Abstract
Background
The PREVENT (Predicting Risk of cardiovascular disease EVENTs risk algorithm was developed to better reflect the impact of metabolic factors on cardiovascular risk.
Objectives
The purpose of this study was to compare the relative performance of PREVENT with standard comparator algorithms (Framingham risk score, pooled cohort equation, SCORE2 [Systematic COronary Risk Evaluation2]) for risk stratification emphasizing the implications of weighing chronic kidney disease.
Methods
A simulated cohort was created of males and females aged 40 to 75 years with and without other traditional risk factors and either normal estimated glomerular filtration rates (eGFR 90 or 60 ml/min/1.73 m2) or abnormal eGFR (45 or 30 ml/min/1.73 m2). The concordance and reclassification rates were calculated for each category of risk with emphasis on subjects characterized as moderate risk by the standard comparator algorithms.
Results
PREVENT demonstrated increased risk with progressive decreases in eGFR. When the standard comparator algorithms identified moderate risk, PREVENT was concordant in 6% to 88% of simulations. In simulations with normal eGFR, PREVENT identified a lower risk in 18% to 88% and a higher risk in 0% to 12% of simulations. Conversely, with abnormal eGFR, PREVENT identified lower risk in 0% to 26% and higher risk in 4% to 94% of simulations.
Conclusions
PREVENT substantially reclassifies risk and has the potential to alter prevention practice patterns. The tendency to assign a lower risk compared to standard algorithms when eGFR is normal may diminish implementation of preventive therapy. National health care systems need to monitor whether such changes improve overall public health.
Key words: estimated glomerular filtration rate, Framingham risk score, pooled cohort equation, PREVENT algorithm, risk algorithm, Systematic Coronary Risk Evaluation
Central Illustration
Most lipid guidelines recommend some form of algorithmic risk stratification to assist in counseling patients regarding the need to address risk factors, particularly lipid risk factors, for the primary prevention of atherosclerotic cardiovascular disease (ASCVD). The introduction of the pooled cohort equation (PCE) in the United States in 2013 initially created controversy due to the perception that the new approach would encourage statin therapy in a much larger number of patients than identified by prior methods.1 The potential impact of this controversy on Canadian guidelines was assessed through modeling of relative performance of the Framingham risk score (FRS) and the new PCE. Contrary to the circumstances facing the practitioner in the United States, a switch from FRS to PCE in Canada would have markedly diminished the number of candidates for statin therapy.2 To avoid such drastic changes in practice patterns, FRS was retained then and even now as the recommended algorithm to use when risk calculation is indicated in Canada.3,4 Conversely, the adoption of the new SCORE2 (Systematic COronary Risk Evaluation2 algorithm in Europe on the premise of augmented relevance and tailored calibration to diverse geographical regions was shown to profoundly diminish the identification of potential candidates for lipid management than endorsed by prior European guidelines, especially women.5
The PREVENT (Predicting Risk of cardiovascular disease EVENTs) risk algorithm has recently been proposed to replace PCE on the premise of better calibration to the U.S. population and better integration of cardiometabolic risk imparted by chronic kidney disease (CKD) and diabetes mellitus (DM).6 Implicit in this is the possibility that identification of patients for statin therapy and perhaps for targeted use of other metabolic agents such as sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and nonsteroidal mineralocorticoid antagonists will be based on the new algorithm. The purpose of this investigation was to compare the relative performance of the PREVENT algorithm to FRS, PCE (both for White and Black subjects), and SCORE2 Moderate Risk Region algorithm used in many European countries (Iceland, Portugal, Sweden, Italy, San Marino, Ireland, Cyprus, Finland, Austria, Malta, Greece, Germany, and Slovenia).7,8 Since only PREVENT allows weighing of CKD in the calculation, we focus on this cardiometabolic risk factor and demonstrate potential practice implications of adoption of PREVENT in international settings.
Methods
We have previously modeled the relative performance of diverse algorithms using simulated patient cohorts representing permutations of sex, age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking, diabetes, and race.2,9 For this analysis, we also simulated cohorts with estimated glomerular filtration rates (eGFR) of 90, 60, 45, and 30 mL/min/1.73 m2 and weighed these categories as indicated in the published PREVENT algorithm.6 Age was 40, 45, 50, 55, 60, 65, 70, or 75 years. Total cholesterol was 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, or 7.0 mmol/L. High-density lipoprotein cholesterol was 0.6, 1.0, 1.4, 1.8, or 2.2 mmol/L. Systolic blood pressure was 100, 110, 120, 130, 140, 150, 160, or 170 mm Hg and either treated or not. Smoking and diabetes were considered present or not. PREVENT accommodates whether or not patients are treated with statins but for this analysis we assumed absence of statin therapy. We did not consider family history of premature ASCVD as a quantitatively defined risk factor. Based on these features, we simulated 20,480 unique cases for each of the 4 categories of eGFR. FRS was applied as previously described.1,3 For PCE, we also modeled White and Black race.1 For SCORE2, we used the algorithms specific to subjects either <50 or ≥50 years of age and calibrated for moderate risk regions of Europe.7,8 Using each algorithm, we calculated % risk/10 years. Furthermore, because each algorithm considers a somewhat different set of events that leads to systematic differences in the numerical value of % risk/10 years, the designations of low, moderate, or high risk were identified based on the risk thresholds specific to each algorithm.1,4,8
Statistical analysis
The primary focus was the percentage of cases that would be assigned a concordant or different risk category by PREVENT as compared to the comparator algorithms calculated. A kappa statistic was calculated to reflect this translation to clinical decision-making. The % risks/10 years were correlated using the “least squares” method (Microsoft Excel 2016) to calculate a straight line that best fit the data after forcing the line to cross at the origin (0, 0% risk/10 years).
Ethics approval was not required for this exercise based on mathematical modeling.
Results
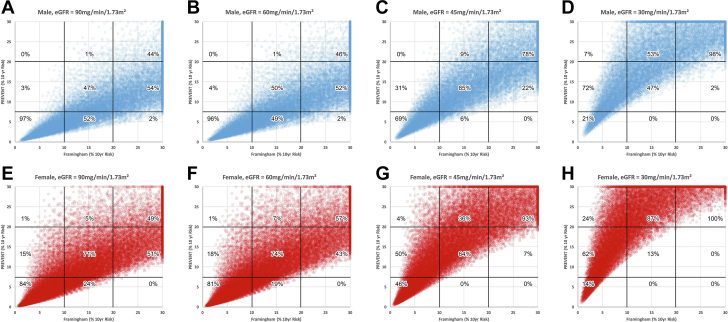
Figure 1 shows plots of the FRS (x-axis) and the PREVENT risk score (y-axis) as well as the thresholds used to define low, moderate, or high risk. Superimposed percentages indicate the percent of simulations that are concordant or discordant within each category of low, moderate, or high risk as defined by FRS. In males (Figures 1A to 1D) and females (Figures 1E to 1H), progressive increase in the slope relationship is evident as the risk imparted by progressive CKD is reflected by the PREVENT algorithm. As noted in Figure 1A, 52% of males considered to have moderate risk using the FRS would be considered to have low risk by the PREVENT algorithm. In Figure 1E, 24% of females considered to have moderate risk by the FRS would be considered to have low risk by the PREVENT algorithm.
Figure 1.
FRS Compared With PREVENT
Comparison of risk categorization by the Framingham risk score and the PREVENT (Predicting Risk of Cardiovascular Disease EVENTs risk algorithm. (A to D) Simulated populations of males with eGFR of 90, 60, 45, or 30 ml/min/1.73 m2, respectively, while (E to H) are for females. Vertical and horizontal lines show the thresholds used to define %10-year risk as either low, moderate, or high. Percentages reflect the proportions within each risk category defined by the current standard (the FRS) that would be concordantly classified or reclassified to a lower/higher risk category by PREVENT. The percentages total 100% when added vertically for each risk category in each panel. eGFR = estimated glomerular filtration rate; FRS = Framingham risk score.
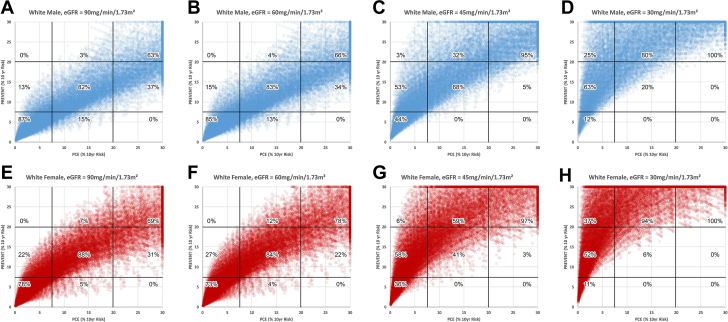
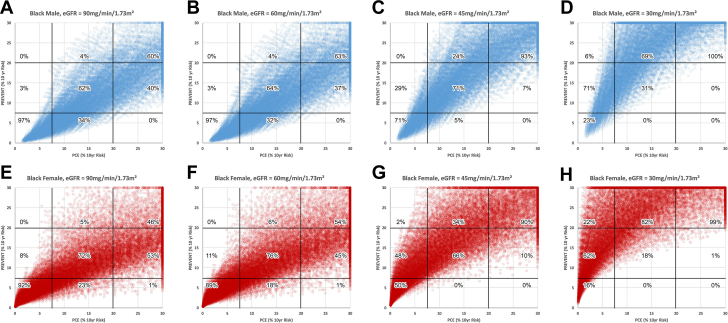
Figures 2 and 3 use the same format as Figure 1 to compare PREVENT to PCE in White subjects and Black subjects, respectively. In White subjects with eGFR of either 90 or 60 ml/min/1.73 m2, 13 to 15% of males (Figures 2A and 2B), and 22 to 27% of females (Figures 2E and 2F) would be reclassified from low to moderate risk using PREVENT. Conversely, 34 to 37% of males and 22 to 31% of females would be reclassified from high risk to moderate risk. When eGFR is either 45 or 30 ml/min/1.73 m2 (Figures 2C, 2D, 2G and 2H) reclassification to a higher level of risk occurs in the majority of subjects in both males and females whereas reclassification to a lower risk level was rare (0-5%). In Black subjects with eGFR of either 90 or 60 ml/min/1.73 m2, 3% of Black males (Figures 3A and 3B), and 8 to 11% of Black females would be reclassified from low to moderate risk using PREVENT. Conversely, 37 to 40% of Black males and 45 to 53% of Black females would be reclassified from high to moderate risk. When eGFR is either 45 or 30 ml/min/1.73 m2 (Figures 3C, 3D, 3G, and 3H) reclassification to a higher level of risk occurs in the majority of Black subjects in both males and females whereas reclassification to a lower risk level was uncommon (0-10%).
Figure 2.
PCE (White Subjects) Compared With PREVENT
Comparison of risk categorization by the pooled cohort equation (PCE) as applied to white subjects and the PREVENT risk algorithm. Format as in Figure 1.
Figure 3.
PCE (Black Subjects) Compared With PREVENT
Comparison of risk categorization by the pooled cohort equation (PCE) as applied to black subjects and the PREVENT risk algorithm. Format as in Figure 1.
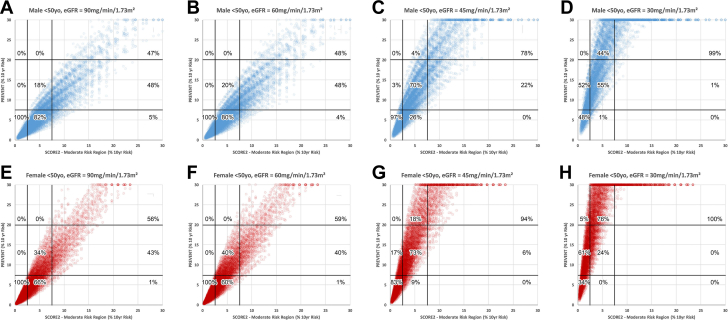
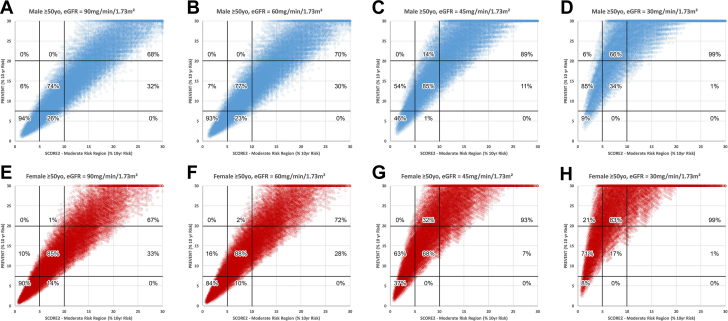
Figure 4 shows comparison of PREVENT and SCORE2 moderate risk region for simulations of subjects <50 years of age. With eGFR of either 90 or 60 ml/min/m2, there is nearly complete concordance in categorizing low risk using either algorithm and in both sexes. When eGFR is either 45 or 30 ml/min/1.73 m2, the PREVENT algorithm downgrades moderate risk to low risk in 1% to 26% of males and 0 to 9% of females, respectively. Similar trends for subjects ≥50 years of age are shown in Figure 5. The supplementary table shows the detailed results of linear correlations between the PREVENT algorithm and all comparator algorithms.
Figure 4.
SCORE2 (Subjects <50 Years Old) Compared With PREVENT
Comparison of risk categorization by the SCORE2 algorithm in subjects <50 years old and the PREVENT risk algorithm. Format as in Figure 1.
Figure 5.
SCORE2 (Subjects ≥50 Years Old) Compared With PREVENT
Comparison of risk categorization by the systematic coronary risk evaluation2 (SCORE2) Algorithm in subjects ≥50 years old and the PREVENT risk algorithm. Format as in Figure 1.
Table 1 provides a summary focusing on subjects identified as moderate risk by FRS, PCE, or SCORE2 which would be subject to reclassification to either a higher or lower risk category by the PREVENT algorithm. PREVENT reclassified FRS-determined moderate risk male patients to a lower risk in 49% to 52% of cases with eGFR of 90 or 60 ml/min/1.73 m2 and to a higher risk in 1% of cases. At an eGFR of 45 or 30 ml/min/1.73 m2, reclassification to a high-risk category using PREVENT occurrs in 9% and 53% of cases, respectively. Similar trends are seen with respect to females. Comparing PCE (white)-determined moderate risk with PREVENT, in both males and females there is modest reclassification to a low risk when eGFR is either 90 or 60 ml/min/1.732 (4%-15% of simulations) but substantial reclassification to high risk in 32% to 94% of simulations when the eGFR is either 45 or 30 ml/min/m2, with the largest effects noted in females. Comparing SCORE2-determined moderate risk in subjects <50 years of age with PREVENT, reclassification to low risk occurs in 60% to 82% of simulations, predominantly in males, when the eGFR is 90 or 60 ml/min/1.73 m2. When eGFR is either 45 or 30 ml/min/1.73 m2, reclassification to low risk is less common (0%-26%) and reclassification to high risk is more common (4%-76%), particularly in females. The Central Illustration summarizes the results of Table 1. The supplementary table provides the kappa statistical analyses of concordance at all risk levels between the PREVENT algorithm and all the comparator algorithms.
Table 1.
Reclassification of Moderate Level of Risk as Determined by Comparator Algorithms
| Moderate Level of Risk by Comparator Algorithms | Sex | eGFR (ml/min/1.73 m2) | Relative Performance of PREVENT Algorithm |
||
|---|---|---|---|---|---|
| % With Concordant Moderate Risk | % Reclassified to Low Risk | % Reclassified to High Risk | |||
| FRS | Male | 90 | 47 | 52 | 1 |
| 60 | 50 | 49 | 1 | ||
| 45 | 85 | 6 | 9 | ||
| 30 | 47 | 0 | 53 | ||
| Female | 90 | 71 | 24 | 5 | |
| 60 | 74 | 19 | 7 | ||
| 45 | 64 | 0 | 36 | ||
| 30 | 13 | 0 | 87 | ||
| PCE (White) | Male | 90 | 82 | 15 | 3 |
| 60 | 83 | 13 | 4 | ||
| 45 | 68 | 0 | 32 | ||
| 30 | 20 | 0 | 80 | ||
| Female | 90 | 88 | 5 | 7 | |
| 60 | 84 | 4 | 12 | ||
| 45 | 41 | 0 | 59 | ||
| 30 | 6 | 0 | 94 | ||
| PCE (Black) | Male | 90 | 62 | 34 | 4 |
| 60 | 64 | 32 | 4 | ||
| 45 | 71 | 5 | 24 | ||
| 30 | 31 | 0 | 69 | ||
| Female | 90 | 72 | 23 | 5 | |
| 60 | 76 | 18 | 6 | ||
| 45 | 66 | 0 | 34 | ||
| 30 | 18 | 0 | 82 | ||
| SCORE2 moderate risk region, age <50 y | Male | 90 | 18 | 82 | 0 |
| 60 | 20 | 80 | 0 | ||
| 45 | 70 | 26 | 4 | ||
| 30 | 55 | 1 | 44 | ||
| Female | 90 | 34 | 66 | 0 | |
| 60 | 40 | 60 | 0 | ||
| 45 | 73 | 9 | 18 | ||
| 30 | 24 | 0 | 76 | ||
| SCORE2 moderate risk region, age ≥50 y | Male | 90 | 74 | 26 | 0 |
| 60 | 77 | 23 | 0 | ||
| 45 | 85 | 1 | 14 | ||
| 30 | 34 | 0 | 66 | ||
| Female | 90 | 85 | 14 | 1 | |
| 60 | 88 | 10 | 2 | ||
| 45 | 68 | 0 | 32 | ||
| 30 | 17 | 0 | 83 | ||
eGFR = estimated glomerular filtration rate; FRS = Framingham risk score; PCE = pooled cohort equation; PREVENT = Predicting Risk of cardiovascular disease EVENTs algorithm; SCORE2 = Systematic COronary Risk Evaluation2.
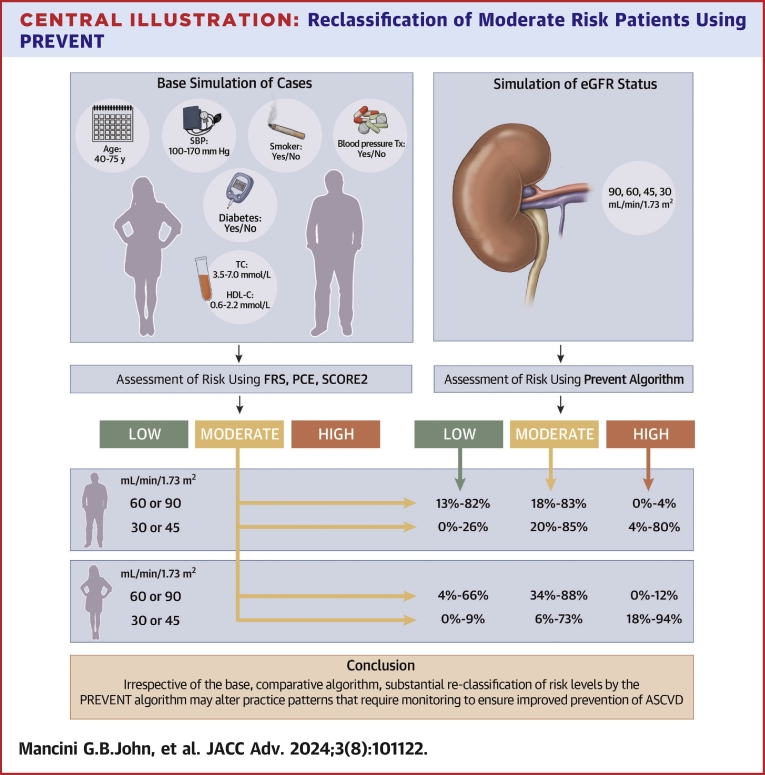
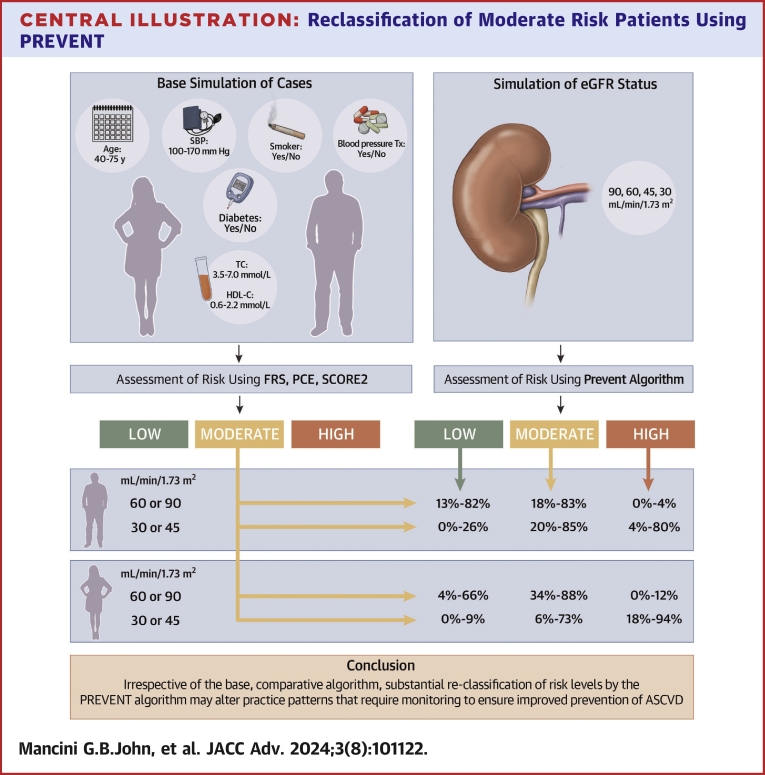
Central Illustration.
Reclassification of Moderate Risk Patients Using PREVENT
Male and female cases were simulated on the basis of age, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), systolic blood pressure (SBP), blood pressure treatment (Tx), smoking, and diabetes. Risk was calculated using the standard comparator algorithms (Framingham risk score [FRS], pooled cohort equation [PCE], Systematic Coronary Risk Evaluation2 [SCORE2]) for stratification to low-, moderate-, or high-risk categories. The PREVENT algorithm was also used to calculate risk assuming estimated glomerular filtration rate (eGFR) of 90, 60, 45, and 30 mL/min/1.73 m2. The concordance and discordance rates are summarized for males and females having relatively normal eGFR and for frankly abnormal eGFR with substantial reclassification irrespective of the standard algorithm investigated. In general, there is a tendency for prevent to reclassify to lower risk in the absence of abnormal eGFR and to reclassify modestly or substantially to higher risk with frankly abnormal eGFR. ASCVD = atherosclerotic cardiovascular disease; PREVENT = Predicting Risk of cardiovascular disease EVENTs.
Discussion
This modeling experiment provides a basis for speculating rationally about the theoretical impact of the adoption of PREVENT in North America and in parts of Europe, specifically with respect to risk factor management in the setting of CKD.
The implications for adoption of PREVENT in Canada are complicated by the structure of the guidelines that give a high degree of weight to the results of specific randomized clinical trials. For example, based on the CARDS (Collaborative Atorvastatin Diabetes Study)10 and the SHARP (Study of Heart and Renal Protection),11 statins are endorsed for most patients with DM and for patients with nondialysis and nontransplant CKD already. Accordingly, in spite of algorithmic risk stratification, the simulations represented in Figures 1C, 1D, 1G, and 1F would all be considered statin-indicated patients in Canada. From this perspective, adoption of the PREVENT approach would markedly change the current pattern of practice modeled upon SHARP and would theoretically result in fewer patients being counseled to use lipid-lowering therapy. Even in the absence of low eGFR, Figures 1A, 1B, 1E, and 1F demonstrate that 52% of males and 24% of women currently felt to have moderate risk would be reclassified as low risk using the PREVENT algorithm. Whether such changes in Canada are endorsed remains to be deliberated upon through the guideline development process of the Canadian Cardiovascular Society. In the past, however, there has been reluctance to dramatically alter well-established practice patterns, particularly if that were to result in fewer candidates for primary prevention.2
In the United States, adoption of PREVENT will have the intended goal of giving weight to the cardiovascular risk imparted by CKD and DM. Specifically based on modeling of degrees of CKD in this article, PREVENT should indeed improve identification of higher risk subjects warranting lipid and other primary prevention interventions. However, in the absence of either moderate or severe degrees of CKD, the algorithm tends to reclassify to a lower risk category and may lead to less aggressive risk factor management in primary prevention subjects without CKD as compared to reliance on the PCE approach. Between 68% and 73% of White males and between 38% and 50% of White females and even higher percentages of Black males and females currently considered to have high risk by PCE and with normal renal function or an eGFR of 60 mL/min/1.73 m2 would be considered to have only moderate risk using the PREVENT algorithm. The impact of this downgrading on actual implementation of preventive therapies warrants monitoring once PREVENT is officially incorporated into new U.S. guidelines.
We demonstrate that the PREVENT algorithm would dramatically alter risk allocation in parts of Europe currently using the SCORE2 Moderate Risk Region algorithm. In general, both algorithms tend to identify low-risk categories similarly but PREVENT reclassifies many moderate risk simulations to low risk and many high risk simulations to moderate risk. A previous study has already highlighted that the SCORE2 approach has potentially decreased the number of candidates for statin therapy, particularly women.5 Therefore, the degree of recalibration and weighting of risk factors in the new PREVENT algorithm is not likely translatable to the European context.
The greatest potential influence of the PREVENT algorithm is in those situations currently considered to represent moderate risk. Table 1 summarizes these potential changes. The largest magnitude lower risk reclassification from moderate risk is occurring when PREVENT is compared with SCORE2 among those with normal eGFR (82%), whereas the largest magnitude change for higher risk reclassification from moderate risk is when PREVENT is compared with the PCE for those with abnormal eGFR, particularly White females (94%). The former may be viewed as contrary to prevention goals whereas the latter may be viewed as an improvement in prevention goals in women. It must be recognized, however, that such conclusions are largely from the perspective of initiation of statin therapy. If PREVENT was adopted to determine therapy for other cardiometabolic drugs such as sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and nonsteroidal mineralocorticoid antagonists, which currently are indicated in the patient phenotypes studied in randomized clinical trials and without risk calculation, eligibility for therapy might be reduced, fostering therapy for only higher risk subjects. This, in fact, was one of the main reasons for originally invoking risk calculations when statins were not generic.
Study limitations
This study is limited by the fact that it is a modeling experiment, it does not take into account clustering of risk factors and predicted categories of risk are not compared to outcomes in a real-world cohort of patients followed for at least 10 years. Nevertheless, the theoretical, relative performances for risk stratification of the different risk algorithms are clearly demonstrable using this modeling approach and allow for rational speculation of changes in practice that may ensue wherever PREVENT is adopted. The study does not explicitly demonstrate the way in which PREVENT weighs diabetes but since all the comparator algorithms provide an option for weighing diabetes, we chose to focus on the more unique, eGFR aspect of PREVENT for which data required for modeling are publicly available. Another limitation, due to both complexity and redundancy, is that we did not evaluate models using SCORE2 for low-, high-, and very high-risk regions as applied, respectively, in subjects <50 years old and ≥50 years old. We felt that a primary focus on SCORE2 for Moderate-Risk Regions would provide sufficient context and would suffice to demonstrate the potential implications of using PREVENT in parts of Europe.
Conclusions
Development of risk algorithms that are relevant to contemporary patients and with appropriate calibration and discriminatory power is a laudable and welcome achievement. But these new approaches have the potential for profound influence on practice patterns that should not be assumed to be beneficial to overall public health, especially with respect to translation of the benefits of lipid lowering, and possibly other cardiometabolic drugs, for reduction of ASCVD. Monitoring of the changes in practice patterns that may emerge after new algorithms are introduced into guidelines should be considered an important responsibility of national health care systems to ensure continued improvement in primary prevention.
Perspectives.
COMPETENCY IN PATIENT CARE: Primary prevention is currently heavily influenced by risk calculations using guideline-sanctioned algorithms that the practitioner must understand and implement appropriately. However, these algorithms are currently different internationally and subject to abrupt change with guideline updates.
TRANSLATIONAL OUTLOOK: Implementation of PREVENT has the potential for markedly altering risk stratification and existing practice patterns. These changes cannot automatically be assumed to improve primary prevention for ASCVD, even for patients with CKD. The real-world effects of the adoption of PREVENT require direct monitoring by national health authorities to ensure overall improvement in public health.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Mancini G.B., Ryomoto A. Comparison of cardiovascular risk assessment algorithms to determine eligibility for statin therapy: implications for practice in Canada. Can J Cardiol. 2014;30:661–666. doi: 10.1016/j.cjca.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 3.D'Agostino RB Sr., Vasan R.S., Pencina M.J., et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 4.Pearson G.J., Thanassoulis G., Anderson T.J., et al. 2021 Canadian cardiovascular Society guidelines for the management of Dyslipidemia for the prevention of cardiovascular disease in Adults. Can J Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen M.B., Tybjaerg-Hansen A., Nordestgaard B.G. Statin eligibility for primary prevention of cardiovascular disease According to 2021 European prevention guidelines compared with other international guidelines. JAMA Cardiol. 2022;7:836–843. doi: 10.1001/jamacardio.2022.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan S.S., Matsushita K., Sang Y., et al. Development and Validation of the American Heart Association's PREVENT equations. Circulation. 2024;149:430–449. doi: 10.1161/CIRCULATIONAHA.123.067626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCORE2 Working Group, ESC. Cardiovascular Risk Collaboration SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Mancini G.B.J., Ryomoto A., Yeoh E., Brunham L.R., Hegele R.A. Recommendations for statin management in primary prevention: disparities among international risk scores. Eur Heart J. 2023;45(2):117–128. doi: 10.1093/eurheartj/ehad539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colhoun H.M., Betteridge D.J., Durrington P.N., et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 11.Baigent C., Landray M.J., Reith C., et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.