Abstract
Joubert Syndrome, manifests in a spectrum of neurological symptoms. This case describes a 7-year-old girl with perinatal complications, and subsequent neurodevelopmental challenges. An MRI confirmed the diagnosis of Joubert syndrome, with the distinctive “molar tooth sign” being a key imaging characteristic. Approximately 25% of cases exhibit nephronophthisis, impacting kidney function, further complicating the clinical picture. Diagnosis relies on imaging and management necessitates a multidisciplinary approach, addressing symptoms and complications, with prognosis linked to the presence of organic disease. The case emphasizes the significance of a multidisciplinary strategy, including genetic counseling, and underscores the diverse manifestations of this syndrome. Prenatal identification through ultrasound and MRI plays a crucial role in diagnosing and treating this rare condition.
Keywords: Joubert syndrome, Vermis, Cerebellum, Cerebellar vermis hypoplasia
Introduction
Joubert Syndrome (JS) is a clinically and genetically heterogeneous group of disorders characterized by hypoplasia of the cerebellar vermis [1], presenting with the characteristic radiologic “molar sign” and accompanying neurological symptoms [2]. These symptoms include dysregulation of respiratory patterns and developmental delays. The estimated incidence is 1 in 100,000 live births [3]. Specific genetic mutations causing this condition are more common in certain ethnic groups, such as Ashkenazi Jewish, French-Canadian, and Hutterite populations, displaying an autosomal recessive pattern and, more rarely, an X-linked pattern. More than 30 genes have been involved in the functions of cellular structures called primary cilia, with greater impact at the neuronal, renal, hepatic, auditory, and olfactory levels [4].
Clinical manifestations may include low muscle tone (hypotonia) in infancy, contributing to difficulty in coordinating movements (ataxia) in early childhood. Other characteristic features of the condition include episodes of unusually rapid (hyperpnea) or slow (apnea) breathing in infancy and abnormal eye movements (ocular motor apraxia). Most affected individuals experience developmental delays and intellectual disability, ranging from mild to severe [4]. The condition is sometimes associated with other ocular abnormalities (such as retinal dystrophy, leading to vision loss, and coloboma, a gap or division in an eye structure), kidney disease (including polycystic kidney disease and nephronophthisis), liver disease, and skeletal anomalies [5].
Case report
A 7-year-old girl presented to the emergency department with: asthenia, adynamia, pale skin, loss of appetite, unquantified fever, odynophagia, and otalgia. Initial management comprised oral antibiotics, yet with no discernible improvement. Subsequently, a 3-day course of amikacin was administered, coupled with paracetamol-naproxen for an additional period, as part of the ongoing medical intervention.
As significant past medical history she had a perinatal period marked by the absence of spontaneous breathing and crying within the first minute, necessitating positive pressure ventilation for 2 cycles. Following this, she underwent a 15-day hospitalization without advanced airway management, which subsequently impacted her neurodevelopment. Notably, she achieved a sitting position at 12 months but exhibited limited communication characterized by bisyllabic utterances.
Laboratory tests 15 days after onset showed some signs of dehydration and low blood count (Table 1). She was then referred from a Basic Community Hospital to a second-level hospital due to dehydration. Initial laboratory findings at the second hospital revealed an acute kidney injury (Table 2). Moreover, a venous blood gas analysis revealed metabolic acidosis. In response, Nephrology recommended a renal ultrasound; however, due to acute criteria, peritoneal dialysis was promptly initiated, involving the successful placement of a Tenckhoff catheter. After the surgical intervention, laboratory values returned to normal.
Table 1.
Laboratory test results with positive findings.
| Parameter | Result | Units |
|---|---|---|
| Hemoglobin (Hb) | 4.8 | g/dL |
| Hematocrit (hct) | 15.2 | % |
| White Blood Cells | 14,200 | µ/L |
| Segmented | 75 | % |
| Lymphocytes | 14 | % |
| Platelets | 283,000 | µ/L |
Table 2.
Laboratory test results confirming acute kidney injury.
| Parameter | Result | Units |
|---|---|---|
| Creatinine | 10.9 | mg/dL |
| Blood urea nitrogen (BUN) | 166.2 | mg/dL |
| Urea | 355.7 | mg/dL |
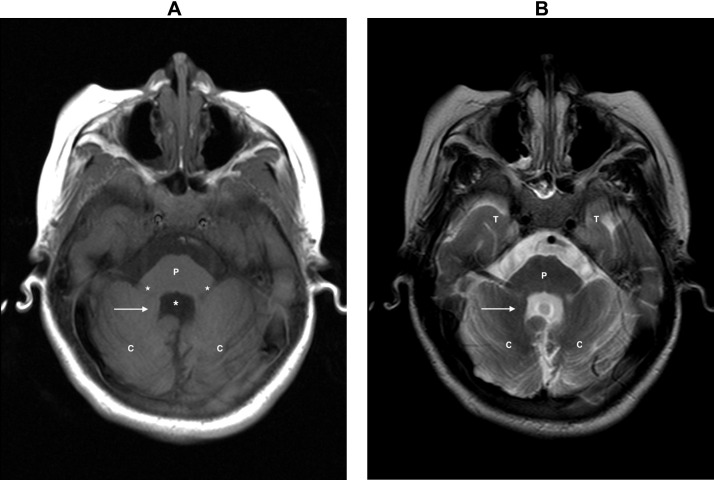
Upon a follow-up neurological assessment conducted several days postsurgery; generalized hypotonia in all 4 limbs, along with +/++ stretch reflexes, was identified. Furthermore, the limbs exhibited hypotrophy with no other evident abnormalities. Consequently, an MRI study was requested due to the patient's acute deterioration which revealed hypoplasia of the cerebellar vermis (Fig. 1), accompanied by an apparent diminished size of the fourth ventricle. Notably, the superior cerebellar peduncles exhibit a pronounced horizontalization and prominent thickened elongated superior cerebellar peduncles, presenting a distinct visual form recognized as the “molar tooth sign” better observed in axial imaging in the midbrain (Fig. 2). Additionally, the fourth ventricle appears compressed, resulting in the characteristic resemblance of a “bad wing” (Fig. 3). And associated hypoplasia of the cerebellar vermis (Fig. 4). Considering these imaging characteristics, the diagnosis of Joubert syndrome was confirmed.
Fig. 1.
Sagittal T1- weighted image. Hypoplasia of the vermis. The fourth ventricle is enlarged and deformed with a wider communication with the cisterna magna (white arrow). With elongated cerebellar peduncles, thickened and horizontally directed superior cerebellar peduncles are seen between the midbrain (M), pons (P), and cerebellum (Cb).
Fig. 2.
(A) Axial T1- weighted image. Prominent superior cerebellar peduncles and the deep interpeduncular fossa (white arrow), the hypoplasia of the vermis is seen (white dotted arrow). Midbrain (M), temporal lobe (T). (B) Axial T2- weighted image with the typical appearance of the molar tooth sign: elongated superior cerebellar peduncles (white arrow) and a deep midline cleft at the pontomesencephalic junction (yellow arrow).
Fig. 3.
Axial T1-weighted image at the level of the posterior fossa. Apposed cerebellar (C) hemispheres (white arrow) with the typical fourth ventricle appearance reminiscent of a bat with wings (white asterisk), with its anterior portion made by the cerebellar peduncles (white star) and the pons (P).
Fig. 4.
Coronal T2-weighted image. Demonstrates the enlarged superior cerebellar peduncles (black arrow) and widening of the fourth ventricle (black star) seen because of the hypoplastic vermis (black dot), between the cerebellum (C). Lateral ventricles (V) and corpus callosum are seen without alteration.
Discussion
Joubert syndrome (JS) was originally described by Marie Joubert in an article detailing the clinical and radiological findings seen in a family with multiple affected members. In this paper, the most important radiological finding during pneumoencephalography was the identification of vermis agenesis (partial or complete), which accounts for the genetic relationship observed in 4 siblings [1]. Nowadays, it's known to be a rare disorder that occurs in approximately 1 in 80,000 to 100,000 live births. It is characterized by neurodevelopmental abnormalities, identifying it as a rare autosomal recessive disorder [3] and is part of a group of pathologies classified as congenital ciliary disorders. A group of genetic diseases caused by abnormal formation or function of the cilia of primary cells [5]. To date, more than 35 disease-causing genes have been identified, of which only one is linked to the X chromosome. Together, these genes are responsible for 62%-94% of cases [4,6].
Clinically, it is characterized by neurological alterations, such as hypotonia, ataxia, and developmental delay, almost always associated with intellectual disability and alterations in the respiratory pattern. This can manifest as alternating episodes of small apnea/hyperpnea, worsening with emotional stress, and usually resolving around the sixth month of life. However, some cases may progress to prolonged apnea attacks requiring ventilatory assistance. Eye movement abnormalities are also common, with oculomotor apraxia and primary position nystagmus being typical presentations. Around 25% of cases present with nephronophthisis, characterized by tubule-interstitial structural alterations involving an irregular and thickened basement membrane of the tubular epithelium. This condition can lead to progressive interstitial fibrosis, resulting in chronic kidney disease or remain asymptomatic for several years. There is a childhood variant thar occurs in the first years of life, characterized by a faster and more aggressive course. Other manifestations include delays in the development of language and motor skills, varying in severity. Our patient exhibits a range of these symptoms. Additionally, a wide range of malformations of the central nervous system has been described, which can impact the development and prognosis of the patients [[4], [5], [6]].
Diagnosis is based on clinical and radiological data. In some cases, a genetic mutation associated with the syndrome is detected [5]. The radiological manifestations usually present the classic “molar tooth sign” (Fig. 5) due to the lack of decussation of the fibers of the superior cerebellar peduncle, which leads to their elongation and horizontalization in their course, together with an enlargement of the interpeduncular cistern. The Shepherd's crook sign becomes evident when obtaining sagittal MRI, CT, and ultrasound images in the posterior cavity. The curvature of the crook is defined by the brainstem, while the arch is shaped by the long, thickened, and unusually oriented superior cerebellar peduncles, followed by the cerebellar hemispheres [6]. Others have highlighted supplementary features, including the enlargement and deformation of the fourth ventricle, which displayed a wing-like structure reminiscent of a bat's wings (Fig. 5) [2,7]. Several additional significant anomalies include hypo/aplasia of the cerebellar vermis and constriction at the pontomesencephalic junction, cortical dysplasias, polymicrogyria, and encephalocele [6].
Fig. 5.
(A and B) Axial T1-weighted image. Shows a “molar tooth” appearance in an enlarge view with its representative schematic. (C and D) axial T1-weighted image showing the characteristic “hadwing” appearance in a zoomed view with its representative schematic.
Management of the condition focuses on treating symptoms and requires a collaborative, multifaceted approach. This usually involves physical therapy, adaptation of educational strategies to cognitive and behavioral aspects, and prevention of complications and associated symptoms [5,6]. The prognosis is determined by the Joubert syndrome and the prevalence of organic disease. In our case, the patient exhibited certain kidney anomalies that require further evaluation, along with developmental delays. Given the 25% risk of recurrence in somatic recessive forms, genetic counseling is crucial. Prenatal identification can be performed by ultrasound at 11–12 weeks of gestation, and anatomical assessment of the cerebellum can be performed using MRI at 20–22 weeks of gestation [3].
Conclusion
In summary, Joubert syndrome is a rare congenital ciliary disorder. It presents with neurodevelopmental abnormalities, eye movement issues, and nephronophthisis in 25% of cases. Diagnosis relies on the “molar tooth sign” and “batwing” sign on imaging, with over 35 identified genes. Comprehensive management addresses symptoms and complications, with prognosis tied to its type and organic disease presence. Our case underscores kidney anomalies and developmental delays, urging further evaluation. Genetic counseling is crucial due to the 25% recurrence risk in autosomal recessive forms. Prenatal identification through ultrasound and MRI aids proactive management. JS requires a multidisciplinary approach, integrating clinical, radiological, and genetic insights but multimodal imaging remains vital for a definitive diagnosis.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used [ChatGPT] in order to [Enhance the syntax and coherence of ideas.]. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Patient consent
I confirm that written, informed consent for the publication of this case report has been obtained from the patient(s) or their legal representative(s). This statement will be included in the article. The actual consent documents will be retained in our records and will not be submitted as part of this publication process.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19(9):813–825. doi: 10.1212/wnl.19.9.813. [DOI] [PubMed] [Google Scholar]
- 2.McGraw P. The molar tooth sign. Radiology. 2003;229(3):671–672. doi: 10.1148/radiol.2293020764. [DOI] [PubMed] [Google Scholar]
- 3.Brancati F, Dallapiccola B, Valente EM. Joubert Syndrome and related disorders. Orphanet J Rare Dis. 2010;5:20. doi: 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parisi M, Glass I. Joubert syndrome. 2003 Jul 9 [Updated 2017 Jun 29]. In: Adam MP, Feldman J, Mirzaa GM, et al., GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1325/. [PubMed]
- 5.Brancati F, Dallapiccola B, Valente E. Joubert syndrome and related disorders. Orphanet J Rare Dis. 2010;5(1):20. doi: 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15(5):511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- 7.Manley AT, Maertens PM. The Shepherd's Crook sign: a new neuroimaging pareidolia in Joubert syndrome. J Neuroimaging. 2015;25(3):510–512. doi: 10.1111/jon.12159. [DOI] [PubMed] [Google Scholar]