Abstract
Repotrectinib, licensed in November 2023, is a novel ROS1 tyrosine kinase inhibitor (TKI) with activity against G2032R, the most common resistance mutation to prior generations of ROS1 TKIs. Here, we report a case of a patient who was heavily pretreated, with advanced L1951R and L2026M mutated ROS1-rearranged NSCLC, who initially responded to repotrectinib but later developed further on-target resistance with the emergence of an L2086F mutation. The disease then responded to cabozantinib, a separate class of ROS1 TKI with preclinical activity against L2086F.
Keywords: ROS1, L2086F, Cabozantinib, Repotrectinib, Case report
Introduction
Chromosomal rearrangements involving the ROS1 receptor tyrosine kinase gene are seen in 1% to 2% of NSCLC.1 Crizotinib and entrectinib are licensed ROS1-directed tyrosine kinase inhibitors (TKIs) associated with excellent and durable responses to therapy.1 A major challenge in the treatment of ROS1-rearranged NSCLC with ROS1 TKIs is the emergence of on-target resistance. Repotrectinib, a novel ROS1 TKI with activity against G2032R and several other crizotinib- and entrectinib-associated ROS1 resistance mutations, was licensed by the Food and Drug Administration on November 15, 2023.2
Case
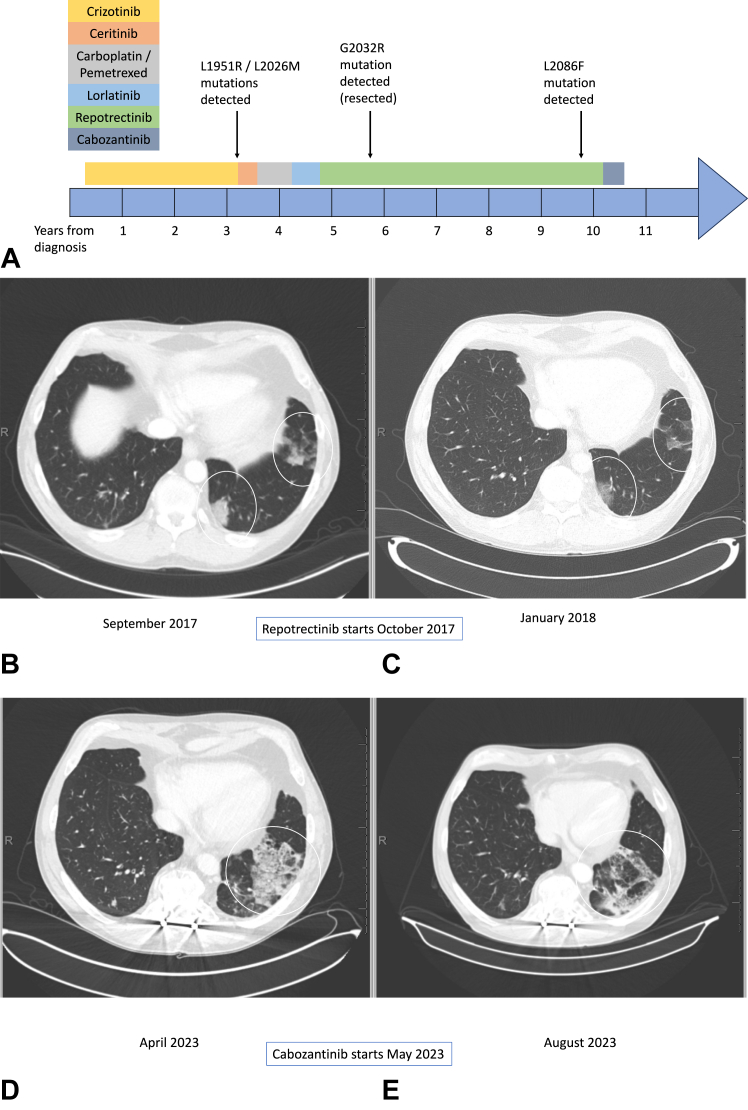
A 48-year-old White male patient, never smoker, presented with cough and shortness of breath. Computed tomography (CT) of the chest found dominant and persistent nodularity in the left lower lobe and smaller nodules in all other lobes. A positron emission tomography/CT found heterogeneous fluorodeoxyglucose activity throughout the left lower lobe. There was no abnormal fluorodeoxyglucose activity elsewhere. Magnetic resonance imaging of the brain did not find any evidence of metastatic disease. The patient underwent a left lower lobe lobectomy that revealed a 6-cm moderately differentiated adenocarcinoma with a mixed papillary and lepidic pattern. Fluorescence in situ hybridization revealed a ROS1 rearrangement. None of the sampled lymph nodes were involved. Owing to the presence of small nodules throughout the lungs, the patient was presumed to have stage IV disease and began receiving crizotinib 250 mg twice daily. His drug treatment timeline is presented in detail in Figure 1A.
Figure 1.
(A) Timeline of patinet's treatment and molecular analyses; (B and C). CT scans during repotrectinib (see text for details); (D and E). CT scans during cabozantinib (see text for details). CT, computed tomography.
The patient achieved a complete radiographic response with crizotinib. After 17 months on crizotinib, a nodule in the left upper lobe began enlarging. Over the next 16 months, he slowly developed additional new nodules throughout the lungs. A wedge resection of the right lung was performed to acquire tissue for next-generation sequencing (NGS), which found L1951R and L2026M resistance mutations in trans.3 Ceritinib found in vitro activity against a cell line generated from this patient, so he was transitioned to off-label ceritinib at 750 mg daily (dose later reduced to 600 mg). His best response over the next four months was stable disease. He next received four cycles of carboplatin and pemetrexed, followed by pemetrexed maintenance, and his disease remained stable for another six months. Treatment with off-label lorlatinib at 75 mg daily was then tried but produced no response and was poorly tolerated.
After eight months on lorlatinib with continued progression of disease, the patient entered a clinical trial of repotrectinib.2 He received repotrectinib at 160 mg (daily for 14 days, then twice daily) and initially had a response to therapy (Fig. 1B and C). After 12 months on repotrectinib, the patient developed progression of disease in the left upper lobe. NGS analyses after wedge resection of the area of oligo-progression reported a previously undetected G2032R resistance mutation but no additional changes. The patient’s remaining disease stayed stable on repotrectinib for another 24 months before he developed another enlarging nodule in the right upper lobe. This area of oligo-progression was treated with stereotactic body radiation therapy. After a total of 36 months on repotrectinib, multifocal progression of disease throughout the chest occurred. NGS on tissue from a biopsy of an area of progression within the right upper lobe revealed a new L2086F mutation with no evidence of the prior ROS1 mutations. Repotrectinib was then stopped, and cabozantinib 40 mg daily was initiated. The patient initially struggled to tolerate this treatment, requiring a brief hold and dose reduction, but was successfully re-escalated to the 40-mg daily dose after a month. The first CT scan on cabozantinib, performed three months after initiating treatment, found a partial response (Fig. 1D and E). This response has been maintained for seven months to date.
Discussion
This is a case of a patient who was heavily pretreated, with advanced ROS1-rearranged NSCLC harboring L1951R and L2026M resistance mutations. He initially responded to repotrectinib. An area of progression harboring a G2032R mutation was resected. Repotrectinib has a reported 59% response rate in G2032R positive cases.2 Nevertheless, it was also noted in five of 43 patients with disease progression who had previously received at least one ROS1 TKI in the registrational TRIDENT-1 repotrectinib trial.2 Whether in such cases G2032R-positive clones arose before repotrectenib and are then driven through an unknown additional repotrectinib resistance mechanism, or whether G2032R alone can still sometimes drive resistance remains not known. In our case, the G2032 mutation was not seen again on testing on tissue from other sites, suggesting that the clone was fully removed by surgery. A clone harboring an L2086F mutation arose later in treatment. It responded to treatment with cabozantinib. In a recent study, characterizing resistance mechanisms to crizotinib and lorlatinib in patients with ROS1-rearranged NSCLC, L2086F was the second most common resistance mutation detected.4 In their in vitro model, the L2086F mutation conferred resistance not just to lorlatinib but also to crizotinib, entrectinib, ceritinib, taletrectinib, and repotrectinib. A clinical response to cabozantinib has been reported in a patient with ROS1-rearranged NSCLC who developed L2086F and F2004V mutations on lorlatinib.5 To our knowledge, this is the first report of the emergence of an L2086F mutation on repotrectinib. We hope that this case will inform TKI treatment for ROS1-rearranged NSCLC.
Conclusion
There seems to be a role for cabozantinib in patients who develop an L2086F mutation on repotrectinib or other ROS1 TKIs. L2086F mutations should be prioritized for new drug development given their potential to be a problematic mechanism of resistance for most available ROS1 TKIs.
CRediT Authorship Contribution Statement
Urs Weber: Conceptualization, Data curation, Formal analysis, Writing - original draft; Writing - review & editing.
Kurtis D. Davies: Conceptualization, Data curation, Formal analysis, Writing - original draft; Writing - review & editing.
D. Ross Camidge: Conceptualization, Data curation, Formal analysis, Writing - original draft; Writing - review & editing.
Disclosure
Dr. Ross Camidge reports ad hoc advisory boards/consultations from Abbvie, Amgen, Anheart, Anchiarno, Appolomics, AstraZeneca/Daiichi, Beigene, Dizal, Bio-Thera, Blueprint, BMS, Eisai, Elevation, Eli Lilly, EMD Serono, GSK, Helsinn, Hengrui, Hummingbird, Janssen, Kestrel, Medtronic, Mersana, Mirati, Nalo Therapeutics, Nuvalent, Onkure, Pfizer, Puma, Qilu, Regeneron, Ribon, Roche, Sanofi, Seattle Genetics, Takeda, Theseus, Turning Point, and Xcovery; research funding from Inivata; company-sponsored trials at institution (Principal Investigator roles) from Abbvie, AstraZeneca, Blueprint, Dizal, Inhibrx, Karyopharm, Nuvalent, Pfizer, Phosplatin, Psioxus, Rain, Roche/Genentech, Seattle Genetics, Takeda, Turning Point, and Verastem. The remaining authors declare no conflict of interest.
Acknowledgments
The work of Dr. Ross Camidge was partially supported by the Joyce Zeff Chair in Lung Cancer Research and by the Thoracic Oncology Research Initiative at the University of Colorado Cancer Center. The patient provided informed consent for this case report to be written.
Footnotes
Cite this article as: Weber U, Davies DD, Ross Camidge D. L2086F mutant ROS1-rearranged NSCLC resistant to repotrectinib responds to cabozantinib: a case report. JTO Clin Res Rep 2024;5:100673
References
- 1.Patil T., Simons E., Mushtaq R., Pacheco J.M., Doebele R.C., Bowles D.W. Targeted therapies for ROS1-rearranged non-small cell lung cancer. Drugs Today (Barc) 2019;55:641–652. doi: 10.1358/dot.2019.55.10.3030646. [DOI] [PubMed] [Google Scholar]
- 2.Drilon A., Camidge D.R., Lin J.J., et al. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390:118–131. doi: 10.1056/NEJMoa2302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCoach C.E., Le A.T., Gowan K., et al. Resistance mechanisms to targeted therapies in ROS1+ and ALK+ non-small cell lung cancer. Clin Cancer Res. 2018;24:3334–3347. doi: 10.1158/1078-0432.CCR-17-2452. Epub 2018 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J.J., Choudhury N.J., Yoda S., et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in ROS1 fusion-positive lung cancer. Clin Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto M., Patil T. Exceptional response to lorlatinib and cabozantinib in ROS1-rearranged NSCLC with acquired F2004V and L2086F resistance. NPJ Precis Oncol. 2023;7:56. doi: 10.1038/s41698-023-00381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]