Summary
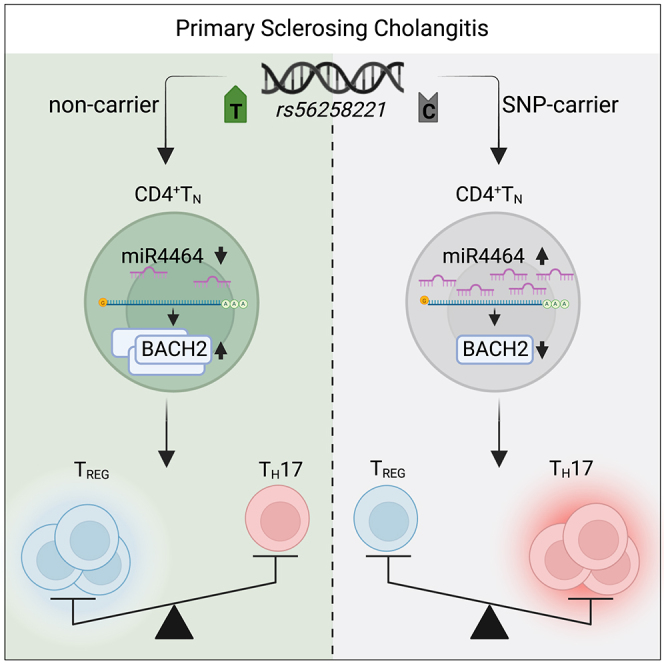
Primary sclerosing cholangitis (PSC) is an immune-mediated liver disease of unknown pathogenesis, with a high risk to develop cirrhosis and malignancies. Functional dysregulation of T cells and association with genetic polymorphisms in T cell-related genes were previously reported for PSC. Here, we genotyped a representative PSC cohort for several disease-associated risk loci and identified rs56258221 (BACH2/MIR4464) to correlate with not only the peripheral blood T cell immunophenotype but also the functional capacities of naive CD4+ T (CD4+ TN) cells in people with PSC. Mechanistically, rs56258221 leads to an increased expression of miR4464, in turn causing attenuated translation of BACH2, a major gatekeeper of T cell quiescence. Thereby, the fate of CD4+ TN is skewed toward polarization into pro-inflammatory subsets. Clinically, people with PSC carrying rs56258221 show signs of accelerated disease progression. The data presented here highlight the importance of assigning functional outcomes to disease-associated genetic polymorphisms as potential drivers of diseases.
Keywords: primary sclerosing cholangitis, immune-mediated liver disease, genetic polymorphism, CD4 T cells, naive T cells, TH17 cells, regulatory T cells, BACH2, miR4464
Graphical abstract
Highlights
-
•
People with PSC carrying rs56258221 bear a pro-inflammatory immunophenotype
-
•
rs56258221 impairs BACH2 expression and skews differentiation of naive CD4+ T cells
-
•
Aberrant CD4+ TN differentiation is linked to elevated BACH2-targeting miR4464 levels
-
•
Carriers of rs56258221 show clinical signs of accelerated disease progression
Poch, Bahn et al. identify the primary sclerosing cholangitis-associated genetic polymorphism rs56258221 to enhance miR4464 expression in naive CD4+ T cells. By targeting BACH2, a gatekeeper of T cell quiescence, elevated miR4464-levels change the fate of naive CD4+ T cells towards pro-inflammatory subsets.
Introduction
Primary sclerosing cholangitis (PSC) is a progressive inflammatory liver disease leading to obliteration of the intra- and/or extrahepatic bile ducts. Due to the widely unknown pathogenesis of PSC, there is no effective pharmacological therapy with a proven impact on the course of disease, and patients frequently develop end-stage liver disease and require liver transplantation.1,2
One presumable key contributor to the likely multifactorial disease pathogenesis is a dysregulated T cell function, including both CD4+ and CD8+ T cells.3,4,5 We previously described increased frequencies of CD4+ T helper (TH) 17 cells and decreased frequencies of regulatory T cells (TREG) within the peripheral blood of people with PSC compared to healthy donors and people with other inflammatory liver diseases.6,7 Moreover, we have recently shown that CD4+ T cells with a naive-like phenotype, showing a high capacity to polarize toward TH17 cells, are increased in the blood and livers of people with PSC.8 This is in line with the observation of an increased TH17 response upon pathogen encounter by PSC-derived T cells in vitro,9 supporting the hypothesis that T cells in PSC are prone to acquire a pro-inflammatory phenotype.1,2
However, the mechanisms causing this misguided immune cell differentiation are poorly understood. Besides environmental factors such as microbiota composition in intestine and bile ducts, genetic predisposition might contribute to T cell phenotype.10,11 Previously, multiple genome-wide association studies have associated PSC to gene polymorphisms related to immune cell and particularly T cell function (e.g., IL2RA, BACH2, FOXP1, and CD28).12,13,14,15,16,17 Of note, most of the PSC-related polymorphisms are also associated with other autoimmune diseases, e.g., type 1 diabetes or rheumatoid arthritis, and only few studies have so far reported on the functional implications of these polymorphisms for T cells, which is particularly true for PSC.1,7,18
We hypothesized that genetic predisposition contributes to T cell dysregulation in PSC and investigated the functional role of selected T cell-related gene polymorphisms (CD28/CTLA4, IL2RA, FOXP1, and BACH2) on the fate and function of T cells. Particularly, BACH2 (BTB domain and CNC homolog 2) has been shown to be a critical transcription factor for differentiation and maturation of both T and B lymphocytes. BACH2, initially identified as a transcriptional repressor in B cells,19 has emerged as a central regulator also of T cells, with its targets including central transcription factors of T cell activation, e.g., PRDM1 and RUNX3.20,21,22 Knockout studies in mice have shown that lack of BACH2 leads to spontaneous T cell differentiation toward effector T cells as well as reduced numbers of TREG.23 In contrast, its overexpression was shown to promote a less differentiated “stem-like” T cell phenotype,24 underlining its potential role as a gatekeeper of quiescence in naive T cells. Of note, the SNP rs56258221 is located in the intergenic region between BACH2 and MIR4464, an miRNA previously imputed to target BACH2 3′ untranslated region (UTR).25
We here identified this intergenic SNP, which was present in approximately 21% of people with PSC and 18% of controls in the study identifying this risk locus,16 to associate with a distinct peripheral blood immunophenotype and skewed polarization of naive CD4+ T (CD4+ TN) cells toward inflammatory TH cell subsets. Functionally, this polymorphism enhanced expression of miR4464 and thereby led to reduced BACH2 protein levels in CD4+ TN, confirming the regulatory role of miR4464 on BACH2 translation.
Overall, these data reveal a potential mechanism by which genetic predisposition shapes T cell function in autoimmunity and identify miRNAs and BACH2 as potentially druggable targets for the treatment of PSC.
Results
People with PSC carrying the risk variant rs56258221 show a distinct T cell phenotype compared to non-carriers
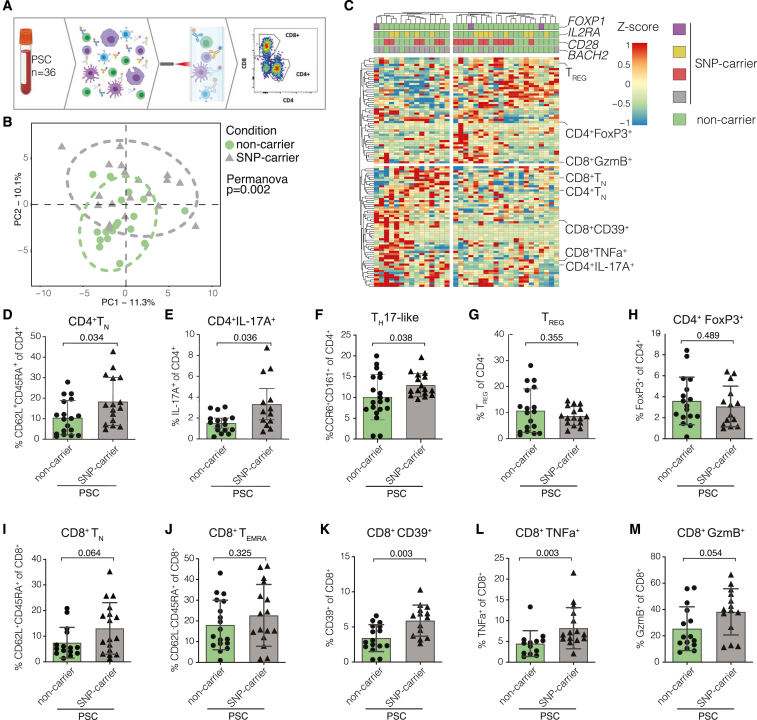
Peripheral blood samples were collected from people with PSC, and comprehensive immunophenotyping via flow cytometry yielded extensive T cell phenotypes of a representative PSC cohort (N = 36). The cohort was then genotyped for the PSC-associated polymorphisms rs56258221 (BACH2/MIR4464), rs80060485 (FOXP1), rs4147359 (IL2RA), and rs7426056 (CD28/CTLA4)16 (Figure 1A). We identified rs56258221 genotypes within the PSC cohort to separate our dataset into two cohorts with distinct immunophenotypes overall (p = 0.002) (Figure 1B), which was not observed for the other SNPs investigated (Figures S1A–S1C). Within this dataset, we detected several subsets of both CD4+ and CD8+ T cells to significantly differ in frequency between SNP carriers and non-carriers (Figures 1B and 1C). CD4+ TN (Figure 1D, p = 0.034) and TH17 cells (Figure 1E, p = 0.036; Figure 1F, p = 0.038) were among the subsets with increased frequencies in SNP carriers,26,27 whereas the frequency of TREG was comparable between the two groups (Figure 1G, p = 0.355; Figure 1H, p = 0.489). Similar to CD4+, CD8+ T cells showed a trend toward increased TN (Figure 1I, p = 0.064) but not for terminally differentiated T cells re-expressing CD45RA (TEMRA) (Figure 1J, p = 0.325), which had previously been associated with changes in BACH2 levels in CD8+ T cells.21,24 However, we observed an increased frequency of CD39-expressing CD8+ T cells, which points toward activated cells28 in SNP carriers compared to non-carriers (Figure 1K, p = 0.003). In line with this, we observed a higher expression of pro-inflammatory cytokine tumor necrosis factor alpha (Figure 1L, p = 0.003) and granzyme B (GzmB) (Figure 1M, p = 0.054) in CD8+ T cells of people with PSC carrying rs56258221. The expression of GzmB has previously been shown to be affected by BACH2.20
Figure 1.
Risk variant rs56258221 (BACH2/MIR4464) entails phenotypic differences in peripheral T cells in people with PSC
(A) Schematic depiction of the workflow for immunophenotyping.
(B) Principal component analysis of the analyzed immunophenotyping data, separated by the genotype for polymorphism rs56258221.
(C) Heatmap illustrating the immunophenotyping dataset (n = 36) and highlighting populations that differed in frequency between carriers of rs56258221 (n = 18) and non-carriers (n = 18).
(D–H) Frequencies of different CD4+ T cell subsets. (D) TN, identified by CD62L/CD45RA. (E) TH17 cells identified by IL-17A expression upon stimulation with PMA (phorbol 12-myristate 13-acetate)/ionomycin. (F) TH17 cells identified by CCR6/CD161. (G) TREG identified by CD127/CD25. (H) TREG identified by FoxP3 expression upon stimulation with PMA/ionomycin.
(I–M) Frequencies of different CD8+ T cell subsets. (I) TN, identified by CD62L/CD45RA. (J) TEMRA, identified by CD62L/CD45RA. (K) CD39 expression on CD8+ T cells. (L) TNF expression upon stimulation with PMA/ionomycin. (M) GzmB expression upon stimulation with PMA/ionomycin. Characteristics of the clinical cohort are included in Table S1. Statistics: normality distribution was tested by Kolmogorov-Smirnov test; normal distribution: Welch’s t test; no normal distribution: Mann-Whitney U test. p < 0.05 was considered statistically significant. Data are presented as mean ± SD and deriving from n ≥ 2 repeats per experiment.
We next followed up on these observations by performing bulk RNA sequencing on liver tissue samples acquired via liver biopsies from people with PSC (n = 12). Notably, in carriers of rs56258221, we found significantly increased expression of C-C chemokine receptor type 6 (Figure S1N, p = 0.034) and killer lectin-like receptor B1 (Figure S1O, p = 0.018), which are commonly used markers to identify T cells that are polarized toward a TH17-like phenotype.29 Most other markers assessed, including genes associated with regulatory and activated phenotype of CD8+ T cells, were not changed between SNP carriers and non-carriers (Figures S1P–S1U).
To generalize our findings about rs56258221 affecting immune cell frequencies in the peripheral blood of people with PSC, we additionally performed immunophenotyping in cohorts of healthy blood donors (HD, n = 18), people with primary biliary cholangitis (PBC, n = 16), people with inflammatory bowel disease without PSC (IBD, n = 12), and people with metabolic dysfunction-associated steatohepatitis (MASH, n = 6). However, we did not observe any significant differences in the populations mentioned previously between carriers and non-carriers of rs56258221 (Figures S1D–S1M).
Developmental trajectories of peripheral blood CD4+ and CD8+ TN are altered in carriers of rs56258221
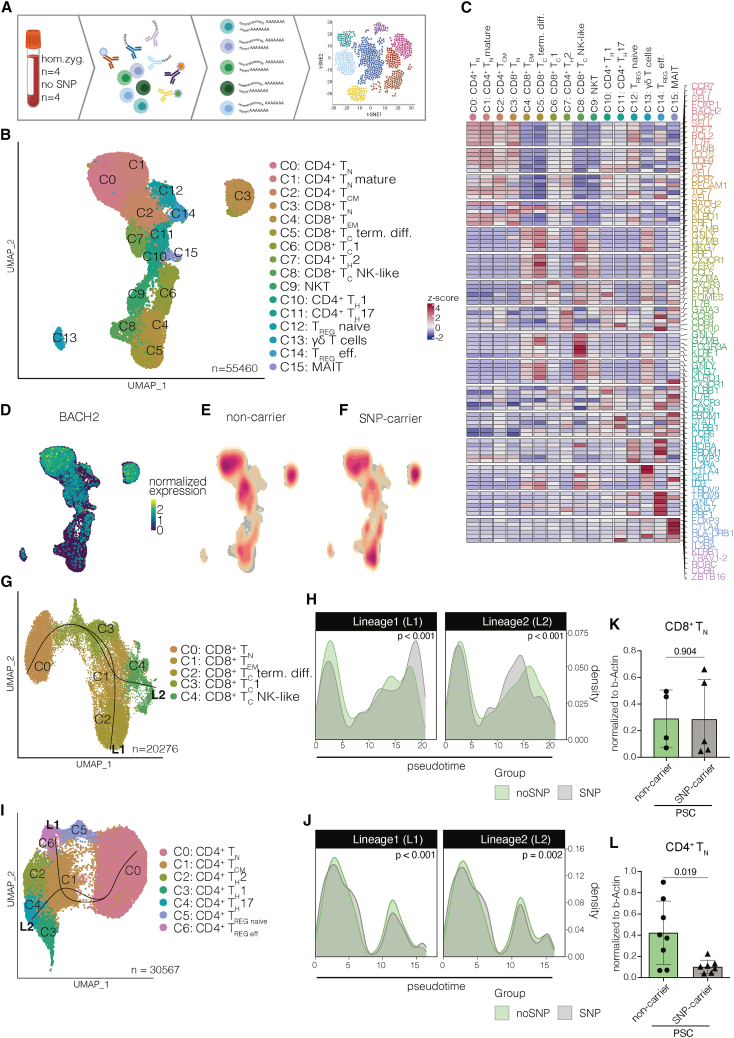
In order to investigate whether carrying rs56258221 affects the overall transcriptome of T cells and thereby shapes their cellular state, we performed cellular indexing of transcriptomes and epitopes by sequencing (CITE-Seq) on peripheral blood-derived T cells from people with PSC either homozygous for rs56258221 (n = 4) or non-carriers (n = 4). We sequenced 55,460 T cells across eight individuals and identified most of the major CD4+ (TN, central memory [TCM], TH1, TH2, TH17, and TREG) and CD8+ T cell subsets (TN, TCM, effector memory [TEM], cytotoxic [TC]), as well as gamma delta T cells (gd T cells), mucosa-associated invariant T cells (MAIT), and natural killer T cells (NKT) via analysis of differentially expressed genes combined with surface protein expression (Figures 2A–2C and S2A). After confirming TN as the major source of BACH2 expression among these clusters (Figure 2D), we compared BACH2 expression levels between carriers of rs56258221 and non-carriers but observed no differences between the groups (Figure S2B). We next compared the cell composition between the two genotypes among the clusters and noted a dissimilar distribution of cells within the CD4+ TN cluster as well as the CD8+ TC terminally differentiated cluster (Figures 2E and 2F). Hypothesizing on the effects of carrying rs56258221 on differentiation of TN, we excluded NKT, MAIT, and gd T cells and split the dataset into CD4+ and CD8+ T cells. We then utilized slingshot combined with condiments30,31 to compare the differentiation capacities between carriers of rs56258221 and non-carriers. Interestingly, we detected significant differences in cellular distribution along the trajectories of both CD4+ and CD8+ T cells (Figures 2G–2J).
Figure 2.
Developmental trajectories of peripheral blood TN are altered in carriers of rs56258221
(A) Schematic depiction of the workflow for CITE-Seq (n = 4 homozygous carrier; n = 4 non-carrier) (all 8 patients cisgender males).
(B) Uniform manifold approximation and projection (UMAP) resembling 55,460 peripheral blood T cells from people with PSC either homozygous for or not carrying rs56258221, subdivided into 16 clusters by Seurat.
(C) Heatmap highlighting differentially expressed genes used to assign cell types to clusters.
(D) Expression of BACH2 mapped onto the UMAP from (B), highlighting TN as the main population expressing BACH2.
(E and F) Cellular density within landscape of peripheral blood T cells from (J), separated into SNP carriers (E) and non-carriers (F).
(G) Subclustering of CD8+ T cells and trajectory analysis via slingshot with CD8+ TN as the selected starting point.
(H) Differential progression analysis via condiments to identify differential distribution of cells along trajectory.
(I) Subclustering of CD4+ T cells and trajectory analysis via slingshot with CD4+ TN as the selected starting point.
(J) Differential progression analysis via condiments to identify differential distribution of cells along trajectory.
(K) Relative BACH2 expression compared to B-actin expression via western blot from FACS-sorted CD8+ TN (n = 9).
(L) Relative BACH2 expression compared to B-actin expression via western blot from FACS-sorted CD4+ TN (n = 15). Characteristics of the clinical cohort are included in Tables S2 and S3. Statistics: normality distribution was tested by Kolmogorov-Smirnov test; normal distribution: Welch’s t test; no normal distribution: Mann-Whitney U test. p < 0.05 was considered statistically significant. Data are presented as mean ± SD and deriving from n ≥ 2 repeats per experiment.
Considering these findings and also previously published studies on the effect of altered BACH2 levels on T cell differentiation and phenotype,21,22,23,24,26 we quantified BACH2 protein levels in fluorescence-activated cell-sorted peripheral blood CD4+ and CD8+ TN. Notably, we detected a strong reduction of BACH2 protein levels in CD4+, but not CD8+ TN (Figure 2K–2L, p = 0.019 and p = 0.904) in homozygous carriers of rs56258221. Combined, we observed phenotypic differences in peripheral blood T cells between the two genotypes. These differences were accompanied by reduced BACH2 protein levels in CD4+ but not CD8+ TN, indicating a possible direct effect of rs56258221 on BACH2 expression in CD4+ TN.
CD4+ TN cells from carriers of rs56258221 show a developmental propensity toward a pro-inflammatory phenotype in vitro
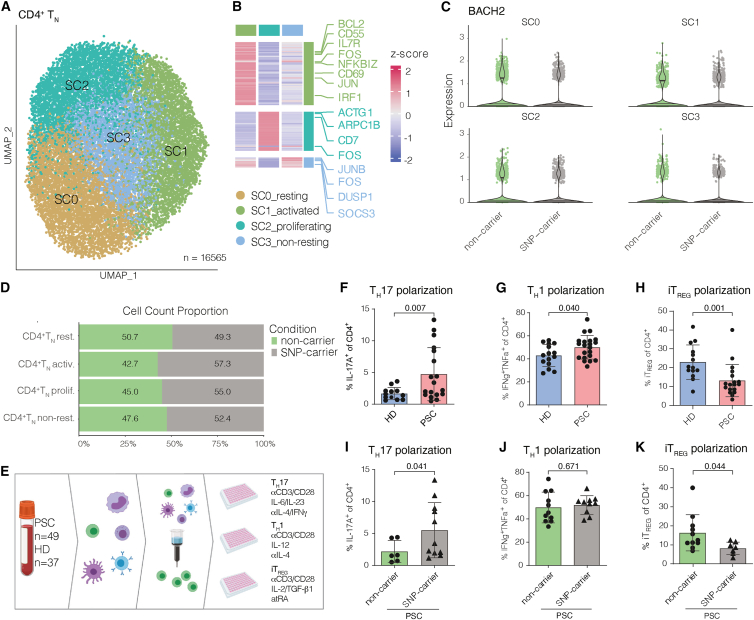
Based on the phenotypical changes in CD4+ T cells and the reduced BACH2 protein levels in CD4+, but not CD8+ TN of SNP carriers, we followed up on this by re-clustering the CD4+ TN clusters from our CITE-Seq dataset in order to investigate potential differences between the genotypes. Re-clustering resulted in four subclusters of CD4+ TN, which we annotated according to their gene expression profiles (SC0_resting state, SC1_activated state, SC2_proliferating state, and SC3_non-resting state) (Figures 3A and 3B). The expression levels of BACH2 mRNA among the subclusters were similar between cells from carriers of rs56258221 and non-carriers (Figure 3C). Interestingly, though, the clusters assigned to activated transcriptomes (SC1 and SC2) showed a trend toward enrichment in SNP carriers (Figure 3D).
Figure 3.
PSC-associated polymorphism rs56258221 (BACH2/MIR4464) contributes to increased polarization of CD4+ TN cells toward pro-inflammatory phenotypes
(A and B) Re-clustering of CITE-Seq data from CD4+ TN (cluster C0 from Figure 1J) resulted in four subclusters of CD4+ TN (SC0-3) (A), which were assigned to cellular states via analysis of differentially expressed genes (B). SC0 did not show differentially expressed genes.
(C) Expression levels of BACH2 among each cluster.
(D) Bar plot comparing the frequencies of SC0-3 between SNP carriers and non-carriers.
(E) Schematic depiction of the workflow for in vitro polarization assays.
(F) After 12 days of polarizing culture of CD4+ TN from people with PSC (n = 18) and HD (n = 12), the frequency of CD4+IL-17A+ cells was determined.
(G) After 7 days of polarizing culture of CD4+ TN from people with PSC (n = 21) and HD (n = 15), the frequency of T CD4+CXCR3+TBET+IFNg+TNFa+ cells was determined.
(H) After 7 days of polarizing culture of CD4+ TN from people with PSC (n = 18) and HD (n = 14), the frequencies of CD4+CD25+CD152+FOXP3+ cells were determined.
(I–K) Data on people with PSC from TH17 (F), TH1 (G), and iTREG (H) polarization were separated by genotype for polymorphism rs56258221. Characteristics of the clinical cohort are included in Tables S4–S6. Statistics: normality distribution was tested by Kolmogorov-Smirnov test; normal distribution: Welch’s t test; no normal distribution: Mann-Whitney U test. p < 0.05 was considered statistically significant. Data are presented as mean ± SD and deriving from n ≥ 2 repeats per experiment.
Next, we decided to perform functional experiments on CD4+ TN in vitro to assess potential differences in response to stimulation (Figure 3E).
We previously demonstrated that CD4+ TN cells from people with PSC show increased TH17 polarization capacities in vitro.6,8 We observed that CD4+ TN cells from people with PSC show a higher propensity to polarize not only toward TH17 cells (Figure 3F, p = 0.007) but also toward TH1 cells, compared to controls (Figure 3G, p = 0.040). Notably, the propensity of PSC-derived CD4+ TN cells to polarize toward pro-inflammatory subsets was accompanied by a significantly lower rate of polarization toward induced TREG (iTREG) (Figure 3H, p = 0.001). These observations suggest a shift in the developmental propensities of CD4+ TN cells in PSC toward a pro-inflammatory phenotype, in line with the previously reported imbalance of TH17 and TREG frequencies in peripheral blood of people with PSC and the propensity of liver-resident naive-like CD4+ T cells to differentiate into TH17 cells.7,8,9 We next assessed whether genetic predisposition contributes to the observed differences by comparing carriers and non-carriers of the PSC-associated genetic polymorphisms assessed in this study (CD28/CTLA4, IL2RA, FOXP1, and BACH2/MIR4464). In line with the previous results, we observed an association of rs56258221 (BACH2/MIR4464) with a pro-inflammatory polarization of CD4+ TN, which was particularly true for the TH17/iTREG dichotomy (Figures 3I–3K; TH17 p = 0.041, iTREG p = 0.044). This association was not present for the other SNPs investigated (Figures S3A–S3H) and was not linked to differences in proliferation of CD4+ TN (Figures S3I–S3K).
Taken together, our findings on the polymorphism rs56258221 (BACH2/MIR4464) support the hypothesis of genetic predisposition contributing to skewed CD4+ TN polarization capacities in PSC.
The risk variant rs56258221 is associated with increased expression of miR4464 in CD4+ TN
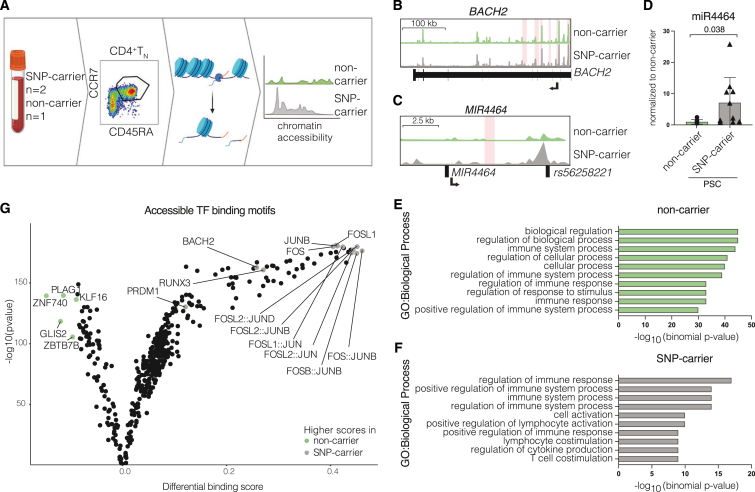
To assess whether epigenetic changes are linked to the observed differences in phenotype and differentiation, we utilized our previously published dataset on single-cell sequencing assay for transposase-accessible chromatin (scATAC-Seq) on CD4+ TN cells from people with PSC8 and retrospectively identified SNP carriers (n = 2) and non-carriers (n = 1) within this dataset (Figure 4A). We detected the BACH2 locus to be more accessible in CD4+ TN from carriers of rs56258221 (Figure 4B), which was unexpected considering the reduction of BACH2 protein. As the intergenic polymorphism rs56258221 is assigned to both BACH2 and MIR4464, we hypothesized on post-transcriptional regulation contributing to the observed reduction in BACH2 protein.
Figure 4.
The risk variant rs56258221 (BACH2/MIR4464) is associated with lower BACH2 protein levels and increased expression of miR4464
(A) Schematic depiction of the workflow for scATAC-Seq analysis (n = 4).
(B) Coverage plot showing the chromatin region of BACH2. Significantly increased accessible chromatin regions in SNP carriers (n = 2) are highlighted in red.
(C) Coverage plot showing the chromatin region surrounding rs56258221, including the MIR4464 locus. Significantly increased accessible chromatin regions in SNP carriers (n = 2) are highlighted in red.
(D) Detection of miR4464 in FACS-sorted CD4+ TN of homozygous carriers and non-carriers of rs56258221.
(E and F) Gene ontology (GO) terms representing total accessible chromatin regions from either SNP carriers (E) or non-carriers (F). The ten GO terms with lowest p values are displayed.
(G) Assignment of transcription factor-binding sites (TFBS) to accessible chromatin regions via TOBIAS. Analysis of differential TFBS accessibility between carriers of rs56258221 (n = 2) and non-carriers (n = 2) is shown. Characteristics of the clinical cohort are included in Table S3. Statistics: normality distribution was tested by Kolmogorov-Smirnov test; normal distribution: Welch’s t test; no normal distribution: Mann-Whitney U test. p < 0.05 was considered statistically significant. Data are presented as mean ± SD and deriving from n ≥ 2 repeats per experiment.
Of note, miR4464 was previously imputed to exert regulatory function on BACH2, as its binding motifs are located within the 3′ UTR of the BACH2 mRNA.25
To address our hypothesis on miR4464 being involved in the observed reduction of BACH2 protein levels, we went back to the scATAC-Seq dataset and observed a pronounced increase of chromatin accessibility spanning the genomic location of rs56258221 (Chr6:90,320,722), which is in close proximity to MIR4464 (Figure 4C). Next, we used fluorescence-activated cell-sorted CD4+ TN from the same people with PSC included in our single-cell RNA sequencing and western blot experiments to determine expression levels of miR4464. Notably, we observed a significantly higher expression of miR4464 in CD4+ TN from carriers of rs56258221 compared to non-carriers (Figure 4D, p = 0.038).
In addition, gene ontology analysis of overall accessible chromatin sites suggested an activated cellular phenotype for CD4+ TN from carriers of rs56258221, indicated by the terms “cell activation,” “positive regulation of lymphocyte activation,” and “T cell costimulation” (Figure 4E). In contrast, CD4+ TN cells from non-carriers were linked to a presumably steady cellular state (Figure 4F). To analyze this finding in more detail, we assigned transcription factor-binding sites to accessible chromatin regions by utilization of TOBIAS (Transcription factor Occupancy prediction By Investigation of ATAC-seq Signal).32 We observed an increase in accessible binding sites of transcription factors associated with T cell activation, particularly members of the AP-1 complex (BATF, FOS, JUN, and JDP), Blimp-1 (PRDM1), and Runx3 (RUNX3), which are all known targets of BACH2,20,21 in carriers of rs56258221 (Figure 4G). In line with this, CD4+ T cells from SNP carriers also showed an increase in BACH2-binding sites, indicating less binding activity of BACH2 as a transcriptional repressor (Figure 4G).
Combined, we show reduced protein levels of the transcriptional repressor BACH2 accompanied by increased expression of miR4464 in carriers of the risk variant rs56258221. This reduction is associated to an increase of accessible motifs of both BACH2 and its targets in the epigenetic landscape of CD4+ TN.
Increased expression of miR4464 attenuates BACH2 translation in CD4+ TN, resulting in a reduced polarization capacity toward iTREG
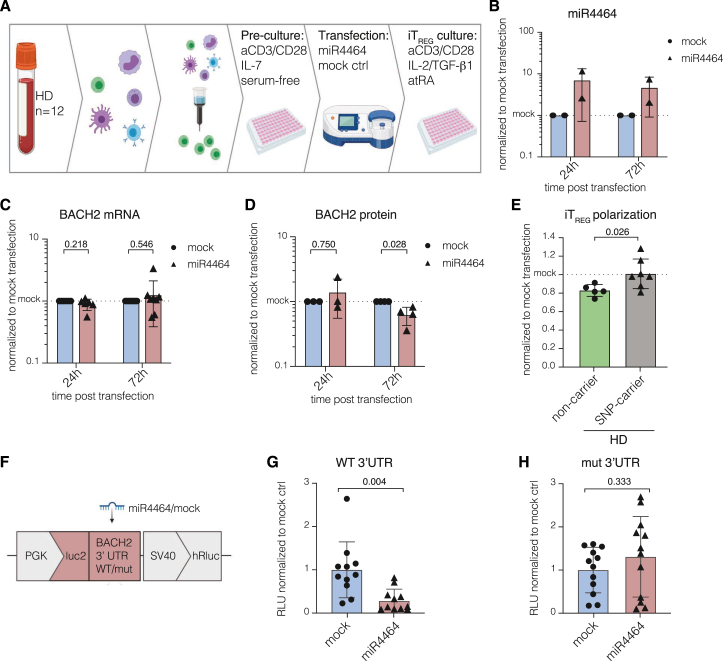
After identifying increased expression of BACH2-targeting miR4464 as a potential cause for the observed reduction of BACH2 protein in CD4+ TN, we investigated whether miR4464 functionally contributes to this effect by downregulating the translation of BACH2 mRNA in carriers of rs56258221. Therefore, we isolated CD4+ TN from peripheral blood of healthy donors (n = 12) and transfected the cells with miR4464. Next, we performed polarizing in vitro culture toward iTREG, using the same experimental setup as previously described (Figure 5A). First, we controlled for successful transfection by determining the level of miR4464, which showed an approximate 10-fold increase compared to cells transfected with non-binding miRNA (mock) and only slightly decreased during 72 h post transfection (Figure 5B). Of note, the observed increase was in a similar range to the changes observed between homozygous carriers of rs56258221 and non-carriers (cf. Figure 4D). Next, we determined the expression levels of both BACH2 mRNA and protein and observed no profound effect of the transfection with miR4464 on BACH2 mRNA but a significant reduction of BACH2 protein (Figures 5C and 5D, p = 0.028). We next cultured transfected CD4+ TN under iTREG-polarizing conditions. Notably, we observed a significant decrease in iTREG formation in healthy non-carriers of rs56258221 upon transfection with miR4464, compared to mock-transfection, which was not seen for SNP carriers (Figure 5E, p = 0.026). This observation is potentially due to the increased miR4464 levels per se in SNP carriers, which we described in the section earlier. To finally prove that an increase in miR4464 directly impacts BACH2 translation, we cloned the fragment from the 3′ UTR region of BACH2 mRNA containing the predicted miR4464-binding site25 or a mutated binding site into the pmirGLO dual-luciferase vector. Transfection of HEK293T cells with the BACH2 tester plasmid in combination with miR4464 resulted in strongly reduced luciferase signals compared to mock transfection, pointing toward an effective targeting of the cloned 3′ UTR region of BACH2 mRNA by miR4464 (Figure 5G, p = 0.004). Importantly, this effect was not observed upon transfection with plasmids containing a mutated miR4464 binding site within the 3′ UTR region of BACH2 (Figure 5H, p = 0.333), confirming the imputed data that were previously reported on miR4464 targeting BACH2 mRNA.25
Figure 5.
miR4464 induces reduced BACH2 protein translation in CD4+ TN and reduces polarization capacity toward iTREG
(A) Schematic depiction of the workflow for the in vitro transfection of CD4+ TN with miR4464.
(B) Detection of miR4464 levels in CD4+ TN cells 24 and 72 h post transfection.
(C) Detection of BACH2 mRNA levels in CD4+ TN cells 24 and 72 h post transfection.
(D) Quantified western blot analysis on BACH2 protein levels from CD4+ TN cells 24 and 72 h post transfection.
(E) In vitro polarization toward iTREG. Frequencies of iTREG 6 days post transfection with miR4464. Data were normalized to the respective mock transfection controls.
(F) Schematic depiction of the experimental setup utilizing the pmirGLO tester plasmid containing the 3′ UTR of BACH2 mRNA or a mutated control site.
(G and H) Luciferase activity was determined from HEK293T cells 24 h post co-transfection of either miR4464 or mock control and the tester plasmid containing a mutated control binding site (G) or the wild-type binding site (H). Luciferase activity was normalized to include Renilla luciferase (hRluc) control. Statistics: normality distribution was tested by Kolmogorov-Smirnov test; normal distribution: Welch’s t test; no normal distribution: Mann-Whitney U test. p < 0.05 was considered statistically significant. Data are presented as mean ± SD and deriving from n ≥ 2 repeats per experiment.
Combined, our data show that miR4464 indeed binds the 3′ UTR region of BACH2 mRNA and thereby directly regulates protein translation of this central transcriptional repressor, which had an immediate impact on the capability of CD4+ TN to polarize into iTREG.
Markers of disease severity in people with PSC carrying rs56258221
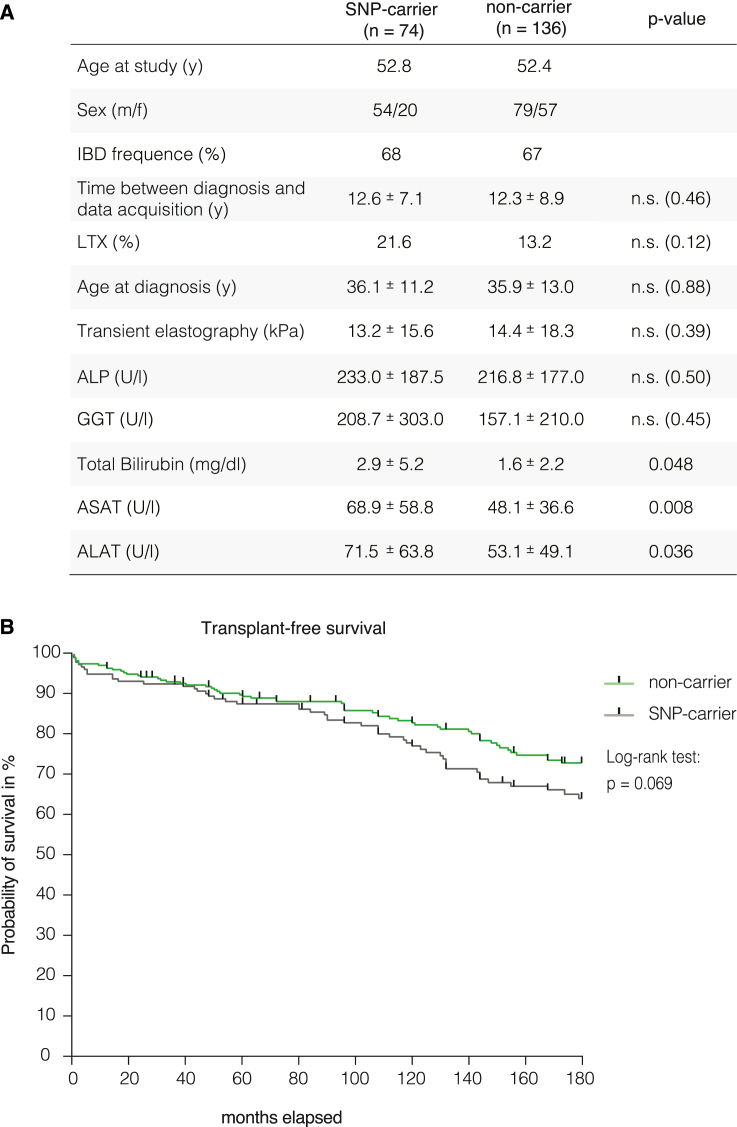
To investigate whether the previously shown differences in T cell differentiation and phenotype associate with variations in the clinical course of people with PSC, we evaluated a selection of routine laboratory parameters of people with PSC attending the outpatient clinic for gastroenterology and hepatology at the University Medical Centre Hamburg-Eppendorf (n = 210). Using SNP genotyping, we identified 74 carriers and 136 non-carriers of rs56258221 in our cohort. Both groups showed similar age at diagnosis (36.1 vs. 35.8 years) and duration of disease at the time of data collection (12.6 vs. 12.3 years) (Figure 6A).
Figure 6.
Clinical phenotype of people with PSC carrying polymorphism rs56258221 (BACH2/MIR4464)
(A) Laboratory parameters and clinical features of people with PSC attending the outpatient clinic for gastroenterology and hepatology of the University Medical Centre Hamburg-Eppendorf (n = 210). The data were separated into carriers and non-carriers of rs56258221. Both groups span a similar time between diagnosis of PSC and collection of laboratory parameters (mean ± SD).
(B) Assessment of transplant-free survival between SNP carriers and non-carriers. The combined independent cohorts from University Medical Centre Hamburg-Eppendorf (Germany) and the Norwegian PSC Centre in Oslo (Norway) are shown. Survival data were analyzed by using log rank test. Tick marks represent censoring.
Intriguingly, biomarkers routinely used to evaluate severity of disease differed between carriers and non-carriers of rs56258221. These included increased levels of aspartate aminotransferase (AST, 69 vs. 48 U/L, p = 0.007), alanine aminotransferase (ALT, 72 vs. 53 U/L, p = 0.035), and total bilirubin (2.9 vs. 1.6 mg/dL, p = 0.047) (Figure 5A) in carriers of rs56258221. In addition, carriers showed a higher rate of liver transplantation (LTx), although this difference was not statistically significant (21.6% vs. 13.2%, p = 0.120).
Consequently, we aimed to further validate a potential effect of rs56258221 on the clinical course of people with PSC. Therefore, we extended our PSC cohort from Hamburg (n = 210) with an independent PSC cohort (n = 137) from the Norwegian PSC Center at the Oslo University Hospital, Rikshospitalet that included 100 people with PSC who had received LTx. In the analysis of a 15-year period post diagnosis in 347 people with PSC, there was a trend of carriers (n = 132) toward a more severe clinical course compared to non-carriers (n = 215) of rs56258221 (log rank test: p = 0.069) (Figure 6B).
Discussion
Both genetic and environmental factors contribute to the pathogenesis of PSC, a dreadful liver disease lacking any effective pharmacological treatment option. In most people with PSC, chronic disease progression inevitably leads to end-stage liver disease, death, or liver transplantation within two decades.1,10 Despite the fact that over 20 gene polymorphisms have been assigned to elevated risk for PSC, data on functional consequences of these polymorphisms are scarce,7,18 which is most likely due to their intronic and intergenic locations.1,12,13,14,15,16,17,33 We here hypothesized that PSC-associated genetic variants contribute to the dysregulation of T cells we and others have described previously in people with PSC.6,7,8,9,18,34
The majority of the PSC-associated polymorphisms is located within or nearby genetic loci related to T cell function. Among these, we identified the intergenic polymorphism rs56258221 at the BACH2/MIR4464 gene locus to affect the phenotype of both CD4+ and CD8+ T cells and, in particular, the fate and function of CD4+ TN. We demonstrate that CD4+ TN cells from people with PSC display skewed polarization capabilities toward pro-inflammatory subsets, i.e., TH1 and TH17. In contrast, the polarization of CD4+ TN toward anti-inflammatory iTREG was impaired. This is of interest as TH17 and its signature cytokine IL-17 are widely recognized as key players in PSC pathogenesis and autoimmunity.1,2,6,8,9 Moreover, the presence of rs56258221 characterized a population with increased frequencies of TH17 cells, and SNP carriers showed a skewed in vitro differentiation of CD4+ TN toward TH17 and away from iTREG cells.
BACH2 is a transcriptional repressor playing an essential role in T cell quiescence and activation, as well as in the maintenance of CD4+ T cell subsets, i.e., TREG, TH1, and TH17 cells.19,20,21,22,23,29 Although the exact mechanism of action of BACH2 remains to be determined, its interference with signaling of both T cell and IL-2 receptor has been described for murine TREG.23,35 Intriguingly, the transfer of BACH2-deficient TREG into recipient mice was shown to induce signs of autoimmunity.35 However, so far, it was unknown whether and how the disease-associated SNP rs56258221 influences the function of BACH2. Consequently, we analyzed mRNA and protein levels of BACH2 in CD4+ TN isolated from people with PSC carrying and not carrying the risk allele rs56258221 and found similar mRNA expression, but significantly lower BACH2 protein levels in SNP carriers.
In line with lowered BACH2 protein levels in CD4+ TN from carriers of rs56258221, we observed more accessible BACH2-binding motifs in chromatin sequencing data, which represents its lower activity as a transcriptional repressor.21,24 In addition to binding motifs of BACH2 itself, we detected more accessible motifs of PRDM1, RUNX3, and members of the AP-1 transcriptional complex (e.g., Jun and Fos), all of which are known targets of BACH221,24,26 and involved in TH17 cell formation and the development of T cell effector function.36,37 Overall, chromatin accessibility suggested a more activated phenotype of CD4+ TN in SNP carriers compared to non-carriers, associating lowered BACH2 protein levels to enhanced T cell activation and being in line with the observation of BACH2-deficient T cells spontaneously developing an activated phenotype.26,38 Combined, these findings demonstrated an association of risk variant rs56258221 with lower BACH2 protein levels and functional consequences in CD4+ T cells. However, and in contrast to these findings, we observed a more accessible BACH2 gene in SNP carriers compared to non-carriers. Hypothesizing on post-transcriptional regulation, we investigated the involvement of miR4464 and were able to link its increased expression and genetic accessibility to the reduced BACH2 protein levels in carriers of rs56258221. The MIR4464 locus is located within the cis-regulatory region upstream of BACH2, and miR4464 has previously been suggested to target the 3′ UTR of BACH2 mRNA using in silico analyses.25 Finally, we were able to confirm the imputed regulation of BACH2 translation through miR4464 by utilizing a luciferase-based plasmid system with mutated miR4464-binding sites.39
From a clinical perspective, our data suggest that rs56258221 may contribute to disease progression and, if validated in independent studies, potentially enables risk stratification of people with PSC in the future. Considering the elevated risk for hepatobiliary malignancies in people with PSC and the function of BACH2 in suppressing transcription factors of the AP-1 family, which are key molecules in tumorigenesis, our data should stimulate research into the effects of rs56258221 in cholangiocytes.
Our study provides evidence for the previously imputed function of miR4464 in regulating BACH2 protein expression. Moreover, we link the autoimmunity-associated polymorphism rs56258221 to dysregulation of CD4+ TN by shifting the balance of TH17 and TREG toward pro-inflammatory TH17 subsets. In addition, CD8+ T cells were found to be more activated in SNP carriers, but the mechanism was not linked to changes in BACH2 protein levels and remains to be elucidated. The data presented here should fuel more studies investigating the effects of disease-associated risk variants that, although mostly located in non-coding regions, may have an impact on cellular function and possibly on disease phenotype.
Limitations of the study
Limitations of our study include the fact that genetic polymorphisms occur in linkage disequilibrium, which complicates the annotation of a functional outcome to one specific polymorphism, especially when working with human material. Notably, rs56258221 is in high linkage disequilibrium with rs72928038, which is an intronic BACH2-SNP that has recently been linked to a reduced expression of BACH2 protein and a more activated T cell phenotype of CD8+ T cells in particular.40,41 Our findings on CD4+ T cells are complementary to this study and suggest that the examined SNPs do affect a wide range of different T cell subsets. In addition, we report on a potentially more severe phenotype of PSC in SNP carriers vs. non-carriers. However, given the lack of validated surrogate biomarkers in PSC42 and the variable disease course, the presented clinical data are based on a rather limited number of genotyped participants and should be interpreted with caution. To generate robust clinical data, controlled multi-centre studies are needed. Overall, rs56258221 represents only one of many polymorphisms contributing to the genetic risk in PSC. Moreover, as is true for most miRNAs, miR4464 has a variety of targets other than BACH2.25 Therefore, we cannot exclude transfected miR4464 to target other molecules in vitro.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Brilliant Violet 605™ anti-human CD103 (clone: Ber-ACT8) | BioLegend | Cat: 350218; RRID: AB_2564283 |
| PE/Dazzle™ anti-human CD11c (clone: 3.9) | BioLegend | Cat: 301641; RRID: AB_2564082 |
| PE/Cyanine7 anti-human CD123 (clone: 6H6) | BioLegend | Cat: 306010; RRID: AB_493576 |
| Brilliant Violet 650™ anti-human CD127 (clone: A019D5) | BioLegend | Cat: 351326; RRID: AB_2562095 |
| Brilliant Violet 711™ anti-human CD14 (clone: M5E2) | BioLegend | Cat: 301838; RRID: AB_2562909 |
| Brilliant Violet 605™ anti-human CD16 (clone: 9G8) | BioLegend | Cat: 302039; RRID: AB_2561354 |
| APC/Cyanine7 anti-human CD16 (clone: 3G8) | BioLegend | Cat: 302018; RRID: AB_314218 |
| PerCP/Cyanine5.5 anti-human CD160 (clone: BY55) | BioLegend | Cat: 341210; RRID: AB_2562874 |
| PE anti-human CD161 (clone: HP-3G10) | BioLegend | Cat: 339904; RRID: AB_1501083 |
| Brilliant Violet 605™ anti-human CD161 (clone: HP-3G10) | BioLegend | Cat: 339916; RRID: AB_2563607 |
| Brilliant Violet 711™ anti-human CD183 (clone: G025H7) | BioLegend | Cat: 353732; RRID: AB_2563533 |
| PE/Dazzle™ 594 anti-human CD19 (clone: HIB19) | BioLegend | Cat: 302252; RRID: AB_2563560 |
| PE/Cyanine7 anti-human CD194 (clone: L291H4) | BioLegend | Cat: 359410; RRID: AB_2562431 |
| PerCP/Cyanine5.5 anti-human CD196 (clone: G034E3) | BioLegend | Cat: 353406; RRID: AB_10918437 |
| Alexa Fluor® 647 anti-human CD197 (clone: G043H7) | BioLegend | Cat: 353218; RRID: AB_10917385 |
| Alexa Fluor® 647 anti-human CD199 (clone: L053E8) | BioLegend | Cat: 358911; RRID: AB_2562523 |
| APC/Cyanine7 anti-human CD1c (clone: L161) | BioLegend | Cat: 331520; RRID: AB_10644008 |
| Brilliant Violet 421™ anti-human CD20 (clone: 2H7) | BioLegend | Cat: 302330; RRID: AB_10965543 |
| Alexa Fluor® 700 anti-human CD20 (clone: 2H7) | BioLegend | Cat: 302322; RRID: AB_493753 |
| Alexa Fluor® 647 anti-human CD223 (clone: 11C3C65) | BioLegend | Cat: 369304; RRID: AB_2566480 |
| PerCP/Cyanine5.5 anti-human CD24 (clone: ML5) | BioLegend | Cat: 311116; RRID: AB_10960741 |
| Brilliant Violet 421™ anti-human CD25 (clone: BC96) | BioLegend | Cat: 302630; RRID: AB_11126749 |
| Alexa Fluor® 647 anti-human CD268 (clone: 11C1) | BioLegend | Cat: 316914; RRID: AB_2203680 |
| APC/Cyanine7 anti-human CD27 (clone: O323) | BioLegend | Cat: 302816; RRID: AB_571977 |
| Brilliant Violet 421™ anti-human CD272 (clone: MIH26) | BioLegend | Cat: 344511; RRID: AB_2566507 |
| Brilliant Violet 605™ anti-human CD279 (clone: EH12.2H7) | BioLegend | Cat: 329924; RRID: AB_2563212 |
| PE/Cyanine7 anti-human CD28 (clone: CD28.2) | BioLegend | Cat: 302926; RRID: AB_10644005 |
| PerCP/Cyanine5.5 anti-human CD3 (clone: OKT3) | BioLegend | Cat: 317336; RRID: AB_2561628 |
| Brilliant Violet 650™ anti-human CD3 (clone: OKT3) | BioLegend | Cat: 317324; RRID: AB_2563352 |
| Alexa Fluor® 488 anti-human CD38 (clone: HIT2) | BioLegend | Cat: 303512; RRID: AB_493088 |
| PE/Cyanine7 anti-human CD39 (clone: A1) | BioLegend | Cat: 328212; RRID: AB_2099950 |
| Alexa Fluor® 700 anti-human CD4 (clone: OKT4) | BioLegend | Cat: 317426; RRID: AB_571943 |
| PE/Dazzle™ 594 anti-human CD4 (clone: RPA-T4) | BioLegend | Cat: 300548; RRID: AB_2563566 |
| PE/Cyanine7 anti-human CD43 (clone: CD43-10G7) | BioLegend | Cat: 343208; RRID: AB_2563698 |
| PE anti-human CD44 (clone: 338808) | BioLegend | Cat: 338808; RRID: AB_2076578 |
| Brilliant Violet 785™ anti-human CD45 (clone: HI30) | BioLegend | Cat: 304048; RRID: AB_2563129 |
| Brilliant Violet 711™ anti-human CD45RA (clone: HI100) | BioLegend | Cat: 304138; RRID: AB_2563815 |
| PE/Cyanine7 anti-human CD49a (clone: TS2/7) | BioLegend | Cat: 328312; RRID: AB_2566272 |
| FITC anti-human CD49b (clone: P1E6-C5) | BioLegend | Cat: 359306; RRID: AB_2562531 |
| PE anti-human CD49d (clone: 9F10) | BioLegend | Cat: 304303; RRID: AB_314429 |
| Brilliant Violet 421™ anti-human CD56 (clone: HCD56) | BioLegend | Cat: 318328; RRID: AB_11218798 |
| PE/Dazzle™ 594 anti-human CD57 (clone: HNK-1) | BioLegend | Cat: 359619; RRID: AB_2564062 |
| Brilliant Violet 510™ anti-human CD62L (clone: DREG-56) | BioLegend | Cat: 304843; RRID: AB_2617002 |
| PerCP/Cyanine5.5 anti-human CD69 (clone: FN50) | BioLegend | Cat: 310926; RRID: AB_2074956 |
| APC/Cyanine7 anti-human CD69 (clone: FN50) | BioLegend | Cat: 310914; RRID: AB_314849 |
| PE anti-human CD73 (clone: AD2) | BioLegend | Cat: 344004; RRID: AB_2298698 |
| Brilliant Violet 510™ anti-human CD8a (clone: RPA-T8) | BioLegend | Cat: 301048; RRID: AB_2561942 |
| PE/Dazzle™ 594 anti-human CD8a (clone: HIT8a) | BioLegend | Cat: 300930; RRID: AB_2629639 |
| Alexa Fluor® 700 anti-human CD8a (clone: RPA-T8) | BioLegend | Cat: 301028; RRID: AB_493745 |
| PE anti-human CD8b (clone: 2ST8.5H7) | BD Biosciences | Cat: 641057; RRID: AB_1645747 |
| Alexa Fluor® 647 anti-human FOXP3 (clone: 259D) | BioLegend | Cat: 320214; RRID: AB_492984 |
| FITC anti-human Granzyme B (clone: GB11) | BioLegend | Cat: 515403; RRID: AB_2114575 |
| FITC anti-human HLA-DR (clone: L243) | BioLegend | Cat: 307604; RRID: AB_314682 |
| APC/Cyanine7 anti-human IFN-γ (clone: 4S.B3) | BioLegend | Cat: 502530; RRID: AB_10663412 |
| PE anti-human IL-10 (clone: JES3-9D7) | BioLegend | Cat: 501404; RRID: AB_315170 |
| Brilliant Violet 711™ anti-human IL-17A (clone: BL168) | BioLegend | Cat: 512328; RRID: AB_2563888 |
| PE/Cyanine7 anti-human IL-4 (clone: MP4-25D2) | BioLegend | Cat: 500824; RRID: AB_2126746 |
| FITC anti-human/mouse integrin β7 (clone: FIB27) | BioLegend | Cat: 121010; RRID: AB_2129310 |
| PE anti-human IgD (clone: IA6-2) | BioLegend | Cat: 348203; RRID: AB_10550096 |
| PE-Cy™7 anti-auman TCRγ/δ (clone: 11F2) | BD Biosciences | Cat: 655410; RRID: AB_2870377 |
| Brilliant Violet 650™ anti-human TNF-α (clone: MAb11) | BioLegend | Cat: 502938; RRID: AB_2562741 |
| APC anti-human TCR Vα7.2 (clone: 3C10) | BioLegend | Cat: 351708; RRID: AB_10933246 |
| FITC anti-human TCR delta (clone: TS-1) | Invitrogen | Cat: TCR2055; RRID: AB_223619 |
| APC anti-human TCR Vd2 (clone: 123R3) | Miltenyi Biotec | Cat: 130-095-803; RRID: AB_10831200 |
| FITC anti-human TCR-Vg9 (clone: IMMU 360) | BeckmanCoulter | Cat: IM1463; RRID: AB_130871 |
| CITEseq: TotalSeq™-C0063 anti-human CD45RA (clone: HI100) | BioLegend | Cat: 304163; RRID: AB_2800764 |
| CITEseq: TotalSeq™-C0087 anti-human CD45RO (clone: UCHL1) | BioLegend | Cat: 304259; RRID: AB_2800766 |
| CITEseq: TotalSeq™-C0147 anti-human CD62L (clone: DREG-56) | BioLegend | Cat: 304851; RRID: AB_2800770 |
| CITEseq: TotalSeq™-C0148 anti-human CD197 (clone: G043H7) | BioLegend | Cat: 353251; RRID: AB_2800943 |
| CITEseq: TotalSeq™-C0124 anti-human CD31 (clone: WM59) | BioLegend | Cat: 303139; RRID: AB_2800757 |
| CITEseq: TotalSeq™-C0154 anti-human CD27 (clone: O323) | BioLegend | Cat: 302853; RRID: AB_2800747 |
| CITEseq: TotalSeq™-C0386 anti-human CD28 (clone: CD28.2) | BioLegend | Cat: 302963; RRID: AB_2800751 |
| CITEseq: TotalSeq™-C0156 anti-human CD95 (clone: DX2) | BioLegend | Cat: 305651; RRID: AB_2800787 |
| CITEseq: TotalSeq™-C0246 anti-human CD122 (clone: TU27) | BioLegend | Cat: 339021; RRID: AB_2814240 |
| CITEseq: TotalSeq™-C0140 anti-human CD183 (clone: G025H7) | BioLegend | Cat: 353747; RRID: AB_2800949 |
| CITEseq: TotalSeq™-C0185 anti-human CD11a (clone: TS2/4) | BioLegend | Cat: 350617; RRID: AB_2800935 |
| CITEseq: TotalSeq™-C0576 anti-human CD49d (clone: 9F10) | BioLegend | Cat: 304345; RRID: AB_2814137 |
| CITEseq: TotalSeq™-C0390 anti-human CD127 (clone: A019D5) | BioLegend | Cat: 351356; RRID: AB_2800937 |
| CITEseq: TotalSeq™-C0085 anti-human CD25 (clone: BC96) | BioLegend | Cat: 302649; RRID: AB_2800745 |
| CITEseq: TotalSeq™-C0159 anti-human HLA-DR (clone: L243) | BioLegend | Cat: 307663; RRID: AB_2800795 |
| CITEseq: TotalSeq™-C0901 anti-human GARP (clone: 7B11) | BioLegend | Cat: 352517; RRID: AB_2819994 |
| CITEseq: TotalSeq™-C0176 anti-human CD39 (clone: A1) | BioLegend | Cat: 328237; RRID: AB_2800853 |
| CITEseq: TotalSeq™-C0577 anti-human CD73 (clone: AD2) | BioLegend | Cat: 344031; RRID: AB_2800916 |
| CITEseq: TotalSeq™-C0151 anti-human CD152 (clone: BNI3) | BioLegend | Cat: 369621; RRID: AB_2801015 |
| CITEseq: TotalSeq™-C0360 anti-human CD357 (clone: 108-17) | BioLegend | Cat: 371227; RRID: AB_2810583 |
| CITEseq: TotalSeq™-C0171 anti-human CD278 (clone: C398.4A) | BioLegend | Cat: 313553; RRID: AB_2800823 |
| CITEseq: TotalSeq™-C0071 anti-human CD194 (clone: L291H4) | BioLegend | Cat: 359425; RRID: AB_2800988 |
| CITEseq: TotalSeq™-C0143 anti-human CD196 (clone: G034E3) | BioLegend | Cat: 353440; RRID: AB_2810563 |
| CITEseq: TotalSeq™-C0141 anti-human CD195 (clone: J418F1) | BioLegend | Cat: 359137; RRID: AB_2810570 |
| CITEseq: TotalSeq™-C0366 anti-human CD184 (clone: 12G5) | BioLegend | Cat: 306533; RRID: AB_2800791 |
| CITEseq: TotalSeq™-C0144 anti-human CD185 (clone: J252D4) | BioLegend | Cat: 356939; RRID: AB_2800968 |
| CITEseq: TotalSeq™-C0125 anti-human CD44 (clone: BJ18) | BioLegend | Cat: 338827; RRID: AB_2800900 |
| CITEseq: TotalSeq™-C0146 anti-human CD69 (clone: FN50) | BioLegend | Cat: 310951; RRID: AB_2800810 |
| CITEseq: TotalSeq™-C0149 anti-human CD161 (clone: HP-3G10) | BioLegend | Cat: 339947; RRID: AB_2810532 |
| CITEseq: TotalSeq™-C0581 anti-human TCR Vα7.2 (clone: 3C10) | BioLegend | Cat: 351735; RRID: AB_2810556 |
| CITEseq: TotalSeq™-C0088 anti-human CD279 (clone: EH12.2H7) | BioLegend | Cat: 329963; RRID: AB_2800862 |
| CITEseq: TotalSeq™-C0378 anti-mouse CD223 (clone: C9B7W) | BioLegend | Cat: 125237; RRID: AB_2832450 |
| CITEseq: TotalSeq™-C0250 anti-mouse/human KLRG1 (clone: 2F1/KLRG1) | BioLegend | Cat: 138433; RRID: AB_2800649 |
| CITEseq: TotalSeq™-C0168 anti-human CD57 (clone: QA17A04) | BioLegend | Cat: 393321; RRID: AB_2801030 |
| CITEseq: TotalSeq™-C0169 anti-human CD366 (clone: F38-2E2) | BioLegend | Cat: 345049; RRID: AB_2800925 |
| CITEseq: TotalSeq™-C0089 anti-human TIGIT (clone: A15153G) | BioLegend | Cat: 372729; RRID: AB_2801021 |
| CITEseq: TotalSeq™-C0139 anti-human TCR γ/δ (clone: B1) | BioLegend | Cat: 331231; RRID: AB_2814199 |
| CITEseq: TotalSeq™-C0584 anti-human TCR Vα24-Jα18 (clone: 6B11) | BioLegend | Cat: 342925; RRID: AB_2810539 |
| Western Blot Antibody: BACH2 | Cell Signaling Technology | Cat: 80775; RRID: AB_2799961 |
| Western Blot Antibody: beta-Actin (C4) | Cell Signaling Technology | Cat: SC-47778; RRID: AB_626632 |
| anti-human CD3 antibody; clone: OKT3 | BioLegend | Cat: 317302; RRID: AB_571927 |
| anti-huamn CD28 antibody; clone: CD28.1 | BioLegend | Cat: 302934; RRID: AB_2616667 |
| anti-human IL-4 antibody | Miltenyi Biotech | Cat: 130-095-753; RRID: AB_10831210 |
| anti-human IFNγ antibody | Miltenyi Biotech | Cat: 130-095-743; RRID: AB_10830868 |
| anti-human IL-12 antibody | PeproTech | Cat: 500-P154G; RRID: AB_2929517 |
| Biological samples | ||
| Human peripheral blood (german cohort) | University Medical Center Hamburg-Eppendorf (I. Dept. of Medicine) (Hamburg, Germany) | N/A |
| Liver tissue for bulk RNA seq | University Medical Center Hamburg-Eppendorf (I. Dept. of Medicine) (Hamburg, Germany) | N/A |
| Human peripheral blood (norwegian cohort) | Norwegian PSC Center (Oslo, Norway) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| IL-2 (cell culture) | R&D Systems | Cat: 202-IL-010 |
| ATRA (cell culture) | Sigma-Aldrich | Cat: R2625 |
| TGFβ (cell culture) | Miltenyi Biotech | Cat: 130-095-066 |
| IL-6 (research grade) | Miltenyi Biotech | Cat: 130-093-929 |
| IL-1β (research grade) | Miltenyi Biotech | Cat: 130-095-374 |
| IL-23 (research grade) | Miltenyi Biotech | Cat: 130-095-758 |
| IL-7 | Miltenyi Biotech | Cat: 130-095-362 |
| RPMI 1640 medium | Gibco | Cat: 11875093 |
| Opti-MEM medium | Gibco | Cat: 31985070 |
| Fetal calve serum (FCS/FBS) | PAN Biotech | Cat: P30-3033 |
| 1% Penicillin-Streptomycin | Sigma Aldrich | Cat: P4333 |
| Fixable Viability Dye eFluor 506 | ThermoFisher | Cat: 65-0866-14 |
| Bovine serum albumine (BSA) | Carl Roth | Cat: 0163.4 |
| Phosphate buffered saline (PBS) | Gibco | Cat: 10010023 |
| Phorbol 12-myristate 13-acetate (PMA) | Sigma Aldrich | Cat: P1585 |
| Ionomycin | Sigma Aldrich | Cat: I24222 |
| Brefeldin A | BD Bioscience | Cat: 55029 |
| Critical commercial assays | ||
| TaqMan SNP Genotyping assay (rs56258221) | ThermoFisher | Cat: C__88670967_10 |
| TaqMan SNP Genotyping assay (rs80060485) | ThermoFisher | Cat: C_103476844_10 |
| TaqMan SNP Genotyping assay (rs4147359) | ThermoFisher | Cat: C___1841422_10 |
| TaqMan SNP Genotyping assay (rs7426056) | ThermoFisher | Cat: C__29052378_10 |
| CellTrace Violet Cell Proliferation Kit | ThermoFisher | Cat: C34557 |
| DNA Blood Midi Kit | Qiagen | Cat: 51104 |
| Ficoll-Paque PLUS density gradient media | Cytiva | Cat: 17144003 |
| MACS: Naive CD4+ T cell Isolation Kit II, human | MIltenyi Biotec | Cat: 130-094-131 |
| Foxp3/Transcription Factor Staining Buffer Set | eBioscience/Invitrogen | Cat: 00-5523-00 |
| NucleoSpin RNA Kit | Macherey-Nagel | Cat: 740955.250 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat: 4368813 |
| KAPA PROBE FAST 1PCR Kit | KAPA Biosystems | Cat: KK4715 |
| HPRT housekeeper | ThermoFisher | Cat: Hs02800695_m1 |
| Chromium Next GEM Single Cell 5′ Reagent Kit v2 | 10x Genomics | Cat: 1000265 |
| Chromium Next GEM Chip K Single Cell Kit | 10x Genomics | Cat: 1000287 |
| Dynabeads MyOne SILAN | ThermoFisher | Cat: 37002D |
| TaqMan MicroRNA Cells-to-CT Kit | ThermoFisher | Cat: 4391848 |
| TaqMan MicroRNA Assay for miR4464 | ThermoFisher | Cat: 463103_mat |
| TaqMan MicroRNA Control Assay (RNU48) | ThermoFisher | Cat: 001006 |
| Amaxa Cell Line Nucleofactor Kit V | Lonza Bioscience | Cat: VCA-1003 |
| Transit-X2-Dynamic delivery system | Mirusbio | Cat: MIR 6004 |
| Dual-Luciferase Reporter Assay System | Promega | Cat: E1910 |
| Deposited data | ||
| CITE-sequencing data | This paper | ArrayExpress: E-MTAB-14013 |
| Bulk RNAseq data | This paper | ArrayExpress: E-MTAB-14103 |
| Experimental models: Cell lines | ||
| HEK293T cells | ATCC | Cat: CRL-3216; RRID: CVCL_0063 |
| Oligonucleotides | ||
| miR4464 (for transfection experiment) | ThermoFisher | ID: MC22259 |
| non-binding (mock) miR | ThermoFisher | ID: 4464058 |
| Recombinant DNA | ||
| BACH2-miR4464 tester plasmids | This paper | Table S4 |
| pmiR-GLO dual luciferase reporter vector | Promega | Cat: E1330 |
| Software and algorithms | ||
| FlowJo v10 | https://www.flowjo.com | RRID: SCR_008520 |
| Graphpad Prism 9.3 | https://www.graphpad.com | RRID: SCR_002798 |
| R 3.6.2 | The R Foundation https://www.r-project.org/ |
RRID: SCR_001905 |
| Seurat 3.1 | https://satijalab.org/seurat/ | RRID: SCR_07322 |
| Cellranger 3.0.2 | 10x Genomics https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
RRID: SCR_017344 |
| tidyverse 2.0.0 | https://www.tidyverse.org/ | RRID: SCR_019186 |
| Other | ||
| ViiA 7 Real-Time PCR System | Applied Biosystems | Cat: 4453536 |
| LSRFortessa | BD Biosciences | RRID: SCR_018655 |
| BD FACS Aria III | BD Biosciences | RRID: SCR_016695 |
| Cytek Aurora | Cytek | Part Number: N7-00003 |
| 2100 Bioanalyzer Instrument | Agilent Technologies | RRID: SCR_018043 |
| QuBit 3.0 Fluorometer | ThermoFisher | RRID: SCR_020311 |
| NovaSeq6000 | Illumina | RRID: SCR_016387 |
| Nucleofector 2b Device | Lonza Bioscience | RRID: SCR_022262 |
| BioTek Synergy H1 microplate luminometer | Agilent | Cat: H1MG |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Christoph Schramm (c.schramm@uke.de).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
-
•
(Section 1: Data) Processed single-cell-sequencing data have been deposited at the ArrayExpress database (European Bioinformatics Institute) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Raw sequencing data reported in this study cannot be deposited in a public repository due to data privacy concerns. Original western blot images and de-identified bulk RNAseq data will be shared by the lead contact upon request.
-
•
(Section 2: Code) This paper does not report original code.
-
•
(Section 3: Statement) Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participants details
People with PSC and clinical data
We conducted a cross-sectional study in adult people (>18 years of age) with PSC either at the University Medical Centre Hamburg-Eppendorf (Hamburg, Germany) (n = 210) or Oslo University Hospital Rikshospitalet (Oslo, Norway) (n = 138). Fresh blood samples from people with PSC were collected via the YAEL outpatient service of the I. Department of Medicine. Gender and age matched healthy blood donors (HD) were used as controls. Liver tissue for bulk RNAseq was obtained from people with PSC who had undergone biopsy at the I. Department of Medicine (University Medical Center Hamburg-Eppendorf). The biopsies were taken during mini-laparoscopy by using TruCut needles. Immediatly after material extraction the tissue was preserved in liquid nitrogen.
All study participants provided written informed consent according to the ethical guidelines of the Institutional Review Board of the medical faculty of the University of Hamburg (PV4081).
Method details
SNP genotyping
Genomic DNA was isolated from peripheral venous blood or serum by using the Qiagen DNA Blood Midi Kit (Qiagen, NL) according to the manufacturer’s instructions. TaqMan 5′-nuclease assays were performed as 10 μL-reactions on 96-well plates using TaqMan Genotyping Mastermix and predesigned TaqMan probes. Each run consisted of a 10 min hold cycle at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Cycling and detection of reporter signals (VIC/FAM) were performed using a ViiA 7 Real-Time PCR System (Applied Biosystems, USA).
Isolation of PBMCs and CD4+ TN cells
Blood samples were collected in EDTA tubes and stored overnight at 4°C. Peripheral Blood Mononuclear Cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Paque PLUS, Cytiva, UK). Subsequently, CD4+ TN cells were isolated from PBMCs using the Naive CD4+ T cell Isolation Kit II, human (Miltenyi Biotec, GER) and purity was determined by flow cytometry (CD4+ CD197+ CD45RA+ cells). Only samples with a purity of ≥90% TN of total CD4+ were used for further experiments.
Immunophenotyping
Heparinized whole blood of people with PSC, PBC, IBD, NAFLD or healthy donors was stained with 8 different antibody cocktails for 30 min at room temperature. Afterward, RBC Lysis/Fixation solution (Biolegend, USA) was added and after 10 min cells were subsequently washed two times with PBS. Stained cells were suspended in PBS containing 2% FCS and 0.01% NaN3. Samples were measured using a BD LSRFortessa (BD Biosciences, USA) or Cytek Aurora (Cytek) Following analyses was performed using FlowJo v10 (BD Biosciences, USA).
In vitro iTREG polarization assay
CD4+ TN cells were cultured in the presence of coated anti-CD3 (2 μg/mL, OKT3, Biolegend, USA), anti-CD28 (1 μg/mL, CD28.2, Biolegend, USA), IL-2 (100 U/ml, R&D Systems, USA), all-trans retinoic acid (ATRA, 10 nM, Sigma Aldrich, GER) and TGF-β1 (7 ng/mL, Miltenyi Biotec, GER) in RPMI1640 medium (Gibco) containing 10% fetal calf serum (FCS) (PAN Biotech, GER) and 1% Penicillin-Streptomycin (Sigma Aldrich, GER). On day 3, medium and cytokines were substituted. Cells were analyzed on day 6 by flow cytometry. Staining was performed by using the eBioscience Foxp3/Transcription Factor Staining Buffer Set (eBioscience, USA). TREG were defined as CD4+ CD25+ CD127lo CD152+ Foxp3+ cells.
In vitro TH1 polarization assay
CD4+ TN cells were cultured in the presence of coated anti-CD3 (2 μg/mL, OKT3, Biolegend, USA), anti-CD28 (1 μg/mL, CD28.2, Biolegend, USA), anti-IL-4 (2.5 μg/mL, Miltenyi Biotec, GER), IL-2 (100 U/ml, R&D Systems, USA) and IL-12 (375 U/ml, Miltenyi Biotec, GER) in RPMI1640 medium (Gibco, USA) containing 10% fetal calf serum (FCS) (PAN Biotech, GER) and 1% Penicillin-Streptomycin (Sigma Aldrich, GER). On day 3, medium and cytokines were substituted. Cells were analyzed on day 6 by flow cytometry. Before staining, cells were restimulated with Phorbol 12-myristate 13-acetate (50 ng/ml) (Sigma Aldrich), Ionomycin (1μg/ml) (Sigma Aldrich) and Brefelding A (1x) (BD Bioscience) for 4 h at 37°. Intracellular staining was performed by using the eBioscience Foxp3/Transcription Factor Staining Buffer Set (eBioscience, USA). TH1 cells were defined as CD4+ TNFα+ IFNγ+ cells.
In vitro TH17 polarization assay
CD4+ TN cells were cultured in the presence of coated anti-CD3 (2 μg/mL, OKT3, Biolegend, USA), anti-CD28 (1 μg/mL, CD28.2, Biolegend, USA), anti-IL-4 (2.5 μg/mL), anti-IFNγ (1 μg/mL, both Miltenyi Biotec, GER), anti-IL-12 (1 μg/mL, PeproTech, GER), IL-6, IL-1b and IL-23 (all 30 ng/mL and all Miltenyi Biotec, GER) in RPMI1640 medium (Gibco, USA) containing 10% fetal calf serum (FCS) (PAN Biotech, GER) and 1% Penicillin-Streptomycin (Sigma Aldrich, GER). On days 5 and 8, medium and cytokines were substituted. Cells were analyzed on day 12 by flow cytometry. TH17 cells were defined as CD4+ IL-17A+ cells.
In vitro proliferation assay
Isolated CD4+ TN cells were stained with the CellTrace Violet Cell Proliferation Kit (ThermoFisher Scientific, USA) according to the manufacturer’s protocol and cultured in the presence of coated anti-CD3 (2 μg/mL, OKT3, Biolegend, USA), anti-CD28 (1 μg/mL, CD28.2, Biolegend, USA) and IL-7 (500 U/ml, Miltenyi Biotec, GER) in RPMI1640 medium (Gibco, USA) containing 10% fetal calf serum (FCS) (PAN Biotech, GER) and 1% Penicillin-Streptomycin (Sigma Aldrich, GER). Cells were analyzed on day 6 by flow cytometry.
Real-time qPCR
RNA was extracted using the NucleoSpin RNA Kit (Macherey-Nagel, GER) according to the manufacturer’s protocol. 0.7μg of extracted RNA was transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). qRT-PCR was performed using KAPA PROBE FAST 1PCR Kit and KAPA PROBE FAST ROX low (KAPA Biosystems, UK). Expression of target genes was normalized to the expression of hypoxanthin-guanine phosphoribosyltransferase (HPRT) (02800695_m1, ThermoFisher Scientific, USA) as a housekeeper. Expression data was examined using the 2−ΔCt method.
FACS sorting and antibody staining for sequencing
Peripheral blood CD3+ T cells were isolated and FACS-sorted from previously cryopreserved peripheral blood mononuclear cells (PBMCs). Previously frozen cells were thawed and diluted with 50 mL of RPMI containing 10% FCS (PAN Biotech, GER) and 1% P/S (Sigma Aldrich, GER). After centrifugation, cells were washed with PBS and then stained with FITC-conjugated anti-CD3 antibody at a 1:200 dilution in combination with live/dead staining using Fixable Viability Dye eFluor 506 (Amcyan) (ThermoFisher Scientific, USA) at a dilution of 1:2000. At the same time, cells were stained with oligo-conjugated antibodies for CITE-Seq using TotalSeq A antibodies, (BioLegend, USA). Staining of cells was performed for 30 min on ice and after washing the cells, subsequent FACS-sorting and utilization of the Chromium Single-Cell platform (10x Genomics, USA) was performed.
Preparation of scRNA-Seq and CITE-Seq libraries
The scRNA-Seq library was prepared using the Chromium Next GEM Single Cell 5′ Reagent Kit v2, according to the manufacturer’s instructions (10x Genomics, USA). FACS-sorted cells were washed once with PBS containing 0.04% bovine serum albumin (BSA) and resuspended in PBS. 20,000 cells were used for GEM generation through the 10x Chromium Controller using the Chromium Next GEM Chip K Single Cell Kit (10x Genomics, USA). Briefly, droplet preparation was followed by reverse transcription and cell barcoding, the emulsions were resolved and cDNA was purified using Dynabeads MyOne SILANE (ThermoFisher Scientific, USA) followed by a PCR amplification with additional use of a primer for amplification of antibody derived tags (ADTs). Amplified cDNA was then used for construction of 5′ gene expression library, V(D)J-library and ADT library using the dual index strategy following the manufacturer’s instructions. Quality control and quantification of the generated libraries was conducted using a 2100 Bioanalyzer Instrument (Agilent Technologies, USA) and a QuBit 3.0 Fluorometer (ThermoFisher Scientific, USA), respectively.
Sequencing
The libraries were sequenced on an Illumina NovaSeq6000 to a minimum sequencing depth of 20,000 reads per cell for gene expression library, as well as a minimum sequencing depth of 5,000 reads for TCR- and ADT-libraries using read lengths of 100 bp read 1 (26 cycles), 8bp i5 index (10 cycles), 8 bp i7 index (10 cycles), 100 bp read 2 (90 cycles).
scRNA-Seq: data processing
For the scRNA-Seq data, after demultiplexing, reads were aligned against the GRCh38 human reference genome (release 93) and summarized using the Cellranger pipeline (version 3.0.2, 10x Genomics, USA). Further analysis steps were performed in R (version 3.6.2; The R Foundation for Statistical Computing, AUT).
scRNA-Seq: quality control and normalization
For each sample, genes not observed in at least 1% of all cells were dropped. Low quality or damaged cells were excluded using a combination of multiple sample dependent quality measures: minimum UMI count (mean 1234, range 800–2000), minimum and maximum number of expressed genes (mean 756, range 450–1000, and mean 2625, range 2500–3000 respectively), and mitochondrial transcript percentage (mean 6%, range 5–7.5%). Additionally, we filtered out doublet cell candidates using Scrublet (41), adjusting its estimated doublet threshold as needed.
For normalization we used Seurat’s SCTransform (40) function. SCTransform combines the usual scRNA workflow of data normalization, identification of highly variable genes (HVG) and data scaling. Briefly, for each gene a regularized negative binomial regression is performed and the resulting pearson residuals (regression residuals divided by expected standard deviation) represent a variance-stabilizing transformation of the expression data that can be used as normalization for downstream analysis. HVGs are selected based on the highest variance in the pearson residuals.
We opted not to correct for possible cell cycle influence during this process since visual inspection of the first two principal components on cell cycle genes (cc.genes included in Seurat) did not show an obvious bias toward these genes. These steps resulted in overall 8 (SNP-carrier and non-carrier, n = 4 each) samples and 56209 cells (SNP-carrier: 31014; non-carrier: 25195).
scRNA-Seq: ADT normalization
For each sample where ADT expression data was available, we applied feature-wise centered log ratio transformation implemented in Seurat’s NormalizeData function.
scRNA-Seq: Dimension reduction, clustering and differential expression analysis
The batched corrected counts for all integration anchors were used as input for PCA. For UMAP embedding and graph-based shared nearest neighbor clustering we used up to 20 principle components. We iteratively increased the clustering resolution parameter until no additional clusters with biological meaningful cluster markers were detected. Cluster marker detection was performed by differential gene expression analysis for each cluster against all remaining cells using logistic regression on the sample-wise normalized RNA matrices. We included the donor variable as covariate in the regression model and tested for significant differential expression against a null model with likelihood ratio test.
Cluster labels were assigned using a combination of detected marker genes, ADT expression and UMAP overlay of the cell populations identified by manual gating of the ADT expression.
We identified 16 clusters, the biggest clusters expectedly comprising naive CD4+ T cells (C0: CD4+ TN, C1: CD4+ TN mature), identified by high expression of CCR7, SELL, TCF7 and FOXP1. Central memory CD4+ T cells (C2: CD4+ TCM) were identified by ICOS, MAL, SELL, IL7R mRNA and CCR7 protein expression. A variety of different T Helper (TH) cell subtypes (C10: CD4+ TH1: CXCR3, PRDM1, CD69, STAT1; C7: CD4+ TH2: GATA3, CD69, CCR4, TNFRSF4; C11: CD4+ TH17: KLRB1, CCR6, RORA, PRDM1) as well as two clusters of regulatory T cells (C12: CD4+ TREG naive: FOXP3, FOXP1, IL2RA, SELL; C14: CD4+ TREG effector: FOXP3, LGALS3, CTLA4, HLA-DRB1) (Figures S1H–S1I). Naive CD8+ T cells (C3: CD8+ TN) were identified by high expression of CCR7, SELL, PECAM1, TCF7. Effector memory CD8+ T cells (C4: CD8+ TEM) were identified by the expression of KLRD1, NKG7, PRF1 and terminally differentiated CD8+ T cells (C5: CD8+ TC term. diff.) by the additional expression of CX3CR1 and ZEB2. Type-1 cytotoxic CD8+ T cells (C6: CD8+ TC1) were identified by the expression of CXCR3, KLRG1, EOMES and NK-like CD8+ T cells (C8: CD8+ TC NK-like) by GNLY, GZMB, FCGR3A. In addition, natural killer T cells (C9: NKT) were identified by similar markers as C8, but expressing CD4 instead of CD8. Other unconventional T cell subsets, as gamma delta T cells (C13: γδ T cells) were identified by the high expression of TRDV2, TRGV9, NKG7 and PRF1, whereas mucosa-associated invariant T cells (MAIT) were identified by the high expression of KLRB1, CCR6, RORC and ZBTB16.
Western Blot
To determine protein levels of BACH2 in FACS-sorted TN cells, we washed cells and resuspended the pellet in glycerol-based lysis buffer including protease inhibitors. After sonification, we determined the protein content and denaturated samples by addition of the respective volume of 5x Laemmli buffer and subsequent heating to 95°C for 5 min. For gel electrophoresis, we used 12% SDS-gels and used 10 μg total protein. For protein transfer on nitrocellulose membranes, we used the wet blotting system. After blocking steps with 5% non-fat dried milk in TBST, we used antibodies against BACH2 (Cell Signaling Technology, USA) and beta-Actin (Cell Signaling Technology, USA).
Detection of miRNAs
To detect miRNAs, we used the Cells-to-CT Kit (ThermoFisher Scientific, USA) on 105 or less FACS-sorted TN cells per reaction, according to the manufacturer’s recommendations and using Single-Primer reactions. To detect the miRNAs of interest, we used TaqMan-based probes against miR4464 (ThermoFisher Scientific, USA) and normalized its expression to ubiquitously expressed RNU48 (ThermoFisher Scientific, USA).
Transfection of CD4+ TN with miR4464
For transfection of CD4+ TN from HD and people with PSC, we used freshly isolated CD4+ TN from peripheral blood, as described above. We seeded 3x105 cells per well in 96-well plates, previously coated with 2 μg/mL anti-CD3 antibodies (clone OKT3, Biolegend, USA), and incubated the cells under serum-free conditions for 4 days in Opti-MEM (Gibco, USA) containing 1 μg/mL anti-CD28 antibodies (clone CD28.1, Biolegend, USA). For transfection, we harvested the cells, counted them and used 2x106 or less cells per transfection, using the Amaxa Cell Line Nucleofector Kit V (Lonza Bioscience, SUI) in combination with the Nucleofector 2b Device (Lonza Bioscience, SUI), according to the manufacturer’s protocol. For each transfection we used miR4464 or mock control at 1 nM. After transfection, we seeded the cells again into 96-well plates coated with 2 μg/mL anti-CD3 antibodies (clone OKT3, Biolegend, USA) in RPMI1640 medium (Gibco,USA) containing 10% fetal calf serum (PAN Biotech, GER), 1% Penicillin-Streptomycin (Sigma Aldrich, GER) and 100 U/ml IL-2 (R&D Systems, USA). After 24 h, we changed to medium to iTREG polarizing conditions and performed further culture and analyses as described above.
RNA sequencing and analyses
RNA quality was assessed using Bioanalyzer, and samples with and RNA integrity number >7 were included for RNA sequencing. Up to 1ug RNA was used to synthesize mRNA libraries using TruSeq stranded mRNA library Preparation Kit (Illumina) on a Hiseq 4000 system (Illumina).
We used FastQC (version 0.11.5, Babraham Institute, United Kingdom) for a general quality check of the raw fastq files. TruSeq2-PE adapter and low quality read trimming was performed with Trimmomatic43 (version 0.36) using the options ILLUMINACLIP:TruSeq2-PE.fa:2:30:10:2:falseSLIDINGWINDOW:4:15. Subsequently, the reads were aligned against the ensemble 87 reference genome and ensemble 87 reference annotation with STAR (version 2.7.3a).44 On average read depth for the 47 samples was 40.310.540 (range: 8.318.152–61.856.384) and on average 92.76% (range: 79.27%–95.88%) of the reads were uniquely mapped to the reference genome. TPM were estimated using RSEM45 (v 1.3.0).
Further analysis was performed with R (version 1.2.1335, The R Foundation for Statistical Computing, Austria). To filter the data, a threshold of ≥10 counts in all samples was set. We used DESeq2 for normalization and differential gene expression analysis.46 Data was inspected for possible confounding effects using PCA based on regularized logarithm transformed counts.
BACH2 tester plasmid
In order to generate the wild type plasmid, a fragment of the BACH2-3′UTR containing the predicted miR4464 binding site (Fr1) was created by PCR using primers Pr1 and Pr2 (Table S7). The predicted miR4464 binding site was obtained from the miRDB database (25). The fragment carrying the mutated miR4464 site in the BACH2-3′UTR sequence (Fr2) was created by two PCR reactions: the first performed with Pr1 and Pr4 and the second performed with the product of the first PCR as forward primer and Pr2 (Table S7). The PCR products were then cloned into the pmirGLO dual luciferase reporter vector (Promega, USA) downstream the firefly luciferase gene.
Dual-Glo luciferase assay
HEK293T cells were transfected with 40ng of wildtype or mutated plasmid together with 61,6nM of miR4464 (ThermoFisher Scientific, USA) or control miR (ThermoFisher Scientific, USA) using Mirus transfection reagent (Transit-X2-Dynamic delivery system). After 48 h, cells were harvested for measuring luciferase activity as well as protein content. Luciferase activity was measured in a microplate luminometer (Synergy H1 BioTek) using the Dual-Luciferase Reporter Assay System (Promega, USA) following the manufacturer’s protocol. The indicated values represent firefly luciferase activities normalized to the Renilla luciferase activities.
Quantification and statistical analysis
Statistical analysis
Statistical analysis was performed using GraphPad Prism software Version 9.3 (GraphPad Software, USA). Differences between two groups were analyzed by unpaired t-Test with Welch’s correction (normal distribution) or by Mann-Whitney test (no normal distribution). Differences between 3 groups or more were evaluated using the ordinary one-way ANOVA (normal distribution) or Kruskal-Wallis test (no normal distribution). p-Values below 0.05 were considered statistically significant. Data is presented as median ± interquartile range, unless otherwise stated. Categorial data (e.g., LTX-frequency) between two groups were analyzed using contingency tables with Fisher’s exact test.
Acknowledgments
We want to thank all participants for taking part in our study and consenting to donate extra blood samples for research use. We thank Sabrina Kreß, Jennifer Wigger, Angela Schmidt, Marko Hilken, and Martina Fahl for excellent technical assistance.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; CRU306 290522633 and 426581255; LFF-FV78), the Helmut and Hannelore Greve Foundation, the YAEL Foundation (C. Schramm), and the Open Access Publication Fund of the University Medical Centre Hamburg-Eppendorf, Germany.
Author contributions
T.P. and J.B. conceptualized the study, performed the experiments, analyzed the data, and wrote the manuscript. J.K., I.E., H.G., L.K.K., L.A.L., N.I., A.-M.S., and A.L. performed the experiments and analyzed the data. C.C. performed analyses of sequencing data. S.S. and M.S. provided blood samples of the Hamburg PSC cohort. T.F., L.K.E., and T.H.K. provided blood samples and clinical data of the Oslo PSC cohort. A.F. provided the sequencing platform. C. Schlein, N.H., E.G., S.H., A.W.L., and N.G. advised the study and reviewed the manuscript. D.S. and C. Schramm conceptualized and supervised the study and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: June 19, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101620.
Supplemental information
References
- 1.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J. Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis K.N., LaRusso N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu C., Boucheron N., Müller A.C., Májek P., Claudel T., Halilbasic E., Baazim H., Lercher A., Viczenczova C., Hainberger D., et al. 24-Norursodeoxycholic acid reshapes immunometabolism in CD8(+) T cells and alleviates hepatic inflammation. J. Hepatol. 2021;75:1164–1176. doi: 10.1016/j.jhep.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liaskou E., Jeffery L.E., Trivedi P.J., Reynolds G.M., Suresh S., Bruns T., Adams D.H., Sansom D.M., Hirschfield G.M. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147:221–232.e7. doi: 10.1053/j.gastro.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer C.L., von Seth E., Buggert M., Strauss O., Hertwig L., Nguyen S., Wong A.Y.W., Zotter C., Berglin L., Michaëlsson J., et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb3107. [DOI] [PubMed] [Google Scholar]
- 6.Kunzmann L.K., Schoknecht T., Poch T., Henze L., Stein S., Kriz M., Grewe I., Preti M., Hartl J., Pannicke N., et al. Monocytes as Potential Mediators of Pathogen-Induced T-Helper 17 Differentiation in Patients With Primary Sclerosing Cholangitis (PSC) Hepatology. 2020;72:1310–1326. doi: 10.1002/hep.31140. [DOI] [PubMed] [Google Scholar]
- 7.Sebode M., Peiseler M., Franke B., Schwinge D., Schoknecht T., Wortmann F., Quaas A., Petersen B.S., Ellinghaus E., Baron U., et al. Reduced FOXP3(+) regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J. Hepatol. 2014;60:1010–1016. doi: 10.1016/j.jhep.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Poch T., Krause J., Casar C., Liwinski T., Glau L., Kaufmann M., Ahrenstorf A.E., Hess L.U., Ziegler A.E., Martrus G., et al. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4(+) T cells in primary sclerosing cholangitis. J. Hepatol. 2021;75:414–423. doi: 10.1016/j.jhep.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katt J., Schwinge D., Schoknecht T., Quaas A., Sobottka I., Burandt E., Becker C., Neurath M.F., Lohse A.W., Herkel J., Schramm C. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58:1084–1093. doi: 10.1002/hep.26447. [DOI] [PubMed] [Google Scholar]
- 10.Kummen M., Thingholm L.B., Ruhlemann M.C., Holm K., Hansen S.H., Moitinho-Silva L., Liwinski T., Zenouzi R., Storm-Larsen C., Midttun O., et al. Altered Gut Microbial Metabolism of Essential Nutrients in Primary Sclerosing Cholangitis. Gastroenterology. 2021;160:1784–1798.e1780. doi: 10.1053/j.gastro.2020.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liwinski T., Zenouzi R., John C., Ehlken H., Rühlemann M.C., Bang C., Groth S., Lieb W., Kantowski M., Andersen N., et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69:665–672. doi: 10.1136/gutjnl-2019-318416. [DOI] [PubMed] [Google Scholar]
- 12.Karlsen T.H., Franke A., Melum E., Kaser A., Hov J.R., Balschun T., Lie B.A., Bergquist A., Schramm C., Weismüller T.J., et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Melum E., Franke A., Schramm C., Weismüller T.J., Gotthardt D.N., Offner F.A., Juran B.D., Laerdahl J.K., Labi V., Björnsson E., et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat. Genet. 2011;43:17–19. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folseraas T., Melum E., Rausch P., Juran B.D., Ellinghaus E., Shiryaev A., Laerdahl J.K., Ellinghaus D., Schramm C., Weismüller T.J., et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 2012;57:366–375. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellinghaus D., Folseraas T., Holm K., Ellinghaus E., Melum E., Balschun T., Laerdahl J.K., Shiryaev A., Gotthardt D.N., Weismüller T.J., et al. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology. 2013;58:1074–1083. doi: 10.1002/hep.25977. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.Z., Hov J.R., Folseraas T., Ellinghaus E., Rushbrook S.M., Doncheva N.T., Andreassen O.A., Weersma R.K., Weismüller T.J., Eksteen B., et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat. Genet. 2013;45:670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellinghaus D., Jostins L., Spain S.L., Cortes A., Bethune J., Han B., Park Y.R., Raychaudhuri S., Pouget J.G., Hübenthal M., et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 2016;48:510–518. doi: 10.1038/ng.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liaskou E., Jeffery L., Chanouzas D., Soskic B., Seldin M.F., Harper L., Sansom D., Hirschfield G.M. Genetic variation at the CD28 locus and its impact on expansion of pro-inflammatory CD28 negative T cells in healthy individuals. Sci. Rep. 2017;7:7652. doi: 10.1038/s41598-017-07967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muto A., Hoshino H., Madisen L., Yanai N., Obinata M., Karasuyama H., Hayashi N., Nakauchi H., Yamamoto M., Groudine M., Igarashi K. Identification of Bach2 as a B-cell-specific partner for small maf proteins that negatively regulate the immunoglobulin heavy chain gene 3' enhancer. EMBO J. 1998;17:5734–5743. doi: 10.1093/emboj/17.19.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labarta-Bajo L., Zúñiga E.I. BAtCHing stem-like T cells during exhaustion. Nat. Immunol. 2021;22:274–276. doi: 10.1038/s41590-021-00891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roychoudhuri R., Clever D., Li P., Wakabayashi Y., Quinn K.M., Klebanoff C.A., Ji Y., Sukumar M., Eil R.L., Yu Z., et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat. Immunol. 2016;17:851–860. doi: 10.1038/ni.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roychoudhuri R., Hirahara K., Mousavi K., Clever D., Klebanoff C.A., Bonelli M., Sciumè G., Zare H., Vahedi G., Dema B., et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidwell T., Liao Y., Garnham A.L., Vasanthakumar A., Gloury R., Blume J., Teh P.P., Chisanga D., Thelemann C., de Labastida Rivera F., et al. Attenuation of TCR-induced transcription by Bach2 controls regulatory T cell differentiation and homeostasis. Nat. Commun. 2020;11:252. doi: 10.1038/s41467-019-14112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao C., Lou G., Sun H.W., Zhu Z., Sun Y., Chen Z., Chauss D., Moseman E.A., Cheng J., D'Antonio M.A., et al. BACH2 enforces the transcriptional and epigenetic programs of stem-like CD8(+) T cells. Nat. Immunol. 2021;22:370–380. doi: 10.1038/s41590-021-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukumo S.i., Unno M., Muto A., Takeuchi A., Kometani K., Kurosaki T., Igarashi K., Saito T. Bach2 maintains T cells in a naive state by suppressing effector memory-related genes. Proc. Natl. Acad. Sci. USA. 2013;110:10735–10740. doi: 10.1073/pnas.1306691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards C.L., de Oca M.M., de Labastida Rivera F., Kumar R., Ng S.S., Wang Y., Amante F.H., Kometani K., Kurosaki T., Sidwell T., et al. The Role of BACH2 in T Cells in Experimental Malaria Caused by Plasmodium chabaudi chabaudi AS. Front. Immunol. 2018;9:2578. doi: 10.3389/fimmu.2018.02578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timperi E., Barnaba V. CD39 Regulation and Functions in T Cells. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22158068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crome S.Q., Wang A.Y., Levings M.K. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010;159:109–119. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street K., Risso D., Fletcher R.B., Das D., Ngai J., Yosef N., Purdom E., Dudoit S. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 2018;19:477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux de Bezieux H., Van den Berge K., Street K., Dudoit S. Trajectory inference across multiple conditions with condiments. Nat. Commun. 2024;15:833. doi: 10.1038/s41467-024-44823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentsen M., Goymann P., Schultheis H., Klee K., Petrova A., Wiegandt R., Fust A., Preussner J., Kuenne C., Braun T., et al. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 2020;11:4267. doi: 10.1038/s41467-020-18035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji S.G., Juran B.D., Mucha S., Folseraas T., Jostins L., Melum E., Kumasaka N., Atkinson E.J., Schlicht E.M., Liu J.Z., et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 2017;49:269–273. doi: 10.1038/ng.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoknecht T., Schwinge D., Stein S., Weiler-Normann C., Sebode M., Mucha S., Otto B., Ellinghaus E., Stahl F., Franke A., et al. CD4+ T cells from patients with primary sclerosing cholangitis exhibit reduced apoptosis and down-regulation of proapoptotic Bim in peripheral blood. J. Leukoc. Biol. 2017;101:589–597. doi: 10.1189/jlb.5A1015-469R. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Dai D., Hu Q., Yang F., Xue Y., Li F., Shen N., Zhang M., Huang C. Bach2 attenuates IL-2R signaling to control Treg homeostasis and Tfr development. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109096. [DOI] [PubMed] [Google Scholar]
- 36.Rutishauser R.L., Martins G.A., Kalachikov S., Chandele A., Parish I.A., Meffre E., Jacob J., Calame K., Kaech S.M. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain R., Chen Y., Kanno Y., Joyce-Shaikh B., Vahedi G., Hirahara K., Blumenschein W.M., Sukumar S., Haines C.J., Sadekova S., et al. Interleukin-23-Induced Transcription Factor Blimp-1 Promotes Pathogenicity of T Helper 17 Cells. Immunity. 2016;44:131–142. doi: 10.1016/j.immuni.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richer M.J., Lang M.L., Butler N.S. T Cell Fates Zipped Up: How the Bach2 Basic Leucine Zipper Transcriptional Repressor Directs T Cell Differentiation and Function. J. Immunol. 2016;197:1009–1015. doi: 10.4049/jimmunol.1600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simerzin A., Zorde-Khvalevsky E., Rivkin M., Adar R., Zucman-Rossi J., Couchy G., Roskams T., Govaere O., Oren M., Giladi H., Galun E. The liver-specific microRNA-122∗, the complementary strand of microRNA-122, acts as a tumor suppressor by modulating the p53/mouse double minute 2 homolog circuitry. Hepatology. 2016;64:1623–1636. doi: 10.1002/hep.28679. [DOI] [PubMed] [Google Scholar]
- 40.Mouri K., Guo M.H., de Boer C.G., Lissner M.M., Harten I.A., Newby G.A., DeBerg H.A., Platt W.F., Gentili M., Liu D.R., et al. Prioritization of autoimmune disease-associated genetic variants that perturb regulatory element activity in T cells. Nat. Genet. 2022;54:603–612. doi: 10.1038/s41588-022-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asimit J.L., Rainbow D.B., Fortune M.D., Grinberg N.F., Wicker L.S., Wallace C. Stochastic search and joint fine-mapping increases accuracy and identifies previously unreported associations in immune-mediated diseases. Nat. Commun. 2019;10:3216. doi: 10.1038/s41467-019-11271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponsioen C.Y., Lindor K.D., Mehta R., Dimick-Santos L. Design and Endpoints for Clinical Trials in Primary Sclerosing Cholangitis. Hepatology. 2018;68:1174–1188. doi: 10.1002/hep.29882. [DOI] [PubMed] [Google Scholar]
- 43.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
(Section 1: Data) Processed single-cell-sequencing data have been deposited at the ArrayExpress database (European Bioinformatics Institute) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Raw sequencing data reported in this study cannot be deposited in a public repository due to data privacy concerns. Original western blot images and de-identified bulk RNAseq data will be shared by the lead contact upon request.
-
•
(Section 2: Code) This paper does not report original code.
-
•
(Section 3: Statement) Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.