Abstract
Treatment of human immunodeficiency virus type 1 (HIV-1)-infected individuals with highly active antiretroviral therapy has effectively decreased viral load to undetectable levels. However, efforts to eliminate HIV-1 from these individuals have been unsuccessful, due to the presence of stable, latent viral reservoirs in resting and active CD4+ T lymphocytes and macrophages. These latent populations have become critical targets in the effort to eradicate HIV-1 from infected individuals. The mechanisms of HIV-1 latency have been studied by using the HIV-1-infected promonocytic cell line U1. The interferon-inducible double-stranded RNA-dependent p68 protein kinase (PKR), a key enzyme in the host-mediated antiviral response, is known to be down-regulated during HIV-1 infection. Therefore, in order to evaluate the role of PKR in the inhibition of replication of reactivated HIV-1 in latently infected U1 cells, we have utilized cDNA constructs containing PKR under the transcriptional control of the HIV-1 long terminal repeat. One PKR-transduced clone, U1/106-4:27, inhibited the tumor necrosis factor alpha (TNF-α)-induced replication of HIV-1 by 99% compared to control U1 cells as measured by syncytium formation and HIV-1 p24 antigen enzyme-linked immunosorbent assay. Western blot analysis showed an increase in PKR expression through 96 h postinduction in the U1/106-4:27 clone, concomitant with maximal increases in phosphorylation of the α subunit of eukaryotic initiation factor 2 and NF-κB activity at 72 h postinduction. These results demonstrate that overexpression of PKR can inhibit the replication of reactivated HIV-1 in latently infected cells and confirm the involvement of PKR in the interferon-associated antiviral pathway against HIV-1 infection. Additionally, treatment of the PKR-transduced U1/106-4:27 clone with the protease inhibitor saquinavir (250 nM) completely inhibited TNF-α-induced HIV-1 replication.
Human immunodeficiency virus type 1 (HIV-1) infection includes an asymptomatic period of clinical latency, which intervenes between infection and the development of AIDS (40). The understanding of the role of viral latency in HIV-1 infection has been markedly advanced by measurement of viral burden in HIV-1-infected individuals receiving highly active antiretroviral therapy (HAART). Although HIV-1-infected individuals receiving HAART can achieve undetectable viral loads (<200 copies of HIV-1 RNA per ml of serum), HIV-1 infection is not likely to be eradicated by this approach due to the presence of a stable copy number in resting and activated CD4+ T cells (14, 22, 24, 59). Further, HIV-1-infected individuals who interrupted drug treatment because of intolerance or noncompliance showed a rapid increase in viral load to pretherapy levels (26). Therefore, innovative strategies are necessary to inhibit the replication of reactivated HIV-1 in latently infected cells. Advances in this area have been made by studying latently infected cell models in which constitutive HIV-1 expression is minimal but can be induced physiologically with cytokines (8).
The approach to inhibiting HIV-1 replication utilized in this laboratory has been to employ the interferon (IFN)-associated antiviral defense pathway. IFNs are produced and secreted by cells in response to various inducers such as double-stranded RNA (dsRNA) or viral infection. IFNs have the capacity to induce a series of gene products through signal transduction pathways which can interfere with viral infection and other events such as proliferation and differentiation (53). IFNs are already clinically used against hepatitis B and C viruses, HIV-1, and papillomavirus (28). A key enzyme in the host-mediated antiviral response is the IFN-inducible, dsRNA-dependent protein kinase PKR, a serine/threonine kinase (42). The binding of two molecules of PKR to one molecule of dsRNA initiates an autophosphorylation event which is followed by phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF-2α), preventing a GDP-for-GTP recycling reaction and leading to inhibition of protein synthesis initiation (38, 50, 56). In vitro studies have also suggested that PKR activates NF-κB by phosphorylation of I-κB, the NF-κB inhibitor (35, 36, 61). However, the direct phosphorylation of I-κB by PKR or the interaction of these two proteins has not been demonstrated in vivo. Activated NF-κB migrates to the nucleus and activates the transcription of many genes implicated in the antiviral and antiproliferative effects of IFN (55). The activation of PKR in infected cells has been shown to result in the death of these cells, the prevention of virus replication, and the subsequent infection of neighboring cells (34, 54). However, many viruses, including HIV-1, have developed strategies to down-regulate PKR levels and/or activity following infection (6, 33, 34, 41). PKR is upregulated immediately following HIV-1 infection by HIV-1 TAR RNA, which acts as a dsRNA activator (6, 32, 47, 48). However, in later stages of HIV-1 infection, PKR is inhibited due to the binding of HIV-1 TAR RNA to HIV-1 Tat protein. The antiviral pathways are thus down-regulated, and infectious HIV-1 particles appear in the supernatant. In humans, this leads to the progression to AIDS (19).
The purpose of the current study was to investigate the role of PKR expression and activity during HIV-1 reactivation by placing PKR cDNA under the transcriptional control of the HIV-1 long terminal repeat (LTR), followed by transduction through a retrovirus-mediated delivery system into the HIV-1 latently infected model promonocytic cell line U1. To accomplish this goal, we have employed intracellular immunization, i.e., the regulated expression of a molecular species designed to interfere with and prevent HIV-1 replication (4). In order to be effective, the introduced genes must (i) be stably expressed in sufficient quantities to inhibit viral replication, (ii) be nontoxic to the target cells, and (iii) be efficiently transferred to the target cells (60). The intracellular immunization approach used in the current study selectively expresses PKR following HIV-1 reactivation. We examined the inhibition of tumor necrosis factor alpha (TNF-α)-induced replication of reactivated HIV-1 in PKR-transduced U1 clones. PKR expression and enzyme activity post-TNF-α induction were also examined.
MATERIALS AND METHODS
Cell culture.
SupT1 (T lymphoblastoid) and U1 (promonocytic) cells were obtained from the National Institutes of Health AIDS Research Reference and Reagent Program and were grown at 37°C and 5% CO2 in RPMI 1640 medium (GIBCO) containing 2 mM glutamine and supplemented with 10% heat-inactivated donor calf serum and 100 U of penicillin-streptomycin (Biofluids, Inc.) per ml. The amphotropic retroviral packaging cell line GP+envAm12 (a generous gift from A. Bank) was grown at 37°C and 5% CO2 in Dulbecco modified Eagle medium (GIBCO) containing 2 mM glutamine and supplemented with 10% heat-inactivated donor calf serum and 100 U of penicillin-streptomycin per ml.
Retrovirus-mediated transduction of U1 cells.
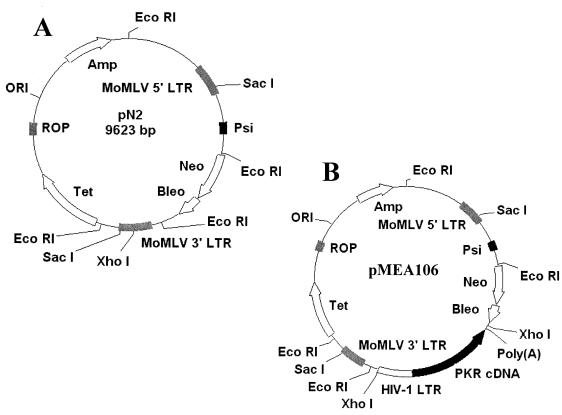
The cloning procedures were performed as previously described (1, 2). Briefly, the plasmid pSP72 (Promega) was digested with XhoI and PstI and a triple ligation was performed to create the plasmid which contains the HIV-1 LTR controlling the expression of wild-type PKR cDNA (1,826-nucleotide HindIII/PstI fragment). The HIV-1 LTR-PKR cDNA fragment (2,813 bp) was directly subcloned into the pN2 vector (Fig. 1A) in the reverse orientation to ensure only HIV-1 LTR-driven transcription of the antiviral PKR cDNA (pMEA106, Fig. 1B). The plasmids (pN2 and pMEA106) were each transfected into GP+envAm12 cells by standard procedures for calcium phosphate coprecipitation (49). Stable transfectants were selected by culturing the cells in the presence of 1 mg of G418 (GIBCO) per ml. Individual clones (producer cell lines N2-20 [backbone vector] and 106-4 [HIV-1 LTR-PKR, reverse orientation]) were isolated by minitrypsinization in cloning wells (Bellco Glass Company) and expanded for further characterization. Supernatants were collected from the retroviral producer cell lines N2-20 and 106-4 and incubated with U1 cells for 2 h. Homogeneous cell populations containing the integrated retroviral vector were obtained by serial dilution of U1 cells followed by selection in the presence of 1 mg of G418 per ml. Integration and copy number of the proviral construct were confirmed by Southern blot analysis with a 923-bp neomycin gene-specific radiolabeled probe (data not shown).
FIG. 1.
Plasmid constructions. Restriction maps of pN2 (A) and pMEA106 (B) plasmids are depicted. The pMEA106 plasmid was obtained by inserting the HIV-1 LTR-PKR-poly(A) cDNA fragment (2,813 bp), in the reverse orientation, into the XhoI site of the pN2 backbone vector. pN2 and pMEA106 were then individually transfected into the GP+envAm12 packaging cell line. The obtained homogeneous retroviral producer cell clones with the highest titers, i.e. N2-20 and 106-4, were used to transduce U1 cells. MoMLV, Moloney murine leukemia virus. ORI, origin; ROP, replication origin.
HIV-1 induction of retrovirally transduced, G418-selected U1 cells.
U1 cells and transduced cells (2 × 105 cells/ml in 10 ml) were induced with 50 ng of TNF-α (Promega) per ml at 37°C and 5% CO2. Cells were collected at 0, 24, 48, 72, and 96 h postinduction (p.i.) for cytoplasmic and nuclear protein extraction.
HIV-1-induced syncytium analysis.
Transduced and control U1 cells (2 × 105 cells/200 μl) were treated with 50 ng of TNF-α per ml and incubated at 37°C and 5% CO2 in a 96-well plate. In the study involving saquinavir (SQV; Roche), 2 × 105 cells were pretreated with SQV at final concentrations of 0.25, 2.5, 25, 250, and 2,500 nM 1 h prior to induction of HIV-1 replication with TNF-α (final volume, 300 μl). Forty-eight hours p.i., the cells were serially diluted through 1:27; 2 × 105 SupT1 indicator cells were then added to each well. Syncytia were scored 96 h p.i. A single syncytium score from triplicate assays was calculated by correcting for each dilution factor and averaging the three values.
Western blot analysis.
Protein preparations were obtained by NP-40 extraction (20 mM HEPES [pH 7.5], 5 mM MgCl2, 120 mM KCl, 10% glycerol, 0.5% NP-40) of transduced cells and U1 cell controls at 0, 24, 48, 72, and 96 h p.i. One hundred micrograms of protein extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blots were then probed with either a 1:200 dilution of our rabbit polyclonal anti-PKR antibody followed by a 1:1,000 dilution of horseradish peroxidase (HP)-conjugated mouse anti-rabbit immunoglobulin G (IgG) (Pierce) or a 1:1,000 dilution of human sera obtained from an HIV-1-infected patient (generously provided by B. Suh) followed by a 1:1,000 dilution of HP-conjugated goat anti-human IgG (Pierce). Blots were developed by the ECL system (Amersham), followed by autoradiography. Densitometric analyses were done by using the NIH Image program, version 1.6. The same protocol was used with 50 μg of nuclear protein extract for the NF-κB expression (52). The blots were then probed with a 1:1,000 dilution of either rabbit polyclonal anti-p50 antibody or rabbit polyclonal anti-p65 antibody (Rockland) followed by a 1:1,000 dilution of HP-conjugated mouse anti-rabbit IgG (Pierce).
Determination of eIF-2α phosphorylation.
Protein preparations of transduced cells and U1 cell controls were obtained by CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) extraction (9.5 M urea, 5% CHAPS, 1% Pharmalyte [pH 4.5 to 5.4], 1% Pharmalyte [pH 5 to 6], 50 mM NaF, 25 mM dithiothreitol) at 0, 24, 48, 72, and 96 h p.i. (51). Fifty micrograms of protein extract was analyzed by vertical slab isoelectric focusing (VSIEF) gel electrophoresis (39). After transfer, Western blot analysis was performed as described above, with a 1:10,000 dilution of mouse monoclonal anti-eIF-2α antibody (a generous gift from J.-J. Chen), followed by a 1:5,000 dilution of HP-conjugated goat anti-mouse IgG (Pierce). The size of eIF-2α was verified by migration alongside authentic purified eIF-2α (generous gifts from W. C. Merrick and S. R. Kimball).
Determination of NF-κB activity.
Protein preparations of transduced cells and U1 cell controls were obtained by nuclear extraction at 0, 24, 48, 72, and 96 h p.i. (52). Five micrograms of nuclear extract was incubated with a 32P-labeled complementary, synthetic oligonucleotide probe corresponding to the NF-κB binding site (sense probe, 5′-ACAAGGGACTTTCCGCTGGGGACTTTCCAGGGA-3′) and analyzed by gel electrophoretic mobility shift assay (GEMSA) (3). Gels were dried and analyzed by autoradiography. Densitometric analysis was performed as described above. Specificity of NF-κB for its DNA oligonucleotide sequence was demonstrated by (i) competition with a 40-fold excess of unlabeled, annealed κB probe and (ii) supershift analysis with rabbit polyclonal antibodies against p65, p50, and I-κB (Rockland), added 30 min prior to incubation with the radiolabeled oligonucleotide probe.
RESULTS
During HIV-1 infection, it has been shown that PKR expression and enzyme activity are down-regulated (47, 48). This down-regulation occurs primarily through the binding of HIV-1 Tat protein to the viral dsRNA element, HIV-1 TAR RNA, thus inhibiting PKR activation. In addition, HIV-1 Tat has been demonstrated to down-regulate PKR activity by direct interaction with this kinase (7, 41).
Retroviral transduction of U1 cells with HIV-1 LTR-PKR cDNA constructs.
To understand the impact of overexpression of PKR on the replication of reactivated HIV-1 in latently infected cells, we describe experiments in which the latently infected promonocytic cell line U1 was stably transduced with an HIV-1 LTR-driven PKR cDNA construct (106-4) via a retrovirus-mediated delivery system (Fig. 1). Nontransduced U1 cells and N2-20 (backbone vector)-transduced clones provided controls for PKR expression. Thirteen N2-20 and 27 106-4-transduced U1 clones were isolated. The clones were verified to contain the correct integrated cDNA by Southern blot analyses (data not shown). Cell numbers and viability of all clones were normal compared to those of the U1 control cell line (data not shown). The clones were characterized by their ability to inhibit TNF-α-induced replication of reactivated HIV-1 as follows.
TNF-α-induced replication of reactivated HIV-1 is inhibited in HIV-1 LTR-PKR cDNA-transduced U1 cells.
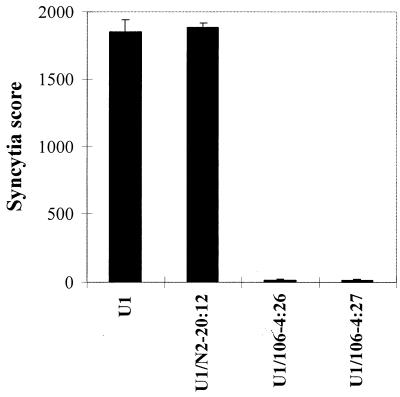
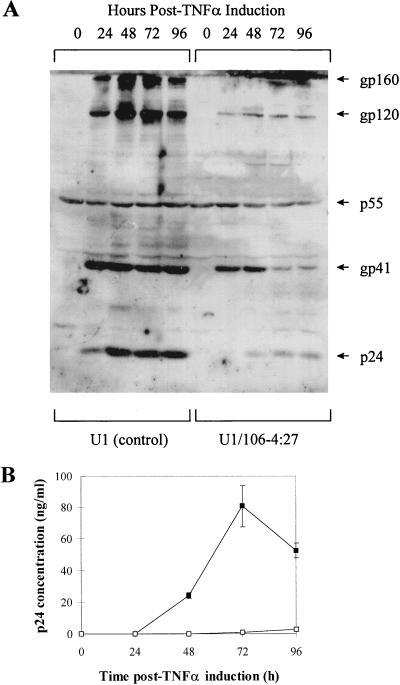
U1 control cells and the PKR-transduced U1 clones were characterized by formation of HIV-1-induced syncytia (Fig. 2), Western blot assay for HIV-1 proteins (Fig. 3A), and HIV-1 p24 antigen capture enzyme-linked immunosorbent assay (ELISA) (Fig. 3B). Of the 27 clones containing the HIV-1 LTR-PKR cDNA, two clones (U1/106-4:26 and U1/106-4:27) inhibited syncytium formation by 99% compared to U1 control cells (Fig. 2). The syncytial score for the U1/N2-20:12 clone was essentially the same as that for the U1 control cells. Based on repeated syncytium assays, the U1/106-4:27 clone was selected as a representative clone for further comparison with the U1 control. Western blot analysis of U1 control cells with sera from an HIV-1-infected individual demonstrated a large induction of HIV-1 p24, gp41, gp120, and gp160 protein expression at 24, 48, 72, and 96 h p.i., compared to uninduced U1 control cells (time zero) (Fig. 3A). However, in the U1/106-4:27 clone, HIV-1 protein expression was dramatically inhibited at 24, 48, 72, and 96 h p.i. compared to that in nontransduced U1 control cells. Finally, quantitative analysis of the inhibition of HIV-1 p24 antigen expression in cell supernatants of the U1/106-4:27 clone by ELISAs revealed a 99% inhibition at 72 h p.i., compared to U1 control cells (Fig. 3B). HIV-1 p24 antigen expression was strongly inhibited up to 7 days p.i. (data not shown). Therefore, we have demonstrated that transduction of an HIV-1 LTR-PKR retroviral cDNA construct into the genome of U1 cells results in the inhibition of the TNF-α-induced replication of reactivated HIV-1.
FIG. 2.
HIV-1-induced syncytium formation in U1 and PKR-transduced U1 cells in response to TNF-α treatment. U1 control cells and the U1/N2-20:12 (backbone vector control), U1/106-4:26, and U1/106-4:27 clones were treated with TNF-α (50 ng/ml), and syncytia were scored in triplicate at multiple dilutions at 96 h p.i. A single syncytium score was calculated as described in Materials and Methods.
FIG. 3.
Expression of HIV-1 proteins in U1 and PKR-transduced U1 cells in response to TNF-α treatment. (A) Protein extracts were prepared at 0, 24, 48, 72, and 96 h p.i., and equivalent amounts (100 μg) were analyzed by Western blotting with human sera obtained from an HIV-1-infected individual. HIV-1 protein sizes were determined by comparison with Rainbow molecular weight markers (Amersham) (B) Cell supernatants were tested for HIV-1 p24 antigen levels by using the HIV-1 p24 antigen capture ELISA kit (SAIC Frederick) (■, U1 control; □, U1/106-4:27 clone). Vertical bars represent the standard deviations obtained with three independent experiments.
PKR expression increases in the PKR-transduced clone following TNF-α treatment.
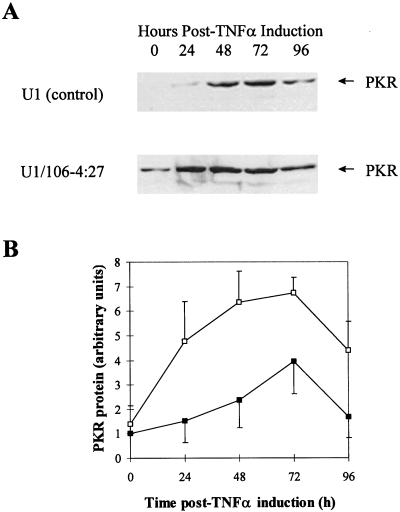
To test whether the inhibition of HIV-1 in the PKR-transduced U1 clone coincided with increased PKR expression, Western blot assays were performed at 0, 24, 48, 72, and 96 h p.i. with a polyclonal anti-PKR antibody. PKR expression in U1 control cells increased 1.5-, 2.4-, and 4-fold at 24, 48, and 72 h p.i., respectively, compared to that at time zero (Fig. 4). Simultaneously, PKR expression in the U1/106-4:27 clone was increased 4.8-, 6.4-, and 6.7-fold at 24, 48, and 72 h p.i., respectively. Thus, the increases in PKR expression in the U1/106-4:27 clone coincide with the inhibition of TNF-α-induced HIV-1 replication shown in Fig. 2 and 3. The data demonstrate that the PKR antiviral pathway is augmented in the U1/106-4:27 clone. At 96 h p.i., the expression of PKR in the U1/106-4:27 clone decreased, which may be attributed to the absence of HIV-1 infectious particles and subsequent return of normal cellular function (Fig. 4). In some experiments, endogenous PKR expression was observed at time zero in controls and clones. This increased PKR expression can be explained by the low basal expression of HIV-1 in the U1 cell line. We have further demonstrated the involvement of PKR in the IFN-associated antiviral pathway in HIV-1 infection; PKR activity is inhibited in U1/106-4:27 cells when the PKR inhibitor 2-aminopurine (10, 57) is added prior to induction of HIV-1 replication with TNF-α (data not shown).
FIG. 4.
PKR expression in U1 and PKR-transduced U1 cells in response to TNF-α treatment. (A) Protein extracts were prepared at 0, 24, 48, 72, and 96 h p.i., and equivalent amounts (100 μg) were analyzed by Western blotting with a polyclonal anti-PKR antibody. PKR was calculated to be 68 kDa by migration alongside bovine serum albumin (66 kDa) contained within the molecular mass markers. (B) Protein levels of PKR were determined by densitometric analysis of Western blots of three independent experiments (■, U1 control; □, U1/106-4:27 clone). Vertical bars represent the standard deviations obtained with three independent experiments.
eIF-2α phosphorylation increases in the PKR-transduced clone following TNF-α treatment.
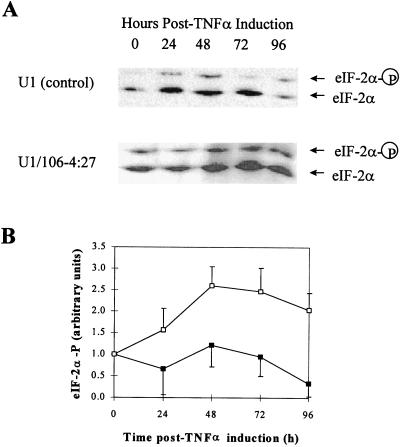
To investigate the mechanism of the anti-HIV-1 effect of overexpression of PKR, we measured PKR activity in the cytoplasm after TNF-α treatment. The phosphorylation state of eIF-2α in nontransduced U1 controls and the U1/106-4:27 clone was determined by VSIEF gel electrophoresis. At 48 and 72 h p.i., levels of phosphorylated eIF-2α in the U1/106-4:27 clone were increased 2.6- and 2.5-fold, respectively, over that at time zero (Fig. 5). A decline in eIF-2α phosphorylation to 2.0-fold was observed for the U1/106-4:27 clone at 96 h p.i. These results indicate that the increased PKR expression found in the TNF-α-treated PKR-transduced U1 clone occurs concomitantly with increased PKR enzyme activity. Thus, the enhancement of the PKR antiviral pathway in TNF-α-treated U1 cells transduced with HIV-1 LTR-PKR cDNA is responsible for the inhibition of the replication of reactivated HIV-1.
FIG. 5.
Phosphorylation of eIF-2α in U1 and PKR-transduced U1 cells in response to TNF-α treatment. (A) Protein extracts were prepared at 0, 24, 48, 72, and 96 h p.i., and equivalent amounts (50 μg) were analyzed by VSIEF gel electrophoresis, followed by Western blot analysis with a monoclonal anti-eIF-2α antibody. A representative gel is shown. (B) Densitometric analyses of gels from three independent experiments are presented. One arbitrary unit corresponds to the amount of eIF-2α in the U1 control (■) and U1/106-4:27 clone (□) at time zero, set at 100%. Vertical bars represent the standard deviations obtained with three independent experiments.
NF-κB activity increases in the PKR-transduced U1 clone following TNF-α treatment.
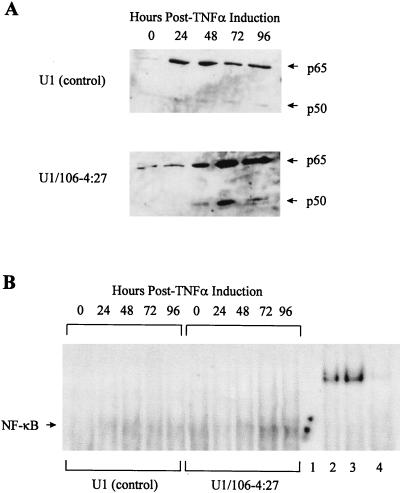
It is known that, in addition to eIF-2α, there are other substrates of PKR. One of these targets for PKR kinase activity, at least in vitro, is the inhibitor of the NF-κB family of transcription factors, I-κB (16, 35). As such, PKR has been shown to be an important factor in the complex regulation of NF-κB (10, 36, 37, 44). Interestingly, NF-κB is also involved in the positive regulation of HIV-1 expression, through interaction with the two κB sites present in the HIV-1 LTR (43). To further investigate the effect of overexpression of PKR on NF-κB activity in the HIV-1 LTR-PKR-transduced U1 cells, the following two assays were used. Western blot analysis of nuclear protein extracts with the prototypic NF-κB monomers p65 and p50 was used to determine levels of the individual monomers present in the nucleus. GEMSAs were used to determine the ability of the classic dimeric form of NF-κB (p65-p50) to bind to its cognate DNA sequence. Western blot analysis demonstrated 20- and 10-fold increases in nuclear p65 and p50 expression, respectively, in U1 control cells at 24 h p.i. and gradual decreases through 96 h p.i., compared to time zero (Fig. 6A). However, in the U1/106-4:27 clone, nuclear p65 and p50 expression increased 62- and 38-fold, respectively, at 72 h, which was maintained through 96 h p.i.
FIG. 6.
Regulation of NF-κB expression and activity in U1 and PKR-transduced U1 cells in response to TNF-α treatment. (A) Nuclear expression of NF-κB p65 and p50 monomers: nuclear protein extracts were prepared at 0, 24, 48, 72, and 96 h p.i., and equivalent amounts (50 μg) were analyzed by Western blotting with polyclonal anti-p65 and anti-p50 antibodies. Protein sizes were determined by comparison to the molecular weight markers. (B) Induction of NF-κB–DNA binding activity: 5 μg of nuclear protein extracts from U1 control cells and the U1/106-4:27 clone treated with TNF-α (50 ng/ml) was analyzed for NF-κB activity by binding to a 5′-end-labeled HIV-1 κB double-stranded DNA oligonucleotide (see Materials and Methods). Verification of the specificity of NF-κB binding was done by antibody supershift assays. Nuclear protein extracts from the TNF-α-treated U1/106-4:27 clone at 72 h p.i. (5 μg) were incubated with a 40-fold excess of unlabeled HIV-1 κB double-stranded DNA oligonucleotide (lane 1), polyclonal anti-p65 (lane 2), anti-p50 (lane 3), or anti-I-κB (lane 4) antibodies.
GEMSA analysis of DNA binding activity in U1 cell controls demonstrated a 4.9-fold increase at 48 h p.i. compared to uninduced U1 cell controls, with a gradual decrease through 96 h p.i. In the U1/106-4:27 clone, there was an 8.4-fold increase over uninduced U1 control cells at 72 h, which was maintained through 96 h p.i. (Fig. 6B). The specificity of the GEMSA was demonstrated by competition for radioactive κB oligonucleotide binding with a 40-fold molar excess of an unlabeled κB oligonucleotide (Fig. 6B, lane 1). In addition, the NF-κB–DNA complex was supershifted with polyclonal anti-p65 and anti-p50 antibodies but not with an anti-I-κB antibody (Fig. 6B, lanes 2, 3, and 4). These data demonstrate that increases in NF-κB expression in the early stages of HIV-1 infection (24 h p.i.) in nontransduced U1 cells are the result of active replication of the virus. However, in the PKR-transduced U1 clone, the later appearance of high levels of active NF-κB (72 h p.i.), in tandem with the near-total absence of HIV-1 replication, indicates that the HIV-1 LTR-PKR cDNA construct is not increasing the expression of HIV-1.
Inhibition of TNF-α-induced replication of HIV-1 in the PKR-transduced U1 clone treated with SQV.
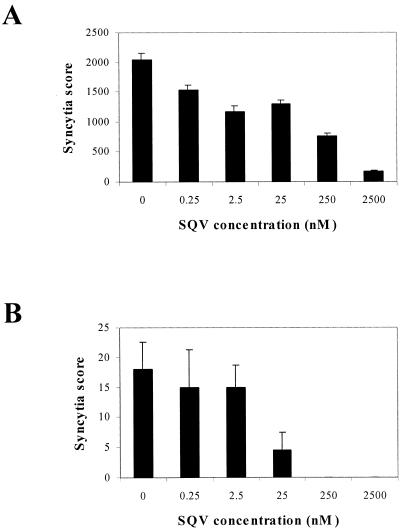
In view of the 99% inhibition by the overexpressed PKR, it was necessary to determine if the remaining 1% HIV-1 expression in the TNF-α-treated U1/106-4:27 clone was a unique variant of HIV-1 or, alternatively, whether this 1% remaining HIV-1 could be inhibited by the approved HIV-1 reverse transcriptase (RT) and/or protease inhibitors. Consistent with published reports, the RT inhibitor zidovudine did not inhibit HIV-1 replication in latently infected U1 cells (data not shown) because the HIV-1 RNA genome is already reverse transcribed and integrated into the U1 cellular genome (46). The HIV-1 protease inhibitor SQV (250 nM) inhibited HIV-1 replication in the U1 controls by 63%, whereas SQV (250 nM) completely inhibited the replication of reactivated HIV-1 in the PKR-transduced clone (Fig. 7). Similar inhibitions of HIV-1 p24 antigen expression were observed in the presence of SQV at 250 nM (data not shown).
FIG. 7.
TNF-α-induced replication of HIV-1 in U1 and PKR-transduced U1 cells treated with SQV. U1 control (A) and U1/106-4:27 (B) cells were incubated with the indicated concentrations of SQV 1 h prior to treatment with TNF-α (50 ng/ml). Syncytia were scored in triplicate at multiple dilutions at 96 h p.i. A single syncytium score was calculated as described in Materials and Methods.
DISCUSSION
In this report, reactivation of latent HIV-1 has been studied in vitro with the U1 cell line. U1, an HIV-1 latently infected cell line derived from the well-characterized promonocytic cell line U937, harbors two integrated provirus copies in which HIV-1 is expressed by TNF-α or tetradecanoyl phorbol acetate (8, 25, 46). It has been reported elsewhere that, in U1 cells, a mutation in the tat gene is involved in postintegration latency (20). In this study, we provide direct evidence that the overexpression of human PKR in U1 cells inhibits HIV-1 replication. This evidence was obtained via the strategy of intracellular immunization. Previous antiretroviral gene therapy approaches in which foreign gene products were introduced into the cell have suffered from the potential rejection of gene-modified cells by host immune surveillance. The retrovirus-mediated transduction of HIV-1 LTR-PKR cDNA constructs described here is an advance over other antiretroviral systems described to date. The HIV-1 LTR-controlled IFN-α2 system relies on the expression of IFN-α, which leads to the induction of many other genes with a myriad of consequences (5). Unlike IFN-α, PKR requires the presence of dsRNA for its activation, thereby adding a second requirement for establishment of an antiviral state. Thus, our approach may ultimately provide an efficient therapeutic strategy to inhibit reactivation of HIV-1 in latently infected cells. This technique ensures that PKR will be transcriptionally silent in the absence of HIV-1 LTR-induced expression and will not activate host immune surveillance, i.e., development of cytotoxic T-cell responses.
Reactivation of HIV-1 in latently infected U1 cells is inhibited by 99% in the HIV-1 LTR-driven PKR cDNA-transduced U1/106-4:27 clone, without cytotoxicity (Fig. 2 and 3). In this clone, following TNF-α induction of HIV-1, we have demonstrated that the overexpression of PKR occurred concomitantly with increases of eIF-2α phosphorylation, NF-κB nuclear expression, and NF-κB–DNA binding activity (Fig. 4, 5, and 6). The experiments reported here were conducted for 96 h, on the basis of the observation that maximal eIF-2α phosphorylation occurred at 72 h p.i. and subsequently decreased at 96 h p.i. (Fig. 5). Similarly, the maximum expression of the nuclear transcription factor NF-κB was at 72 h with a marked decrease at 96 h (Fig. 6). The role of NF-κB in this system must be complex in that there is competition for transcription of the HIV-1 genome, normal cellular genes, and the HIV-1 LTR-driven PKR cDNA. The inhibition of replication of reactivated HIV-1 by the overexpression of PKR resulted in the inhibition of HIV-1 protein synthesis and, therefore, the decrease in HIV-1 infectious particles. These results support the report that, in cells which express PKR, virus replication is inhibited and infection of neighboring cells is prevented (54). Finally, an examination of the effect of the HIV-1 protease inhibitor SQV on TNF-α-induced HIV-1 replication in the PKR-transduced U1 clone demonstrated a 100% inhibition of syncytium formation at 250 nM SQV, compared to a 63% inhibition in the U1 control cells (Fig. 7). In the control U1 cells, a 10-fold-higher SQV concentration (2,500 nM) was insufficient for complete inhibition (92%). In a related study from this laboratory, we reported a 90% inhibition of HIV-1 replication in SupT1 cells transduced with HIV-1 LTR-PKR cDNA constructs. The addition of 250 nM zidovudine completely inhibited the replication of the remaining HIV-1 (2).
Current anti-HIV-1 strategies which include HIV-1 RT inhibitors (nucleoside analogs) and protease inhibitors have had limited success as evidenced by the evolution of resistant retroviral strains and the inability to suppress HIV-1 replication following maintenance regimens after an initial response to combination antiretroviral therapy (29, 45). Several laboratories have reported the existence of reservoirs (sanctuaries) which contain either latent HIV-1 in resting cells, slowly replicating HIV-1, hidden HIV-1 virions, HIV-1 in compartments that are not accessible to anti-HIV-1 drugs, or latently infected memory CD4+ cells that carry integrated provirus (11, 14, 15, 17, 21, 24). Recent estimates of the decay rates of reservoirs of resting CD4+ T cells infected with HIV-1 indicate that an individual would need to receive HAART for over 60 years before eradication of the virus would occur (23). Further, while these reservoirs are not comprised of HIV-1 drug-resistant variants, the possibility of their reactivation poses a problem at the time of termination of treatment of HIV-1-infected individuals (29, 45, 58, 59). Latently infected cells have been isolated from antiretroviral therapy-naive patients, as well as those receiving HAART (11, 14, 15, 24, 58, 59). Because of the predominance of these HIV-1 reservoirs in the microenvironment of the lymphoid tissue, they are subject to activation by a variety of endogenous cytokines (12). Thus, the discontinuation of HAART may allow for the reactivation of HIV-1 and reestablishment of productive HIV-1 infection. Recent studies have shown that induction of latent reservoirs to a productive HIV-1 replicative state with a combination of proinflammatory and immunoregulatory cytokines allows these reservoirs to be targeted and effectively removed with HAART (12, 13). However, the potential disadvantages of this approach cannot be overlooked. The success of this therapeutic strategy is contingent upon the removal of HAART, to allow the reactivation of HIV-1 in the latent compartments to take place. Allowing the reestablishment of productive HIV-1 infection may have serious consequences, in that it does not consider other compartments of latent infection, such as macrophages or dendritic cells (11). Furthermore, the use of cytokines (e.g., interleukin-2) can have toxic effects and therefore may not be amenable to an in vivo therapy regimen (9, 18).
Regardless of the mechanism of reactivation of latent HIV-1 (i.e., by long-range transmission from infected T cells or by proximal activation, transmission, and interaction with an antigen-presenting cell), latently infected cells play an essential role in sustaining HIV-1 infection, even if they do not contribute substantially to viral load (27). We present here an alternative approach to the inhibition of HIV-1 replication, namely, prevention of the reactivation of latently infected cells by the strategy of intracellular immunization. The studies with latently infected U1 cells described are in agreement with a previous report from our laboratory in which PKR is overexpressed in SupT1 cells transduced with HIV-1 LTR-PKR cDNA constructs (2). We have also reported that transduction of an HIV-1 LTR-driven cDNA construct containing the natural antiviral gene product 2-5A synthetase into the chromosome of SupT1 cells effectively inhibits HIV-1 replication (30, 31). The complete inhibition of the replication of HIV-1 in the U1/106-4:27 clone by the combination of overexpressed PKR and a low-dose antiretroviral compound (SQV) is especially significant. The strategy of intracellular immunization coupled with decreased dosages (and associated toxicities) of approved antiretroviral drugs may have great potential in achieving complete inhibition of HIV-1 in latent reservoirs, as well as reducing the risk of generating viral escape mutants.
ACKNOWLEDGMENTS
We thank E. E. Henderson (Temple University School of Medicine) for assistance in the handling of HIV-1 latently infected cell lines, B. Suh (Temple University Hospital) for providing the HIV-1-infected human sera, J.-J. Chen (Harvard University-MIT) for the mouse monoclonal anti-eIF-2α antibody, W. C. Merrick (Case Western Reserve University) and S. R. Kimball (Pennsylvania State University College of Medicine) for the purified eIF-2α, D. P. Bednarik (Human Genome Sciences) for providing the pLTRα2IFN vector, and A. Bank (Columbia University) for the GP+envAm12 retroviral producer cell line. Finally, we are grateful to Kathryn T. Iacono, Nancy L. Reichenbach, Joseph W. Homan, and Susan E. Horvath for technical assistance and advice.
This work was supported by United States Public Health Service grant R01-AI34765 (awarded to R.J.S.), federal Work Study awards (N.F.M.), and Daniel Swern Memorial fellowships (awarded to N.F.M. and M.E.A.).
REFERENCES
- 1.Adelson M E. Ph.D. thesis. Philadelphia, Pa: Temple University; 1997. [Google Scholar]
- 2.Adelson, M. E., C. Martinand-Mari, K. T. Iacono, N. F. Muto, and R. J. Suhadolnik. Inhibition of human immunodeficiency virus (HIV-1) replication in SupT1 cells transduced with an HIV-1 LTR-driven PKR cDNA construct. Eur. J. Biochem., in press. [DOI] [PubMed]
- 3.Bachelerie J, Alcami J, Arenzana-Seisdedos F, Virelizier J-L. HIV enhancer activity perpetuated by NF-κB induction on infection of monocytes. Nature. 1991;350:709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- 4.Baltimore D. Intracellular immunization. Nature. 1988;335:7395–7396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- 5.Bednarik D P, Mosca J D, Raj N B, Pitha P M. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated α2-interferon. Proc Natl Acad Sci USA. 1989;86:4958–4962. doi: 10.1073/pnas.86.13.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benkirane M, Neuveut C, Chun R F, Smith S M, Samuel C E, Gatignol A, Jeang K-T. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 1997;16:611–624. doi: 10.1093/emboj/16.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand S R, Kobayashi R, Mathews M B. The tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem. 1997;272:8388–8395. doi: 10.1074/jbc.272.13.8388. [DOI] [PubMed] [Google Scholar]
- 8.Butera S T, Folks T M. Application of latent HIV-1 infected cellular models to therapeutic intervention. AIDS Res Hum Retroviruses. 1992;8:991–995. doi: 10.1089/aid.1992.8.991. [DOI] [PubMed] [Google Scholar]
- 9.Carr A, Emery W, Lloyd A, Hoy J, Garsia R, French M, Stewart G, Fyfe G, Cooper D A. Outpatient continuous intravenous interleukin-2 or subcutaneous, polyethylene glycol-modified interleukin-2 in human immunodeficiency virus-infected patients: a randomized, controlled, multicenter study. J Infect Dis. 1998;178:992–999. doi: 10.1086/515653. [DOI] [PubMed] [Google Scholar]
- 10.Cheshire J L, Williams B R G, Baldwin A S., Jr Involvement of double-stranded RNA-activated protein kinase in the synergistic activation of nuclear factor-κB by tumor necrosis factor-α and γ-interferon in preneuronal cells. J Biol Chem. 1999;274:4801–4806. doi: 10.1074/jbc.274.8.4801. [DOI] [PubMed] [Google Scholar]
- 11.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;187:183–187. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 12.Chun T-W, Engel D, Mizell S B, Ehler L A, Fauci A S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun T-W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 14.Chun T W, Finzi D, Margolick J, Chadwirk K, Schwartz D, Siliciano R F. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 15.Chun T-W, Stuyver L, Mizell S B, Ehler L A, Mican J A M, Baseler M, Lloyd A L, Mowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 17.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 18.Davey R T, Jr, Chaitt D G, Piscitelli S C, Wells M, Kovacs J A, Walker R E, Falloon J, Polis M A, Metcalf J A, Masur H, Fyfe G, Lane H C. Subcutaneous administration of interleukin-2 in human immunodeficiency virus type-1-infected persons. J Infect Dis. 1997;175:781–799. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]
- 19.DeVita V T Jr, Hellman S, Rosenberg S A, editors. AIDS: etiology, diagnosis, treatment and prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven; 1997. [Google Scholar]
- 20.Emiliani S, Fischle W, Ott M, VanLint C, Amella C A, Verdin E. Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the U1 cell line. J Virol. 1998;72:1666–1670. doi: 10.1128/jvi.72.2.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci, A. S. 1998. 35th Annual Meeting, Infectious Diseases Society of America, Denver, Colo., 12 to 15 November 1998.
- 22.Fauci A S, Schnittman S M, Poli G, Koenig S, Panteleo G. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991;114:678–698. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- 23.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Kisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 24.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 25.Folks T M, Justement J, Kinter A, Cinarello A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 26.Gervaix A, Schwarz L, Law P, Ho A D, Looney D, Lane T, Wong-Staal F. Gene therapy targeting peripheral blood CD34+ hematopoietic stem cells of HIV-1 infected individuals. Hum Gene Ther. 1997;8:2229–2238. doi: 10.1089/hum.1997.8.18-2229. [DOI] [PubMed] [Google Scholar]
- 27.Grossman Z, Feinberg M B, Paul W E. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci USA. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutterman J U. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91:1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havlir D V, Marschner I C, Hirsch M S, Collier A C, Tebas P, Bassett R L, Ioannidis J P A, Holohan M K, Leavitt R, Boone G, Richman D D. Maintenance antiretroviral therapies in HIV-infected subjects with undetectable plasma HIV RNA after triple-drug therapy. N Engl J Med. 1998;339:1261–1268. doi: 10.1056/NEJM199810293391801. [DOI] [PubMed] [Google Scholar]
- 30.Iacono K T, Adelson M E, Suhadolnik R J. Inhibition of HIV-1 replication through the over-expression of the 40 kDa 2′,5′-oligoadenylate synthetase. J Interferon Cytokine Res. 1997;17(Suppl. 2):S104. [Google Scholar]
- 31.Iacono K T, Adelson M E, Suhadolnik R J. Abstracts of the Keystone Symposia on Molecular & Cellular Biology, AIDS Pathogenesis, Keystone, Colo., 7 to 14 January 1999. 1999. Inhibition of HIV-1 replication through the retroviral delivery of the 40 kDa 2′,5′-oligoadenylate synthetase antiviral enzyme, abstr. 416; p. 126. [Google Scholar]
- 32.Judeware R, Li J, Petryshyn R. Inhibition of the dsRNA-dependent protein kinase by a peptide derived from the human immunodeficiency virus type 1 tat protein. J Interferon Res. 1993;13:153–160. doi: 10.1089/jir.1993.13.153. [DOI] [PubMed] [Google Scholar]
- 33.Katze M. The war against the interferon-induced dsRNA-activated protein kinase: can viruses win? J Interferon Res. 1992;12:241–248. doi: 10.1089/jir.1992.12.241. [DOI] [PubMed] [Google Scholar]
- 34.Katze M G. Games viruses play: a strategic initiative against the interferon-induced dsRNA activated 68,000 Mr protein kinase. Semin Virol. 1993;4:259–268. [Google Scholar]
- 35.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Yang Y-L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maran A, Maitra R K, Kumar A, Dong B, Xiao W, Ki G, Williams B R G, Torrence P F, Silverman R H. Blockage of NF-κB signaling by selective ablation of an mRNA target by 2-5A antisense chimeras. Science. 1994;265:789–792. doi: 10.1126/science.7914032. [DOI] [PubMed] [Google Scholar]
- 38.Mathews M B, Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurides P A, Akkaraju G R, Jagus R. Evaluation of protein phosphorylation state by a combination of vertical slab gel isoelectric focusing and immunoblotting. Anal Biochem. 1989;183:144–151. doi: 10.1016/0003-2697(89)90182-6. [DOI] [PubMed] [Google Scholar]
- 40.McCune J M. Viral latency in HIV disease. Cell. 1995;82:183–188. doi: 10.1016/0092-8674(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 41.McMillan N A J, Chun R F, Siderovski D P, Galabru J, Toone W M, Samuel C E, Mak T K, Hovanessian A G, Jeang K-T, Williams B R G. HIV-1 tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology. 1995;213:413–424. doi: 10.1006/viro.1995.0014. [DOI] [PubMed] [Google Scholar]
- 42.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 43.Nabel G J, Baltimore D. An inducible transcription factor that activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 44.Offermann M K, Zimring J, Mellits K H, Hagan M K, Shaw R, Medford R M, Mathews M B, Goodbourn S, Jagus R. Activation of the double-stranded-RNA-activated protein kinase and induction of vascular cell adhesion molecule-1 by poly(I)-poly(C) in endothelial cells. Eur J Biochem. 1995;232:28–36. doi: 10.1111/j.1432-1033.1995.tb20777.x. [DOI] [PubMed] [Google Scholar]
- 45.Pialoux G, Raffi F, Brun-Vezinet F, Meiffredy V, Flandre P, Gastaut J A, Dellamonica P, Yeni P, Delfraissy J F, Aboulker J P. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. N Engl J Med. 1998;339:1269–1276. doi: 10.1056/NEJM199810293391802. [DOI] [PubMed] [Google Scholar]
- 46.Poli G, Orenstein J M, Kinter A, Folks T M, Fauci A S. Interferon-α but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- 47.Roy S, Agy M, Hovanessian A G, Sonenberg N, Katze M G. The integrity of the stem structure of human immunodeficiency virus type 1 Tat-responsive sequence RNA is required for interaction with the interferon-induced 68,000-Mr protein kinase. J Virol. 1991;65:632–640. doi: 10.1128/jvi.65.2.632-640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy S, Katze M, Parkin N, Edery I, Hovanessian A, Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990;247:1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Samuel C E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603–7606. [PubMed] [Google Scholar]
- 51.Savinova O, Jagus R. Use of vertical slab isoelectric focusing and immunoblotting to evaluate steady-state phosphorylation of eIF2α in cultured cells. Methods. 1997;11:419–425. doi: 10.1006/meth.1996.0438. [DOI] [PubMed] [Google Scholar]
- 52.Schreiber E, Matthais P, Muller M M, Schattner W. Rapid detection of octamer binding proteins with “mini-extracts” prepared from a small number of cells. Nucleic Acids Res. 1989;11:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark G H, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 54.Tan S-L, Katze M G. The emerging role of the interferon-induced PKR protein kinase as an apoptotic effector: a new face of death? J Interferon Cytokine Res. 1999;19:543–554. doi: 10.1089/107999099313677. [DOI] [PubMed] [Google Scholar]
- 55.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 56.Thomis D C, Samuel C E. Mechanism of interferon action: characterization of the intermolecular autophosphorylation of PKR, the interferon-inducible, RNA-dependent protein kinase. J Virol. 1995;69:5195–5198. doi: 10.1128/jvi.69.8.5195-5198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wathelet M G, Clauss I M, Paillard F C, Huez G A. 2-Aminopurine selectively blocks the transcriptional activation of cellular genes by virus, double-stranded RNA and interferons in human cells. Eur J Biochem. 1989;184:503–509. doi: 10.1111/j.1432-1033.1989.tb15043.x. [DOI] [PubMed] [Google Scholar]
- 58.Wong J K, Günthard H F, Havlir D V, Zhang Z, Haase A T, Ignacio C C, Kwok S, Emini E, Richman D D. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci USA. 1997;94:12574–12579. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 60.Wong K K, Jr, Chatterjee S. Controlling herpes simplex virus infections: is intracellular immunization the way of the future? Curr Top Microbiol Immunol. 1992;179:159–174. doi: 10.1007/978-3-642-77247-4_10. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y L, Reis L F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissmann C M. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]