HIV viraemia in humanized mice. J. Antimicrob. Chemother. 2017;72:2570–2573. doi: 10.1093/jac/dkx195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sillman B., Bade A.N., Dash P.K., Bhargavan B., Kocher T., Mathews S., Su H., Kanmogne G.D., Poluektova L.Y., Gorantla S., et al. Creation of a long-acting nanoformulated dolutegravir. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-02885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilmore J.C., Hoque M.T., Dai W., Mohan H., Dunk C., Serghides L., Bendayan R. Interaction between dolutegravir and folate transporters and receptor in human and rodent placenta. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabrera R.M., Souder J.P., Steele J.W., Yeo L., Tukeman G., Gorelick D.A., Finnell R.H. The antagonism of folate receptor by dolutegravir: Developmental toxicity reduction by supplemental folic acid. AIDS. 2020;34:162–163. doi: 10.1097/QAD.0000000000002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan H., Brunham R.C., McClarty G. Acquisition and synthesis of folates by obligate intracellular bacteria of the genus Chlamydia. J. Clin. Invest. 1992;90:1803–1811. doi: 10.1172/JCI116055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taha H., Das A., Das S. Clinical effectiveness of dolutegravir in the treatment of HIV/AIDS. Infect. Drug Resist. 2015;8:339–352. doi: 10.2147/IDR.S68396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams J.L., Patterson K.B., Prince H.M.A., Sykes C., Greener B.N., Dumond J.B., Kashuba A.D.M. Single and Multiple Dose Pharmacokinetics of Dolutegravir in the Genital Tract of HIV Negative Women. Antivir. Ther. 2013;18:1005–1013. doi: 10.3851/IMP2665.Single. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovarova M., Benhabbour S.R., Massud I., Spagnuolo R.A., Skinner B., Baker C.E., Sykes C., Mollan K.R., Kashuba A.D.M., García-Lerma J.G., et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-06490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W.J., Mao L.F., Lai H.L., Wang Y.W., Jiang Z.B., Li W., Huang J.M., Xie Y.J., Xu C., Liu P., et al. Dolutegravir derivative inhibits proliferation and induces apoptosis of non-small cell lung cancer cells via calcium signaling pathway. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105129. [DOI] [PubMed] [Google Scholar]
- 56.Krakower D.S., Jain S., Mayer K.H. Antiretrovirals for Primary HIV Prevention: The Current Status of Pre- and Post-Exposure Prophylaxis. Curr. HIV AIDS Rep. 2015;12:127–138. doi: 10.1053/j.gastro.2016.08.014.CagY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Puysseleyr L., De Puysseleyr K., Braeckman L., Morré S.A., Cox E., Vanrompay D. Assessment of Chlamydia suis Infection in Pig Farmers. Transbound. Emerg. Dis. 2017;64:826–833. doi: 10.1111/tbed.12446. [DOI] [PubMed] [Google Scholar]
- 58.De Puysseleyr K., De Puysseleyr L., Dhondt H., Geens T., Braeckman L., Morré S.A., Cox E., Vanrompay D. Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 2014;14:560–566. doi: 10.1186/s12879-014-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanninger S., Donati M., Di Francesco A., Hässig M., Hoffmann K., Seth-Smith H.M.B., Marti H., Borel N. Selective pressure promotes tetracycline resistance of Chlamydia suis in fattening pigs. PLoS One. 2016;11:e0166917. doi: 10.1371/journal.pone.0166917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marti H., Kim H., Joseph S.J., Dojiri S., Read T.D., Dean D. Tet(C) Gene Transfer between Chlamydia suis Strains Occurs by Homologous Recombination after Co-infection: Implications for Spread of Tetracycline-Resistance among Chlamydiaceae. Front. Microbiol. 2017;8:156. doi: 10.3389/fmicb.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suchland R.J., Sandoz K.M., Jeffrey B.M., Stamm W.E., Rockey D.D. Horizontal Transfer of Tetracycline Resistance among Chlamydia spp. In Vitro. Antimicrob. Agents Chemother. 2009;53:4604–4611. doi: 10.1128/AAC.00477-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B., Pachaiyappan B., Gruber J.D., Schmidt M.G., Zhang Y., Woster P.M. Antibacterial Diamines Targeting Bacterial. Membranes. 2018;59:3140–3151. doi: 10.1021/acs.jmedchem.5b01912.Antibacterial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrera-Espejo S., Cebrero-Cangueiro T., Labrador-Herrera G., Pachón J., Pachón-Ibáñez M.E., Álvarez-Marín R. In vitro activity of pentamidine alone and in combination with antibiotics against multidrug-resistant clinical Pseudomonas aeruginosa strains. Antibiotics. 2020;9:885. doi: 10.3390/antibiotics9120885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cebrero-Cangueiro T., Álvarez-Marín R., Labrador-Herrera G., Smani Y., Cordero-Matía E., Pachón J., Pachón-Ibáñez M.E. In vitro Activity of Pentamidine Alone and in Combination With Rifampicin, and Doripenem Against Clinical Strains of Carbapenemase-Producing and/or Enterobacteriaceae. Front. Cell Infect. Microbiol. 2018;8:363. doi: 10.3389/fcimb.2018.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y., Zhao H., Lin J., Li Z., Tian G., Yang Y.Y., Yuan P., Ding X. Repurposing Non-Antibiotic Drugs Auranofin and Pentamidine in Combination to Combat Multidrug-Resistant Gram-Negative Bacteria. Int. J. Antimicrob. Agents. 2022;59 doi: 10.1016/j.ijantimicag.2022.106582. [DOI] [PubMed] [Google Scholar]
- 66.Wu C., Xia L., Huang W., Xu Y., Gu Y., Liu C., Ji L., Li W., Wu Y., Zhou K., Feng X. Pentamidine sensitizes FDA-approved non-antibiotics for the inhibition of multidrug-resistant Gram-negative pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1771–1779. doi: 10.1007/s10096-020-03881-0. [DOI] [PubMed] [Google Scholar]
- 67.MacNair C.R., Farha M.A., Serrano-Wu M.H., Lee K.K., Hubbard B., Côté J.-P., Carfrae L.A., Tu M.M., Gaulin J.L., Hunt D.K., et al. Preclinical Development of Pentamidine Analogs Identifies a Potent and Nontoxic Antibiotic Adjuvant. ACS Infect. Dis. 2022;8:768–777. doi: 10.1021/acsinfecdis.1c00482. [DOI] [PubMed] [Google Scholar]
- 68.Maciejewska D., Żabiński J., Kaźmierczak P., Wójciuk K., Kruszewski M., Kruszewska H. In vitro screening of pentamidine analogs against bacterial and fungal strains. Bioorg. Med. Chem. Lett. 2014;24:2918–2923. doi: 10.1016/j.bmcl.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 69.Wesseling C.M.J., Slingerland C.J., Veraar S., Lok S., Martin N.I. Structure − Activity Studies with Bis-Amidines That Potentiate Gram- Positive Speci fi c Antibiotics against Gram-Negative Pathogens. ACS Infect. Dis. 2021;7:3314–3335. doi: 10.1021/acsinfecdis.1c00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hafiz S., Kyriakopoulos C. StatPearls Publishing; 2023. Pentamidine. [PubMed] [Google Scholar]
- 71.Muñoz B.Y., Mantilla J.C., Escobar P. Therapeutic response and safety of the topical, sequential use of antiseptic, keratolytic, and pentamidine creams (3-PACK) on leishmania (viannia) braziliensis-infected mice. Mem. Inst. Oswaldo Cruz. 2019;114 doi: 10.1590/0074-02760180535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chu H., Slepenkin A., Elofsson M., Keyser P., de la Maza L.M., Peterson E.M. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseria gonorrhoeae. Int. J. Antimicrob. Agents. 2010;36:145–150. doi: 10.1016/j.ijantimicag.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osaka I., Hefty P.S. Lipopolysaccharide-Binding Alkylpolyamine DS-96 Inhibits Chlamydia trachomatis Infection by Blocking Attachment and Entry. Antimicrob. Agents Chemother. 2014;58:3245–3254. doi: 10.1128/AAC.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Kahane S., Cutcliffe L.T., Skilton R.J., Lambden P.R., Clarke I.N. Development of a Transformation System for Chlamydia trachomatis: Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scidmore M.A. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Curr. Protoc. Microbiol. 2005;11 doi: 10.1002/9780471729259.mc11a01s00. Unit 11A.1. [DOI] [PubMed] [Google Scholar]
- 76.Faris R., Weber M.M. Propagation and Purification of Chlamydia trachomatis Serovar L2 Transformants and Mutants. Bio. Protoc. 2019;9:e3459. doi: 10.21769/bioprotoc.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klasinc R., Reiter M., Digruber A., Tschulenk W., Walter I., Kirschner A., Spittler A., Stockinger H. A Novel Flow Cytometric Approach for the Quantification and Quality Control of Chlamydia trachomatis Preparations. Pathogens. 2021;10:1617. doi: 10.3390/pathogens10121617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ragland S.A., Criss A.K. Protocols to Interrogate the Interactions Between Neisseria gonorrhoeae and Primary Human Neutrophils. Methods Mol. Biol. 2019;1997:319–345. doi: 10.1007/978-1-4939-9496-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J.-H., Chung T., Oldenburg K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 80.Warf M.B., Nakamori M., Matthys C.M., Thornton C.A., Berglund J.A. Pentamidine reverses the splicing defects associated with myotonic dystrophy. Proc. Natl. Acad. Sci. USA. 2009;106:18551–18556. doi: 10.1073/pnas.0903234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stary G., Olive A., Radovic-moreno A.F., Gondek D., Alvarez D., Basto P.A., Perro M., Vrbanac V.D., Tager A.M., Shi J., et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348 doi: 10.1126/science.aaa8205.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2,167 compounds were tested for their ability to inhibit Ct growth for more than 50% compared to controls in serovar L2. Azithromycin served as a positive control and DMSO as a negative control. Nuclei and Ct inclusions per FOV were determined.
The non-antibiotics inhibiting Ct growth identified in the primary drug screen were tested in serovar L2 in an 8-point dose response with duplicates in 3-fold dilutions, typically starting at 13.5 μM. Number of nuclei and inclusions were determined in one FOV per well.
The non-antibiotics which were validated to inhibit Ct growth in serovar L2 were applied to serovar E and serovar F in 4-point serial dilutions. Number of nuclei and inclusions were determined in one FOV per well.
Cells were infected with Ct, and compounds were added at indicated concentrations only 1 h after infection.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.